Abstract
In order to develop an effective and economical method for removing U(VI) from the low concentration radioactive wastewater with the U(VI) concentration of less than 1 mg L−1, the biomass of Aspergillus niger was prepared and modified with ethylenediamine, and the biosorption of uranium from the low concentration radioactive wastewater by the unmodified and the modified biomasses was investigated in a batch system. The modified biomass exhibited the adsorption efficiency of 99.25 % for uranium under the optimum conditions that pH was 5.0, the contact time was 150 min, and the biosorbent dose was 0.2 g L−1. The adsorption fitted well to Langmuir isotherm, and the maximum sorption capacity of the modified biomass for U(VI) was determined to be 6.789 mg g−1 which increased by 36.45 % compared with the unmodified biomass. The adsorption kinetics was better depicted by pseudo-second-order kinetic model. The Gibbs free energy change (ΔG 0), enthalpy change (ΔH 0), and entropy change (ΔS 0) showed that the process of U(VI) adsorption was spontaneous, endothermic, and feasible. The changes in the groups, morphology, and the presence of U(VI) on the surface of the adsorbents which were characterized by FT-IR, SEM, and EDS, demonstrated that the U(VI) was successfully adsorbed onto the modified biomass. Moreover, the UO2 2+ absorbed on the modified biomass can be released by 0.1 mol L−1 HNO3 with high desorption efficiency of 99.21 %. The results show that the modified biomass can remove U(VI) from low concentration radioactive wastewater more effectively than the unmodified biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Uranium mining and hydrometallurgy usually produce large volumes of low concentration radioactive wastewater with U(VI) concentration of lower than 1 mg L−1 (Xu et al. 2010) which is 20 times the maximum acceptable concentration of uranium for drinking water (GB 23727-2009) stipulated by the standardization technology committee of the national nuclear industry of People’s Republic of China (SAC/TC 58). Such low concentration radioactive wastewater can cause damage to kidney, liver, and bone and even result in cancer (Wang et al. 2010; Kushwaha and Sudhakar 2013), and Canada, USA, and People’s Republic of China stipulate that the maximum acceptable concentrations of uranium for drinking water are 20, 30, and 50 μg L−1, respectively (Wang et al. 2011; Anirudhan et al. 2009). Therefore, the low concentration radioactive wastewater is of concern for public health (Elsabawy et al. 2011) and the effective and economical methods for its treatment should be developed.
The conventional methods such as chemical precipitation, membrane separation, solvent extraction, and ion exchange are both ineffective and uneconomical for removing U(VI) from the low concentration radioactive wastewater (Luo et al. 2006). Although some researchers have studied the biosorption methods (Wang et al. 2010; Li et al. 2004; Bai et al. 2010; Bhat et al. 2008; Ghasemi et al. 2011; Ding et al. 2012; Cheng et al. 2011; Xie et al. 2008; Genç et al. 2003; Akhtar et al. 2007) and other researchers have dealt with the modification methods for biosorbents (Bai et al. 2010; Bayramoğlu et al. 2006; Chen et al. 2014a, b; He et al. 2014; Pillai et al. 2013), the methods they proposed are only for high concentration radioactive wastewater with the U(VI) concentration of much higher than 1 mg L−1.
The objective of this research was to develop an effective and economical biosorption method for removing and recovering U(VI) from the low concentration radioactive wastewater. Since Aspergillus niger has been proved to have many carboxyl and phosphate groups on its surface capable of adsorbing heavy metals and may be an effective and economical biosorbent for heavy metals (Khambhaty et al. 2009; Kapoor and Viraraghavan 1997; Tsekova et al. 2010; Ding et al. 2012), it was selected to accomplish this objective. In this research, the biomass of A. niger was prepared and modified with ethylenediamine; the experiments on adsorbing U(VI) from the low concentration radioactive wastewater were conducted; the adsorption mechanism was analyzed by SEM-EDS and FT-IR; and the experimental data were fitted with equilibrium isotherm, kinetic and the thermodynamic models. Furthermore, experiments on adsorption-desorption cycling were conducted.
2 Materials and Methods
2.1 Preparation of Unmodified Biomass
The A. niger for the present research was isolated from uranium wastewater and identified by Microbial Type Culture Collection (MTCC), Guangdong Institute of Microbiology, People’s Republic of China. The microorganism was inoculated on the potato-dextrose agar (PDA) plates and was cultivated at 30 °C until the plates were covered with spores. The liquid medium was obtained by adding 20 g sucrose into 1 L potato filtrate which was obtained by putting 200 g of potato into 1 L distilled water and boiling for 10 min (Ding et al. 2012). The microbial suspension with OD600 value of approximately 0.1, where OD600 indicates the absorbance, or optical density, of a sample measured at a wavelength of 600 nm, was then prepared with pipette on the sterile console. The inoculated medium was continuously cultured on a rotary shaker at 25 °C and 200 rpm for 3 days. The culture fluid was filtrated for the mycelium, and the mycelium was then washed with sterile water until the filtrate became neutral. After that, the biomass was dried in an oven at 50 °C for 24 h. The completely dried mycelium was ground to powder, and the powder was sieved through a 100 mesh standard test sieve to obtain the unmodified biomass of A. niger for the adsorption experiments.
2.2 Chemical Modification of the Biomass
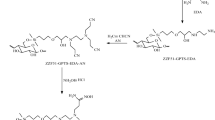
Ten grams of the unmodified biomass was suspended in 200 mL of N,N-dimethylformamide (DMF), heated and stirred continuously in a three-necked flask. Thirty-five milliliters of sulfur dichloride (SOCl2) was added dropwise to the suspension. The temperature of the mixture was kept between 95 and 100 °C by controlling the heating rate carefully after the temperature of the mixture reached 80 °C. Then, the mixture was stirred for another 4 h. With the reaction mixture cooling to room temperature, it was poured into a beaker containing 500 mL ice water and was stirred continuously. The precipitate was obtained by filtering, then washed with distilled water and 3 % ammonium hydroxide successively to remove the residual sulfite remained on the A. niger chloride until the supernatant became neutral. Finally, after the filtered solid was dried at 50 °C for 10 h under vacuum (Mwangi and Ngila 2012), it was ground into powder and stored in a desiccator.
Five grams of the neutralized chlorinated biomass was mixed with 25 mL of ethylenediamine, and the mixture was stirred at 80~85 °C for at least 3 h under reflux conditions. The solution was filtered; the resultant solid was washed three times with distilled water and ethyl alcohol respectively and then dried under vacuum at room temperature for 24 h; and the modified biomass of A. niger was obtained for the adsorption experiments. The general reaction occurs as follows:
2.3 Reagents
The standard stock solution of uranium (1 g L−1) was prepared by putting 1.1792 g U3O8 into a 100-mL beaker, adding 10 mL of hydrochloric acid, 3 mL of hydrogen peroxide, and two drops of nitric acid into the beaker, and dissolving the U3O8 completely under heating on a sand bath. The solution was then diluted with distilled water to 1 L. The standard solution of uranium was prepared by diluting the standard stock solution to 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8 mg L−1. All the reagents were of analytical grade.
2.4 Instrumentation
The concentration of uranium in solution was measured using trace uranium analyzer (WGJ-III, China). The precision pH meter (PB-20 (PB-S), Germany) calibrated by the buffer solutions (pH 4 and 6.86) was used to adjust the pH of the solution. The liquid medium was autoclaved at 121 °C for 20 min in an autoclave (VB-40, Germany). The plates which cultured A. niger were placed in the water-jacket thermostatic constant incubator (GSP-9080MBE, China) at 30 °C for 4 days. The vacuum drying oven (BZF-50, China) was used to dry the modified biomass in the study. The centrifuge (TGL-16H, China) was used to treat the solution from the adsorption experiments. All the distilled water for the experiments was produced on the central distilled water system platform (CN120RDM1-230, UK).
2.5 Adsorption Experiments
In the batch adsorption experiments, the unmodified and the modified biomass, each weighing 0.02 g, were added into the two flasks, respectively, each containing 100 mL of uranium solution, and the two flasks were shaken on a rotary shaker at 25 °C and 200 rpm for 2.5 h unless otherwise stated. After the adsorption reached equilibrium, the solution was separated by centrifugation at 8000 rpm for 5 min, and the concentration of U(VI) in the supernatant was determined by WGJ-III type trace uranium analyzer at λ = 652 nm. In measuring the concentration, special J-22 fluorescence enhancement agent was put into the solution in order that it reacted with the uranyl ions to form a compound with higher fluorescence intensity. The determined metal ion content was used to evaluate the adsorption capacity and efficiency. All the experiments were conducted in triplicate.
The adsorption capacity of the adsorbent was calculated by Eq. (3); the removal efficiency (%) was determined using Eq. (4); and the adsorption distribution coefficient K d (mL g−1) was determined by Eq. (5):
where q (mg g−1) is the adsorption capacity of the adsorbent, C 0 (mg L−1) and C e (mg L−1) represent the initial concentration of U(VI) and the concentration of U(VI) at equilibrium, respectively, R (%) is the removal efficiency, V (L) is the volume of the uranium solution, m (g) is the mass of the adsorbent, and K d (mL g−1) is the adsorption distribution coefficient.
All the data were analyzed using OriginPro8.0 software.
3 Equilibrium and Kinetic Studies
3.1 Equilibrium Isotherm Models
The adsorption equilibrium between the metal ions adsorbed by the adsorbent and metal ions in the solution can be described by the Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) models.
For the Langmuir model, it is hypothesized that all of the adsorption sites on the surface of the adsorbent were uniformly distributed and the adsorption equilibrium was balanced dynamically. Therefore, the surface of the adsorbent was covered with monomolecular layer of solute.
A linear form of the Langmuir isotherm equation is given as follows (Kiran et al. 2005):
where q e (mg g−1) is the amount of the adsorbed metal ions at equilibrium, C e (mg L−1) is the concentration of uranium at equilibrium, q max (mg g−1) is the maximum adsorption capacity, and b (L mg−1) is the Langmuir isotherm coefficient related to the energy of adsorption.
The essential characteristics of the Langmuir isotherm can be described by the separation factor R L , which is defined by the following equation (Bai et al. 2013):
R L indicate that the type of the isotherms is either irreversible (R L = 0), favorable (0 < R L < 1), linear (R L = 1), or unfavorable (R L > 1).
The Freundlich isotherm model is an empirical one for adsorption on irregular surfaces, and its linear equation is given by the following equation (Kiran et al. 2005):
where K F and n are the Freundlich constants for adsorption capacity and adsorption intensity, respectively. The adsorption is favorable when \( 0<\frac{1}{n}<1 \)
The D-R isotherm model, which is used to separate the physical adsorption process from the chemical one, describes a single adsorption of an adsorbent which has the homogeneous pore structure. The linear form of D-R isotherm equation is expressed as follows (Guler and Sarioglu 2013):
where q m (mol g−1) is the amount of single saturated adsorption, β (mol2 J−2) is the activity coefficient related to the adsorption energy, and ε (J mol−1) is related to the concentration of metal ions at equilibrium (\( \varepsilon =RTl\mathrm{n}\left(1+\frac{1}{C_e}\right) \)).
The free energy E (kJ mol−1) for the adsorption is calculated as follows (Bai et al. 2013):
E can indicate that the adsorption process is a physical one (E <8 kJ mol−1) or a chemical one (E >8 kJ mol−1).
3.2 Kinetic Studies
Dynamic models are extensively used to discuss the mechanism of the adsorption and the factors controlling the adsorption rate. In this research, the Lagergren’s pseudo-first-order and pseudo-second-order kinetic models and the intraparticle diffusion model were used to analyze the experimental data.
The Lagergren’s pseudo-first-order kinetic equation is expressed as follows (Yuvaraja et al. 2014; Pillai et al. 2013):
The Lagergren’s pseudo-second-order kinetic equation is expressed as follows (Bai et al. 2012):
The initial adsorption rate equation is expressed as follows:
The intraparticle diffusion equation is expressed as follows (Liu et al. 2013):
where q t (mg g−1), q e (mg g−1), and q e1 (mg g−1) denote the adsorption capacity at time t, the theoretical amount of metal ions adsorbed at equilibrium, and the experimental adsorption capacity, respectively; k 1 (min−1), k 2 (g mg−1 min−1), and k id (g mg−1 min−1) are the rate constants for the Lagergren’s pseudo-first-order, the pseudo-second-order, and the intraparticle diffusion models, respectively.
3.3 Thermodynamic Studies
The thermodynamic parameters including the standard Gibbs free energy change (ΔG°), standard enthalpy change (ΔH°), and standard entropy change (ΔS°) are the basic indicators for determining whether the adsorption reaction is spontaneous and whether the adsorption reaction is endothermic or exothermic one. The three thermodynamic parameters can be calculated respectively as follows (Aslani et al. 2012; Vijayaraghavan and Yun 2008):
where K d represents adsorption distribution coefficient, T denotes the absolute temperature (K), and R is the gas constant (R = 8.314 × 10−3 kJ (mol K)−1).
3.4 Characterization
The functional groups on the surface of the unmodified and the modified biomasses were analyzed and identified qualitatively by Fourier transform infrared spectrometer (IR Prestige-21, Japan). All the infrared spectra were measured over the range 4,000–400 cm−1 region with a resolution of ±4 cm−1. Surface morphology of the unmodified and the modified biomasses was obtained using scanning electron microscope (SEM) (JSM-6360LV, Japan), which is considered as the most reliable tool for the determination of physical structure of biosorbents (Ahmed et al. 2014). The elements on the modified biomass after adsorption treatment were analyzed by EDS spectrum using energy-dispersive spectroscopy (EDX-GENESIS60S, Germany).
3.5 The Elution of Uranium and Regeneration of Biomass
Different desorption solutions such as HCl, HNO3, NaOH, Na2CO3, NaHCO3, and ultrapure water were used to release the uranium absorbed on the modified biomass. The modified biomass samples were mixed with 50-mL regenerating solution in concentration of 0.1 mol L−1 and kept shaking in a rotary shaker at 25 °C and 200 rpm for 2.5 h. The best desorption solution can be determined based on the amount of desorbed uranium calculated in the following formula (Akhtar et al. 2007):
where q d \( {q}_d \)(mg g−1) is the amount of the desorbed U(VI), V (L) is the volume of the desorption solution, and q (mg g−1) is the amount of the adsorbed U(VI).
The adsorption-desorption process was repeated for five cycles in order to study the performance of the regenerated adsorbent.
4 Results and Discussion
4.1 Material Characterization
4.1.1 FT-IR
Figure 1 shows the FT-IR spectra for the unmodified A. niger, modified A. niger, and the modified A. niger after adsorption treatment. Curve 1 shows that a strong band at 3381 cm−1 was dominated by –OH stretching groups in carboxylic and –NH stretching groups (Erkaya et al. 2014). The peaks observed at 2925 and 2854 cm−1 were due to the C–H stretching (Anirudhan et al. 2012), and the peak at 1641 cm−1 was attributed to the C=O stretching in carboxyl groups (Saeed et al. 2005). Curve 2 shows that the modification did not generate great changes in the peaks of the major groups capable of adsorbing U(VI). Compared with curve 1, a new peak at 1053 cm−1 in curve 2 was assigned to the C–N stretching (Deng and Ting 2005). Curve 2 also shows a new characteristic peak at 1548 cm−1, which was ascribed to the angular deformation of the N–H (amine group) of ethylenediamine from the A. niger (Mwangi and Ngila 2012). Curve 3 shows that the intensity of the FT-IR spectrum of the modified biomass loaded with U(VI) was higher than that of the modified biomass unloaded with U(VI), indicating that the groups of –OH, –C=O, and –CONH could interact with U(VI).
4.1.2 SEM
Figure 2a shows the SEM image of the unmodified biomass whose surface was smooth. Figure 2b shows that the surface of the modified biomass became rough; many foam-like structures occurred on the surface of biomass; and the biomass became loose and porous due to the modification. As a result, the specific surface area of the biomass was significantly increased and the groups were uniformly distributed on its surface. As shown in Fig. 2c, after the modified biomass adsorbed U(VI), its surface became more flat. The reason for this may be that the U(VI) ions combined with the groups on the surface of the biomass and the gaps on the surface was then filled with U(VI) (Saeed et al. 2005).
4.1.3 EDS
Figure 3 shows the SEM-EDX images of the modified biomass after adsorption treatment. In order to make the surface of film be conductive, the surface was coated with a layer of gold before the detection. From the analysis of the elemental composition of the sample showed in Fig. 3, it can be seen that the U(VI) adsorbed on the biomass amounted to 27.64 %. This indicated that the modified biomass can absorb U(VI) efficiently.
4.2 Effect of Initial pH
Figure 4 shows the effect of pH on the removal efficiency of U(VI) by the unmodified and the modified biomass. For the unmodified biomass, the removal efficiency of U(VI) increased from 1.65 % at pH 2 to 77.8 % at pH 7, and when the pH was greater than 7, it decreased. For the modified biomass, the removal efficiency of U(VI) increased from 49.51 % at pH 2 to 99.25 % at pH 5, and when the pH was greater than 5, it decreased. The reason for this variation trend may be that the presence status of uranium was changed in the solution, and the active sites were protonated. At low pH, the predominant species of U(VI) were UO2 2+, the concentration of H+ ions was greater, and they competed with UO2 2+ for binding sites. As pH increased, the UO2 2+ shifted to (UO2)2(OH)2 2+, (UO2OH)+, and (UO2)3(OH)5 + (Moghaddam et al. 2013), which combined more easily with adsorption sites than H+, and the biomass carried more negative charges; thus, they could attract more U(VI) ions (Chen et al. 2014a, b). With the further increase of pH, the removal efficiency decreased with the increase of the concentration of the dissolved carbonate which could compete with the adsorption site (Aytas et al. 2011). Therefore, further experiments on the unmodified and the modified biomass were carried out for pH values greater than their optimum pH values.
4.3 Effect of Contact Time
It is obviously shown in Fig. 5 that the removal efficiency of the modified biomass was up to 99.25 % at 150 min and the concentration of U(VI) could rapidly meet the national standard requirements (GB 23727-2009) within 5 min. The adsorption capacities of the unmodified and the modified biomass increased rapidly during the first phase, and they decelerated and reached equilibrium after 120 and 150 min, respectively. It can be concluded that the adsorption during the first phase may be physical adsorption or ion exchange, and the adsorption during the second phase may be complexation, microprecipitation, or saturation of the binding sites (Ronda et al. 2013; Kulkarni et al. 2013).
4.4 Effect of Initial U(VI) Concentration
Although lots of previous research shown in Table 1 has investigated the removal of U(VI) from mining industry wastewater, comparatively little research has studied the adsorption of low concentration of U(VI) for it is more difficult than high levels of U(VI) concentration, and this may due to that the driving force provided by the initial U(VI) concentration could overcome mass transfer resistance in the adsorption process (Kulkarni et al. 2013).
In our study, the effect of different initial U(VI) concentrations varying from 0.2 to 0.8 mg L−1 on the adsorption of U(VI) by the unmodified and the modified biomass is shown in Fig. 6. As shown in Fig. 6, the adsorption capacities of the unmodified and the modified biomass increased from 0.82 to 2.62 mg g−1 and from 0.99 to 3.85 mg g−1 with the increase of the initial U(VI) concentration, respectively. It can also be observed in Fig. 6 that the adsorption capacity of the modified biomass was larger than that of the unmodified biomass at different initial U(VI) concentrations. It should be noted that neither the adsorption capacity of the unmodified biomass nor that of the modified biomass have reached the saturation point. Then, the results could only demonstrate that the U(VI) biosorption capacity of the modified biomass is higher than that of the unmodified biomass in low concentration radioactive wastewater.
4.5 Effect of Adsorbent Dose on Adsorption
As can be seen from Fig. 7, the removal efficiency of U(VI) by the modified biomass increased from 89.7 to 99.25 % when the adsorbent dose increased from 0.05 to 0.2 g L−1, and this was due to the increase of the number of the active sites for adsorbing U(VI) with the increase of the adsorbent dose. The removal efficiency decreased slightly when the dose was larger than 0.2 g L−1, and this could be attributed to the screening effect or partial aggregation on the surface of the biomass (Prodromou and Pashalidis 2013). Although the variation trend of the removal efficiency of U(VI) by the unmodified biomass was almost the same as that by the modified biomass, the maximum removal efficiency by the unmodified biomass was only 78.88 %.
4.6 Adsorption Isotherms
Table 2 lists the adsorption isotherm constants which were deduced from the intercepts and the slopes of the linear plots of 1/q e versus 1/C e (Fig. 8a), lnq e versus lnC e (Fig. 8b), and lnq e versus ε 2 (Fig. 8c), respectively. The results of correlation coefficient exhibited in Table 1 demonstrated the data of both biosorbents fitted the Langmuir isotherm best with a satisfactory correlation coefficient values higher than 0.96. This can be concluded that the adsorption was a monolayer one (Ahmed et al. 2014) and mainly controlled as chemically (Resmi et al. 2012). The maximum U(VI) sorption capacity was 4.314 mg g−1 for unmodified biomass and 6.789 mg g−1 for modified biomass. This phenomenon showed that the U(VI) sorption capacity of modified biomass was relative higher than that of unmodified biomass for the ethylenediamine treatment provided more amine groups and then more sites could be used in U(VI) sorption. The value of b for the modified biomass was also higher than that of unmodified biomass, which implied the ability of bonding uranium for the unmodified biomass was stronger. Since the value of \( {\mathrm{K}}_F \)of the modified biomass was higher than the unmodified biomass, it can be considered that the adsorption capacity of the modified biomass was higher than the unmodified biomass.
The results of Table 2 indicate the adsorption processes were favorable. For both kinds of the biosorbents, although\( E \) were less than 8, their correlation coefficients of Langmuir isotherms were larger than D-R isotherms, which indicated that the adsorption process of U(VI) was given priority to with chemical adsorption accompanied by physical adsorption.
4.7 Kinetic Modeling
versus t 1/2 (Fig. 9c) are summarized in Table 3. As can be seen, the adsorption kinetic data show a very good agreement with the pseudo-second-order model as the correlation coefficients for the two adsorbents were over 0.999. The theoretical values of q e were 1.97 mg g−1 for the unmodified biomass and 2.45 mg g−1 for the modified biomass, and they were very close to the experimental data (q e1,unmodified = 2 mg g−1, q e1,modified = 2.48 mg g−1). Based on these results, it could be concluded that the adsorption process of U(VI) was mainly dominated by chemical adsorption, which could be attributed to ion exchange, chelation, and complex between the adsorbent and the adsorbate (Kushwaha and Sudhakar 2013).
4.8 Thermodynamic Studies
Figure 10 shows the plot of lnK d versus 1/T, and the thermodynamic parameters obtained from the plot are presented in Table 4. It can be seen that all the values of ΔG 0 for the unmodified and the modified biomass were negative, indicating that the adsorption of U(VI) by both the adsorbents were spontaneous. Meanwhile, for both adsorbents, the values of ΔG 0 decreased gradually with the increase of the temperature, indicating that rising temperature was conducive to U(VI) adsorption at high temperature (Gok and Aytas 2009). The positive value of ΔH 0 for the unmodified and the modified biomass were found to be 5.09 and 27.52 kJ mol−1, respectively, indicating that the adsorption processes were endothermic.
The positive value of ΔS 0 suggested that the degree of randomness was increasing at the solid-liquid interface attributed to the liberation of the free water molecules to the solution during the adsorption of U(VI) by both biosorbents (Bai et al. 2013).
4.9 Desorption, Regeneration, and Reuse Studies
HCl (0.1 mol L−1), HNO3, NaOH, Na2CO3, NaHCO3, and ultrapure water were used to conduct experiments on the desorption, and the results were given in Table 5. It can be seen that 0.1 mol L−1 HNO3 was the optimum eluant for the desorption, and the desorption rate of U(VI) reached 99.21 %.
Experiments on five cycles of adsorption-desorption were conducted using 0.1 mol L−1 HNO3, and the results are presented in Fig. 11. In the desorption experiments, the desorption rates maintained between 95.38 and 99.09 %. The results indicated that the modified biomass could be regenerated and reused for extracting U(VI) from low concentration radioactive wastewater.
5 Conclusions
The adsorption experiments showed that under the experimental conditions that the initial U(VI) concentration of the low concentration radioactive wastewater was 0.5 mg L−1, pH was 5, and the dose of the modified biomass of A. niger was 0.2 g L−1, the adsorption arrived at equilibrium in 150 min, the removal efficiency of U(VI) amounted to 99.25 %, and the U(VI) concentration decreased to the level lower than the maximum acceptable concentration of uranium for drinking water. The equilibrium isotherm of the adsorption was found to be in good agreement with Langmuir model, and the maximum adsorption capacity of the modified biomass for U(VI) was calculated to be 6.789 mg g−1. The adsorption kinetics was found to follow the pseudo-second-order model, indicating that the adsorption process of U(VI) was dominated by chemical adsorption between the modified biomass and the U(VI) ions. The thermodynamic studies showed that the adsorption process of U(VI) was feasible, spontaneous, and endothermic. FT-IR analysis was conducted, and the amide and hydroxyl groups on the surface of the modified biomass were found to be responsible for the adsorption of U(VI). The desorption and regeneration studies showed that 0.1 mol L−1 HNO3 was the most effective desorption agent, and the desorption rate was still as high as 95.38 % after five cycles of adsorption and desorption. The results show that the biosorption method based on the ethylenediamine-modified biomass of A. niger was an effective and economical one for treating the low concentration radioactive wastewater.
References
Ahmed, S. H., Sheikh, E. M. E. I., & Morsy, A. M. A. (2014). Potentiality of uranium biosorption from nitric acid solutions using shrimp shells. Journal of Environmental Radioactivity, 134, 120–127.
Akhtar, K., Akhtar, M. W., & Khalid, A. M. (2007). Removal and recovery of uranium from aqueous solutions by Trichoderma harzianum. Water Research, 41(6), 1366–1378.
Anirudhan, T. S., Divya, L., & Suchithra, P. S. (2009). Kinetic and equilibrium characterization of uranium (VI) adsorption onto carboxylate-functionalized poly (hydroxyethylmethacrylate)-grafted lignocellulosics. Journal of Environmental Management, 90(1), 549–560.
Anirudhan, T. S., Jalajamony, S., & Sreekumari, S. S. (2012). Adsorption of heavy metal ions from aqueous solutions by amine and carboxylate functionalized bentonites. Applied Clay Science, 265, 67–71.
Aslani, M. A. A., Yusan, S., Yenil, N., & Kuzu, S. (2012). Sorption profile of uranium (VI) from aqueous medium onto 3-O-acetyl-(S)-1,2-O-trichloroethylidene-5,6,8-trideoxy-a-D-xylo-oct-5(E)-eno-1,4-furano-7-ulose (OASOTCETDOXDXOEEFU). Chemical Engineering Journal, 200, 391–398.
Aytas, S., Turkozu, D. A., & Gok, C. (2011). Biosorption of U(VI) by bi-functionalized low cost biocomposite adsorbent. Desalination, 280, 354–362.
Bai, J., Yao, H. J., Fan, F. L., Lin, M. S., Zhang, L. N., Ding, H. J., Lei, F. A., Wu, X. L., Li, X. F., Guo, J. S., & Qin, Z. (2010). Biosorption of uranium by chemically modified Rhodotorula glutinis. Journal of Environmental Radioactivity, 101(11), 969–973.
Bai, J., Wu, X. L., Fan, F. L., Tian, W., Yin, X. J., Zhao, L., Fan, F. Y., Li, Z., Tian, L. L., Qin, Z., & Guo, J. S. (2012). Biosorption of uranium by magnetically modified Rhodotorula glutinis. Enzyme and Microbial Technology, 51(6), 382–387.
Bai, J., Fan, F. L., Wu, X. L., Tian, W., Zhao, L., Yin, X. J., Fan, F. Y., Li, Z., Tian, L. L., Wang, Y., Qin, Z., & Guo, J. S. (2013). Equilibrium, kinetic and thermodynamic studies of uranium biosorption by calcium alginate beads. Journal of Environmental Radioactivity, 126, 226–231.
Bayramoğlu, G., Celik, G., & Arica, M. Y. (2006). Studies on accumulation of uranium by fungus Lentinus Sajor-caju. Journal of Hazardous Materials, 136(2), 345–353.
Bhat, S. V., Melo, J. S., Chaugule, B. B., & D’Souza, S. F. (2008). Biosorption characteristics of U(VI) from aqueous medium onto Catenella repens, a red alga. Journal of Hazardous Materials, 158(2), 628–635.
Chen, F., Tan, N., Long, W., & Yang, S. K. (2014a). Enhancement of U(VI) biosorption by chemically modified marine-derived mangrove endophytic fungus Fusarium sp. #ZZF51. Journal of Radioanalytical and Nuclear Chemistry, 299(1), 193–201.
Chen, F., Tan, N., Yan, X. M., Yang, S. K., She, Z. G., & Lin, Y. C. (2014b). Uranium(VI) removal from aqueous solution by Poly(amic acid)-modified marine fungus. Separation Science and Technology, 49, 1251–1258.
Cheng, Y. M., Sun, X., Liao, X. P., & Shi, B. (2011). Adsorptive recovery of uranium from nuclear fuel industrial wastewater by titanium loaded collagen fiber. Chinese Journal of Chemical Engineering, 19(4), 592–597.
Deng, S. B., & Ting, Y. P. (2005). Polyethylenimine-modified fungal biomass as a high-capacity biosorbent for Cr(VI) anions: sorption capacity and uptake mechanisms. Environmental Science & Technology, 39, 8490–8496.
Ding, D. X., Tan, X., Hu, N., Li, G. Y., Wang, Y. D., & Tan, Y. (2012). Removal and recovery of uranium (VI) from aqueous solutions by immobilized Aspergillus niger powder beads. Bioprocess and Biosystems Engineering, 35(9), 1567–1576.
Ding, D. X., Fu, P. K., Li, L., Xin, X., Hu, N., & Li, G. Y. (2014). U(VI) ion adsorption thermodynamics and kinetics from aqueous solution onto raw sodium feldspar and acid-activated sodium feldspar. Journal of Radioanalytical and Nuclear Chemistry, 299(3), 1903–1909.
Elsabawy, K. M., Sekkina, M. M. A., & Tawfik, A. T. (2011). Green synthesis of nano-v-biotite for removal of toxic heavy metals, Th (IV) and U (VI) from aqueous solutions. Journal of Science and Technology, 2(8), 686–696.
Erkaya, I. A., Arica, M. Y., Akbulut, A., & Bayramoglu, G. (2014). Biosorption of uranium (VI) by free and entrapped Chlamydomonas reinhardtii: kinetic, equilibrium and thermodynamic studies. Journal of Radioanalytical and Nuclear Chemistry, 299(3), 1993–2003.
Genç, Ö., Yalçınkaya, Y., Büyüktuncel, E., Denizli, A., Arıca, M. Y., & Bektaş, S. (2003). Uranium recovery by immobilized and dried powdered biomass: characterization and comparison. International Journal of Mineral Processing, 68(1), 93–107.
Ghasemi, M., Keshtkar, A. R., Dabbagh, R., & Safdari, S. J. (2011). Biosorption of uranium (VI) from aqueous solutions by Ca-pretreated Cystoseiraindica alga: breakthrough curves studies and modeling. Journal of Hazardous Materials, 189(1), 141–149.
Gok, C., & Aytas, S. (2009). Biosorption of U(VI) from aqueous solution using calcium alginate beads. Journal of Hazardous Materials, 168(1), 369–375.
Guler, U. A., & Sarioglu, M. (2013). Single and binary biosorption of Cu(II), Ni(II) and methylene blue by raw and pretreated Spirogyra sp.: equilibrium and kinetic modeling. Journal of Environmental Chemical Engineering, 1(3), 369–377.
He, S., Ruan, B., Zheng, Y. P., Zhou, X. B., & Xu, X. P. (2014). Immobilization of chlorine dioxide modified cells for uranium absorption. Journal of Environmental Radioactivity, 137, 46–51.
Kapoor, A., & Viraraghavan, T. (1997). Heavy metal biosorption sites in Aspergillus niger. Bioresource Technology, 61, 221–227.
Khambhaty, Y. K., Mody, K., Basha, S., & Jha, B. (2009). Kinetics equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus nigerniger. Chemical Engineering Journal, 145(3), 489–495.
Kiran, I., Akar, T., & Tunali, S. (2005). Biosorption of Pb(II) and Cu(II) from aqueous solutions by pretreated biomass of Neurosporacrassa. Process Biochemistry, 40(11), 3550–3558.
Kulkarni, R. M., Shetty, K. V., & Srinikethan, G. (2013). Cadmium (II) and nickel (II) biosorption by Bacillus laterosporus (MTCC 1628). Journal of the Taiwan Institute of Chemical Engineers, 45(4), 1628–1635.
Kushwaha, S., & Sudhakar, P. P. (2013). Sorption of uranium from aqueous solutions using palm-shell-based adsorbents: a kinetic and equilibrium study. Journal of Environmental Radioactivity, 126, 115–124.
Li, P. F., Mao, Z. Y., Rao, X. J., Wang, X. M., Min, M. Z., Qiu, L. W., & Liu, Z. L. (2004). Biosorption of uranium by lake-harvested biomass from a cyanobacterium bloom. Bioresource Technology, 94(2), 193–195.
Liu, Y. G., Liao, T., He, Z. B., Li, T. T., Wang, H., Hu, X. J., Guo, Y. M., & He, Y. (2013). Biosorption of copper (II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. Transactions of Nonferrous Metals Society of China, 23(6), 1804–1814.
Luo, F., Liu, Y. H., Li, X. M., Xuan, Z. X., & Ma, J. T. (2006). Biosorption of lead ion by chemically-modified biomass of marine brown algae Laminaria japonica. Chemosphere, 64(7), 1122–1127.
Mahmoud, M. A. (2013). Removal of Uranium (VI) from aqueous solution using low cost and eco-Friendly adsorbents. Chemical Engineering and Process Technology, 4(6), 169.
Moghaddam, M. R., Fatemi, S., & Keshtkar, A. (2013). Adsorption of lead (Pb2+) and uranium cations by brown algae; experimental and thermodynamic modeling. Chemical Engineering Journal, 231, 294–303.
Mwangi, I. W., & Ngila, J. C. (2012). Removal of heavy metals from contaminated water using ethylenediamine-modified green seaweed (Caulerpaserrulata). Physics and Chemistry of the Earth, Parts A/B/C, 50, 111–120.
Pillai, S. S., Mullassery, M. D., Fernandez, N. B., Girija, N., Geetha, P., & Koshy, M. (2013). Biosorption of Cr(VI) from aqueous solution by chemically modified potato starch: equilibrium and kinetic studies. Ecotoxicology and Environmental Safety, 92, 199–205.
Prodromou, M., & Pashalidis, I. (2013). Uranium adsorption by non-treated and chemically modified cactus fibres in aqueous solutions. Journal of Radioanalytical and Nuclear Chemistry, 298(3), 1587–1595.
Resmi, G., Thampi, S. G., & Chandrakaran, S. (2012). Removal of lead from wastewater by adsorption usingacid-activated clay. Environmental Technology, 33(3), 291–297.
Ronda, A., Martín-Lara, M. A., Calero, M., & Blázquez, G. (2013). Analysis of the kinetics of lead biosorption using native and chemically treated olive tree pruning. Ecological Engineering, 58, 278–285.
Saeed, A., Iqbal, M., & Akhtar, M. W. (2005). Removal and recovery of lead (II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). Journal of Hazardous Materials, 117(1), 65–73.
Saifuddin, N. M., & Dinara, S. (2012). Immobilization of Saccharomyces Cerevisiae onto cross-linked Chitosan coated with magnetic nanoparticles for adsorption of Uranium (VI) ions. Advances in Natural and Applied Science, 6(2), 249–267.
Saleem, N., & Bhatti, H. N. (2011). Adsorptive removal and recovery of U(VI) by citrus waste biomass. Bioresources, 6(3), 2522–2538.
Satpati, S. K., Pal, S., Roy, S. B., & Tewari, P. K. (2014). Removal of uranium(VI) from dilute aqueous solutions using novel sequestering sorbent poly-acryl hydroxamic acid. Journal of Environmental Chemical Engineering, 2(3), 1343–1351.
Şimşek, S., & Ulusoy, U. (2013). Adsorptive properties of sulfolignin-polyacrylamide graft copolymer for lead and uranium: effect of hydroxylamine-hydrochloride treatment. Reactive and Functional Polymers, 73(1), 73–82.
Tsekova, K., Todorova, D., Dencheva, V., & Ganeva, S. (2010). Biosorption of copper(II) and cadmium(II) from aqueous solutions by free and immobilized biomass of Aspergillus niger. Bioresource Technology, 101, 1727–1731.
Vijayaraghavan, K., & Yun, Y. S. (2008). Biosorption of CI Reactive Black 5 from aqueous solution using acid-treated biomass of brown seaweed Laminariasp. Dyes and Pigments, 76(3), 726–732.
Wang, J. S., Hu, X. J., Liu, Y. G., Xie, S. B., & Bao, Z. L. (2010). Biosorption of uranium(VI) by immobilized Aspergillus fumigatus beads. Journal of Environmental Radioactivity, 101(6), 504–508.
Wang, J. S., Bao, Z. L., Chen, S. G., & Yang, J. H. (2011). Removal of uranium from aqueous solution by chitosan and ferrous ions. Journal of Engineering for Gas Turbines and Power, 133(8), 1–3.
Xie, S. B., Yang, J., Chen, C., Zhang, X. J., Wang, Q. L., & Zhang, C. (2008). Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. Journal of Environmental Radioactivity, 99(1), 126–133.
Xu, L. C., Zhang, G. F., Gao, J., Zhang, X. L., & Wei, G. Z. (2010). Recycling and reuse of wastewater from uranium mining and milling. Uranium Mining and Metallurgy, 29(2), 78–81.
Yuvaraja, G., Krishnaiah, N., Subbaiah, M. V., & Krishnaiah, A. (2014). Biosorption Pb(II) from aqueous solution by Solanum melongena leaf powder as a low-cost biosorbent prepared from agricultural waste. Colloids and Surfaces B: Biointerfaces, 114, 75–81.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (91326106 and 114055081) and the Development Program for Science and Technology for National Defense (B3720132001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, D.X., Xin, X., Li, L. et al. Removal and Recovery of U(VI) from Low Concentration Radioactive Wastewater by Ethylenediamine-Modified Biomass of Aspergillus niger . Water Air Soil Pollut 225, 2206 (2014). https://doi.org/10.1007/s11270-014-2206-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2206-4