Abstract
The immobilized Aspergillus niger powder beads were obtained by entrapping nonviable A. niger powder into Ca-alginate gel. The effects of pH, contact time, initial uranium (VI) concentration and biomass dosage on the biosorption of uranium (VI) onto the beads from aqueous solutions were investigated in a batch system. Biosorption equilibrium data were agreeable with Langmuir isotherm model and the maximum biosorption capacity of the beads for uranium (VI) was estimated to be 649.4 mg/g at 30 °C. The biosorption kinetics followed the pseudo-second-order model and intraparticle diffusion equation. The variations in enthalpy (26.45 kJ/mol), entropy (0.167 kJ/mol K) and Gibbs free energy were calculated from the experimental data. SEM and EDS analysis indicated that the beads have strong adsorption capability for uranium (VI). The adsorbed uranium (VI) on the beads could be released with HNO3 or HCl. The results showed that the immobilized A. niger powder beads had great potential for removing and recovering uranium (VI) from aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of uranium mining industry, a large amount of wastewater containing uranium has been discharged into the environment. In addition, malfunctions of nuclear reactors and leaks of cooling water have also endangered the environment [1, 2]. Uranium (VI) is soluble and mobile and its accumulation in water will have tremendous impact on environment [3, 4]. Furthermore, the uranium compounds can enter into human bodies through food chain and cause severe damage to their kidney and even cause them to die [5, 6]. On the other hand, uranium is the prerequisite material for nuclear energy and full use should be made of the uranium in solutions. Therefore, it is imperative to remove and recover the uranium from the aqueous solutions, not only for the remediation of environment and the health of the human beings, but also for the development of nuclear energy.
Conventional methods for removing uranium (VI) from wastewaters include chemical precipitation, coagulation and ion-exchange process. However, these conventional methods are extremely expensive and ineffective particularly at low metal concentrations [7, 8].
Recent research has revealed that different groups of microorganisms, such as algae, bacteria, yeasts, fungi and industrial wastes, could be used as natural adsorbents for uranium (VI) from aqueous solutions, and, among these, fungi and algae have the greatest potential due to their high adsorption capacity and low cost [3, 9, 10]. However, the physical and mechanical characteristics of the microbial biomass such as small particle size, difficult separation of solids and liquids and poor mechanical strength have impeded their commercial application as a biosorbent [11, 12]. Fortunately, researchers have developed the immobilization methods for biomass and the immobilized biomass has advantages over the free biomass in that it has higher mechanical strength, and can more easily be separated from the aqueous solutions and the adsorbed metal ions can more easily be recovered [13, 14].
Most previous experiments on adsorption of uranium (VI) onto the immobilized biomass were focused on the immobilization of viable microorganisms [13, 15], and few experiments were carried out on the immobilization of nonviable powdered fungi.
In the present research, nonviable powdered A. niger was immobilized using alginate, the effects of initial pH, contact time, initial uranium (VI) concentration and biomass dosage on the adsorption of uranium (VI) from the aqueous solutions onto the immobilized biomass were investigated, the experimental data were analyzed using different models and the process parameters were evaluated.
Materials and methods
Microorganism, medium and preparation of nonviable fungal biomass
A. niger was used for the present research. It was isolated from uranium ore powders and was identified by microbial type culture collection (MTCC), Guangdong Institute of Microbiology, China. Spores from the established culture (4–5 days old) were incubated on potato-dextrose agar (PDA) plates and kept culturing at 30 °C for 4 days. The cultured spores were used to prepare inocula. The inocula were inoculated into the liquid medium. The medium was prepared by adding 20 g sucrose into 1 L of potato leach solution which was obtained by taking 200 g of potato to a stainless pot, kept boiling for 10 min, filtering out the potato and making the liquid from the filtration be 1 L of constant volume.
The A. niger was cultivated in Erlenmeyer flasks, each with 250 ml of liquid medium, which were put on a shaker and the A. niger was kept culturing at 30 °C and 200 rpm for 72 h. The mycelia were filtered with a nylon cloth and washed three times with ultrapure water. The freshly harvested mycelia were dried in an oven at 45 °C for 24 h. The dried biomass was then ground and sieved through a 160-mesh sieve. The powdered biomass was stored in a refrigerator at 4 °C and ready for immobilization.
Reagents and instruments
All the reagents used in the experiment were of analytical reagent (AR) grade. CaCl2 and sodium alginate powder were bought from Tianjin Guangfu Fine Chemical Research Institute, China. The black powdered U3O8 (provided by 272 Uranium Industry Limited Company of China National Nuclear Corporation) was used to prepare stock uranium (VI) solution. Ultrapure water was used throughout the study.
In this study, the JSM-6360LV (JEOL, Japan) instrument coupled with energy dispersive X-ray (EDAX, USA) analysis, the CL-32L autoclave (ALP, Japan), the SPH-200D thermostated shaker bath (SHIPING Temperature, China) and the TKA-GenPure water purification systems were used. Uranium (VI) concentrations were determined by means of a T6 UV–vis spectrophotometer (Pgeneral, China).
Methods
Preparation of immobilized A. niger powder beads
The powdered biomass A. niger was immobilized by entrapment into the sodium alginate according to [11]. 2 % (w/v) of sodium alginate was dissolved in heated ultrapure water. After the sodium alginate solution was cooled, 2 % (w/v) of A. niger powder was added to the sodium alginate solution. The mixture was further stirred to make the powder distribute uniformly within the sodium alginate solution. After that, the mixture was added dropwise into 1.5 % (w/v) of CaCl2 solution using 5 ml injector. The resultant beads of 2 ± 0.2 mm in diameter were cured in this solution at 4 °C for 1.5 h. Then the beads were filtered out and washed three times with 300 ml of ultrapure water. After that, these biomass entrapped beads were collected by filtration with a piece of nylon cloth for 0.5 h at 4 °C. Blank beads with 2 % (w/v) of sodium alginate were also prepared in the same way as the biomass entrapped beads were prepared. A part of beads were weighed and then dried in a oven at 45 °C for 24 h and the dry/wet weight ratio was determined. The wet biomass was stored in a refrigerator at 4 °C and used throughout the study unless otherwise mentioned.
Preparation of uranium (VI) solution and concentration measurements
The stock uranium (VI) solution (1,000 mg/L) was prepared according to [16]. The working solutions were prepared by diluting the stock solution as required. The concentrations of uranium (VI) in the solution before and after biosorption treatment were determined using TOPO (trioctylphosphine oxide) extraction and Br-PADAP (2-(5-Bromo-2-pyridylazo)-5-(diethylamino) phenol) by spectrophotometry at λmax of 578 nm stipulated in EJ267.4-1984 (The People’s Republic of China Nuclear Industry Standards).
Batch biosorption experiments
Experiments were performed on the biosorption of uranium (VI) from aqueous solutions onto the immobilized A. niger powder beads in a batch system. The effects of initial pH of solution, contact time, initial uranium (VI) concentration and biomass dosage were investigated.
The effect of initial pH of solution in the range of 2–9 on the adsorption processing of uranium (VI) onto the immobilized A. niger powder beads was investigated, the pH of the working solutions was adjusted to the required value with 0.1 M HNO3 or 0.1 M NaOH solution before the biosorption experiments were begun and not controlled during the experimentation. Experiments were performed with 150 mL Erlenmeyer flasks. 0.03 g dry weight of wet beads and 100 mL of 50 mg/L uranium (VI) solution were added to each flask and the flasks were put on the shaker and kept shaken at 200 rpm and 30 °C for 9 h. The effect of contact time on the biosorption rate of uranium (VI) onto the immobilized A. niger powder beads was investigated at pH 5. At the prescribed time interval, samples were withdrawn and centrifuged at 15,000 rpm for 1 min and the liquid supernatant was taken to determine uranium (VI) concentration. The effect of initial uranium (VI) concentration in the range of 10–200 mg/L on the adsorption capacity of the immobilized A. niger powder beads for uranium (VI) was studied at pH 5 for 9 h and the rest of the procedures were the same as described above. The effect of biomass dosage (dry weight) in the range of 0.1–1.2 g/L was also investigated in the same way as described above. The experiments on biosorption of uranium (VI) onto free A. niger powder and blank Ca-alginate beads were also performed at pH 5 and the contact time was 9 h.
The adsorption percentage (%), biosorption capacity, Q e (mg/g), and adsorption distribution constant [17], K d (mL/g), were calculated using the following equations:
Where, C i (mg/L) and C f (mg/L) are the initial and final concentrations of uranium (VI), respectively, V (L) is the volume of solution, M (g) is the dry weight of biosorbent, M b (g) and M s (g) are the amount of uranium (VI) in the biosorbent and solution, respectively.
All the experiments were conducted in triplicate and repeated three times. Experimental data were analyzed by Origin software (Version v8.1, USA).
Equilibrium and kinetics studies
Equilibrium isotherm models
In order to describe the sorption equilibrium of uranium (VI) onto the immobilized A. niger powder beads, the experimental data obtained at 30 °C were analyzed using Langmuir, Freundlich and Redlich–Peterson equations.
The Langmuir model is based on monolayer sorption, which assumes the specific homogeneous sites and solute monolayer coverage on the surface of adsorbent [18]. The Langmuir model is expressed by:
A linear form of this equation is:
Where, C e (mg/L) is the equilibrium concentration; Q e (mg/g), the equilibrium biosorption capacity; Q max (mg/g), the maximum adsorption capacity of uranium (VI) onto the biosorbent and b (L/mg), the Langmuir constant.
The Freundlich isotherm assumes a heterogeneous surface on the biosorbent [17, 19]. Freundlich model is given as linearized form [3]:
Where, n and K f are the Freundlich isotherm constants that indicate the biosorption intensity and the extent of the biosorption.
The Redlich–Peterson model incorporates the features of the Langmuir and the Freundlich isotherms and is expressed as [19]:
Where, K r (L/mg), αr and β are the Redlich–Peterson isotherm constants, and the exponent, β lies between 0 (Henry’s law equation) and 1 (Langmuir form).
Kinetic studies
Kinetic models have been used to explain the controlling mechanism such as mass transfer and chemical reactions involved in adsorption process [20]. Lagergren pseudo-first, pseudo-second-order models and intraparticle diffusion equation were used to test the experimental data involved in the biosorption of uranium (VI) onto the immobilized A. niger powder beads at 30 °C.
The Lagergren pseudo-first-order equation is represented by [3, 21]:
Where, Q t (mg/g) are the biosorption capacity at time t, k 1 (h−1) is the pseudo-first-order rate constant.
The pseudo-second-order model describing chemical sorption is expressed as [19, 22]:
As t is tending to zero, the initial biosorption rate (H) is given as:
Where, k 2 (g/mg h) is the pseudo-second-order rate constant.
Intraparticle diffusion model is used to describe the behavior of solute diffusing and binding to interior surfaces of biosorbent. The rate constant for intraparticle diffusion (k id) is given as [19, 23]:
Where, t (h) is the contact time and k id is the intraparticle diffusion rate constant.
Thermodynamic studies
The thermodynamic parameters including Gibbs free energy change (ΔG o), enthalpy change (ΔH o) and entropy change (ΔS o) are the fundamental criterion of the spontaneity of a process [19]. In order to determine the thermodynamic parameters, experimental data obtained at different temperatures of 20 °C (293 K), 30 °C (303 K), 35 °C (308 K) and 40 °C (313 K) for biosorption of uranium (VI) onto the immobilized A. niger powder beads were analyzed using the following equations [17, 19]:
Where K d (mL/g) is distribution constant, T (K) is temperature in Kelvin and R (8.314 J/mol K) is the gas constant. Thermodynamic parameters can be determined from the slope and intercept of ln K d versus 1/T plots.
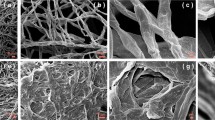
SEM and EDS analysis
Scanning electron microscopy (SEM) coupled with EDX was used to analyze the immobilized A. niger powder beads before and after biosorption treatment. For this purpose, the samples of the immobilized A. niger powder beads were collected by filtration and washed three times with ultrapure water. A small number of the immobilized A. niger powder beads were placed on cover slip and given discission with pipette tips to expose their internal surfaces. All of the samples were vacuum dried for 24 h at room temperature (25 °C). The dried samples were then transferred to stub and sputter-coated with 4 nm of gold particles. After that, the stub was put in SEM holder, and photographs and elemental composition of the sample were obtained. In this study, the content of the detected elements was determined using the classic ZAF model.
Uranium (VI) elution and regeneration of biomass
Elution experiments were carried out using different regenerating solutions including HCl, HNO3, NaOH, Na2CO3, NaHCO3, (NH4)2CO3, (NH4)2SO4 and ultrapure water to release the loaded uranium (VI) from the immobilized A. niger powder beads. Biomass samples (corresponding to 0.03 g dry weight) were washed by ultrapure water and mixed with 50 mL of 0.1 M regeneration solution in flasks and the flasks were put in the shaker and kept shaken for 3 h at 200 rpm and 30 °C. All experiments were conducted in duplicates and mean values were noted.
The amount of eluted uranium (VI) (Q d) and desorption percentage (%) were determined in the following equation [3]:
Where Q d (mg/g) is the amount of eluted uranium (VI) from the biosorbent, C d (mg/L) is the concentration of uranium (VI) desorbed into the eluent, V (L) is the volume of solution, M (g) is the dry weight of biosorbent and Q (mg/g) is biosorption capacity.
Results and discussion
Properties of immobilized A. niger powder beads
The biosorption of uranium (VI) from aqueous solutions using the free A. niger powder, the blank Ca-alginate beads and the immobilized A. niger powder beads were investigated in a batch system. Biosorbents (corresponding to 0.03 g dry weight) were added into flasks, 100 mL of 50 mg/L uranium (VI) solution (pH 5) was added into each of the flasks, and the flasks were put in the shaker and kept shaking at 200 rpm and 30 °C for 9 h. The uranium (VI) biosorption percentages (%) for the free A. niger powder, the blank Ca-alginate beads and the immobilized A. niger powder beads were calculated to 52.53, 58.44 and 88.9 %, respectively. The results indicated that the immobilized A. niger powder beads had better adsorption capability for uranium (VI) than its free powdered biomass and the blank Ca-alginate beads. Akhtar et al. [13] studied the adsorption behavior of the free and immobilized Trichoderma harzianum mycelia and reported that, although Ca-alginate could adsorb uranium (VI) due to the existence of carboxylic groups in the alginate polymer, its adsorption capability was much lower than the immobilized T. harzianum mycelia by Ca-alginate. According to the earlier uranium (VI) biosorption investigations, this experiment was examined at single temperature and pH [13, 15, 17]. Although the experiment had its limitation, this did suggest that the sodium alginate could be used as suitable immobilization matrix for further studies.
Effect of initial pH of solution
Previous studies have showed that biosorption process of biomass for metals was strongly affected by the pH of aqueous solution. The experimental results of the effect of the initial pH of the solution on the adsorption of uranium (VI) onto the immobilized A. niger powder beads were shown in Fig. 1a. As shown in Fig. 1a, the highest biosorption percentage of uranium (VI) onto the immobilized A. niger powder beads amounted to approximately 90 % at pH 5. A sudden increase in uranium (VI) uptake with a slight increase in pH from 4 to 5, referred to as an “adsorption edge”, was observed [24]. Small uranium (VI) uptake occurred at pH 2.0. The reason for this was that pH 2.5 was the onset for hydrolysis of the uranyl ions. When pH was below 2.5 only the simple UO2 2+ ions existed in the solution and protons (H+) occupied a large number of the adsorption sites. When pH was above 2.5, uranium in the solution were mostly in the form of hydrolyzed composites such as (UO2)2(OH) 2+2 , UO2OH+, (UO2)3(OH)5+, and these hydrolyzed species could more easily be adsorbed than the free hydrated ions [25]. As a result, uranium (VI) uptake increased with the rise of pH. However, as the pH was over 5.0 dissolved carbonate and atmospheric CO2 would result in the formation of carbonate complexes, which would compete with the adsorption site of the biosorbent for uranium (VI), resulting in a decrease in the adsorption amount of uranium (VI) [16]. Another reason, besides carbonates complexes competition, should be that over pH 5.0 the neutral forms of uranium (VI) such as UO2(OH)2·H2O are the major species [26]. As the pH increased to 8.0, visual precipitates of schoepite (4UO3·9H2O) were formed [27]. Therefore, subsequent experiments were conducted using the solution at initial pH 5.0.
a Effect of initial pH of solution on the biosorption of uranium (VI) by the immobilized A. niger powder beads (C i 50 mg/L, M 30 mg, t 9 h, T 303 K). b Effect of contact time on the biosorption of uranium (VI) by the immobilized A. niger powder beads (C i 50 mg/L, pH 5, M 30 mg, T 303 K). c Effect of initial uranium concentration on the biosorption of uranium (VI) by the immobilized A. niger powder beads (pH 5, M 30 mg, t 9 h, T 303 K). d Effect of the dosage on the biosorption of uranium (VI) by the immobilized A. niger powder beads (C i 50 mg/L, pH 5, t 9 h, T 303 K)
Effect of contact time
Figure 1b showed the effect of contact time on the sorption of uranium (VI) onto the immobilized A. niger powder beads. The rapid increase in uranium (VI) biosorption was observed within 1.5 h, and this might be due to the fact that the biosorption occurred in the exterior surface of the biomass. The biosorption capacity tended to a constant after 9 h, and the uranium (VI) uptake process could be considered to have reached equilibrium.
Effect of initial uranium (VI) concentration
Figure 1c showed the variation in biosorption capacity and distribution constant of the biomass for the adsorption of uranium (VI) onto the immobilized A. niger powder beads as a function of initial uranium (VI) concentration. The experiments on the adsorption of uranium (VI) onto the immobilized A. niger powder beads were performed using solutions (pH 5) with different uranium (VI) concentrations ranging from 10 to 200 mg/L. As shown in Fig. 1c, uranium (VI) uptake increased with the rise of initial concentrations of the solutions, and the amount of uranium (VI) adsorbed was approximately 470 mg/g when the initial concentration of uranium (VI) in the solution was 200 mg/L. The reason for this was that the initial concentration provided an important driving force to overcome all mass transfer resistance of uranium (VI) between the aqueous and solid phases [17]. Akhtar et al. [13] found that the uranium (VI) biosorption capacity of T. harzianum was less than 400 mg/g dry weight at equilibrium uranium (VI) concentration of 200 mg/L, and the maximum value of 612 ± 6 mg/g was obtained at a equilibrium uranium (VI) concentration of 808 ± 7 mg/L. It should be noted that the biosorpion capacity of uranium (VI) onto the immobilized A. niger powder beads did not reach its equilibrium. The results could not be used to determine the maximum value of the biosorption capacity, but they could clearly indicate that the immobilized A. niger powder beads had high uranium (VI) biosorption capacity in dilute concentration solutions.
The adsorption distribution constant (K d) was shown in Fig. 1c. A high value of distribution constant is the characteristic of a good biosorbent. The immobilized A. niger powder beads exhibited a K d value of 29,845 ml/g dry weight at a residual uranium (VI) concentration of 5.1 mg/L when the biomass dosage was 0.3 g/L. The calcium alginate beads, T. harzianum and Aspergillus fumigates were reported to have the maximum K d of approximately 4,500, 80,744 and 10,000 ml/g at residual uranium (VI) concentrations of 1.9, 1.5 and 19 mg/L, respectively [3, 17, 28]. As compared, the immobilized A. niger powder beads were good enough to be used as a biosorbent to remove uranium (VI) from aqueous solutions.
Effect of biosorbent dosage
Figure 1d showed the variation in biosorption of uranium (VI) onto the immobilized A. niger powder beads as a function of the biomass dosage ranging from 0.1 to 1.2 g/L. Results showed that the removal percentage of uranium (VI) increased from 55.2 to 93.4 % with an increase of biomass dosage from 0.1 to 0.5 g/L, and the maximum adsorption distribution constant Kd was calculated to be 46,957 mL/g at a residual uranium (VI) concentration of 3.3 mg/L when the biomass dosage was 0.5 g/L. This indicated that the number of the binding sites for uranium (VI) increased with the increase of the biomass dosage in the range of 0.1–0.5 g/L. However, when the biomass dosage was more than 0.5 g/L, the removal percentage of uranium (VI) from the solution decreased. This might be due to the fact that higher biomass dosage could produce a ‘screen’ between the solid and bulk phases, and that the screen reduced the effective contact area for uranium (VI) uptake [17, 19].
Biosorption isotherms
The equilibrium isotherm constants determined using Langmuir, Freundlich and Redlich–Peterson models were shown in Table 1. The results showed that the experimental data fitted the Langmuir isotherms better than Freundlich. When β = 1.0, the Redlich–Peterson model had the highest correlation coefficient and the model was the Langmuir form, indicating that the biosorption isotherms of uranium (VI) onto the immobilized A. niger powder beads exhibited Langmuir behavior. The biosorption isotherms of uranium (VI) exhibited Langmuir behavior, indicating that the biosorption was a monolayer adsorption [17]. The value of b was calculated to be between 0 and 1 indicating that the reaction was favorable [19]. The value of 1/n was smaller than 1, and this indicated that heterogeneous surfaces existed on the immobilized A. niger powder beads, and that the biosorption of uranium (VI) occurred only in the active site with a monolayer pattern [3]. The Q max represented the maximum adsorption capacity of the immobilized A. niger powder beads when they were covered by uranium (VI) with a monolayer pattern [13]. As indicated in the reported studies, T. harzianum, calcium alginate beads and A. fumigates have the maximum uranium (VI) adsorption capacities of 612, 400 and 423 mg/g, respectively, the immobilized A. niger powder beads used in this study has the highest Q max for uranium (VI) uptake [3, 17, 28].
Kinetic models
Table 2 showed the kinetic constants obtained using Lagergren pseudo-first, pseudo-second-order models and intraparticle diffusion equation. The results showed that the biosorption reaction could be approximated with the pseudo-second-order model since the correlation coefficient was larger than 0.999. On the other hand, the Lagergren pseudo-first-order model was not suitable for describing the biosorption behavior since the theoretical Q e values were too small as compared to the experimental Q e values although the correlation coefficient was as large as 0.987 [3].
Plot of Q t versus t 1/2 for uranium (VI) was shown in Fig. 2. From this figure, an initial steep-sloped portion (from 0 to 1.5 h), which was attributed to external surface biosorption, was followed by a gentle-sloped portion (from 1.5 to 6 h) to the intraparticle diffusion and a plateau to the equilibrium. The gentle-sloped portion was attributed to gradual adsorption, where the intraparticle diffusion is rate controlled. And the intraparticle diffusion began to slow down, leading to a plateau to the equilibrium, due to the extremely low solute concentration in the solution [23]. The intraparticle diffusion rate (k id) was calculated from the slope of the intermediate gentle-sloped portion. The uranium (VI) was initially adsorbed by the exterior surface of the immobilized A. niger powder beads. When the biosorption at the exterior surface reached saturation, the uranium (VI) entered into the immobilized A. niger powder beads by the pores within the beads and was adsorbed by the interior surfaces of the beads. When the uranium (VI) diffused into the pores of the beads, the diffusion resistance was increased, which caused the diffusion rate to decrease. With the decrease of the uranium (VI) concentration in the solution, the diffusion rate became increasingly lower, and consequently, the diffusion processes reached equilibrium. On the other hand, according to the theoretical equation for diffusion, the initial rate constant obtained from the slope of the steep-sloped portion was not directly related to the square root of the initial uranium (VI) concentration. This confirmed that intraparticle diffusion was not the only rate-determining step for uranium (VI) biosorption [29]. Therefore, the biosorption process of uranium (VI) by the immobilized A. niger powder beads was controlled by mass transfer, sorption of uranium (VI) onto sites and intraparticle diffusion together.
Thermodynamic studies
When the temperatures for reaction were 293, 298, 303 and 313 K, the Gibbs free energy change (ΔG o) for the biosorption of uranium (VI) by the immobilized A. niger powder beads were −22.49, −23.32, −24.16 and −25.84 kJ/mol, respectively. The negative values of Gibbs free energy change indicated that the biosorption of uranium (VI) was of spontaneous nature. These values decreased with an increase in temperature shown that the uranium (VI) biosorption is more favorable at higher temperatures [17]. The enthalpy change (ΔH o) and entropy change (ΔS o) calculated were 26.45 kJ/mol and 0.167 kJ/mol K, respectively. The positive value of enthalpy change (ΔH o) indicated the biosorption process of uranium (VI) onto the immobilized A. niger powder beads was of endothermic nature. The positive value of entropy change (ΔS o) showed that complexation randomness increased at the interface between the solid and the solution during the biosorption of uranium (VI) onto the immobilized A. niger powder beads [19].
SEM and EDS results
Figure 3 showed the SEM images of the exterior and interior surfaces of the immobilized A. niger powder beads before and after biosorption treatment at 1,000× magnification. As seen from the figures, the surfaces of the biosorbent before biosorption were heterogeneous, porous and clean. After biosorption, a great deal of precipitates was observed on both the exterior and the interior surfaces of the biosorbent. Figure 4 showed the results of EDS analysis. It indicated that precipitates covered on the biosorbent surfaces contained uranium since the higher peak of uranium on the exterior surfaces and the lower one on the interior surfaces were observed. This was because the immobilized A. niger powder beads were the porous materials and the pores offered many surface areas with binding sites which facilitated pore diffusion during the biosorption of uranium (VI) [16, 17] (Fig. 4).
Uranium elution and regeneration of biosorbents
Table 3 listed the elution efficiency with various regenerating solutions in desorption studies of uranium (VI) from the loaded immobilized A. niger powder beads. It was found that nitric acid and hydrochloric acid performed better than other regenerating solutions in the uranium elution. Table 3 showed the mass losses of the biosorbent in dry weight for different desorbents. It was found that the Ca-alginate matrix completely dissolved in the sodium carbonate, sodium bicarbonate and ammonium carbonate eluents. The similar phenomenon was observed with sodium carbonate and sodium bicarbonate eluents in the desorption of uranium (VI) from loaded T. harzianum but the opposite one, with the ammonium carbonate eluent [3]. Although the sorption–desorption experiment was performed only one cycle, this study indicated that nitric acid and hydrochloric acid could be used as suitable uranium (VI) desorbing agents in further elution studies due to their high elution efficiency and low biomass loss.
Conclusion
The immobilized A. niger powder beads were efficient as a biosorbent for removal of uranium (VI) from aqueous solutions. The maximum biosorption capacity for uranium (VI) has been found as 649.4 mg/g. SEM coupled with EDS analysis and kinetic studies revealed that intraparticle diffusion occurred in the biosorption process and delayed the equilibrium for uranium (VI) biosorption. The elution of the loaded uranium (VI) in 50 mL of 0.1 M HCl reached 93.09 %. Therefore, the immobilized A. niger powder beads had great potential for removing uranium (VI) from aqueous solutions.
References
Kazy SK, D’Souza SF, Sar P (2009) Uranium and thorium sequestration by a Pseudomonas sp.: mechanism and chemical characterization. J Hazard Mater 163:65–72
Preetha CR, Gladis JM, Rao TP, Venkateswaran G (2006) Removal of toxic uranium from synthetic nuclear power reactor effluents using uranyl ion imprinted polymer particles. Environ Sci Technol 40:3070–3074
Akhtar K, Akhtar MW, Khalid AM (2007) Removal and recovery of uranium from aqueous solutions by Trichoderma harzianum. Water Res 41:1366–1378
Fletcher KE, Boyanov MI, Thomas SH, Wu Q, Kemner KM, Löffler FE (2010) U(VI) reduction to mononuclear U(IV) by Desulfitobacterium species. Environ Sci Technol 44:4705–4709
Milja TE, Prathish KP, Rao TP (2011) Synthesis of surface imprinted nanospheres for selective removal of uranium from stimulants of Sambhar salt lake and ground water. J Hazard Mater 188:384–390
Xie S, Yang J, Chen C, Zhang X, Wang Q, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter Freudii. J Environ Radioact 99:126–133
Martins M, Faleiro ML, Rose da Costa AM, Chaves S, Tenreiro R, Matos AP, Costa MC (2010) Mechanism of uranium (VI) removal by two anaerobic bacterial communities. J Hazard Mater 184:89–96
Lloyd JR, Macaskie LE (2000) In: Lovley DR (ed) Environmental metal microbe interaction. American Society of Microbiology, Washington DC, pp 277–327
Ghasemi M, Keshtkar AR, Dabbagh R, Safdari SJ (2011) Biosorption of uranium (VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: Breakthrough curves studies and modeling. J Hazard Mater 189:141–149
Tsuruta T (2002) Removal and recovery of uranyl ion using various microorganisms. J Biosci Bioeng 94:23–28
Kumar R, Singh R, Kumar N, Bishnoi K, Bishnoi NR (2009) Response surface methodology approach for optimization of biosorption process for removal of Cr(VI), Ni (II) and Zn (II) ions by immobilized bacterial biomass sp. Bacillus brevis. Chem Eng J 146:401–407
McHale AP, McHale S (2000) Microbial biosorption of metals: potential in the treatment of metal pollution. Biotechnol Adv 12:647–652
Akhtar K, Khalid AM, Akhtar MW, Ghauri MA (2009) Removal and recovery of uranium from aqueous solutions by Ca-alginate immobilized Trichoderma harzianum. Bioresour Technol 100:4551–4558
Arica MY, Kaçar Y, Genç Ö (2001) Entrapment of white-rot fungus Trametes versicolor in Ca-alginate beads: preparation and biosorption kinetic analysis for cadmium removal from an aqueous solution. Bioresour Technol 80:121–129
Wang J, Hu X, Liu Y, Xie S, Bao Z (2010) Biosorption of uranium (VI) by immobilized Aspergillus fumigatus beads. J Environ Radioact 101:504–508
Pang C, Liu YH, Cao XH, Li M, Huang GL, Hua R, Wang CX, Liu YT, An XF (2011) Biosorption of uranium (VI) from aqueous solution by dead fungal biomass of Penicillium citrinum. Chem Eng J 170:1–6
Gok C, Aytas S (2009) Biosorption of uranium (VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375
Khani MH, Keshtkar AR, Ghannadi M, Pahlavanzadeh H (2008) Equilibrium, kinetic and thermodynamic study on the biosorption of uranium onto Cystoseria indica algae. J Hazard Mater 150:612–618
Yahaya YA, Don MM, Bhatia S (2009) Biosorption of copper (II) onto immobilized cells of Pycnoporus sanguineus from aqueous solution: Equilibrium and kinetic studies. J Hazard Mater 161:189–195
Chiron N, Guilet R, Deydier E (2003) Adsorption of Cu(II) and Pb(II) onto a grafted silica: isotherms and kinetic models. Water Res 37:3079–3086
Benguella B, Benaissa H (2002) Cadmium removal from aqueous solution by chitin: kinetic and equilibrium studies. Water Res 36:2463–2474
Ho SH (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Res 40:119–125
Kim Y, Kim C, Choi I, Rengaraj S, Yi J (2004) Arsenic removal using mesoporous alumina prepared via a templating method. Environ Sci Technol 38:924–931
Kapoor A, Viraraghavan T (1997) Heavy metal biosorption sites in Aspergillus niger. Bioresour Technol 61:221–227
Khani MH, Keshtkar AR, Meysami B, Zarea MF, Jalali R (2006) Biosorption of uranium from aqueous solution by nonliving biomass of marine algae Cystoseira indica. Electron J Biotechnol 9(2):100–106
Yang J, Volesky B (1999) Biosorption of uranium on Sargassum biomass. Water Res 33:3357–3363
Saxena S, Prasad M, D’Souza SF (2006) Radionuclide sorption onto low-cost mineral adsorbent. Ind Eng Chem Res 45:9122–9128
Bhainsa KC, D’Souza SF (1999) Biosorption of uranium (VI) by Aspergillus fumigates. Biotechnol Tech 13:695–699
Keskinkan O, Goksu MZL, Basibuyuk M, Forster CF (2004) Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour Technol 92:197–200
Acknowledgments
The present work was supported by National Natural Science Foundation of China (Grant No. 50774047), Department of Science and Technology of Hunan Province of China (Grant No. 2010GK2025) and Department of Education of Hunan Province of China (Grant No. 10C1134).
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Tan contributed equally to the work.
Rights and permissions
About this article
Cite this article
Ding, DX., Tan, X., Hu, N. et al. Removal and recovery of uranium (VI) from aqueous solutions by immobilized Aspergillus niger powder beads. Bioprocess Biosyst Eng 35, 1567–1576 (2012). https://doi.org/10.1007/s00449-012-0747-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0747-8