Abstract
Rice grassy stunt virus (RGSV) is a tenuivirus posing a threat to rice production in many South, Southeast, and East Asian countries. To date, no host factor interacting with RGSV has been reported. In this study, we screened a rice cDNA library with the GAL4-based yeast two-hybrid system using RGSV p5 as the bait. One of the candidate host factors interacting with RGSV p5 was found to be CBL-interacting protein kinase 25 (OsCIPK25), a member of the plant-specific CBL–CIPK Ca2+ signaling network. The interaction between RGSV p5 and OsCIPK25, as well as OsCIPK5, which is closely related to OsCIPK25, was confirmed by their cellular co-localization and by a bimolecular fluorescence complementation assay in Nicotiana benthamiana cells. Given the importance of CIPKs in the regulation of ion homeostasis and the resemblance of RGSV symptoms to potassium deficiency in rice, we evaluated potassium content of RGSV-infected rice and found it to be much lower than that in the healthy rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viruses need to use and manipulate host cellular functions to accomplish their life cycles because of their limited coding capacity [1]. To do this, viruses have developed a plethora of protein–protein interactions (PPIs) with their hosts [1–4]. As intracellular pathogens, viruses may also interact with host proteins coincidentally. In this case, the PPIs have no beneficial effects on the virus. However, some of them interfere with the host PPI network and disturb host physiology [5, 6]. A large number of plant–virus PPIs have been identified in the past decades. Characterization of these PPIs contributed significantly to our current understanding of plant–virus interaction and has the potential to invoke novel antiviral strategies [1].
Similar to other eukaryotes, plants respond to environmental or endogenous stimuli by signaling cascades involving a small set of secondary messengers, the most well-known of which is calcium (Ca2+) [7]. Ca2+ concentration in the cytoplasm or other sub-cellular compartments fluctuates in response to a stimulus. The spatially, temporally and quantitatively distinct fluctuations termed Ca+2 signatures are decoded by Ca+2 sensor and effector proteins [8–10]. Plants are equipped with a more complex Ca2+ decoding system than other eukaryotes by encoding a greater number of evolutionarily conserved Ca2+ sensors such as calmodulin and evolving some plant-specific Ca2+ sensor-effector modules [11–13]. One of the plant-specific Ca2+ sensor-effector modules is formed by the calcineurin-B like (CBL) proteins and the CBL-interacting protein kinases (CIPKs) [12, 13]. In this module, CBLs have EF hand calcium binding domains and upon binding to Ca2+ interact with specific CIPKs to activate their kinase activity. The model plants Arabidopsis and rice genomes each encodes a complement of 10 distinct CBL proteins with different sub-cellular localizations, expression patterns, and affinities to the 25 (Arabidopsis) or 30 (rice) CIPKs, respectively, forming a specific, robust and versatile Ca2+ decoding network [12, 13]. Recently, this network has been revealed to play central roles in plant development, hormone signaling, and plant responses to biotic and abiotic stresses [12, 13]. Notably, several CBL–CIPK modules were found to regulate membrane ion transporters or anti-transporters, implying an important role of CBL–CIPKs in cellular ion homeostasis [13, 14].
Rice grassy stunt virus (RGSV) is a member of the genus Tenuivirus [15]. It has a genome consisting of six single-stranded RNAs called RNA1 to 6 in the decreasing order of their size, all of which employ an ambisense coding strategy. RNA5 encodes the nucleoprotein (NP) in the negative sense RNA [16]. The NP encapsidates the six species of RGSV genomic RNAs to form distinct filamentous ribonucleoproteins (RNP) [15]. Besides NP, the RNPs also contain a polymerase protein, which is the RNA-dependent RNA polymerase (RdRp) of RGSV encoded by RNA1 in the negative sense [16]. Other RNA segments, as well as the viral sense RNA of RNA1 and 5, encode non-structural proteins whose functions remain poorly characterized [17, 18]. RGSV, transmitted by the brown planthopper Nilaparvata lugens (Stal) in a persistent-propagative manner, infects rice (Oryza sativa L.) and causes excess tillering and stunting of the plants. In some South, Southeast, and East Asian countries, RGSV poses a serious threat to rice production [19].
In spite of the economic importance of RGSV, no host factor interacting with this virus has been reported. In this paper, we report our findings that RGSV p5, which we have shown previously to be a RNA silencing suppressor and enhances pathogenicity of PVX in N. benthamiana, interacts with rice CIPK25 (OsCIPK25) [20]. Given the importance of CBL–CIPKs in the regulation of potassium homeostasis and the resemblance of RGSV symptoms to potassium deficiency in rice, we evaluated potassium content of RGSV-infected rice and found it to be much lower than that in the healthy rice [21, 22].
Materials and methods
Plant and virus
RGSV was collected from Haikou, Hainan province, China. The virus was maintained on rice plants by transmission using N. lugens (Stal).
Cloning and vector construction
Total RNA was extracted from healthy or RGSV-infected rice leaves using EasyPure Plant RNA Kit manufactured by Bejing Transgen Biotech Co. Ltd (Beijing, China). Reverse transcription was carried out using Fast Quant RT Kit with gDNase (Tiangen biotech CO., LTD, Beijng, China). Yeast two-hybrid vectors pGBKT7 and pGADT7 were purchased from Clontech (Takara, Dalian, China). The ORFs of the gene of interest were PCR amplified with gene-specific primers containing restriction sites for Nde I (forward primer) and BamH I (reverse primer) at the 5, termini. The PCR products were cloned with a T-vector pMD 20T and introduced to pGBKT7 and pGADT7 by enzyme digestion and ligation to obtain pGBKT7-p5, pGBKT7-OsCIPK5(25), pGADT7-p5, or pGADT7-OsCIPK5(25). Vectors (pEarleyGate 101-p5/OsCIPK5(25), pEarleyGate 102 p5/OsCIPK 5(25), pEarleyGate 201-YC-p5/OsCIPK5(25) and pEarleyGate 201-YN-p5/OsCIPK5(25) used in cellular localization and BiFC assays were generated using Gateway recombination technology (Invitrogen) by firstly inserting the genes of interest into the entry vector pDONR 221 [23].
Yeast two-hybrid assay
A rice seedling yeast two-hybrid cDNA library from rice cv Wuyujing 3 was constructed following CLONTECH protocols. Matchmaker Gal4 Two-Hybrid System 3 and libraries were used to screen the rice cDNA library as described previously [24]. The bait plasmid (after verifying that it has no autoactivation activity, data not shown) and cDNA library plasmid were transformed into yeast AH109 cells using the sequential transformation protocol. Colonies were selected on SD/Leu–Trp–His-medium and then positive colonies were isolated on SD/Leu–Trp–His–Ade–/X-a-gal medium according to the instruction manual. Primary positive candidate plasmids containing the rice cDNAs were isolated and then co-transformed into AH109 with bait plasmid pGBK-p5 to repeat the two-hybrid assay. The final positive candidate plasmids were selected and determined by sequencing analysis. The sequences of positive clones were subsequently used for an advanced BLAST search within the database of GenBank.
Cellular co-localization and BiFC
Agrobacterium tumefaciens strain EHA105 or GV3101 carrying genes of interest were grown separately to OD600 = 0.8 at 28 °C on Luria–Bertani liquid medium supplemented with 50 μg/μl of rifampicin and kanamycin. The cultures were centrifuged at 12,000×g for 1 min and resuspended in induction media (10 mM MES, pH 5.6, 10 mM MgCl2, and 150 μM acetosyringone). In co-localization and BiFC assays, A. tumefaciens containing different constructs ((pEarleyGate101-p5/pEarleyGate102-OsCIPK5(25), pEarleyGate102-p5/pEarleyGate101-OsCIPK5(25), pEarleyGate201-YC-p5/pEarleyGate 201-YN-OsCIPK5(25), pEarleyGate 201-YN-p5/pEarleyGate 201-YC-OsCIPK5(25)) were mixed in equal volumes. The mixtures of the bacterial cultures were incubated at room temperature for 3 h before infiltrated onto fully grown upper leaves. Six-week-old N. benthamiana was used for the experiment.
Potassium content determination
Rice potassium content was determined by flame photometry essentially as described by Cavell [25]. Briefly, serially diluted K solutions were measured with flame photometry. Readings of serially diluted potassium solutions were used to develop a standard curve. Potassium concentration in digested dried rice was determined by referring to the standard curve with readings from flame photometry. To digest rice plants, grounded rice material was treated sequentially with concentrated H2SO4, heating and H2O2. In each experiment, leaves of three RGSV-infected or healthy rice (about 2-month old) were pooled and the experiment was done in triplicates.
Confocal imaging analysis
The fluorophores in CFP and YFP were excited at 458 and 514 nm and images were taken using BA480–495 and BA535–565-nm emission filters, respectively.
Results
RGSV p5 interacts with CIPK25 and related CIPKs of rice in yeast
To identify potential host interactors of RGSV p5, the recombinant plasmid containing the 576-bp long ORF encoding RGSV p5 inserted in-frame with the GAL4 activation domain (pGBKT7-p5) was used as bait to screen a yeast two-hybrid library of rice. A total of 35 positive colonies were obtained among the approximately 3 × 105 independent transformants screened. Plasmids were isolated from these yeast colonies and the cDNA inserts within the pGADT7 vector were sequenced. Sequence analysis revealed that one of the cDNA inserts corresponded to the C-terminal part of OsCIPK25 (GQ380690) [26].
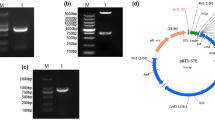
To test the interaction of RGSV p5 with CIPK25 in yeast, the full-length ORF of OsCIPK25 was cloned and inserted into the vector pGADT7 or pGBKT7, respectively. The recombinant plasmid (pGADT7–CIPK25/pGBKT7–CIPK25) was co-transformed into yeast cells with pGBKT7-p5/pGADT7-p5. As shown in Fig. 1a, co-transformants of pGBKT7-p5 and pGADT7–CIPK25, as well as those of pGBKT7–CIPK25 and pGADT7-p5 or pGBKT7-53 and pGADT7-T, which were used as positive controls grew well and turned blue in plates containing SD/–Ade/–His/–Leu/–Trp-dropout medium supplemented with x-α-gal (QDO-x-α-gal) [27]. In contrast, yeast cells co-transformed with pGBKT7-Lam and pGADT7-T, pGBKT7-p5, and pGADT7 empty vector, or pGBKT7 empty vector and pGADT7–CIPK25, which were used as negative controls, did not grow on the medium, although they grew well in plates containing the SD/Leu–Trp-dropout medium (DDO). The interaction between pGBKT7-p5 with two other CIPKs, namely OsCIPK5 (XM_015766105), which is closely related to OsCIPK25, and OsCIPK23 (EU703802.1), which is more distantly related to OsCIPK25 than OsCIPK5, was tested similarly. The results showed that RGSV p5 could interact with OsCIPK5, but not OsCIPK23 in yeast (Fig. 1b and data not shown).
RGSV p5 interacts with OsCIPK25 (a) and OsCIPK5 (b) in yeast. Yeast strain Y2H Gold was co-transformed with pGBK-P5/pGAD–CIPK25 (1), pGAD-P5/pGBK–CIPK25 (2), pGBK–CIPK25/pGADT7 (3), pGBK-P5/pGADT7 (4), pGADT7-T/pGBKT7-T53 (5), pGADT7-T/pGBKT7-Lam (6), pGBK-P5/pGAD-CIPK5 (7), pGAD-P5/pGBK-CIPK5 (8), pGBK-P5/pGAD-CIPK23 (9), or pGBK-CIPK5/pGADT7 (10). The co-transformants were spread on the selection medium SD/Leu-Trp (DDO), SD/–Ade/–His/–Leu/–Trp (QDO), or QDO supplemented with x-α-gal (QDO-x-a-gal). Images were photographed 5 days after the spreading
RGSV p5 interacts with CIPK25 and CIPK5 of rice in plant cells
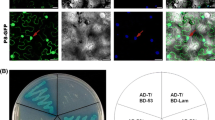
Cellular co-localization and bimolecular fluorescence complementation (BiFC) assay were used to confirm the interaction between RGSV p5 and rice OsCIPK5(25) in plant cells. For the cellular co-localization assay, RGSV p5 fused with yellow fluorescent protein (p5-YFP) or cyan fluorescent protein (p5-CFP) were co-expressed with OsCIPK5(25) fused with CFP (OsCIPK5(25)-CFP) or YFP (CIPK5(25)-YFP) in epidermal cells of N. benthamiana, respectively. As shown in Fig. 2, when individually expressed, p5 seemed to have a similar sub-cellular localization with CIPK5(25). Fluorescence of p5-CFP overlapped with that of CIPK5(25)-YFP respectively when the two fusion proteins were expressed together in tobacco cells. This indicated that RGSV p5 and rice CIPK5(25) were localized to similar sub-cellular compartments and thus would have chances to interact with each other.
Co-localization studies of OsCIPK25 (a), OsCIPK5 (b) and RGSV P5: Leaves of Nicotiana benthamiana were infiltrated with Agrobacterium EHA105 harboring plasmids as indicated to the left. Fluorescence in epidermal cells of the infiltrated area was observed 48 h after infiltration. Bars 10 μm. YFP yellow fluorescent protein, CFP cyan fluorescence protein, BF bright field
For the BiFC assay, RGSV p5 and OsCIPK5(25) were fused with the C- and N-terminal half of YFP, respectively. RGSV p5-YN/RGSV p5-YC was co-expressed with OsCIPK5(25)-YC/OsCIPK5(25)-YN in N. benthamiana. YFP fluorescence was observed 2 days after the co-expression by confocal microscopy. As shown in Fig. 3, reconstitution of YFP fluorescence was readily observable at this time. As negative controls, YFP fluorescence was not observed in cells co-expressing RGSVp5-YN/RGSVp5-YC and YC/YN (using the empty vector), OsCIPK5(25)-YC/OsCIPK5(25)-YN and YN/YC, or RGSV p5-YN/RGSVp5-YC and OsCIPK23-YC/OsCIPK23-YN (data not shown).
Bimolecular fluorescence complementation (BiFC) assay showing in planta interactions between OsCIPK25 (a, b)/OsCIPK5 (c, d) and RGSV P5: Leaves of Nicotiana benthamiana were infiltrated with Agrobacterium EHA105 harboring the indicated constructs. Fluorescence in epidermal cells of the infiltrated area was observed 48 h after infiltration. Bars 10 μm
RGSV-infected plant has a lower content of potassium
The above data clearly showed an interaction between RGSV p5 and two protein components of the plant-specific CBL–CIPK Ca+2 signaling network of rice. To date, the most well-established role of CBL–CIPKs is the regulation of ion metabolism, particularly those of sodium and potassium [13, 14, 21, 22]. In addition, RGSV-infected rice plants exhibit some phenotypes reminiscent of those caused by potassium deficiency. Therefore, we evaluated the effects of RGSV infection on rice total potassium content. To do this, two different rice varieties, Taizhong No. 1 and nipponbare, which show differences in their susceptibility to RGSV (with the former being more susceptible than the later according to our personal observation) were used. The results showed that potassium content in RGSV-infected rice was 52 and 32% lower than that in the healthy plants in the rice varieties Taizhong No. 1 (TN1) and nipponbare, respectively (Fig. 4).
RGSV infection results in lower content of potassium in infected rice. Potassium content in rice varieties Nipponbare and Taizhong No. 1 (TN1) showing symptoms typical of RGSV infection was measured and compared to that of the healthy rice. Student’s t test (*P < 0.05 and **P < 0.01) was used to analyze statistical significance of difference from the wild. DW dry weight
Discussion
We detected and confirmed a PPI between RGSV p5 and two protein components of the plant-specific CBL–CIPK Ca+2 signaling network of rice. To the best of our knowledge, this is the first report of host factors interacting with RGSV, an important rice infecting virus [15, 19]. Cellular proteins are interlinked with direct or indirect interactions to form a PPI network with key signaling proteins serving as the hub [28]. Pathogens tend to hijack hub proteins to manipulate host functions [29, 30]. The interaction between RGSV p5 and rice CIPK5/25 is consistent with this idea.
Presently, the biological implications of the interaction remain elusive. However, two non-mutually exclusive explanations are plausible based on our current understanding of plant–virus interaction [1]. First, RGSV may recruit OsCIPK5/25 functions by the interaction. For example, RGSV p5 may need to be phosphorylated to function properly. Many viral proteins such as the movement protein of Tobacco mosaic virus (TMV) have been shown to need a phosphrylation for proper cellular localization or maximum enzymatic activities [31, 32]. Direct interaction with a protein kinase may facilitate the phosphrylation. In addition, our recent studies have shown that RGSV p5 is a silencing suppressor [20]. There is evidence showing that some protein components of the plant Ca+2 signaling network are endogenous RNA silencing regulators and can be recruited by plant viruses including the potyvirus Tobacco etch virus (TEV) and the geminiviruses Tomato golden mosaic virus (TGMV) and Tomato yellow leaf curl China virus (TYLCCNV) [33–35]. Second, RGSV p5 may inactivate OsCIPK5/25 or disturb their normal function to interfere with the Ca+2 signaling pathway of rice. As it has been mentioned, CIPKs need special PPIs with CBLs. RGSV p5 may disturb the PPIs between OsCIPK5/25 and their cognate CBLs. Considering the emerging role of the CBL–CIPK Ca+2 signaling network in plant innate immunity, this may be a strategy used by RGSV to counteract the defense responses of rice [36–38]. Alternatively, OsCIPK5/25 may not be involved in rice immune responses and the inactivation of OsCIPK5/25 has no effect on viral infection. However, this may disturb the normal physiology of rice [26].
Currently, we cannot test these possibilities because of the lack of an infectious clone for RGSV and the low efficiency with which we can inoculate this virus to rice in our lab. Nevertheless, it is intriguing to note that the most well-established role of the CBL–CIPK Ca+2 signaling network in plants is the regulation of ion homeostasis including that of potassium, while RGSV infection causes symptoms such as brown spots on leaves which somewhat resemble those caused by potassium deficiency [13, 14, 21, 22]. With this in mind, we tested the effects of RGSV infection on potassium metabolism of rice. Also due to our inability to inoculate RGSV to rice with a high efficiency, we were unable to do time point studies. Instead, we measured potassium content of RGSV-infected rice at a rather late time point. Nevertheless, the results were striking and revealed a significant decrease of potassium content after RGSV infection. Specially, the extent of the decreasing corresponds with the severity of symptoms (Fig. 4). Although it is premature now to propose a correlation between p5-CIPK5(25) interaction, potassium deficiency and RGSV symptom induction because transgenic rice lines in which the expression of CIPK5 or CIPK25 was down-regulated by RNA interference (RNAi) exhibited no abnormal phenotype (data not shown), further experiments to investigate this possibility would be interesting.
References
A. Wang, Annu. Rev. Phytopathol. 53, 45–66 (2015)
C. Hipper, V. Brault, V. Zieglergraff, F. Revers, Front. Plant Sci. 4, 3141–3144 (2013)
P.D. Nagy, J. Pogany, Nat. Rev. Microbiol. 10, 137–149 (2012)
N. Pumplin, O. Voinnet, Nat. Rev. Microbiol. 11, 745–760 (2013)
J.N. Culver, M.S. Padmanabhan, J.N. Culver, Annu. Rev. Phytopathol. 45, 221–243 (2007)
V. Pallas, J.A. García, J. Gen. Virol. 92, 2691–2705 (2011)
D.S. Bush, Ann. Rev. Plant Physiol. Plant Mol. Biol. 46, 95–122 (1995)
P.J. White, M.R. Broadley, Ann. Bot.-Lond. 92, 487–511 (2003)
J. Kudla, O. Batistič, K. Hashimoto, Plant Cell 22, 541–563 (2010)
A.N. Dodd, J. Kudla, D. Sanders, Annu. Rev. Plant Biol. 61, 593–620 (2010)
T. Yang, B.W. Poovaiah, Trends Plant Sci. 8, 505–512 (2003)
L. Sheng, Trends Plant Sci. 14, 37–42 (2009)
S. Weinl, J. Kudla, New Phytol. 184, 517–528 (2009)
L. Sheng, W. Lan, S.C. Lee, Curr. Opin. Plant Biol. 12, 339–346 (2009)
B.W. Falk, J.H. Tsai, Annu. Rev. Phytopathol. 36, 139–163 (1998)
S. Toriyama, T. Kimishima, M. Takahashi, J. Gen. Virol. 78(Pt 9), 2355–2363 (1997)
O. Netsu, A. Hiraguri, T. Uehara-Ichiki, K. Komatsu, T. Sasaya, Virus Res. 203, 10–19 (2015)
T.D. Nguyen, S. Lacombe, M. Bangratz, H.A. Ta, D.N. Vinh, P. Gantet, C. Brugidou, Virus Genes 51, 267–275 (2015)
H. Hibino, Annu. Rev. Phytopathol. 34, 249–274 (1996)
C. Zhang, X.J. Liu, K.C. Wu, L.P. Zheng, Z.M. Ding, F. Li, P. Zou, L. Yang, J.G. Wu, Z.J. Wu, Arch. Virol. 160, 2769–2779 (2015)
J. Xu, H.D. Li, L.Q. Chen, Y. Wang, L.L. Liu, L. He, W.H. Wu, Cell. 125(7), 1347–1360 (2006)
Y. Wang, W.H. Wu, Annu. Rev. Plant Biol. 64, 451–476 (2013)
M. Karimi, D. Inzé, A. Depicker, Trends Plant Sci. 7, 193–195 (2002)
L. Lu, Z. Du, M. Qin, P. Wang, H. Lan, X. Niu, D. Jia, L. Xie, Q. Lin, L. Xie, Z. Wu, Virus Genes 38, 320–327 (2009)
A.J. Cavell, J. Sci. Food Agric. 5, 195–200 (1954)
P. Kanwar, S.K. Sanyal, I. Tokas, A.K. Yadav, A. Pandey, S. Kapoor, G.K. Pandey, Cell Calcium 56, 81–95 (2014)
J.A. Mietz, T. Unger, J.M. Huibregtse, P.M. Howley, EMBO J. 11, 5013–5020 (1993)
P. Braun, S. Aubourg, J. Van Leene, G. De Jaeger, C. Lurin, Annu. Rev. Plant Biol. 64, 161–187 (2013)
R. Weßling, P. Epple, S. Altmann, Y. He, Y. Li, S. Henz, N. Mcdonald, K. Wiley, K.C. Bader, C. Gläßer, M.S. Mukhtar, S. Haigis, L. Ghamsari, A.E. Stephens, J.R. Ecker, M. Vidal, J.D.G. Jones, K.F.X. Mayer, T.E.V. Loren, D. Weigel, P. Schulze-Lefert, J.L. Dangl, R. Panstruga, B. Pascal, Cell Host Microbe 16, 364–375 (2014)
M.S. Mukhtar, A.R. Carvunis, M. Dreze, P. Epple, J. Steinbrenner, J. Moore, M. Tasan, M. Galli, T. Hao, M.T. Nishimura, Science 333, 596–601 (2011)
E. Waigmann, M.H. Chen, R. Bachmaier, S. Ghoshroy, V. Citovsky, EMBO J. 19, 4875–4884 (2000)
V. Citovsky, B.G. Mclean, J.R. Zupan, P. Zambryski, Gene Dev 7, 904–910 (1993)
R. Anandalakshmi, R. Marathe, X. Ge, J.M. Herr, C. Mau, A. Mallory, G. Pruss, L. Bowman, V.B. Vance, Science 290, 142–144 (2000)
H.Y. Chung, G. Lacatus, G. Sunter, Virology 460–461, 108–118 (2014)
F. Li, C. Huang, Z. Li, X. Zhou, PLoS Pathog. 10, 304–310 (2014)
K. Xie, Y. Yang, Plant Cell 26, 3077–3089 (2014)
T. Asano, N. Hayashi, M. Kobayashi, N. Aoki, A. Miyao, I. Mitsuhara, H. Ichikawa, S. Komatsu, H. Hirochika, S. Kikuchi, Plant J. 69, 26–36 (2012)
L. Fu, X. Yu, C. An, Plant Physiol. Biochem. 73, 202–210 (2013)
Acknowledgements
This work was supported by Grants from the National Basic Research Program 973 (2014CB138402), Natural Science Foundation of Fujian, China (2016J05071), Natural Science Foundation of China (31301640), and FAFU Science Fund for Distinguished Young Scholars (xjq201622).
Author contributions
G.H.X. and X.J.L. conducted the yeast two-hybrid experiment; G.H.X. and P.Q. conducted the BiFC and cellular localization experiments; X.Y.W. conducted the K+ quantification experiments; J.Z. contributed in vector construction; Z.G.D. and Z.J.W. designed the experiments and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Seung-Kook Choi.
Rights and permissions
About this article
Cite this article
Xiong, G., Liu, X., Qiu, P. et al. Rice grassy stunt virus p5 interacts with two protein components of the plant-specific CBL–CIPK Ca+2 signaling network of rice. Virus Genes 53, 446–453 (2017). https://doi.org/10.1007/s11262-017-1437-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-017-1437-z