Abstract
Rice stripe virus (RSV) infects rice and causes great yield reduction in some Asian countries. In this study, rice cDNA library was screened by a Gal4-based yeast two-hybrid system using pc4, a putative movement protein of RSV, as the bait. A number of positive colonies were identified and sequence analysis revealed that they might correspond to ten independent proteins. Two of them were selected and further characterized. The two proteins were a J protein and a small Hsp, respectively. Interactions between Pc4 and the two proteins were confirmed using coimmunoprecipitation. Implications of the findings that pc4 interacted with two chaperone proteins were discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection cycle of plant viruses involves a phase of movement from initially infected cell into adjacent neighboring cells via plasmodesmata (PD). This cell-to-cell movement is aided by virus-encoded proteins termed movement proteins (MPs). Sometimes, the MPs could form tubules to replace PD to facilitate passage of virions. But, more often, the MPs only transiently and reversibly dilate PD openings to mediate transport of viral nucleic acids or ribonucleic acids–protein complexes [1–4]. In the second case, the MPs usually share many features with a set of endogenous host factors named non-cell autonomous proteins (NCAPs) in terms of cell-to-cell movement [1–12]. Therefore, it is widely accepted that viral MPs exploit pre-existing cellular pathways to fulfill their function. Supporting this, crosscompetition experiments have demonstrated that the viral MPs and host NCAPs likely utilize a common receptor in the pathway for cell-to-cell transport [13]. Additionally, expression of a dominant-negative mutant form of NtNCAPP1, a non-cell-autonomous pathway protein (NCAPP), abolished cell-to-cell transport of TMV MP as well as specific NCAPs such as CmPP16 [14]. The plant non-cell-autonomous pathway involves a set of cellular players that work coordinately [5–12]. Therefore, it is envisionable that many functions of viral MPs are dependent on a chain of interactions with these host factors [1–12].
It was found that phloem proteins ranging from 10 to 200 kDa induced an increase in size exclusion limit (SEL) to the same extent, greater than 20 but less than 40 kDa, yet they all could move from cell to cell [15]. This suggested that protein unfolding might be an essential step in plasmodesmal trafficking. Consistent with this, chemical-crosslinked KN1 that was unable to undergo conformational changes failed to mediate its own cell-to-cell transport [13]. The involvement of a phase of protein unfolding implicated a role of chaperone proteins in the NCAP pathway. Involvement of chaperone proteins in viral cell-to-cell movement was best illustrated by beet yellow virus (BYV), a member of the closteroviridae. This virus encodes an hsp70 homologue in its genome. The hsp70 homologue targets PDs and has been shown to be essential for cell-to-cell movement of BVY [16–18]. However, most viruses do not encode chaperones in their genomes. Instead, it seems that they have adapted to use the existing host cellular chaperone network. To do this, they could manipulate the host transcriptional network to induce expressions of a particular set of chaperone proteins [19]. Alternatively, they could recruit a chaperone protein directly from the host. For example, several viral movement proteins or proteins involved in viral movement were shown to interact with a set of DnaJ proteins [20–23]. Interestingly, it was shown recently that several HSP cognate 70 (hsc70) chaperones isolated from PD-rich wall fractions and from Cucubita pholem exudates could interact with PD and modify the PD SEL. Introduction of a common motif identified in these hsc70s allowed a human hsp70 protein to modify the PD SEL and move from cell to cell [24]. This raised the possibility that hsp70s might play a more direct role in viral movement than previously expected. For example, the Hsp70s, which have intrinsic ATPase activity, could serve as motor proteins facilitating the transport of viral materials through the PD. Noteworthily, no chaperone proteins other than the hsp70s family or their intimate partners have been reported to be involved in plant viral cell-to-cell movement.
RSV is the type species of the genus Tenuivirus, which has not been assigned to any family. It is transmitted transovarily in a circulative manner by some planthopper species (Delphacidae family), primarily the small brown planthopper [25–28]. The genome of RSV comprises four RNAs, named RNA1 to RNA4 in the decreasing order of their molecular weight [25, 28]. RNA1 is of negative sense and encodes a putative protein with a molecular weight of 337 kDa, which was considered to be part of the RNA dependent RNA polymerase associated with the RSV filamentous ribonucleoprotein (RNP) [29–31]. RNAs 2–4 are ambisense, each containing two ORFs, one in the 5′ half of viral RNA (vRNA, the proteins they encoded named p2–p4) and the other in the 5′ half of the viral complementary RNA (vcRNA, the proteins they encoded named pc2–pc4). Pc2 shows stretches of weak amino acid similarity with membrane protein precursor of members of the Bunyaviridae that is processed into two membrane-spanning glycoproteins; however, there is no evidence that RSV forms enveloped particles that could incorporate such glycoproteins [32]. P3 of RSV shares 46% identity with its counterpart, gene-silencing suppressor NS3 protein of the tenuivirus rice hoja blanca virus (RHBV) [33]. Pc3 is the nucleocapsid protein (CP) and p4 the major non-structural protein (NSP), whose accumulation in infected plants correlates with symptom development [34–37]. Pc4 shares some common structures with the viral 30k superfamily movement proteins [38]. Recently, Xiong et al. showed that this protein localized predominantly near or within the cell walls, could move from cell to cell and complement movement defective PVX [39]. This suggested that Pc4 might be a movement protein of RSV. However, as a negative strand RNA virus that does not seem to form intact virions, it can be envisioned that cell-to-cell movement of RSV would be a very complex process, which deserves further research.
RSV infects agriculturally important crop plants such as rice and causes significant yield losses in east Asia. However, our knowledge about RSV, especially its interactions with host factors, remains sparse, partially owning to its reluctance to traditional virological methods such as infectious cloning. To bypass such obstacles, we have used Yeast two-hybrid system to investigate all the potential interactions between RSV encoded proteins and host factors [40]. Here, we report our identification of the interactions of pc4, the putative movement protein of RSV, with a DnaJ protein and an hsp20 family protein of rice.
Materials and methods
Plasmid construction
Total RNA was extracted from RSV-infected rice leaves with Trizol and the RSV gene segment NSvc4 was amplified by RT-PCR with primer pairs F1 and R1. PCR products were cloned into pMD18-T and then digested with NdeI and BamHI, followed by ligation into pGBKT7 vector. The recombinant vector containing the RSV NSvc4 segment was designated as pGBK-NSvc4, and was used as the bait plasmid for following screening by yeast two-hybrid. Two cDNA fragments identified during the yeast two-hybrid screening had sequence identity with two genes encoding an hsp20 and DnaJ protein, respectively. The full-length ORF of the hsp20 and DnaJ were amplified by RT-PCR with primer pairs F2/R2, F3/R3, which were designed according to rice cDNA sequences. The specific fragments were cloned into pMD18-T. Construct containing hsp20 ORF was digested with NdeI and BamHI, and fragment was ligated into NdeI/BamHI-linearized pGADT7 vector. Construct containing DnaJ ORF was digested with SpeI and XhoI, and fragment was ligated into SpeI/XhoI-linearized pGADT7-CP vector containing SpeI site that was previously constructed. The recombinant plasmids were designated as pGAD-hsp20 and pGAD-DnaJ, respectively. There is a c-Myc-epitope tag at the 5′ terminus of NSvc4 ORF in the pGBK-NSvc4 and a HA-epitope tag at the 5′ terminus of hsp20 ORF in the pGAD-hsp20 and DnaJ ORF in the pGAD-DnaJ, respectively. The fusion gene c-Myc-NSvc4 was amplified by PCR with primer pairs F4/R4 using pGBK-NSvc4 as template and cloned into pMD18-T. Construct containing c-Myc-NSvc4 was digested with EcoRI and BamHI, and fragment was ligated into a cauliflower mosaic virus 35S-based pEGAD transient-expression vector linearized by EcoRI and BamHI and the recombinant plasmid was designated as pEGAD-Myc-NSvc4. The c-Myc-NSvc4 fragment digested with Hind and SacI was inserted into Hind/SacI-linearized pKYLX35S2 vector and recombinant plasmid was designated as pKYLX-Myc-NSvc4. The fusion genes HA-hsp20 and HA-DnaJ were amplified by PCR with primer pairs F5/R5 using pGAD-hsp20 and pGAD-DnaJ as templates, respectively, and cloned into pMD18-T. The HA-hsp20 restriction fragment digested with EcoRI and BamHI was ligated into EcoRI/BamHI-linearized pEGAD vector, and the HA-hsp20 and HA-DnaJ restriction fragments digested with Hind III and XbaI were ligated into Hind/XbaI-linearized pKYLX35S2 vector. The recombinant plasmids were designated as pEGAD-HA-hsp20, pKYLX-HA-hsp20, and pKYLX-HA-DnaJ, respectively. The HA-hsp20 had been not ligated into Pegad vector because there were no suitable restriction enzyme sites. In addition, the following recombinant vectors constructed previously were used in this study: pGBK-CP containing RSV CP segment, pGBK-SP containing RSV NSP segment, pEGAD-Myc-CP and pKYLX-Myc-CP containing fusion gene Myc-CP, pEGAD-Myc-SP and pKYLX-Myc-SP containing fusion gene Myc-SP.
Yeast two-hybrid assay
A rice seedling yeast two-hybrid cDNA library from rice cv Wuyujing 3 was constructed with CLONTECH protocols. The titer of the library was determined after amplification and was approximately 1.0 × 1011 cfu/ml. Matchmaker Gal4 Two-Hybrid System 3 and libraries were used to screen the rice cDNA library. The bait plasmid and cDNA library plasmid were transformed into yeast AH109 cells using sequential transformation or simultaneous cotransformation protocol. Colonies were selected on SD/Leu-Trp-His-Ade- medium and then Ade+/His+ positive colonies were isolated on SD/Leu-Trp-His-Ade-/X-α-gal+ medium according to the instruction manual. Primary positive candidate plasmids containing the rice cDNAs were isolated and then co-transformed into AH109 with bait plasmid pGBK-NSvc4 to repeat the two-hybrid assay. The final positive candidate plasmids were selected and determined by sequencing analysis. The sequences of positive colons were subsequently used for an advanced BLAST search within the database of GenBank.
Agrobacterium-mediated transient expression
Agrobacterium strain EHA105 carrying the gene of interest expressed from a binary vector was infiltrated into leaves of Nicotiana benthamiana. Agrobacterium tumefaciens was grown overnight at 28°C on Luria-Bertani agar containing 50 μg/μl of rifampicin and 50 μg/μl of kanamycin. Cells were resuspended in induction media (10 mM MES, pH 5.6, 10 mM MgCl2, and 150 µM acetosyringone) and incubated at room temperature for 3–5 h before inoculation.
Immunoprecipitation
After agrobacterium-mediated transient expression for 24 h, N. benthamiana leaves (approximately 0.3 g) were harvested and ground to a powder in liquid nitrogen. Ground tissues were resuspended in 3.0 ml of IP buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol, 0.1% Nonidet P-40, 5 mM dithiothreitol, and 1.53 Complete Protease Inhibitor [Roche]). The crude lysates were then spun at 20,000g for 15 min at 4°C. After centrifugation, 1 ml of supernatant was incubated with 0.5 μg of the indicated monoclonal antibody for each immunoprecipitation. After a 1-h incubation at 4°C, immunocomplexes were collected by the addition of 50 μl of protein G Sepharose-4 fast flowbeads and incubated end over end for 3–5 h at 4°C. After incubation, the immunocomplexes were washed four times with 1 ml of IP buffer and the pellet was resuspended in 1× SDS-PAGE loading buffer.
Protein separation and western blotting
Protein samples were separated by SDS-PAGE on 10% polyacrylamide gels and transferred by electroblotting to PVDF membranes. Membranes were probed with anti-HA horseradish peroxidase (Roche) or anti-Myc peroxidase (Sigma-Aldrich) to detect HA- and Myc-epitope-tagged proteins, respectively. All immunoprecipitation experiments were repeated at least three times, and the identical results were obtained.
Primers used in this study
Primers used in this study are listed in Table 1.
Results
Identification of RNB8 and RNB5 that interact with RSV pc4
To identify rice proteins that interacted with RSV pc4, the rice cDNA library was screened by a Gal4-based yeast two-hybrid system. A number of positive colonies were identified among the approximately 3.6 × 106 clones that were screened. Sequence analysis of these colonies showed that they might correspond to ten independent proteins. Two of them, designated NB8 and NB5, shared high degree of identity with a DnaJ protein (NM_001060020) and a heat shock protein 20 (hsp20, NM_001056192) from Oryza sativa, respectively. The full-length ORFs corresponding to NB8 and NB5 (hereafter we use RNB8 and RNB5 to represent the genes corresponding to NB8 and NB5, respectively) were cloned from rice using primers designed according to available rice sequences in NCBI. Specific interactions between the two proteins and RSV pc4 were then confirmed using entire ORFs of the two genes by yeast two-hybrid experiments (data not shown).
Sequence analysis of the coding regions indicated that the ORF of the RNB8 gene contained 1,251 nucleotides and encoded a protein of 416 amino acids; the deduced amino acids sequence of RNB8 contained conserved cysteine-rich domains and several glycine-rich regions in addition to the typical J domain (Fig. 1a), thus it represented a member of the group I dnaJ proteins [41–43]. The ORF of the RNB5 gene contained 486 nucleotides and encoded a protein of 161 amino acids with conserved alpha-crystallin domain (ACD) typical of a class of small Hsps [44–47]. The cDNA fragment of the NB8 we initially retrieved from screening of the rice library encoded a polypeptide of 165 amino acid residues that located on the C-terminus of the RNB8 gene (Fig. 1a). This region might be responsible for the interactions between RNB8 and RSV pc4. The cDNA fragment of the NB5 encompasses the entire ORF of the RNB5 (Fig. 1b).
Alignments of the DnaJ and hsp20 proteins from diverse species. The alignments were made using DNAMAN (4.0). a Alignment of DnaJ proteins from Oryza sativa (RNB8 and EAZ24062.1), Arabidopsis (NP_850653.1), Triticum aestivum(ABG78615.1), and zea mays (ACF82216.1). The conserved J domain is marked by a black line. Glycine-rich regions and 4 repeats of C-X-X-C-X-G-X-G motif typical of type I J proteins are boxed and marked by a red line, respectively. Regions of identity or similarity are colored and gaps introduced for alignment are indicated by dots. b Alignment of hsp20 proteins Oryza sativa (RNB5 and AAC78393.1), Pennisetum glaucum (CAA63903.1), and Zea mays (ACF78669.1). Black line indicates the conserved alpha-crystallin domain
Pc4 interacts with the rice dnaJ and hsp20 in plant cells
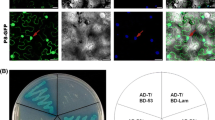
Specific interactions of pc4 with rice DnaJ and hsp20 in yeast suggest functional significance. To test the interactions further, coimmunoprecipitation was used to determine whether such interactions occur in plant cells. As shown in Fig. 2, the c-Myc-epitope-tagged pc4 coimmunoprecipitated with the HA-epitope-tagged RNB8 and HA-epitope-tagged RNB5 after Agrobacterium-mediated transient expression in Nicotiana benthamiana. The interactions were confirmed with the reciprocal experiments, in which HA-epitope-tagged RNB8 and RNB5 were coimmunoprecipitated with the c-Myc-epitope-tagged pc4, respectively. These results provided evidence that RSV pc4 interacts with the DnaJ and hsp20 in plant cell, whereas there were no such interactions of the two rice proteins with RSV CP and SP (Fig. 2).
RSV Pc4, but not CP and NSP, interacted with RNB5 and RNB8 proteins in plant cells. a Immunoblot showing NSvc4 coimmunoprecipitated with the hsp20 (RNB5); for lanes 1–3, the total proteins were extracted from Agrobacterium-infiltrated Nicotiana benthamiana leaves expressing Myc-CP/HA-hsp20, (lane 1), Myc-SP/HA-hsp20 (lane 2), or Myc-pc4/HA-hsp20 (lane 3) and immunoprecipitated with anti-HA (top) or anti-c-Myc (bottom) antibodies. For lanes 4–7, the total proteins were extracted from Agrobacterium-infiltrated Nicotiana benthamiana leaves expressing only Myc-pc4 (lanes 4 and 6) or HA-hsp20 (lanes 5 and 7) and immunoprecipitated with anti-c-Myc (top) or anti-HA (bottom) antibodies. b Immunoblot showing pc4 coimmunoprecipitated with DnaJ (RNB8)
Discussion
Most plant viruses encode specific proteins dedicated to movement of their infectious materials. These movement proteins exploit host NCAP pathway to fulfill their function. Identification of host factors interacting with viral MPs is essential to understand viral movement [1–12]. Here, using pc4, a putative movement protein of RSV, as bait, we identified two rice proteins, i.e., a J protein and a member of the hsp20 family. The two proteins interacting with pc4 did not interact with CP and NSP in yeast and in plant cell, indicating that the interactions between the two proteins and Pc4 were specific.
J proteins, featured by a 70-amino acid signature sequence through which they bind to their partner Hsp70s, are key regulators of the ATP cycle of hsp70s [41–43]. Three groups of J proteins have been characterized. Type I proteins are similar to E. coli DnaJ with the J domain, the Gly/Phe-rich region, and the cysteine repeats. Typ II proteins contain the J domain and the Gly/Phe-rich region, but lack the cysteine repeats. Typ III proteins do not have any of the conserved regions other than the J domain [41–43].
It is believed that transport of the NCAPs to and through the PD involves a phase of conformational change of the NCAPs [13, 15]. The need of a conformational change entails the availability of a putative chaperone protein. The most promising candidate of such a chaperone is a member of the hsp70s, a family of versatile proteins that have been implicated in protein translation, folding, unfolding, translocation, and degradation [48, 49]. Thus, it would be reasonable to speculate that the interaction of Pc4 with a J protein would allow the protein to locally concentrate Hsp70s. By analogy with models proposed for protein translocation into ER and mitochondria [50], unfolding of the movement protein could occur firstly at a conformationally flexible region, the hsp70s then might bind this region and promote further unfolding through trapping and sliding. For more tightly folded domains within the movement protein, the hsp70s could also provide a vigorous force to bias the equilibrium to an unfolded state [50, 51]. The hsp70s that were recently identified to move from cell to cell are attracting candidates to fulfill this function [24].
Another possibility is that Hsp70s could present the viral MPs to host ubiquitin-proteosome pathway for degradation. Degradation of MP by host ubiquitin-proteosome pathway has been observed in TMV [52–54]. It was suggested that the degradation might function to avoid extreme damage to the host and futile movement of the viral materials [52–55]. This was consistent with the observation that Pc4 could only be detected in infected rice plants at a very early stage of infection [56, 57]. In this scenario, the observed larger size of Pc4 when detecting with antisera to the protein in a previous report could be the result of polyubiquination [56, 57].
Previously, NSm, the movement protein of tomato spotted wilt tospovirus (TSWV), has been shown to interact with several members of J proteins from Nicotiana tabacum and Arabidopsis thaliana [20, 21]. The capsid protein (CP) of potyviruses, which is involved in movement of the virus, has been shown to interact with a set of J proteins from tobacco [22]. In the later case, transgenic plants that ectopically overexpress dominant-negative mutants of NtCPIPs showed significantly enhanced virus resistance to PVY, and the resistance was most likely due to strongly reduced cell-to-cell transport [22]. Taking these into account, the recruitment of Hsp70s through interactions with a J protein to facilitate movement protein function seems to be a widely used mechanism of plant viruses. This raises two questions; the first is why most plant viruses do not encode an Hsp70 themselves. The second is why the MPs do not interact with an Hsp70 directly. The answer to the first question is obvious. Recruiting an Hsp70 from the host is more economically reasonable than to encode one. The answer to the second question might lie in the fact that the host encodes more J proteins than Hsp70s, which implies that the functional specificity of a J protein/Hsp70 combination is determined by the J protein [41–43]. It is noteworthy that the J proteins interacting with TSWV NSm and PVY CP belong to type III J proteins. Yet, the J protein identified in this study was a type I DnaJ protein [41–43].
This study also identified a small hsp that interacted with Pc4. sHSPs, defined by possessing a conserved alpha-crystallin domain (ACD), are the most abundant and complex subset of HSPs in plants [44, 45]. Key function of the sHSPs is to prevent aggregation of denatured proteins. By forming a soluble complex with substrate proteins, they can create a transient reservoir of substrates for subsequent refolding by ATP-dependent chaperone systems [44–47]. It is tempting to assume that the Hsp20 forms a complex with Pc4 when the protein is partially unfolded for transport through PD. The presence of the Hsp20 keeps the denatured Pc4, and perhaps the entire viral material for movement, soluble. And when the viral material entered the neighboring cell, the presence of the Hsp20 would allow for an immediate and efficient renature of the viral material. To our knowledge, this is the first report that a plant viral MP interacts with a small Hsp. By analogy with other negative RNA viruses, the ribonucleoprotein particles (RNPs) represent the only structures responsible for transcription and replication for RSV [58–60]. Thus, the infectious materials that move from cell to cell for RSV must be entire RNPs. The RSV RNPs are very complex in structure, containing at least the RdRps, CPs, and genome-length viral RNAs [25, 28]. This might be responsible for our results that MP of RSV needed to interact with an hsp20 in addition to a J protein.
RSV infects rice, one of the most important crop plants in the world, and poses a major threat to rice production in some Asian countries. The identification of the two host factors interacting with a putative movement protein of RSV will undoubtedly propel a step forward of our understanding of RSV. Perhaps more importantly, as has been mentioned, expression of a mutant form of a J protein that interacts with CP of PVX in tobacco dramatically increased the viral resistance of various transgenic lines [22]. Transgenic rice plants expressing RSV CP have been developed and were shown to be efficient for RSV resistance [61]. But the introduction of a viral gene to food crops would inevitably invoke safety concerns. It is intriguing to engineer transgenic rice plants expressing a mutant form of the J protein identified in this study and test their resistance to RSV.
References
J.C. Carrington, K.D. Kasschau, S.K. Mahajan, M.C. Schaad, Plant Cell 8, 1669–1681 (1996)
W.J. Lucas, Virology 344, 169–184 (2006)
H.B. Scholthof, Trends Plant Sci. 10, 376–382 (2005)
E. Waigmann, S. Ueki, K. Trutnyeva, V. Citovsky, Crit. Rev. Plant Sci. 23, 195–250 (2004)
S. Ghoshroy, R. Lartey, J.S. Sheng, V. Citovsky, Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 25–48 (1997)
J.Y. Kim, Curr. Opin. Plant Biol. 8, 45–52 (2005)
K.L. Gallagher, P.N. Benfey, Genes Dev. 19, 189–195 (2005)
T. Kurata, K. Okada, T. Wada, Curr. Opin. Plant Biol. 8, 600–605 (2005)
P. Boevenik, K. Oparka, Plant Physiol. 138, 1815–1821 (2005)
K.J. Oparka, Trends Plant Sci. 9, 33–41 (2004)
W.J. Lucas, J.-Y. Lee, Nat. Rev. Mol. Cell Biol. 5, 712–726 (2004)
R. Ruiz-Medrano, B. Xoconostle-Ca′zares, F. Kragler, Curr. Opin. Plant Biol. 7, 641–650 (2004)
F. Kragler, J. Monzer, K. Shash, B. Xoconostle-Ca′zares, W.J. Lucas, Plant J. 15, 367–381 (1998)
J.Y. Lee, B.C. Yoo, M.R. Rojas, N. Gomez-Ospina, L.A. Staehelin, W.J. Lucas, Science 299, 392–396 (2003)
S. Balachandran, Y. Xiang, C. Schobert, G.A. Thompson, W.J. Lucas, Proc. Natl Acad. Sci. USA 94, 14150–14155 (1997)
D.V. Alzhanova, A.J. Napuli, R. Creamer, V.V. Dolja, EMBO J. 20, 6997–7007 (2001)
V.V. Peremyslov, Y. Hagiwara, V.V. Dolja, PNAS 96, 14771–14776 (1999)
A.A. Agranovsky, V.P. Boyko, A.V. Karasev, E.V. Koonin, V.V. Dolja, J. Mol. Biol. 217, 603–610 (1991)
S.A. Whitham, S. Quan, H.S. Chang, B. Cooper, B. Estes, T. Zhu, X. Wang, Y.M. Hou, Plant J. 33, 271–283 (2003)
S. von Bargen, K. Salchert, M. Paape, B. Piechulla, J.W. Kellmann, Plant Physiol. Biochem. 39, 1083–1093 (2001)
T.R. Soellick, J.F. Uhrig, G.L. Bucher, J.W. Kellmann, P.H. Schreier, Proc. Natl Acad. Sci. USA 97, 2373–2378 (2000)
D. Hofius, A.T. Maier, C. Dietrich, I. Jungkunz, F. Bornke et al., J. Virol. 81, 11870–11880 (2007)
S. Haupt, G.H. Cowan, A. Ziegler, A.G. Roberts, K.J. Oparka, L. Torrance, Plant Cell 17, 164–181 (2005)
K. Aoki, F. Kagler, B. Xoconostle-Ca′zares, W.J. Lucas, Proc. Natl Acad. Sci. USA 99, 16342–16347 (2002)
B.W. Falk, J.H. Tsai, Annu. Rev. Phytopathol. 36, 139–163 (1998)
B.W. Falk, Tenuiviruses, in Encyclopedia of Virology, ed. by R.G. Webster, A. Granoff (Academic, London, 1994), pp. 1410–1416
H. Hibino, Annu. Rev. Phytopathol. 34, 249–274 (1996)
B.-C. Ramirez, A.-L. Haenni, J. Gen. Virol. 75, 467–475 (1994)
S. Toriyama, M. Takahashi, Y. Sano, T. Shimizu, A. Ishihama, J. Gen. Virol. 75, 3569–3579 (1994)
S. Toriyama, J. Gen. Virol. 67, 1247–1255 (1986)
P. Barbier, M. Takahashi, I. Nakamura, S. Toriyama, A. Ishihama, J. Virol. 66, 6171–6174 (1992)
M. Takahashi, S. Toriyama, C. Hamamatsu, A. Ishihama, J. Gen. Virol. 74, 769–773 (1993)
E. Bucher, T. Sijen, P. De Haan, R. Goldbach, M. Prins, J. Virol. 77, 1329–1336 (2003)
Y. Zhu, T. Hayakawa, S. Toriyama, M. Takahashi, J. Gen. Virol. 72, 763–767 (1991)
T. Kakutani, Y. Hayano, T. Hayashi, Y. Minobe, J. Gen. Virol. 72, 465–468 (1991)
T. Kakutani, Y. Hayano, T. Hayashi, Y. Minobe, J. Gen. Virol. 71, 1427–1432 (1990)
A.M. Espinoza, R. Pereira, A.V. Macaya-Lizano, M. Hernandez, M. Goulden, C. Rivera, Virology 195, 156–166 (1993)
U. Melcher, J. Gen. Virol. 81, 257–266 (2000)
R. Xiong, J. Wu, Y. Zhou, X. Zhou, J. Virol. 82, 12304–12311 (2008)
S. Fields, O. Song, Nature 340, 245–246 (1989)
W.L. Kelley, Trends Biochem. Sci. 23, 222–227 (1998)
X.B. Qiu, Y.M. Shao, S. Miao, L. Wang, Cell. Mol. Life Sci. 63, 2560–2570 (2006)
P. Walsh, D. Bursac, Y.C. Law, D. Cyr, T. Lithgow, EMBO Rep. 5, 567–571 (2004)
E.R. Waters, G.J. Lee, E. Vierling, J. Exp. Bot. 47, 325–338 (1996)
R.S. Boston, P.V. Viitanen, E. Vierling, Plant Mol. Biol. 32, 191–222 (1996)
H. Nakamoto, L. Vígh, Cell. Mol. Life Sci. 64, 294–306 (2007)
Y. Sun, T.H. MacRae, Cell. Mol. Life Sci. 62, 2460–2476 (2005)
B. Bukau, A.L. Horwich, Cell 92, 351–366 (1998)
M.P. Mayer, B. Bukau, Cell. Mol. Life Sci. 62, 670–684 (2005)
M. Pilon, R. Schekman, Cell 97, 679–682 (1999)
R. Sousa, E.M. Lafer, Traffic 7, 1596–1603 (2006)
C. Reichel, R.N. Beachy, J. Virol. 74, 3330–3337 (2000)
M. Heinlein, H.S. Padgett, J.S. Gens, B.G. Pickard, S.J. Casper, B.L. Epel, R.N. Beachy, Plant Cell 10, 1107–1120 (1998)
C. Reichel, R.N. Beachy, Proc. Natl Acad. Sci. USA 95, 11169–11174 (1998)
E. Waigmannl, M. Curin, M. Heinlein, Plant Cell Monogr. 7, 29–62 (2007)
Z. Qu, D. Shen, Y. Xu, J. Tan, R. Hull, Chin. J. Genet. 26, 331–337 (1999)
D.L. Liang, X.Q. Ma, Z.C. Qu, Virus Genes 31, 203–209 (2005)
R.M. Elliott, J. Gen. Virol. 71, 501–522 (1990)
F. Baudin, C. Bach, S. Cusack, R.W. Ruigrok, EMBO J. 13, 3158–3165 (1994)
K. Klumpp, R.W.H. Ruigrok, F. Baudin, EMBO J. 16, 1248–1257 (1997)
T. Hayakawa, Y. Zhu, K. Itoh, Y. Kimura, T. Izawa, K. Shimamoto, S. Toriyama, Proc. Natl Acad. Sci. USA 89, 9865–9869 (1992)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lianming Lu and Zhenguo Du contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, L., Du, Z., Qin, M. et al. Pc4, a putative movement protein of Rice stripe virus, interacts with a type I DnaJ protein and a small Hsp of rice. Virus Genes 38, 320–327 (2009). https://doi.org/10.1007/s11262-008-0324-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-008-0324-z