Abstract
The present research evaluated the positive effects of dietary thiamin (vitamin B1) levels on the growth performance, serum biochemistry factors, immune response, and antioxidant activity of great sturgeon (Huso huso) juveniles. Thiamin was included in diets with levels of 0 (control, T0), 7 (T7), 15 (T15), and 25 (T25) mg/kg diet. Measurements of thiamin levels in diets indicated that they contained 1.80 (T0), 8.02 (T7), 16.2 (T15), and 26.6 (T25) mg thiamin/kg feed. Sturgeon juveniles (240 individuals) with average weight of 44.8 ± 1.96 g were distributed into 12 tanks, and fed with the experimental diets for 8 weeks. Final weight, body weight gain (%), specific growth rate, and feed conversion ratio (FCR) of great sturgeon were significantly influenced by dietary thiamin levels, and the maximum fish performance (P < 0.05) was obtained at a level of 15 mg/kg diet. The trypsin, chymotrypsin, creatine kinase, lipase, α-amylase, and alkaline phosphatase activities were notably (P < 0.05) affected by the dietary thiamin levels. The glucose content was not significantly (P > 0.05) different among the experimental treatments. Diets supplemented with thiamine increased significantly (P < 0.05) triglyceride, cholesterol, and total protein levels accompanied with significant (P < 0.05) decreases in aminotransferase aspartate and alanine aminotransferase activities. Serum antioxidant enzymes were remarkably (P < 0.05) higher, while serum malondialdehyde was significantly (P < 0.05) lower in the thiamin-treated fish compared with the control group. Total immunoglobulin, lysozyme, and ACH50 values were significantly (P < 0.05) higher in fish fed with thiamin-supplemented diets than in the control group. The results of the present study demonstrated that dietary thiamin have an important role in enhancing the growth performance, immune response, and antioxidant activity of great sturgeon. Based on the regression fitting curve of final weight, weight gain, specific growth rate, and FCR values, the optimal level of thiamin is found to be 15.0–17.5 mg/kg diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Great sturgeon (Huso huso) is an endemic valuable sturgeon species in the Caspian basin prized mainly for their caviar, meat, and aquaculture potential (Chebanov and Billard 2001; Pourkazemi 2006; He et al. 2017; Matani Bour et al. 2018; Bakhshalizadeh et al. 2021). It is a candidate for aquaculture due to its high growth rate, high adaptability to manufactured feeds and farming conditions, and high resistance to stressful conditions (Falahatkar et al. 2012). In sturgeon culture, special attention should be paid to their nutritional issues that make their farming a profitable production. A suitable diet for sturgeon aquaculture should contain various functional substances especially vitamins, which are reported to be among the most essential ones (De Andrade et al. 2007; Ghiasi et al. 2014, 2017).
Vitamins play important roles in enhancing the growth, disease resistance, maturation, and reproduction processes. on the other hand, vitamins deficiency reduces growth, intestinal weight, microvillus membrane protein, and brush border alkaline phosphatase activity in organisms (Wen et al. 2022). One of the highly important vitamins is thiamin (vitamin B1), which is an essential nutrient for fish (Aoe et al. 1969). Thiamin is vital for the fish metabolism and acts as a coenzyme in enzymatic pathways of pyruvate dehydrogenase, transketolase, ketoglutarate dehydrogenase, and glucose-6-phosphate dehydrogenase (Jenco et al. 2017; Ghiasi et al. 2014, 2017). Since thiamin cannot be synthesized in the body, the fish diets must be managed to prevent thiamin deficiency in fish. Several studies demonstrated that dietary thiamin positively affected the fish growth performance via increasing the activity of digestive enzymes and improving intestinal tissue integrity in Jian carp (Cyprinus carpio var. Jian), golden pompano (Trachinotus ovatus), grass carp (Ctenopharyngodon idella), and major carp (Catla catla) (Huang et al. 2011; Feng et al. 2011; Jiang et al. 2014; Xun et al. 2019; Mohd Khan and Khan 2022; Wen et al. 2022). For sturgeons, although data are available on vitamin C (Moreau et al. 1999a; Falahatkar et al. 2006, 2015), vitamin E (Moreau et al. 1999b; Amlashi et al. 2011), and vitamin A (Fontagné et al. 2006; Wen et al. 2008), enough information is rare about the optimal thiamin levels in a practical diet for sturgeons, including H. huso (Ghiasi et al. 2017; Mohseni et al. 2023). Due to the importance of great sturgeon (H. huso), the present study was done to investigate the positive effects of dietary thiamin on growth indices, biochemical factors, digestive enzymes, innate immunity, and antioxidant capacity in juvenile great sturgeon.
Materials and methods
Ethics statement
All experimental protocols were approved by the Faculty of Sciences, University of Tehran, Tehran, Iran (357; 8 November 2000).
Diets formulation
In this research, a basal control diet (Table 1) was formulated based on scientific information to meet the nutritional requirement of great sturgeon (Matani Bour et al. 2018; Mirzakhani et al. 2018), which contained 460 g/kg of protein and 196 g/kg of fat. Doses of thiamin in experimental diets were selected based on information available in other species (NRC 2011). Then, thiamin (thiamin hydrochloride) was added to the basal control diet at levels of 0 (T0), 7 (T7), 15 (T15), and 25 (T25) mg/kg diet. Measurements of thiamin levels in diets indicated that these diets contained 1.80, 8.02, 16.2, 26.6 mg thiamin/kg feed.
Feed items were obtained from a sturgeon feed company (Caspian Yaqoot Talaei, Eslami, Joybar, Iran). Thiamin-free vitamin supplement and thiamin were respectively procured from Hashtgerd Pharmaceutical Company (Alborz, Iran) and Sigma Company (Vitamin B1 hydrochloride, Sigma, Germany). The calculated amounts of diet items were mechanically stirred in an electric stirrer (Pars Khazar, Tehran, Iran) for 30 min. Thiamin hydrochloride (thiamin) at doses of 0, 7, 15, and 25 mg/kg feed was added to experimental diets. Then, thiamin was well mixed with vitamins and minerals supplements for 30 min using a binder. This mixture was added to the experimental diets, mixed again for 30 min during which, oil and water were added, and stirred again by an electrical stirrer (Pars Khazar, Tehran, Iran). The prepared diets were extruded in an electric meat grinder, and the feeds were broken to a diameter of 3 mm. The pellets were spread and allowed to dry in the air at room temperature for 24 h. Then, the pellets were packed and kept at − 20 °C until feeding.
To determine the protein, crude lipids, moisture, ash, and energy contents, experimental diets were analyzed chemically (AOAC 2005) in the laboratory of Qareburoon Sturgeon Culture and Propagation Center (Sari, Iran). Moisture content was measured by drying food in an oven at 105 °C for 24 h. Crude protein and crude lipids contents were determined using an automatic Kjeldahl device and a Soxhlet system, respectively. Ash content was obtained by burning diets in an electric furnace at 550 °C for 6 h. The amounts of energy of diets was calculated according to the AOAC method (AOAC 2005).
Fish and culture conditions
This research was carried out in the Qareburoon Sturgeon Culture and Propagation Center (Sari, Iran). Great sturgeon juveniles were adapted to the tanks conditions for two weeks, during which they were fed on the basal control diet up to the saturation level three times a day (8:00, 12:00, and 16:00 h). After the acclimation period, 12 round concrete tanks (1.7 × 1.5 × 0.6 m) were randomly assigned to four experimental treatments with three replications each. The experimental tanks were located indoors, and the photoperiod was 16 h of light and 8 h of dark. First, 240 pieces of juvenile great sturgeon (44.98 ± 1.96 g) were randomly stored in tanks (with a density of 20 fish per tank) that were supplied with continuous water flow, and the flow rate was set at 13 ± 0.3 L/min. Fish groups were fed on diets containing 0 (T0), 7 (T7), 15 (T15), and 25 (T25) mg thiamin/kg feed. During the experiment, fish were fed on the experimental diets up to apparent satiety three times (8:00, 12:00, and 16:00 h) every day for 8 weeks. Following every fish feeding, a screening mechanism was implemented for a duration of one hour at the outlet of the tank to impede the flushing away of any feeds. Uneaten feeds were collected one hour after each feeding time, dried at 60 °C, and used in the calculations of feed intake. Water quality parameters were monitored every week as described in Boyd (1984). The values of water quality parameters were controlled during the experimental running and they were; temperature 21.82 ± 0.6 °C, dissolved oxygen 7.8 ± 0.5 mg/L, and pH = 7.3 ± 0.2, total ammonia 0.43 ± 0.05 mg/L, nitrate 19.5 ± 1.5 mg/L, nitrite 0.017 ± 0.001 mg/L.
At the end of feeding trial, fish feeding was stopped for 24 h and all the fish in each tank were anesthetized with clove powder (400 mg/L, Najafi et al. 2017) and separately weighed. Final weight (FW), body weight gain (BWG), specific growth rate (SGR), survival rate (SR), feed intake (FI), and feed conversion ratio (FCR) were calculated as below:
Body weight gain (BWG, %) = 100 × [final weight (g) – (initial weight (g)] / initial weight (g);
Specific growth rate (SGR; g/day) = 100 × [Ln (final weight) - Ln (initial weight)] / days;
Feed conversion ratio (FCR) = dry diet feed (g) / wet weight gain (g);
Survival rate (%) = 100 x (final number of fish / initial number of fish).
Blood and tissue sampling
At the end of the feeding experiment, three fish were randomly selected from each repetition (n = 9 fish per treatment), anesthetized with clove powder (400 mg/L, Najafi et al. 2017), and blood was sampled from the caudal vein. Blood samples were refrigerated at 4 °C and then centrifuged at 3000 g for 10 min. The serum was collected and kept at − 80 °C for further analyses. Then, fish were dissected and the liver from the fish were removed and frozen in liquid nitrogen, and stored at -80 ° C for determining thiamin contents.
Thiamin determination
The total thiamin contents in the experimental diets as well as fish liver tissues were determined by high performance liquid chromatography (HPLC) after acid and enzymatic hydrolysis as described in Velimatti et al. (1993).
Digestive enzymes
Digestive enzymes were measured in fish sera using commercial kits, which were applied to measure trypsin (Cat. No: CK-E91540, Eastbiopharm, CO. China), chymotrypsin (Cat. No: CK-E92024, Eastbiopharm, CO. China), lipase (Cat. No: 1,050,024, Pars Azmon, Alborz, Iran), and α-amylase (Cat. No: 104,050, Pars Azmon, Alborz, Iran).
Serum biochemistry assay
Glucose (GLU), triglyceride (TG), Cholesterol (T-CHO), and total protein (TP) were quantified using assay kits from Pars Azmun Company (Pars Azmun, Karaj, Alborz, Iran) according to the mentioned protocols. Aspartate aminotransferase (AST), alanine aminotransferase (ALT) activities, alkaline phosphatase (ALP), and creatine kinase (CK) were determined using commercial kits (Pars Azmon, Karaj, Alborz, Iran) according to the mentioned protocols.
Immune response assay
Lysozyme (LYZ) activity was measured using modified turbidimetric method according to Ellis et al. (2001) by using Micrococcus luteus (Sigma) as a target in 0.05 M phosphate buffer (pH = 6.2). Serum alternative complement (ACH50) activity was measured based on Yano et al. (1988), in which rabbit red blood cells were used as a target. The protocol of Siwicki and Anderson (1993) was used to assay total immunoglobulin (total Ig) after polyethylene glycol precipitation of Ig and subtraction of initial and final total protein.
Serum antioxidant indices
Enzyme-linked immunosorbent assay (ELISA) commercial kits was used to measure serum superoxide dismutase (SOD, Cat. No: ZB-SOD96A, ZellBio, GmbH, Germany), catalase activity (CAT, Cat. No: ZB-CAT96A, V405, ZellBio, GmbH, Germany), glutathione peroxidase activity (GPx, Cat. No: ZB-GPX-A96, ZellBio, GmbH, Germany), malondialdehyde (MDA, Cat. No: ZB-MDA96A, V405, ZellBio, GmbH, Germany), glutathione S-transferase (GST, Cat. NO: ZX-33103-96, ZellBio, GmbH, Germany), and glutathione reductase (GR, Cat. NO: ZX-33104-96, ZellBio, GmbH, Germany) according to the guide protocols.
Statistical analysis
This research was conducted in a completely randomized design with three replications (n = 3) for all analyses. All data were analyzed statistically using SPSS version 26.00 for windows. First, the normality of the data was tested using the Kolmogorov-Smirnov test, and Levin’s test was used for the homogeneity of variances. Accordingly, significant differences between treatments were compared using one-way analysis of variance (ANOVA). Differences between treatments were evaluated by Tukey’s HSD test. The linear or quadratic effects of dietary thiamin were determined by an orthogonal polynomial contrast analysis (Yossa and Verdegem 2015).
Results
Growth performance and hepatic thiamin content
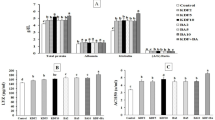
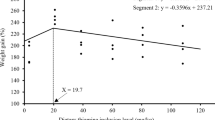
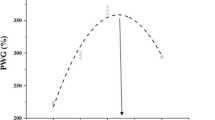
Compared with the control diet, dietary thiamin levels significantly (P < 0.05) prompted the fish performance parameters in linear or quadratic trends (Table 2). However, highest values of FW, BWG%, SGR, and FCR were observed at T15 after which no significant (P > 0.05) difference was observed with T25. Significant (P < 0.05) correlations were observed for dietary thiamin levels effects on FW, BWG%, SGR, and FCR (Fig. 1). Hepatic thiamin content was significantly (P < 0.05) correlated with the dietary thiamin levels, and the highest amount was obtained in T15 and T25 (5.33 and 5.38 mg/kg) with no significant (P > 0.05) difference between them (Table 2; Fig. 2). Dietary thiamin levels and hepatic thiamin followed significant (P < 0.05) linear and quadratic trends. In this study, the optimal dietary thiamin level for beluga fish was estimated to be 15.0–17.5 mg/kg feed based on the fitting curves (Fig. 1) as follows:
FW (y = -10.325 × 2 + 64.375x + 133.58; R2 = 0.935),
BWG% (y = -26.682 × 2 + 156.82x + 199.12; R2 = 0.935),
SGR (y = -0.092 × 2 + 0.5424x + 1.9808; R2 = 0.9788),
FCR (y = 0.0932 × 2 – 0.6444x + 2.2555; R2 = 0.9234).
Digestive enzymes activities
A significant (P < 0.05) difference was observed among treatments in regards of trypsin, chymotrypsin, lipase, and α-amylase activities (Table 3). Their highest values were observed at T15 after which no significant (P > 0.05) difference was observed with T25. Significant (P < 0.05) linear and quadratic trends were obtained for dietary thiamin levels and the activity of above-mentioned enzymes. These enzymes were more active in the thiamin-supplemented treatments compared to the control fish group (Fig. 3).
Biochemical factors
The amount of GLU was not significantly (P > 0.05) differed among the experimental treatments, meanwhile TG, T-CHO, and TP levels were significantly (P < 0.05) higher in the thiamin-fed fish groups than in the control fish group (Table 4). Their highest values were observed at T15 after which no significant (P > 0.05) difference was observed with T25. Significant (P < 0.05) linear and quadratic trends were obtained for dietary thiamin levels and TG, T-CHL, and TP levels. Significant (P < 0.05) differences in activities of CK, ALP, ALT and AST were observed among experimental treatments. Increasing the dietary thiamin linearly enhanced CK and ALP activities but reduced the activity of ALT and AST (Table 4; P < 0.05).
Immunity and antioxidant biomarkers
Total Ig, LYZ, and ACH50 levels were significantly (P < 0.05) higher in thiamin-supplemented diets than in the control group and their highest values were observed at T15 after which no significant (P > 0.05) difference was observed with T25 (Fig. 4). As shown in Table 5, serum antioxidant enzymes (SOD, CAT, GPX, GST, and GR) were significantly (P < 0.05) higher in the thiamin-treated fish than in the control fish group. Significant (P < 0.05) linear and quadratic trends were obtained for dietary thiamin levels and CAT, GPX, GST, and GR levels. These enzymes rose with increasing dietary thiamin levels up to 15 mg/kg diet after which no significant (P > 0.05) difference was observed with T25. On the other hand, serum MDA levels were significantly (P < 0.05) declined in the fish fed with thiamin-enriched diets than in the control (thiamin-free) group. Serum MDA decreased up to 15 mg/kg diet (T15) with no significant (P > 0.05) difference with T25 (Table 5).
Discussion
Vitamins are organic compounds having vital importance functions for fish welfare, and their deficiency causes severe disorders in the fish body where they play important roles in growth, physiology, and metabolism (Lonsdale 2006). The essentiality of dietary thiamin for optimal growth, improvement of immune factors, antioxidant status, and liver thiamin saturation for great sturgeon juveniles was clearly demonstrated in the present study. Our results showed that dietary thiamin levels positively stimulated the fish performance factors in linear or quadratic trends. Similar results were reported in young golden pompano (Xun et al. 2019), Jian carp (Huang et al. 2011), and great sturgeon larvae (Mohseni et al. 2023). The regression analysis indicated that juvenile beluga needed a level of 15.0–17.5 mg/kg diet of thiamin for optimization their growth. This result indicates that fish cannot utilize the higher thiamin levels may because the saturation of thiamin-encaged active sites. Additionally, the secretions of digestive enzymes were maximized at those thiamin levels limiting the further digestion of the feeds nutrients. Zehra and Khan (2017) stated that higher levels of dietary thiamin cannot be accumulated in the liver and may be excreted through urine.
These recommended thiamin levels for great sturgeon juveniles are approximately near that was recommended by Mohseni et al. (2023) who reported 10–20 mg/kg diet of thiamin for optimizing growth in the beluga weaning stage until the fingerling stage. This thiamin requirement is higher than the values reported for common carp (0.5 mg/kg feed: Halver 2002), Nile tilapia (3.5 mg/kg diet; Lim et al. 2011), rainbow trout (1–10 mg/kg feed: NRC 2011), and Channa punctatus (2.34–2.59 mg/kg feed: Zehra and Khan 2018). The wide variation in required amounts of thiamin may be attributed to differences in species, fish size, laboratory conditions, diet quality, methodology, and assessment criteria among others.
The inclusion of appropriate amount of thiamin in aqua-feeds can improve the fish performance by improving the feed utilization. Additionally, the growth improving in thiamin-fed fish, in the present study, could be linked to the secretion of digestive enzymes, which are responsible for the feed digestion and nutrients absorption (Hakim et al. 2006; Zhao et al. 2020). In this regard, our data revealed significant (P < 0.05) increments in trypsin, chymotrypsin, α-amylase, and lipase activities with the consumption of thiamin-supplemented diets. These results are consistent with findings reported in Jian carp (Huang et al. 2011), golden pompano (Xun et al. 2019), yellow catfish (Pelteobagrus fulvidraco) juveniles (Zhao et al. 2020), and great sturgeon larvae (Mohseni et al. 2023). The clear mechanisms by which thiamine enhances digestive enzymes activities in fish remain unknown. Nevertheless, investigations on mammals have evidenced that thiamine plays a crucial role in facilitating the optimal operation of a specific area of the central nervous system that controls the functionality of the gastrointestinal tract (Van der Zanden et al. 2009; Mohseni et al. 2023).
Hepatic vitamin concentration is used as an indicator of vitamin status in fish (Xiang et al. 2016). In the present study, liver thiamin concentrations were proportionally increased with rising dietary thiamin levels up to 15 mg/kg feed with no significant difference with T25. Similarly, Xun et al. (2019) reported that dietary thiamin increased up to 12 mg/kg diet in golden pompano juveniles and then decreased gradually. This suggests that balanced amounts of thiamin in diets enable the liver to develop and absorb thiamin more efficiently (Xun et al. 2019). More thiamin inclusion in the diet did not significantly improve liver thiamin concentration, suggesting that higher levels of dietary thiamin cannot be accumulated in the liver and may be excreted through urine (Zehra and Khan 2017).
Creatine kinase (CK) is involved in the energy metabolism of cells and catalyzes the transfer of phosphate to creatine in an ATP-dependent manner (Zhao et al. 2020). ALP is an important enzyme in the absorption of nutrients such as lipids, glucose, calcium, and inorganic phosphate (Tengjaroenkul et al. 2000). In the present study, the activity of CK and ALP significantly (P < 0.05) increased with the increasing levels of thiamin in diets as compared with the control group. Huang et al. (2011) and Zhao et al. (2020) observed that different levels of dietary thiamin increased the activity of CK and ALP in Jian carp and yellow catfish, respectively, compared to the control group. The increase in brush border enzymes after thiamin supplementation to fish can be attributed to the coenzyme form of thiamin, which acts as an important coenzyme for transketolase and several other enzymes involved in the conversion of carbohydrates and fat into energy (Huang et al. 2007; Zhao et al. 2020).
Various studies have shown that blood biochemistry can be affected by diets composition and are good indicators to show the nutritional status, stress, and overall health of fish. In the present study, TP, TG, and CHO were significantly (P < 0.05) affected by different levels of thiamin. The results obtained herein from the evaluation of blood metabolites show that the concentration of thiamin in the diet at 15 and 25 mg/kg feed increases serum TP contents with no significant (P > 0.05) difference between them. Higher values of serum protein in great sturgeon fed with a level of 15 mg/kg diet of thiamin may be due to sufficient protein synthesis required for optimal growth of this fish species. Huang et al. (2011) also reported that deficient or excessive dietary thiamin inhibited carbohydrate metabolism, resulting in nutrient depletion during protein and lipid synthesis from the glycolytic pathway. TG and CHO are generally affected by carbohydrate, lipid, and protein metabolism. In the present investigation, serum TG and CHO concentrations were significantly higher in fish fed on thiamin-supplemented diets than in the control group. Similar results were reported in Sclizothorax prenanti (Xiang et al. 2016) and yellow catfish juveniles (Zhao et al. 2020). These results probably suggest that thiamin supplementation to fish increases thiamine pyrophosphate formation in the liver, which increases oxidative decarboxylation of α-ketoacids and consequently lipid synthesis (Xiang et al. 2016). An in vitro study showed that thiamin-deficiency glia decreased cholesterol biosynthesis (Volpe and Marasa 1978).
Serum activity of ALT and AST can be used as general indicators of vertebrate liver function where higher levels of both enzymes reflect liver dysfunction or damage (Pan et al. 2003; Linhua et al. 2009). In our study herein, ALT and AST activity significantly (P < 0.05) decreased with increasing thiamin levels in diets as compared with the control fish group. The higher ALT and AST activities in the control fish group might result from a lack of thiamin to act as an antioxidant to protect liver cells from damage. The reduction of ALT and AST by dietary vitamins has been reported in different fish species (Yadollahi et al. 2021).
The health status and nutritional metabolism in fish can be reflected by serum biochemical and innate immunity parameters (Abdel-Tawwab et al. 2023). In the present study, LYZ, total Ig, and ACH50 values were significantly higher in the thiamin-fed fish than suggesting that thiamin supplementation to fish can improve their immune mechanism. Our results are also consistent with those reported in Jian carp (Huang et al. 2011). Likewise, Xun et al. (2019) observed higher activity of LYZ and C4 in golden pompano juveniles fed with different thiamin levels than in the control group.
MDA is known as an indicator of lipid peroxidation and can be defined as an important consequence of lipid oxidative deterioration (Lee and Dabrowski 2003). In the current investigation, serum MDA gradually decreased with increasing thiamin levels up to 15 mg/kg feed and then remained unchanged at T25. This corresponds to the results obtained in young grouper (Huang et al. 2007) and young common carp (Li et al. 2014). The beneficial effects of dietary thiamin in inhibiting lipid peroxidation and protein oxidation may be because it blocks the production of harmful mediator metabolites in fish (Li et al. 2014). Limited data are available on the effect of thiamin on lipid peroxidation in fish. Therefore, further investigation is required for the mechanism by which dietary thiamin reduces oxidative lipid damage. SOD, CAT, GPX and GST are important antioxidant enzymes that implicated to counteract the oxidative stress through maintenance of redox homeostasis and mitigated the overproduction of free radicals (Martinez-Alvarez et al. 2005; Abdel-Tawwab and Wafeek 2017; Hoseinifar et al. 2021). Reduced activity of these enzymes causes lipid peroxidation and the increase in MDA production (Yousef et al. 2018; Abdel-Tawwab et al. 2023). Our results herein indicated that increasing dietary thiamin up to 15 mg/kg feed significantly increased the activity of SOD, CAT, GPX, and GST and then remained unchanged at T25. These results suggest that an adequate level of thiamin in fish diets improved the antioxidant activity. The effect of thiamin on the activity of antioxidant enzymes in fish has been investigated in limited studies. In mammals, thiamin can affect the activity of antioxidant enzymes in different ways. Thiamin may modulate GPx activity by blocking triphosphate accumulation (Park et al. 2003) and may increase antioxidant enzyme activity by suppressing the expression of the transcription factor p53 (Yang et al. 2004; Faraonio et al. 2006). In general, the elevated antioxidant enzymes activity in fish fed on thiamin-enriched diets may be partially related to these pathways, which needs further investigation. The obtained results herein are in line with the results reported in young common carp fed with different levels of thiamin (Li et al. 2014). In contrast with our study, Huang et al. (2007) reported that the SOD activity was not affected by thiamin in the grouper liver. A study conducted on rats demonstrated that the addition of thiamin prevented the occurrence of lipid peroxidation, glutathione depletion, and glyoxal-induced formation of reactive oxygen species. Moreover, it resulted in a reduction of mitochondrial membrane potential in rat hepatocytes (Shangari et al. 2003). They had been observed that vitamin B1 has the ability to directly interact with free radicals and hydroperoxides, and can consistently transmit (2 H+ +2e−) on the pyrimidine ring NH2 group to free radicals, thereby eliminating them. On the other hand, thiamine deficiency has been noted to exert a diminishing effect on NADPH concentration, which serves as a radical scavenger, owing to a reduction in transketolase activity (Huang et al. 2007). Additionally, thiamine deficiency has been observed to impede antioxidant signaling pathways such as Nrf2 and Keap1 (Wen et al. 2016). Consequently, the enhancement of antioxidant capacity in the thiamine-treated great sturgeon may be attributed to the amelioration of tissue health (Mohseni et al. 2023).
Conclusion
The present study demonstrate that dietary thiamin plays an essential role in enhancing the growth performance and welfare status of great sturgeon, H. huso, juveniles. Additionally, dietary thiamin caused significant improvements in digestive enzymes, immune and antioxidant biomarkers. The optimal level of thiamin was 15.0–17.5 mg/kg diet based on growth parameters. The data of the present study will be useful for the inclusion of optimal thiamin levels (15.0–17.5 mg/kg diet) in commercial and practical feeds in great sturgeon farms. Further studies are needed to explore the effects of dietary thiamin on growth, immune, and antioxidant genes expression.
Data Availability
All data of this study are included in this article.
References
Abdel-Tawwab M, Wafeek M (2017) Fluctuations in water temperature affected waterborne cadmium toxicity: Hematology, anaerobic glucose pathway, and oxidative stress status of Nile tilapia, Oreochromis niloticus (L). Aquaculture 477:106–111
Abdel-Tawwab M, Abo Selema TAM, Abotaleb MM, Khalil RH, Sabry NM, Soliman AM, Eldessouki EAA (2023) Effects of a commercial feed additive (Sanacore® GM) on immune-antioxidant profile and resistance of Gilthead seabream (Sparus aurata) against Vibrio alginolyticus infection. Ann. Anim Sci 23:185–193
Amlashi AS, Falahatkar B, Sattari M, Gilani MT (2011) Effect of dietary vitamin E on growth, muscle composition, hematological and immunological parameters of sub yearling beluga Huso huso L. Fish shellfish immunol. 30(3):807–814. https://doi.org/10.1016/j.fsi.2011.01.002
AOAC (2005) Association of Official Analytical Chemists. The Official Methods of Analyses Association of Official Analytical Chemists International. The 18th edition, Gaithersburg, Maryland 20877–2417, USA
Aoe H, Masuda I, Mimura T, Saito T, Komo A, Kitamura S (1969) Water-soluble vitamin requirements of carp. 6. Requirement for thiamine and effects of antithiamines. Bull Jap Soc Sci Fish 35:459–465
Bakhshalizadeh S, Zykov LA, Nasibulina BM, Kurochkina TF, Fazio F (2021) Influence of growth on the biological and commercial productivity of Caspian great sturgeon Huso huso. Aquaculture 533:736139
Boyd CE (1984) Water quality in warm water fishponds. Auburn University Agriculture Experimental Station, Auburn, AL, USA
Chebanov M, Billard R (2001) The culture of sturgeons in Russia: production of juveniles for stocking and meat for human consumption. Aquat Living Resour 14:375–381
De Andrade JIA, Ono EA, de Menezes GC, Brasil EM, Roubach R, Urbinati EC, Tavares-Dias M, Marcon JL, Affonso EG (2007) Influence of diets supplemented with vitamins C and E on Pirarucu (Arapaima gigas) blood parameters. CBPA 146(4):576–580. https://doi.org/10.1016/j.cbpa.2006.03.017
Ellis AE (2001) Innate host defense mechanisms of fish against viruses and bacteria. DCI 25(8–9):827–839. https://doi.org/10.1016/S0145-305X(01)00038-6
Falahatkar B, Soltani M, Abtahi B, Kalbassi MR, Pourkazemi M (2006) Effects of dietary vitamin C supplementation on performance, tissue chemical composition and alkaline phosphatase activity in great sturgeon (Huso huso). J Appl Ichthyol 22:283–286
Falahatkar B, Amlashi AS, Conte F (2012) Effect of dietary vitamin E on cortisol and glucose responses to handling stress in juvenile beluga Huso huso. J Aquat Anim Health 24(1):11–16. https://doi.org/10.1080/08997659.2011.647235
Falahatkar B, Soltani M, Abtahi B, Kalbassi MR, Pourkazemi M (2015) The role of dietary L-ascorbyl‐2‐polyphosphate on the growth and physiological functions of beluga, Huso huso (L innaeus, 1758). Aquac Res 46:3056–3069. https://doi.org/10.1111/are.12468
Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, Russo T, Cimino F (2006) p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem 281(52):39776–39784. https://doi.org/10.1074/jbc.M605707200
Feng L, Huang HH, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Effect of dietary thiamin supplement on immune responses and intestinal microflora in juvenile Jian carp (Cyprinus carpio var. Jian) Aquacult Nutr 17(5):557–569. https://doi.org/10.1111/j.1365-2095.2011.00851.x
Fontagné S, Bazin D, Brèque J, Vachot C, Bernarde C, Rouault T, Bergot P (2006) Effects of dietary oxidized lipid and vitamin A on the early development and antioxidant status of siberian sturgeon (Acipenser baeri) larvae. Aquaculture 257(1–4):400–411. https://doi.org/10.1016/j.aquaculture.2006.01.025
Ghiasi S, Falahatkar B, Dabrowski K, Abasalizadeh A, Arslan M (2014) Effect of thiamine injection on growth performance, hematology and germinal vesicle migration in sterlet sturgeon Acipenser ruthenus L. Aquacult. Int 22:1563–1576. https://doi.org/10.1007/s10499-014-9765-7
Ghiasi S, Falahatkar B, Arslan M, Dabrowski K (2017) Physiological changes and reproductive performance of Sterlet sturgeon Acipenser ruthenus injected with thiamine. Anim Reprod Sci 178:23–30. https://doi.org/10.1016/j.anireprosci.2017.01.005
Hakim Y, Uni Z, Hulata G, Harpaz S (2006) Relationship between intestinal brush border enzymatic activity and growth rate in tilapias fed diets containing 30% or 48% protein. Aquaculture 257(1–4):420–428. https://doi.org/10.1016/j.aquaculture.2006.02.034
Halver JE (2002) The vitamins. In: Halver JE, Hardy RW (eds) Fish Nutrition, 3rd edn. Academic Press, California, USA, pp 61–141
He F, Zarfl C, Bremerich V, Henshaw A, Darwall W, Tockner K, Jaehnig SC (2017) Disappearing giants: a review of threats to freshwater megafauna. Wiley Interdiscip Rev. https://doi.org/10.1002/wat2.1208
Hoseinifar SH, Yousef M, Van Doan H, Ashouri G, Gioacchin G, Maradonn F, Carneval O (2021) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquac 29:198–217
Huang JW, Tian LX, Du ZY, Yang HJ, Liu YJ (2007) Effects of dietary thiamin on the physiological status of the grouper Epinephelus coioides. FISH 33:167–172. https://doi.org/10.1007/s10695-007-9127-8
Huang HH, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Effects of dietary thiamin supplement on growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 17(2):233–240. https://doi.org/10.1111/j.1365-2095.2010.00756.x
Jenco J, Krcmova LK, Solichova D, Solich P (2017) Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogr A 1510:1–12. https://doi.org/10.1016/j.chroma.2017.06.048
Jiang M, Huang F, Zhao Z, Wen H, Wu F, Liu W, Yang C, Wang W (2014) Dietary thiamin requirement of juvenile grass carp, Ctenopharyngodon idella. J World Aquacult Soc 45(4):461–468. https://doi.org/10.1111/jwas.12132
Lee KJ, Dabrowski K (2003) Interaction between vitamins C and E affects their tissue concentrations, growth, lipid oxidation, and deficiency symptoms in yellow perch (Perca flavescens). Br J Nutr 89(5):589–596. https://doi.org/10.1079/BJN2003819
Li XY, Huang HH, Hu K, Liu Y, Jiang WD, Jiang J, Li SH, Feng L, Zhou XQ (2014) The effects of dietary thiamin on oxidative damage and antioxidant defence of juvenile fish. Fish Physiol Biochem 40:673–687. https://doi.org/10.1007/s10695-013-9875-6
Lim C, Yildirim-Aksoy M, Barros MM, Klesius P (2011) Thiamin requirement of Nile tilapia, Oreochromis niloticus. J World Aquacult Soc 42(6):824–833. https://doi.org/10.1111/j.1749-7345.2011.00531.x
Linhua HA, Zhenyu WA, Baoshan XI (2009) Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). J Environ Sci 21(10):1459–1466. https://doi.org/10.1016/S1001-0742(08)62440-7
Lonsdale D (2006) A review of the biochemistry, metabolism and clinical benefits of thiamin (e) and its derivatives. eCAM 3(1):49–59. https://doi.org/10.1093/ecam/nek009
Martinez-Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88. https://doi.org/10.1007/s11160-005-7846-4
Matani Bour HA, Esmaeili M, Abedian Kenari A (2018) Growth performance, muscle and liver composition, blood traits, digestibility and gut bacteria of beluga (Huso huso) juvenile fed different levels of soybean meal and lactic acid. Aquacult Nutr 24(4):1361–1368. https://doi.org/10.1111/anu.12673
Mirzakhani MK, Kenari AA, Motamedzadegan A (2018) Prediction of apparent protein digestibility by in vitro pH-stat degree of protein hydrolysis with species-specific enzymes for siberian sturgeon (Acipenser baeri, Brandt 1869). Aquaculture 496:73–78. https://doi.org/10.1016/j.aquaculture.2018.07.014
Mohd Khan Y, Khan MA (2022) Dietary thiamin requirement of fingerling major carp Catla catla (Hamilton). J Anim Physiol Anim Nutr 106(4):939–946. https://doi.org/10.1111/jpn.13644
Mohseni M, Ghelichpour M, Sayed Hassani MH, Pajand Z, Ghorbani Vaghei R (2023) Effects of dietary thiamine supplementation on growth performance, digestive enzymes’ activity, and biochemical parameters of beluga, Huso huso, larvae. J Appl Ichthyol 30:2023. https://doi.org/10.1155/2023/6982536
Moreau R, Dabrowski K, Czesny S, Cihla F (1999a) Vitamin C-vitamin E interaction in juvenile lake sturgeon (Acipenser fulvescens R.), a fish able to synthesize ascorbic acid. J Appl Ichthyol 15(4–5):250–257. https://doi.org/10.1111/j.1439-0426.1999.tb00245.x
Moreau R, Dabrowski K, Sato PH (1999b) Renal L-gulono-1, 4-lactone oxidase activity as affected by dietary ascorbic acid in lake sturgeon (Acipenser fulvescens). Aquaculture 180(3–4):359–372. https://doi.org/10.1016/S0044-8486(99)00211-2
Najafi M, Falahatkar B, Safarpour Amlashi A, Tolouei Gilani MH (2017) The combined effects of feeding time and dietary lipid levels on growth performance in juvenile beluga sturgeon Huso huso. Aquacult Int 25(1):31–45. https://doi.org/10.1007/s10499-016-0011-3
NRC (2011) Nutrient requirements of fish and shrimp. National Academy Press, Washington DC, USA
Pan CH, Chien YH, Hunter B (2003) The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. J Exp Mar Biol Ecol 297(1):107–118. https://doi.org/10.1016/j.jembe.2003.07.002
Park YS, Koh YH, Takahashi M, Miyamoto Y, Suzuki K, Dohmae N, Takio K, Honke K, Taniguchi N (2003) Identification of the binding site of methylglyoxal on glutathione peroxidase: methylglyoxal inhibits glutathione peroxidase activity via binding to glutathione binding sites arg 184 and 185. Free Radic Res 37(2):205–211. https://doi.org/10.1080/1071576021000041005
Pourkazemi M (2006) Caspian Sea sturgeon conservation and fisheries: past present and future. J Appl Ichthyol 22:12–16
Shangari N, Bruce W, Poon R et al (2003) Toxicity of glyoxals–role of oxidative stress, metabolic detoxification and thiamine deficiency. Biochem Soc Trans 31:1390–1393
Siwicki A, Anderson D (1993) Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin level in serum. In: Anderson A, Waluga D J. (eds) Fish Disease diagnosis and Prevention Methods, Siwicki. Wydawnictwo Instytutu Rybactwa Śródlądowego, Olsztyn, Poland, pp 105–112
Tengjaroenkul B, Smith BJ, Caceci T, Smith SA (2000) Distribution of intestinal enzyme activities along the intestinal tract of cultured Nile tilapia, Oreochromis niloticus L. Aquaculture 182(3–4):317–327. https://doi.org/10.1016/S0044-8486(99)00270-7
Van der Zanden EP, Snoek SA, Heinsbroek SE, Stanisor OI, Verseijden C, Boeckxstaens GE, Peppelenbosch MP, Greaves DR, Gordon S, De Jonge WJ (2009) Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor α4β2. Gastroenterology 31029–1039. https://doi.org/10.1053/j.gastro.2009.04.057
Velimatti O, Liisa V, Antti U-R, Pertti V, Pekka K, Jussi H (1993) The HPLC determination of total thiamin (vitamin B1) in foods. J Food Comp Ana 6(2):152–165
Volpe J, Marasa JC (1978) Dissociation of the regulation of fatty acid synthesis and cyclic amp levels in cultured glial and neuronal cells1. J Neurochem 31(1):277–281. https://doi.org/10.1111/j.1471-4159.1978.tb12460.x
Wen H, Yan AS, Gao Q, Jiang M, Wei QW (2008) Dietary vitamin A requirement of juvenile Amur sturgeon (Acipenser schrenckii). J Appl Ichthyol 24(5):534–538. https://doi.org/10.1111/j.1439-0426.2008.01105.x
Wen LM, Feng L, Jiang WD, Liu Y, Wu P, Zhao J, Jiang J, Kuang SY, Tang L, Tang WN, Zhang YA (2016) Thiamin deficiency induces impaired fish gill immune responses, tight junction protein expression and antioxidant capacity: roles of the NF-κB, TOR, p38 MAPK and Nrf2 signaling molecules. Fish Shellfish Immunol 51:373–383. https://doi.org/10.1016/j.fsi.2015.12.038
Wen LM, Lin F, Wei-Dan J, Wu P, Yang L, Sheng‐Yao K, Li SW, Ling T, Zhang L, Mi HF, Zhou XQ (2022) Effects of dietary thiamin on flesh quality in grass carp (Ctenopharyngodon idella). Aquacult Res 53(18):6671–6682. https://doi.org/10.1111/are.16136
Xiang X, Zhou XQ, Chen GF, Wu P, Zheng ZL (2016) Effect of graded levels of dietary thiamine on the growth performance, body composition and haemato-biochemical parameters of juvenile Sclizothorax prenanti. Aquacult Nutr 22(3):691–697. https://doi.org/10.1111/anu.12291
Xun P, Lin H, Wang R, Huang Z, Zhou C, Yu W, Huang Q, Tan L, Wang Y, Wang J (2019) Effects of dietary vitamin B1 on growth performance, intestinal digestion and absorption, intestinal microflora and immune response of juvenile golden pompano (Trachinotus ovatus). Aquaculture 506:75–83. https://doi.org/10.1016/j.aquaculture.2019.03.017
Yadollahi F, Soltani M, Modarresi MH, Akhondzadeh Basti A (2021) Efficacy of vitamin E with or without probiotic, astaxanthin or rosemary extract on growth performance, survival, haematological parameters, antioxidant activity and liver enzymes in rainbow trout (Oncorhynchus mykiss). Aquacult Res 52(11):5606–5616. https://doi.org/10.1111/are.15436
Yang Z, Ge J, Yin W, Shen H, Liu H, Guo Y (2004) The expression of p53, MDM2 and Ref1 gene in cultured retina neurons of SD rats treated with vitamin B1 and/or elevated pressure. Yan Ke Xue Bao 20(4):259–263
Yano T, Hatayama Y, Matsuyama H, Nakao M (1988) Titration of the alternative complement pathway activity of representative cultured fishes. Nippon Suisan Gakkaishi (Japanese Edition) 54(6):1049–1054
Yossa R, Verdegem M (2015) Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 437:344–350
Yousef M, Hoseini SM, Vatnikov YA, Nikishov AA, Kulikov EV (2018) Thymol as a new anesthetic in common carp (Cyprinus carpio): efficacy and physiological effects in comparison with eugenol. Aquaculture 495:376–383
Zehra S, Khan MA (2017) Dietary thiamin and pyridoxine requirements of fingerling indian major carp, Cirrhinus mrigala (Hamilton). Aquacult Res 48(9):4945–4957. https://doi.org/10.1111/are.13313
Zehra S, Khan MA (2018) Dietary thiamin requirement of fingerling Channa punctatus (Bloch) based on growth, protein gain, liver thiamin storage, RNA/DNA ratio and biochemical composition. Aquacult Nutr 24(3):1015–1023. https://doi.org/10.1111/anu.12638
Zhao H, Chen B, Huang Y, Cao J, Wang G, Chen X, Mo W (2020) Effects of dietary vitamin B1 on growth performance, blood metabolites, body composition, intestinal enzyme activities and morphometric parameters of juvenile yellow catfish (Pelteobagrus fulvidraco). Aquacult Nutr 26(5):1681–1690. https://doi.org/10.1111/anu.13113
Acknowledgements
The research has been conducted with the constant support of the Qareburoon sturgeon culture center regarding the provision of fish and rearing. The authors express gratefulness to Dr. Morteza Yousefi for his help during the final editing of manuscript.
Funding
Authors self-funded this research.
Author information
Authors and Affiliations
Contributions
Zahra behbodi: Data curation, writing; Somayeh bahram: formal analysis, methodology, reviewing and editing; Masoumeh Bahrekazemi: reviewing and editing; Seyed Rohollah Javadian: reviewing and editing; Abas Bozorgnia: reviewing and editing, Mohsen Abdel-Tawwab: analysis and : reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Behbodi, Z., Bahram, S., Bahrekazemi, M. et al. Effects of dietary thiamin (vitamin B1) on the growth performance, serum biochemical factors, immune response, and antioxidant activity of great sturgeon (Huso huso) juveniles. Vet Res Commun 48, 485–496 (2024). https://doi.org/10.1007/s11259-023-10227-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-023-10227-6