Abstract
Juvenile beluga sturgeon, Huso huso (initial weight 50 ± 0.2 g), were fed with two lipid levels (120 g kg−1 lipid as low-energy diet, LE; 240 g kg−1 lipid as high-energy diet, HE) at different feeding times for 8 weeks, and the effects on growth, hematology, muscle composition, and apparent digestibility coefficients of dry matter, lipid, and protein were examined. Feeding times were as follows: (1) at 09:00 and 16:00 (under light regime), (2) at 21:00 and 04:00 (under dark regime), and (3) at 13:00 and 20:00 (under light–dark regime). Dietary lipid levels did not affect the hematological indices or apparent digestibility coefficients of dietary nutrients (P > 0.05); however, they affected the growth and muscle composition (P < 0.05). The fish fed with HE diet had the highest final weight (174.2 – 175.9 g), specific growth rate (2.44 – 2.47 % day−1), weight gain (246.5 – 252.6 %), and the lowest feed conversion ratio (1.10 – 1.12). The fish fed with HE diet had the highest muscle lipid content (26.6 – 35.2 g kg−1). Unlike the lipid levels, feeding times did not significantly affect growth, hematological parameters, nutrient digestibility, and muscle composition (P > 0.05). Accordingly, juvenile beluga sturgeon can be fed at different times of the day without suffering growth loss, because probably, they have a low dependency on vision to detect food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Feeding time is an important factor to be considered in aquaculture, but is often ignored and/or paid little attention (Boujard et al. 1995; Azzaydi et al. 1999). The highest rate of feeding activity occurs in the highest activity times for all fish types (Bolliet et al. 2000). In some fish species, either optimal feeding or the highest activity may occur in different day–night times (Sánchez-Vázquez et al. 1996). However, the mechanisms involved are not very clear.
In addition to feeding time, energy requirements of fish are crucial in fish nutrition. Lipids, carbohydrates, and protein supply the energy needs of fish. Dietary lipids are an important energy source, providing essential fatty acids, phospholipids, sterols, and fat-soluble vitamins necessary for proper functioning of physiological processes which are responsible for maintenance and integrity of cellular membranes (Sargent et al. 2002). The addition of lipids to a diet contributes to effective utilization of dietary protein through the sparing effect in fish (Skalli et al. 2004). However, fish can utilize dietary lipids to a certain level, beyond which growth may be retarded because of reduced feed consumption (Ellis and Reigh 1991) and may increase body lipid deposition and influence carcass quality (Bjerkeng et al. 1997).
A significant interaction has been reported between feeding times and dietary lipid levels to improve performance and energy metabolism, so fish can digest and assimilate more energy in active times than passive times (Bolliet et al. 2000). It has been also found that hormones or metabolites involved in feeding, growth, and energy show significant daily fluctuations (Gélineau et al. 2002). Thus, fish have different physiological moods at different times of the day, showing different responses to dietary treatments at different times (Boujard 2001; Madrid et al. 2001). Therefore, it is required to find the best feeding time when fish are in the best physiological condition for lipid/energy metabolism to achieve the best growth performance. Feeding at optimal times can lower feed loss to the environment, reduce rearing costs, and provide greater profit for fish producers.
The beluga Huso huso is the largest and most expensive species of sturgeon owing to its precious caviar and meat (Vecsei et al. 2002; Pikitch et al. 2005). Beluga is suitable for aquaculture due to its high adaptability to artificial food and rearing systems, high growth rate, and resistance against stress conditions (Falahatkar et al. 2012a). Despite economic importance and high potential of beluga sturgeon in aquaculture, there is little information on feeding behavior of this valuable species. Most studies have focused on the optimal feeding rate and frequency to achieve better growth performance (Deng et al. 2003; Giberson and Litvak 2003; Mohseni et al. 2006); however, no research has been conducted on sturgeon’s feeding time. Thus, there is a need for more studies on feeding times to improve the feeding management and optimize growth performance.
To date, few studies have focused on feeding time impacts on apparent digestibility and growth performance in fish (Boujard and Leatherland 1992; Bolliet et al. 2000, 2004; Hossain et al. 2001), and interestingly, they all have focused on teleost fish. Therefore, for the first time, the present study set to investigate the combined effects of feeding times and dietary levels of lipid on growth performance, hematological parameters, muscle composition, and digestibility of dry matter, lipid, and protein in juvenile beluga sturgeon.
Materials and methods
Experimental diets
Ingredients, compositions, and proximate analysis of the diet are presented in Table 1. Two isonitrogenous diets were formulated containing either 120 g kg−1 lipid as low-energy diet, LE, or 240 g kg−1 lipid as high-energy diet, HE (Table 1). The ingredients were obtained from Behparvar feed manufacturing company (Karaj, Iran). Since there is less information on nutritional requirement of sturgeon and due to similarity to salmonid fish, rainbow trout diet could be a good rationale for these fish (Hung and Deng 2002); hence, a main part of the diet consisted of rainbow trout-formulated diet. Chromic oxide (Cr2O3; Merck Company, Darmstadt, Germany) was added to the experimental diet at 10 g kg diet−1 as a non-digestible indicator to evaluate the nutrient digestibility of the prepared diets. The ingredients were mixed and homogenized thoroughly before moistened via adding 25 % boiling water and then pelleted using a 3-mm-diameter experimental feed mill. Before using, the pellets were dried in a warm-air cabinet at 35 °C for 48 h and stored in a freezer at −17 °C. The pellets were broken into similar and appropriate size and then stored in a refrigerator at 5 °C during the feeding days.
Experimental fish and feeding trial
The juvenile beluga sturgeons (50 ± 0.2 g, mean ± SE) were obtained from the Shahid Dr. Beheshti Sturgeon Fish Propagation and Rearing Complex in Guilan, Iran, where the fish were hatched and reared. Prior to the trial, 360 fish were acclimated for 2 weeks in a flow-through system with artificial diet (with 120 g kg−1 lipid). The fish were distributed into eighteen 785-l circular concrete tanks (20 fish in each tank with a near-uniform biomass of 994.0 ± 2.8 g tank−1) with a flow rate of 13.1 ± 0.9 l min−1 for 8 weeks applying a completely randomized design with six treatments and three replicates per treatment.
Fish were hand-fed with one of the two experimental diets to apparent satiation twice a day, based on checking the presence of uneaten pellets in the tank outlet. To this purpose, a net in the tank outlet was set up for collecting the feed waste. Uneaten feed was dried and weighed to calculate the feed intake. For each tank, feeding lasted 5 min. The feeding times considered in three treatments were as follows: (1) at 09:00 and 16:00 (under light regime), (2) at 21:00 and 04:00 (under dark regime), and (3) at 13:00 and 20:00 (under light–dark regimes). Artificial light–dark cycle, created by black plastic covers, was 12D:12L (light set on at 06:00). The light source was provided by a 205-lx fluorescent lamp on the water surface. Average temperature, pH, nitrite, and dissolved oxygen were 16.7 ± 0.5 °C, 8 ± 0.2, 0.04 ± 0.001 mg l−1, and 6.8 ± 0.1 mg l−1, respectively.
Growth performance
Every 2 weeks, the body weight of all fish was measured. To decrease stress and evacuate the digestive tract contents, the fish starved for 24 h and then were anesthetized with 400 mg l−1 clove powder extract for biometric measurements. Afterward, all fish from each tank were counted and weighed, individually, to calculate the growth and survival rate. Growth performance parameters including weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR), and survival rate (SR) were calculated using the following formulas:
Wf and Wi are final and initial fish weights, respectively; t is the experimental duration in day; DFI is dry feed intake; wet WG is Wf–Wi; Nf and Ni are final and initial numbers of fish in each replicate, respectively.
Three fish per tank (nine fish/treatment) were randomly sampled after 8 weeks of the feeding trial. They were killed by a blow on head (Poli et al. 2005); the blood samples were taken; their livers were dissected and weighed for hepatosomatic index (HSI) assessment according to following formula and were also considered for proximate muscle composition.
Proximate composition
The proximate composition of the belugas and diets were analyzed according to Association of Official Analytical Chemists (AOAC 1998). At the end of the trials, after collecting three fish from each tank (nine fish/treatment), two pieces of dorsal muscles between the lateral and dorsal scutes were sliced and minced for proximate analysis. The samples were dried at 105 °C for almost 24 h to determine dry matter. Crude protein was measured via Kjeldahl method (using Kjeltec Auto Analyser, 2300 Tecator, Sweden). Crude fat was determined by extraction with petroleum ether using Soxhlet apparatus; ash by incineration at 600 °C for 6 h; and energy through bomb calorimeter (Parr Instrument Co, Moline, IL, USA).
Hematological analysis
At the end of the feeding trial, for hematological and biochemical tests, blood samples of three fish from each tank (nine fish/treatment) were taken gently after anesthetization from the caudal vein by 5-ml plastic syringes (2 ml blood fish−1). For each fish, blood sampling took <1 min. Two different aliquots of blood were used for different analyses. An aliquot of whole blood was transferred to a heparinized tube for measuring the hematocrit (Hct), hemoglobin (Hb), and differential white blood cells count. The second aliquot was used for plasma analysis, centrifuged at 1500g for 10 min and preserved at −70 °C. The obtained plasma was used to determine cholesterol and total protein contents. Hct was determined using a microhematocrit centrifuge (3500g for 10 min) according to Řehulka (2000). Hb concentration was determined applying a cyanmethemoglobin method (Řehulka 2000). Differential white blood cells’ count was determined under light microscope as the percentage of lymphocytes, neutrophils, eosinophils, and monocytes after air-drying and fixing of the smear with 96 % ethanol and staining by Giemsa for 30 min (Klontz 1994). Total protein level was determined according to biuret method (Tietz 1986) using a Ziestchem commercial kit (Tehran, Iran). Cholesterol level was determined using colorimetric method (Tietz 1986) and a Pars Azmoon kit (Karaj, Iran).
Digestibility
For digestibility, from the second week to the end of the trial, the feces of the fish were collected. Three fish from each tank (nine fish from each treatment) were slightly caught without any stress and transferred, separately, to six special conical tanks (volume 200 l) 2 h after the second feeding of the day, and the feces, almost 4–6 h after the second feeding, were collected. The water inflow and outflow were on the top and bottom of the tanks, respectively. To prevent nutrient leaching, the feces were collected repeatedly at 20-min intervals. Feces samples from each treatment were labeled and were immediately frozen and kept at −80 °C before analysis. They were analyzed for dry matter, protein, and lipid according to AOAC (1998), and chromic oxide by a spectrophotometric procedure developed using ashing at 450 °C followed by acid digestion in beakers and reading the diluted digests at 440 nm (Fenton and Fenton 1979). Apparent digestibility coefficients (ADC) of the diets were calculated according to the following equation (Degani et al. 1997):
Statistical analysis
All data are presented as mean ± standard error (SE), considering three replicate groups (n = 3). Data on the combined effects of feeding times and dietary lipid levels on growth performance, hematology, biochemistry, and digestibility in juvenile beluga sturgeon were analyzed via two-way analysis of variance (ANOVA) using SPSS 13.0 (Chicago, IL, USA), assuming feeding time and dietary lipid level as independent factors. By observing a significant effect, one-way ANOVA and Tukey’s comparison test determined individual mean differences. All percentage data recognized as non-homogeneous (determined by Kolmogorov–Smirnov test) were transformed by arc-sin prior to the statistical analysis. Differences were considered significant at P < 0.05.
Results
Survival and growth performance
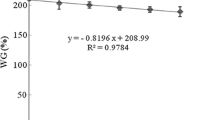
No mortality, deficiency, or illness signs were observed during the feeding trial. Mean weight of fish was not affected by diets 28 days after the experiment; the lowest weight was found in fish fed LE diet under dark at day 42; and the highest weight was observed in fish fed with HE diet at the end of day 56 (P < 0.05; Fig. 1). The growth parameters of fish fed with the experimental diets for 8 weeks are presented in Table 2. The results showed that the growth of belugas was affected by dietary lipid level (P < 0.05), while not affected by the feeding time (P > 0.05; Table 2). FW, WG, and SGR significantly increased, while FCR significantly decreased as the level of dietary lipid increased. The fish fed with HE diet showed the highest FW (from 174.2 ± 5.1 to 175.9 ± 5.0 g), SGR (from 2.44 ± 0.03 to 2.47 ± 0.03 % day−1), WG (from 246.5 ± 9.0 to 252.6 ± 4.5 %), and the lowest FCR (1.10 ± 0.03 to 1.12 ± 0.04). HSI was uninfluenced by feeding time and/or lipid level (P > 0.05). There was no interaction between feeding time and lipid level in terms of growth indices and HSI (P > 0.05; Table 2).
Growth of juvenile beluga sturgeon Huso huso fed under different feeding times (D dark, L light, DL dark and light) and different dietary levels of lipid (LE low energy, HE high energy) during 8 weeks of experiment. Values are mean ± SE of triplicate groups of fish (n = 3). Values within the same time, not sharing common superscript letters, are significantly different (P < 0.05)
Hematological and biochemical indices
Neither feeding times, nor dietary lipid levels did result in significant changes on hematological parameters including Hct, Hb, cholesterol, and total protein levels and the percentage of lymphocyte, neutrophil, eosinophil, and monocyte (P > 0.05; Table 3). There was no interaction between feeding time and lipid level (P > 0.05; Table 3).
Proximate composition
The proximate composition of the beluga muscle was uninfluenced by feeding times (P > 0.05), but influenced by dietary lipid levels (P < 0.05; Table 4). As lipid dietary levels increased, lipid content of the muscle significantly increased, but protein, ash, and dry matter contents showed no significant increase (P > 0.05). The fish fed with HE diet showed the highest muscle lipid content (26.6 ± 5.3 to 35.2 ± 7.8 g kg−1). There was no interaction between feeding time and dietary lipid level (P > 0.05; Table 4).
Digestibility
Neither dietary lipid level, nor feeding time did induce any significant change on digestibility of dry matter, lipid, and protein in beluga (P > 0.05; Table 5). Generally, digestibility of the test diets was high (>90 %). The highest dry matter (92.8 %), protein (95.7 %), and lipid (96.5 %) digestibility was found in complete darkness in the HE diet. However, no significant difference was observed among the treatments (P > 0.05).
Discussion
The combined effects of feeding times and dietary levels of lipid significantly increased the growth performance of fish that fed with high dietary lipid levels, which is possibly due to a protein-sparing effect (Higgs and Dong 2000). In addition, there was less fish oil in LE diet compared with HE diet, so LE diet may have been deficient in essential n-3 fatty acids. In previous studies on Persian sturgeon, Acipenser persicus (Mohseni et al. 2007), and beluga sturgeon (Keramat Amirkolaie et al. 2012), high dietary lipid levels increased significantly the growth performance. Fish use lipids and carbohydrates as energy sources; dietary protein is used to improve cell structure and growth, and thus, the rearing period is reduced (Skalli et al. 2004). On the other hand, high-lipid diets increase energy levels which may cause reduction in both feed consumption and total protein intake and ultimately fish growth (Wang et al. 2005; Chatzifotis et al. 2010). This is probably related to the ability of the fish to utilize dietary lipids (Higgs and Dong 2000). Some species such as beluga can utilize lipid levels to 24 %, as seen by a better growth performance of the fish fed HE diet in this study; however, some species such as white seabass, Atractoscion nobilis, cannot utilize lipid level more than 10 % (Jirsa et al. 2013). In the present experiment, the diets were not isocaloric; diets with high lipid levels create more calories for beluga metabolism, which can lead to an increase in growth rate of the fish.
High levels of lipid/energy in the diet significantly increased the muscle lipid content of beluga sturgeon, which is in agreement with the results on Persian sturgeon (Mohseni et al. 2007) and beluga sturgeon (Keramat Amirkolaie et al. 2012) in which dietary lipid levels increased from 10 to 25 % and 15 to 30 %, respectively. A similar trend has been reported in grouper Epinephelus coioides (Luo et al. 2005) and pike-perch Sander lucioperca (Schulz et al. 2008); in line with the current experiment, these studies on teleosts showed an increase in body lipid content, but a decrease in body moisture content when dietary lipid increased.
Hematological indices can vary between species owing to changes in environmental factors (Barton 2000, 2002; Iwama et al. 2006; Rafatnezhad et al. 2008); nevertheless, in the present study, hematology was not significantly influenced by changes in feeding time or dietary lipid level. These findings are in agreement with those of Safarpour Amlashi et al. (2011) and Falahatkar et al. (2009, 2012a), suggesting that the hematology of beluga sturgeon is relatively unresponsive to diet alteration and/or handling and confinement. The reason for this is not known, but it may be due to the evolutionary history of sturgeon (Falahatkar and Barton 2007; Falahatkar et al. 2012b) and behavioral or endocrinological mechanisms (Kieffer et al. 2001). Further studies are required to understand the low hematological response of sturgeons.
In the present study, it was found that belugas could be fed at all day and night times. Sturgeons are benthic cruisers and light independent somehow with very small eyes in relation to their body size, which do not apparently contribute much to food detection (Billard and Lecointre 2000; Devitsina et al. 2011). Therefore, they have developed a few specialized organs including rostrum, ampulla of lorenzini, and barbels to compensate for visual system’s weakness (Kasumyan 1997). Both olfactory and gustatory systems of sturgeons have high sensibility level (Pavlov and Kasumyan 1990). Kasumyan and Devitsina (1997) found that olfaction is a fundamental sense for feeding behavior in sturgeons; thus, they mostly find the food items with this sense. Hence, they can easily detect their food even in a dark environment.
The findings revealed that feeding time did not affect digestibility and growth of fish. A few studies have demonstrated that feeding time affects diet digestibility and, consequently, results in a change in growth performance and/or physiological parameters (Bolliet et al. 2000; Hossain et al. 2001). The feeding time might also influence growth performance through interactions with both photoperiodical internal cycles and feed intake. Therefore, feeding time must be in line with physiological rhythm to increase growth (Bolliet et al. 2004). The results are consistent with those of Bolliet et al. (2004) who found no significant effect of feeding time on the growth and proximate body composition of rainbow trout. Unlike the supposition, there was no interaction between feeding time and dietary lipid level. These findings explain that beluga can be fed all day/night times without any adverse effect on digestion, growth, and physiological status. Using olfactory and gustatory organs for foraging behavior is likely a major reason behind nonsignificant effects of feeding time on growth and digestion in beluga sturgeon. Probably, this valuable species has circadian locomotor activity and circadian feeding activity. There is limited information on foraging behavior of beluga sturgeon, so further studies on the current topics are therefore needed.
Conclusion
Findings of the current study on beluga sturgeon indicated that feeding times did not influence the growth performance, blood parameters, muscle proximate composition, and diet digestibility. Generally, it is concluded that beluga can be fed at any time of the day or night since this species possesses a few specialized organs including rostrum, ampulla of lorenzini, and barbels to detect food at different times and conditions, thus possessing a circadian feeding activity. Moreover, the data demonstrated that increasing the dietary lipid to 240 g kg−1 would improve the growth performance. It should be noted that this increase in dietary lipid leads to an increase in lipid deposition in the muscle of beluga.
References
AOAC (1998) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Arlington
Azzaydi M, Martinez FJ, Zamora S, Sanchez-Vazquez FJ, Madrid JA (1999) Effect of meal size modulation on growth performance and feeding rhythms in European sea bass Dicentrarchus labrax, L. Aquaculture 170:253–266. doi:10.1016/S0044-8486(98)00411-6
Barton BA (2000) Stress. In: Stickney RR (ed) Encyclopedia of aquaculture. Wiley, NY, pp 892–898
Barton BA (2002) Stress in fishes: a diversity of response with particular reference to changes in circulating corticostreroids. Integr Comp Biol 42:517–525. doi:10.1093/icb/42.3.517
Billard R, Lecointre G (2000) Biology and conservation of sturgeon and paddlefish. Rev Fish Biol Fish 10:355–392. doi:10.1023/A:1012231526151
Bjerkeng B, Refstie S, Fjalestad KT, Storebakken T, Rødbotten M, Roem AJ (1997) Quality parameters of the flesh of Atlantic salmon (Salmo salar) as affected by dietary fat content and full-fat soybean meal as a partial substitute for fish meal in the diet. Aquaculture 157:297–309. doi:10.1016/s0044-8486(97)00162-2
Bolliet V, Cheewasedtham C, Houlihan D, Gelineau A, Boujard T (2000) Effect of feeding time on digestibility, growth performance and protein metabolism in the rainbow trout Oncorhynchus mykiss: interaction with dietary fat levels. Aquat Living Resour 13:107–113. doi:10.1111/j.0022-1112.2004.00418.x
Bolliet V, Jarry M, Boujard T (2004) Rhythmic pattern of growth and nutrient retention in response to feeding time in the rainbow trout. J Fish Biol 64:1616–1624. doi:10.1111/j.0022-1112.2004.00418.x
Boujard T (2001) Daily rhythms and fish physiology. Vie Milieu 51:237–245
Boujard T, Leatherland JF (1992) Circadian rhythms and feeding time in fishes. Environ Biol Fish 35:109–131. doi:10.1007/bf00002186
Boujard T, Gelineau A, Corraze G (1995) Time of a single daily meal influences growth performance in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 26:341–349. doi:10.1111/j.1365-2109.1995.tb00922.x
Chatzifotis S, Panagiotidou M, Papaioannou N, Pavlidis M, Nengas I, Mylonas CC (2010) Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 307:65–70. doi:10.1016/j.aquaculture.2010.07.002
Degani G, Viola S, Yehuda Y (1997) Apparent digestibility coefficient of protein sources for carp (Cyprinus carpio L). Aquac Res 28:23–28. doi:10.1046/j.1365-2109.1997.00825.x
Deng DF, Koshio S, Yokoyama S, Bai SC, Shao Q, Cui Y, Hung SSO (2003) Effects of feeding rate on growth performance of white sturgeon (Acipenser transmontanus) Larvae. Aquaculture 217:589–598. doi:10.1016/s0044-8486(02)00461-1
Devitsina GV, Golovkina TV, Rod’kin MM (2011) Features of gustatory system morphology in early juveniles of Siberian sturgeon Acipenser baerii (Acipenseridae, Acipenseriformes). J Ichthyol 51:1104–1116. doi:10.1134/s0032945211110038
Ellis SC, Reigh RC (1991) Effects of dietary lipid and carbohydrate-levels on growth and body composition of juvenile red drum, Sciaenops ocellatus. Aquaculture 97:383–394. doi:10.1016/0044-8486(91)90330-a
Falahatkar B, Barton BA (2007) Preliminary observations of physiological responses to acute handing and confinement in juvenile beluga Huso huso: preliminary observations. Aquac Res 38:1786–1789. doi:10.1111/j.1365-2109.2007.01855.x
Falahatkar B, Poursaeid S, Shakoorian M, Barton B (2009) Responses to handling and confinement stressors in juvenile great sturgeon Huso huso. J Fish Biol 75:784–796. doi:10.1111/j.1095-8649.2009.02334.x
Falahatkar B, Safarpour Amlashi A, Conte F (2012a) Effect of dietary vitamin E on cortisol and glucose responses to handling stress in juvenile beluga sturgeon Huso huso L. J Aquat Anim Health 24:11–16. doi:10.1080/08997659.2011.647235
Falahatkar B, Akhavan SR, Efatpanah I, Meknatkhah B (2012b) Primary and secondary responses of a teleostean, pikeperch Sander lucioperca, and a chondrostean, Persian sturgeon Acipenser persicus juveniles, to handling during transport. N Am J Aquac 74:241–250. doi:10.1080/15222055.2012.675988
Fenton TW, Fenton M (1979) An improved procedure for the determination of chromic oxide in feed and feces. Can J Anim Sci 59:631–634. doi:10.4141/cjas79-081
Gélineau A, Bolliet V, Corraze G, Boujard T (2002) The combined effects of feeding time and dietary fat levels on feed intake, growth and body composition in rainbow trout. Aquat Living Resour 15:225–230. doi:10.1016/s0990-7440(02)01180-4
Giberson AV, Litvak MK (2003) Effect of feeding frequency on growth, food conversion efficiency, and meal size of juvenile Atlantic sturgeon and shortnose sturgeon. N Am J Aquacult 65:99–105. doi:10.1577/1548-8454(2003)65<99:EOFFOG>2.0.CO;2
Higgs DA, Dong FM (2000) Lipids and fatty acids. In: Stickney RR (ed) The encyclopedia of aquaculture. Wiley, NY, pp 476–496
Hossain MAR, Haylor GS, Beveridge MCM (2001) Effect of feeding time and frequency on the growth and feed utilization of African catfish Clarias gariepinus (Burchell 1822) fingerlings. Aquac Res 32:999–1004. doi:10.1046/j.1365-2109.2001.00635.x
Hung SSO, Deng D-F (2002) Sturgeon, Acipenser spp. In: Webster CD, Lim C (eds) Nutrient requirements and feeding of finfish for aquaculture. CAB International, USA, pp 344–357. doi:10.1079/9780851995199.0344
Iwama GK, Afonso LOB, Vijayan MM (2006) Stress in fishes. In: Evans DH, Claiborne JB (eds) The physiology of fishes. CRC Press, Boca Raton, pp 319–342
Jirsa D, Deng D-F, Davis DA, Wang W-F, Hung SSO, Drawbridge M (2013) The effects of dietary lipid levels on performance and heat-shock protein response of juvenile white seabass, Atractoscion nobilis. Aquac Nutr 19:227–232. doi:10.1111/j.1365-2095.2012.00965.x
Kasumyan AO (1997) Gustatory reception and feeding behavaior of fish. J Ichthyol 37:72–86
Kasumyan AO, Devitsina GV (1997) The effect of olfactory deprivation on chemosensory sensitivity and the state of taste receptors of Acipenserids. J Ichthyol 37:786–798
Keramat Amirkolaie A, Mahdavi S, Hosseini SA (2012) Dietary fat content and feed supply influence growth and body composition in juvenile beluga sturgeon (Huso huso). Aquacult Int 20:859–867. doi:10.1007/s10499-012-9507-7
Kieffer JD, Wakefield AM, Litvak MK (2001) Juvenile sturgeon exhibit reduced physiological responses to exercise. J Exp Biol 204:4281–4289
Klontz GW (1994) Fish hematology. In: Stolen JS, Fletcher TC, Rowley AF, Kelikoff TC, Kaattari SL, Smith SA (eds) Techniques in fish immunology. SOS Publications, NY, pp 121–131
Luo ZHI, Liu Y-J, Mai K-S, Tian L-X (2005) Effect of dietary lipid level on growth performance, feed utilization and body composition of grouper Epinephelus coioides juveniles fed isonitrogenous diets in floating netcages. Aquacult Int 13:257–269. doi:10.1007/s10499-004-2478-6
Madrid JA, Boujard T, Sanchez-Vazquez FJ (2001) Feeding rhythm. In: Houlihan D, Boujard T, Jobling M (eds) Food intake in fish. Blackwell Science Ltd, Oxford, pp 189–215. doi:10.1002/9780470999516
Mohseni M, Pourkazemi M, Bahmani M, Falahatkar B, Pourali HR, Salehpour M (2006) Effects of feeding rate and frequency on growth performance of yearling great sturgeon, Huso huso. J Appl Ichthyol 22:278–283. doi:10.1111/j.1439-0426.2007.00968.x
Mohseni M, Sajjadi M, Pourkazemi M (2007) Growth performance and body composition of sub-yearling Persian sturgeon, Acipenser persicus (Borodin, 1897), fed different dietary protein and lipid levels. J Appl Ichthyol 23:204–208. doi:10.1111/j.1439-0426.2007.00866.x
Pavlov DV, Kasumyan AO (1990) Study of fish behavior and sensory systems in Russia. J Ichthyol 34:1–26
Pikitch EK, Doukakis P, Lauck L, Chakrabarty P, Erickson DL (2005) Status, trends and management of sturgeon and paddlefish fisheries. Fish Fish 6:233–265. doi:10.1111/j.1467-2979.2005.00190.x
Poli BM, Parisi G, Scappini F, Zampacavallo G (2005) Fish welfare and quality as affected by pre-slaughter and slaughter management. Aquacult Int 13:29–49. doi:10.1007/s10499-004-9035-1
Rafatnezhad S, Falahatkar B, Tolouei Gilani MH (2008) Effects of stocking density on hematological, growth indices and fin erosion of great sturgeon (Huso huso) juveniles. Aquac Res 39:1506–1513. doi:10.1111/j.1365-2109.2008.02020.x
Řehulka J (2000) Influence of astaxanthin on growth rate, condition and some blood indices of rainbow trout. Aquaculture 190:27–47. doi:10.1016/s0044-8486(00)00383-5
Safarpour Amlashi A, Falahatkar B, Sattari M, Tolouei Gilani MH (2011) Effect of dietary vitamin E on growth, muscle composition, hematological and immunological parameters in sub-yearling beluga, Huso huso. Fish Shellfish Immunol 30:807–814. doi:10.1016/j.fsi.2011.01.002
Sánchez-Vázquez FJ, Madrid JA, Zamora S, Iigo M, Tabata M (1996) Demand feeding and locomotor circadian rhythms in the goldfish, Carassius auratus: dual and independent phasing. Physiol Behav 60:665–674. doi:10.1016/S0031-9384(96)80046-1
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, pp 181–257
Schulz C, Huber M, Ogunji J, Rennert B (2008) Effects of varying dietary protein to lipid ratios on growth performance and body composition of juvenile pike perch (Sander lucioperca). Aquacult Nutr 14:166–173. doi:10.1111/j.1365-2095.2007.00516.x
Skalli A, Hidalgo MC, Abellan E, Arizcun M, Cardenete G (2004) Effects of the dietary protein/lipid ratio on growth and nutrient utilization in common dentex (Dentex dentex L.) at different growth stages. Aquaculture 235:1–11. doi:10.1016/j.aquaculture.2004.01.014
Tietz NW (1986) Textbook of clinical chemistry. WB Saunders Co, Philadelphia
Vecsei P, Sucui R, Peterson D (2002) Threatened fishes of the world: Huso huso (Linnaeus, 1758) (Acipenseridae). Environ Biol Fish 65:363–365. doi:10.1023/A:1020544505748
Wang JT, Liu YJ, Tian LX, Mai KS, Du ZY, Wang Y, Yang HJ (2005) Effect of dietary lipid level on growth performance, lipid deposition, hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture 249:439–447. doi:10.1016/j.aquaculture.2005.04.038
Acknowledgments
We acknowledge staff at the Shahid Dr. Beheshti Sturgeon Fish Propagation and Rearing Complex for providing the juvenile sturgeon for this experiment. We are also grateful to our Faculty of Natural Resources, University of Guilan, for financial support to M. N., Veterinary General Directory of Guilan Laboratory for their assistance during muscle analysis, and Dr. Fadaei pathology laboratory for their helps during blood analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Najafi, M., Falahatkar, B., Safarpour Amlashi, A. et al. The combined effects of feeding time and dietary lipid levels on growth performance in juvenile beluga sturgeon Huso huso . Aquacult Int 25, 31–45 (2017). https://doi.org/10.1007/s10499-016-0011-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0011-3