Abstract
Obesity, an extremely important factor in feline clinical practice, is estimated to affect up to one third of the feline population. Moreover, it can trigger chronic inflammation, which could predispose to oxidative stress by increasing reactive oxygen species, thereby generating potentially irreversible cellular damage. This study analyzed hematological, biochemical and oxidative stress profiles at various degrees of feline obesity. Forty-five cats were selected and divided into three groups: control (n = 17), overweight (n = 13) and obese (n = 15), after clinical and laboratory evaluation and body condition score. Biochemical and oxidative stress analyses were performed using a photocolorimeter and hematological analyses were performed in a veterinary cell counter. Obese cats showed increased mean corpuscular volume (MCV), red cell distribution width (RDW), HDL cholesterol and triglycerides and decreased activity of gamma-glutamyl transferase (GGT) than control cats, although within the reference ranges for the species. As for oxidative stress, obese cats showed higher total antioxidant capacity (TAC), by the inhibition of 2,2’-Azino-Bis-3-Ethylbenzthiazoline-6-Sulfonic Acid (ABTS), inhibition of ABTS associated with horseradish peroxidase (ABTS + HRP), cupric ion reducing antioxidant capacity (CUPRAC) and ferric reducing antioxidant power (FRAP) methods, while overweight cats had a higher TAC-ABTS + HRP and TAC-FRAP than control cats. We conclude that the conditions of natural obesity and overweight in the feline species alter its hematological, biochemical and oxidative stress parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sedentary lifestyle and inadequate nutrition are undesirable results of the domestication of felines, which commonly leads to obesity, a condition observed in 25 to 35% of cats (Butterwick 2000; Zoran 2009; German 2010). Obesity leads to a predisposition for numerous secondary diseases, such as diabetes mellitus (Biourge et al. 1997; Osto et al. 2013), cardiorespiratory (Chandler 2016), dermatological (Lund et al. 2005) and oral abnormalities (German 2010), lower urinary tract and orthopedic diseases (Öhlund et al. 2018) in addition to hepatic lipidosis (Blanchard et al. 2004) and neoplasms (Chandler et al. 2017).
Adipose tissue is an active endocrine organ, releasing several important hormones that play a role in the development of obesity-associated changes, such as metabolic syndrome and insulin resistance (Kil and Swanson 2010). Considering the human and canine species, obesity causes a chronic inflammatory condition, responsible for the increase in platelets, neutrophils, lymphocytes and monocytes, in addition to elevated levels of pro-inflammatory cytokines (Nemet et al. 2005; Radakovich et al. 2017; Zaldivar et al. 2006). The pro-inflammatory state can trigger the excessive production of reactive oxygen species (ROS) (Dandona et al. 2001, 2004; Tanner et al. 2007) and although ROS are necessary in physiological processes such as cell signaling and defense against microorganisms, excessive production leads to oxidative damage as a result of oxidative stress (Pacher et al. 2007). In obese cats, the association of intense protein and lipid oxidation with an increase in inflammatory cytokines such as interleukins 1 and 6, C-reactive protein and tumor necrosis factor-alpha, constitutes a typical picture of chronic inflammatory disease (Coppack 2001).
Thus, oxidative stress occurs when there is an imbalance between antioxidants and oxidants in the body, a condition associated with several pathological conditions in cats, such as diabetes mellitus (Webb and Falkowski 2009), chronic kidney failure (Keegan and Webb 2010), infection with feline immunodeficiency virus (FIV) (Webb et al. 2008), and a carcinogenic potential (Biezus et al. 2017; Shacter et al. 1989). There is a paucity of studies that evaluate oxidative stress in feline obesity. A single study that induced obesity in cats over a short period of 12 weeks and maintained it for an additional 8-week period revealed increased oxidative stress through increased oxidation of proteins, lipids and DNA (Tanner et al. 2007). However, as obesity was induced, it is difficult to compare these results with actual clinical conditions found in chronically obese cats. Furthermore, the total antioxidant and oxidant capacities were not evaluated. In this context, our study aimed to evaluate the hematological, biochemical and oxidative stress profiles at various levels of feline obesity.
Materials and methods
Animal selection
The study was conducted according to the ethical principles of the Animal Research Ethics Committee of the University Center of the Integrated Faculties of Ourinhos (Protocol no. 007/2019). The participation of each cat was authorized by its owner, who signed a free and informed consent form.
After clinical and laboratory evaluation, 45 domiciled Brazilian Shorthair cats were selected and allocated to three groups, according to the body condition score (BCS) and the characteristics listed in Table 1:
− Control group: 17 adult cats (mean age 4.62 ± 1.70 years), 9 females and 8 males, 15 neutered and 2 unneutered, with BCS 5 (mean weight 3.61 ± 0.67 kg), clinically healthy, with no changes in the clinical examination and laboratory evaluation (complete blood count – CBC, albumin, alanine aminotransferase – ALT, creatinine, total cholesterol, gamma-glutamyl transferase – GGT, glucose, urea, total protein and triglycerides).
− Overweight group: 13 adult cats (mean age 5.04 ± 2.93 years), 8 females and 5 males, all neutered, with BCS 6–7 (mean weight 4.5 ± 0.78 kg) for at least one year.
− Obese group: 15 adult cats (mean age 6.07 ± 2.88 years), 6 females and 9 males, all neutered, presenting BCS 8–9 (mean weight 5.89 ± 0.94 kg) for at least one year.
BCS was proposed previously (Laflamme 1997): cats on control group with BCS 5 presented well-proportioned, ribs could be felt with slight fat covering, waist could be seen behind ribs, but was not pronounced and abdominal fat pad was minimal; cats on overweight group presented BCS 6 and 7, ribs were felt with slight excess of fat covering or were not easily felt through moderate fat covering, waist was not easily seen, abdomen may be slight rounding and moderate abdominal fat pad could be seen; cats on obese group presented BCS 8 and 9, ribs could not be felt due to excessive fat covering, waist was absent, abdomen presented obvious rounding with prominent fat pad and fat deposits were also present over lower back area. All estimates were disclosed by the same evaluator. All animals were photographed for a second blind opinion. In case of divergence, a third blind researcher was consulted and the most prevalent classification was considered.
The only cats included in this study were those fed exclusively with commercial cat food containing similar compositions, and which accepted physical restraint to draw blood samples without struggling. Animals treated in the preceding month with any type of medication, particularly drugs that lead to obesity or that have antioxidant and/or anti-inflammatory action, were not included in this study.
Collection of blood samples and laboratory analysis
The cats were fasted for 12 h, after which 5 mL of blood was drawn by jugular venipuncture into tubes with K2EDTA (BD Vacutainer®, Becton-Dickson, New Jersey, USA) for complete blood count (CBC), tubes with sodium fluoride (Injex Vacuo, Injex Indústrias Cirúrgicas, São Paulo, Brazil) for the biochemical determination of glucose, and tubes with clot activator (BD Vacutainer®, Becton-Dickson, New Jersey, USA) to obtain serum for other analyses. The fluoridated blood was immediately centrifuged (3,000 rpm for 10 min), while the tube used to obtain serum was centrifuged 20 min after collection. All serum processing took place in the absence of light and samples were stored away from light at -20 °C until analysis, for a maximum period of 20 days.
CBC was performed as previously described (Costa et al. 2020; Oliveira et al. 2020). Briefly, red blood cells (RBC), white blood cells (WBC) and platelet (PLT) concentrations, mean corpuscular volume (MCV), red cell distribution width (RDW), mean platelet volume (MPV) and hemoglobin were examined in a veterinary automated cell counter (ABX Micros ESV 60, Paris, France) previously calibrated and checked with commercial controls (ABX Minotrol 16, Paris, France). Hematocrit (HCT) was determined by the Strumia microcapillary method (11,400 rpm for 5 min), the differential leukocyte count was performed using a blood smear stained with commercial hematological dye (Instant-Prov, Newprov, Pinhais, PR, Brazil), and the icterus index test was performed as recommended by Jain (1986). Total plasma protein (TPP) was determined in a portable clinical refractometer (ATAGO, Mod. Master-SUR-NM, Tokyo, Japan).
Biochemical analyses were performed in a semi-automated photocolorimeter (BIO 2000, BioPlus, Barueri, SP, Brazil) in duplicate, using a set of commercial reagents (Labtest Diagnóstica SA, Lagoa Santa, MG, Brazil) according to the manufacturer’s instructions, after calibration with calibrator (Calibra H, Labtest Diagnóstica SA, Lagoa Santa, MG, Brazil) and verification with commercial control levels I (Qualitrol 1 H, Labtest Diagnóstica SA, Lagoa Santa, MG, Brazil) and II (Qualitrol 2 H, Labtest Diagnóstica SA, Lagoa Santa, MG, Brazil). HDL cholesterol levels were determined after precipitation with phosphotungstic acid and magnesium chloride, with subsequent determination of cholesterol. Uric acid, total cholesterol and triglyceride levels were determined by the enzymatic Trinder method, glucose by the glucose oxidase Trinder method, and ALT and AST by the ultraviolet (UV) kinetic method. Albumin was analyzed by the colorimetric method using bromocresol green, total calcium by the colorimetric cresolphthalein method, creatinine by the alkaline picrate colorimetric method, and ALP by Bowers and McComb’s modified kinetic procedure. Phosphorus was evaluated by UV determination according to Daly and Ertingshausen’s modified method, GGT by the Szasz modified method, amylase by the substrate 2-chloro-p-nitrophenyl-alpha-D-maltotrioside, lipase by colorimetric enzymatic method, total protein by biuret colorimetric method, urea by UV enzymatic method and fructosamine by nitroblue tetrazolium (NBT) reduction. The globulin content was quantified by subtracting the albumin from total proteins.
Oxidative stress was determined as previously described (Almeida et al. 2021; Bonatto et al. 2021), by measuring total antioxidant capacity (TAC) using four different methods, total oxidant capacity (TOC), lipid peroxidation and the antioxidants uric acid and albumin. TAC was determined in a semi-automated photocolorimeter (BIO 2000, BioPlus, Barueri, SP, Brazil) by inhibiting the reduction of the ABTS cation alone (TAC-ABTS) (Erel 2004) or in association with peroxidase (TAC-ABTS + HRP) (Rubio et al. 2016a), using the cupric ion reducing antioxidant capacity assay (TAC-CUPRAC) (Rubio et al. 2016b) and the ferric reducing antioxidant power assay (TAC-FRAP) (Benzie and Strain 1996). TOC was determined by the colorimetric method of xylenol orange (Erel 2005), while lipid peroxidation was determined using thiobarbituric acid reactive substances (TBARS) (Hunter et al. 1985). All the reagents were from Sigma-Aldrich Chemical Co.
Statistical analysis
The data were tested for normality using the Shapiro-Wilk test and for homoscedasticity using the Bartlett test. Differences between groups were verified by ANOVA and Tukey’s post-hoc test or Kruskal-Wallis and Dunn’s post-hoc test. All the statistical analyses were performed using a computer program (GraphPad Prism, v.6.00 for Windows, GraphPad Software, La Jolla, CA, USA, www.graphpad.com" target=“_blank”>www.graphpad.com) and differences were considered significant when p < 0.05.
Results
Hematological and biochemical parameters
With regard to hematological parameters, obese cats showed higher RDW than control cats and higher MCV than cats in the overweight group, but no differences were found in the other hematological variables (Table 1).
As for biochemical parameters, obese cats had higher levels of HDL cholesterol and triglycerides than control cats and lower GGT activity than overweight cats, but showed no significant differences in other biochemical parameters (Table 1).
Hematological and biochemical changes still remain within the species reference ranges (Table 1).
Oxidative stress parameters
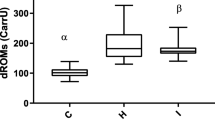
Obese cats showed higher TAC by the ABTS (Fig. 1 C), ABTS + HRP (Fig. 1D), CUPRAC (Fig. 1E) and FRAP (Fig. 1 F) methods than the control group. Overweight cats presented only higher TAC- ABTS + HRP (Fig. 1D) and TAC-FRAP (Fig. 1 F) than the control group and presented similar levels of TAC-ABTS (Fig. 1 C) and TAC-CUPRAC (Fig. 1E). No differences were observed among the groups with respect to the antioxidants albumin (Fig. 1 A) and uric acid (Fig. 1B), TOC (Fig. 1G) and to lipid peroxidation (Fig. 1 H).
Oxidative stress markers albumin (A), uric acid (B), total antioxidant capacity (TAC) determined by ABTS cation inhibition method alone (ABTS, C) or in association with peroxidase (ABTS + HRP, D), cupric reducing antioxidant capacity assay (CUPRAC, E) and by ferric reducing antioxidant power assay (FRAP, F), total oxidant capacity (TOC, G) and lipid peroxidation determined by thiobarbituric acid reactive substances (TBARS, H) in cats with body score condition 5 (Control, n = 17), 6/7 (Overweight, n = 13) and 8/9 (Obese, n = 15) according to Laflamme (1997). Bars indicate minimum and maximum values and boxes represent the first and third quartiles. Statistically significant difference is indicated by * (P < 0.05), ** (P < 0.01), *** (P < 0.001) or **** (P < 0.0001) according to Kruskal-Wallis and Dunn’s multiple comparisons test (TAC-CUPRAC) or ANOVA with Tukey’s multiple comparisons test (TAC-ABTS, TAC-ABTS + HRP and TAC-FRAP)
Discussion
Studies evaluating laboratory changes in different stages of feline obesity are scant. We observed increased TAC by four different methods, with no alteration in TOC and lipid peroxidation in obese cats. Moreover, changes in CBC such as increased MCV and RDW and in biochemical parameters such as increased triglycerides and HDL cholesterol levels, as well as decreased GGT activity, were also observed in different stages of obesity. In this regard, the current study sheds greater light on the pathophysiological mechanisms of feline obesity.
The average age of obese cats in this study was 6 years. Several authors have linked obesity to increasing age (Colliard et al. 2009; Courcier et al. 2010; Laflamme 2012) states that cats between 5 and 10 years old are more prone to obesity, and that this risk increases greatly from the age of 10 years onwards. Thus, we show that as soon as 3 years old cats start out overweight and become obese.
All the animals of the obese group were neutered and this group was predominantly composed of males. Previous studies have demonstrated that neutered male cats are more prone to obesity (Cave et al. 2012; Rowe et al. 2015). Male cats are approximately 13 times more likely to develop obesity than females, and if neutered, that chance increases to 15 times (Lund et al. 2005; Robertson 1999; Russell et al. 2000). In dogs, obesity is usually more common among neutered females, as males have a higher resting metabolic rate than females (Courcier et al. 2010; German 2006; Kil and Swanson 2010).
Obese cats showed increased MCV and RDW, showing not only an increase in the size of red blood cells but also greater variation in cell size, although within the reference ranges for the feline species (Rizzi et al. 2010). To date, we have not found studies that have related the increased RDW with obesity in cats. In humans, the relationship between obesity with metabolic syndrome (MS) and RDW alterations has been explored (Tsuda et al. 2001; Fujita et al. 2013; Laufer Perl et al. 2015; Vayá et al. 2015; Farah and Khamisy-Farah 2015; Yan et al. 2019). Sanchez-Chaparro et al. (2010) observed that the inflammatory state in human MS impaired erythrocyte maturation, leading to anisocytosis and increased RDW. Farah and Khamisy-Farah et al. (2015) reported a positive correlation between RDW and MS, and RDW increased with the severity of MS. Fujita et al. (2013) observed higher RDW in overweight adolescents than in normal-weight, with no significant difference in the mice experimental model. Other study reported that high RDW was associated with increased risk of MS and long-term mortality (Laufer Perl et al. 2015). It is possible that, in humans, changes in lipid, glucose and insulin concentrations lead to reduced RBC deformability through the impact on the erythrocyte membrane, which could predispose to a higher erythrocyte replacement rate (Tsuda et al. 2001; Vayá et al. 2015; Yan et al. 2019). Furthermore, changes in these constituents would cause greater morphologic alterations (shape/volume), leading to an increase in RDW (Vayá et al. 2015). Further studies are needed to expand our understanding of the results reported here, since these changes are discrete and are insufficient to exceed the reference range for cats.
As for the WBC count, considering the groups examined in this pilot study no changes were observed in the population of blood leukocytes. Previous studies have shown that obesity leads to a pro-inflammatory state with increased inflammatory cytokines in humans (Nemet et al. 2005; Zaldivar et al. 2006), dogs (Radakovich et al. 2017) and cats (Tanner et al. 2007), and changes in WBC, such as increased neutrophils, monocytes and lymphocytes in obese children (Nemet et al. 2005; Zaldivar et al. 2006) and obese dogs due to increased levels of neutrophils and monocytes (Radakovich et al. 2017).
As for lipid metabolism, obese cats showed higher levels of triglycerides still within the reference ranges for the feline species (Kaneko et al. 2008), as described in the literature of different species (Alberti et al. 2006; Hoenig 2006; Mori et al. 2012; Vasan 2003). Lipid alterations are relatively common in veterinary medicine, especially in obese animals, often as a result of excessive intake of high calorie diets containing large amounts of carbohydrates and lipids (Bailhache et al. 2003; Barrie et al. 1993; Chikamune et al. 1995; Jeusette et al. 2005; Johnson 2005; Hoenig 2006). However, in the present study, all the owners reported feeding their cats solely with commercial cat food in the amount indicated by each manufacturer. In addition, considering the cats selected in our study there was no significant change of total cholesterol levels between obesity levels, corroborating the findings of previous studies that found no change in cholesterol levels in feline obesity (Aguiar et al. 2018; de Freitas et al. 2017). Higher HDL cholesterol levels in obese cats have been previously demonstrated (de Freitas et al. 2017). Unlike humans, cats have predominantly circulating HDL lipoprotein (Bauer 1996; Hoenig et al. 2003) emphasizes that obese cats have high levels of HDL cholesterol, which suggests the presence of cholesteryl ester transfer protein deficiency.
Obese cats showed reduced GGT activity. Increased GGT activity has been reported in obese humans, which is directly related to metabolic syndrome and its comorbidities (Saely et al. 2008). However, the reasons that led to the reduction in the activity of this enzyme in feline obesity are still unknown, and further studies are needed to clarify this issue.
Few earlier studies have evaluated oxidative stress in feline obesity. All the methods employed in this study indicated that obese cats had higher TAC, although TOC and lipid peroxidation remained unchanged in this pilot study. On the other hand, overweight cats showed an increase in TAC only by the ABTS + HRP and FRAP methods. Thus, oxidative stress markers varied according to animal weight, with increased TAC concentration in obese and overweight cats, depending on the method of analysis. Other authors have observed protein oxidation and lipid peroxidation in cats that had obesity induced and maintained for an 8-weeks period, as well as an increase in inflammatory cytokines, indicating an inflammatory condition induced by obesity (Tanner et al. 2007). In our study, the inclusion criterion was that the animals had to have been obese for at least one year, which means that they had already experienced obesity for a long period, making the process more chronic. Therefore, it was assumed that the animals were already adapted to obesity and that the TAC increased in order to fight oxidative damage during this condition.
The TAC evaluation methods showed differences, with overweight cats showing an increase in TAC only by the ABTS + HRP and FRAP methods and obese cats an increase in TAC by the four evaluated methods. A comparison of overweight and obese cats showed a difference only by the CUPRAC method, with obese cats showing higher CUPRAC than overweight animals. The differences observed in the TAC can most likely be attributed to the biochemical assays used in each method. The FRAP method primarily assesses uric acid, bilirubin, vitamin C and polyphenols (Benzie and Strain 1996). The CUPRAC method predominantly assesses non-enzymatic antioxidants from the thiol group (Rubio et al. 2016b), while the ABTS method assesses protein-based antioxidants such as glutathione and albumin (Erel 2004). The difference found between the methods may be related to antioxidant compounds not evaluated in the present study, since albumin and uric acid did not differ between groups. It is known that about 60% of TAC in human plasma is composed of uric acid (Benzie and Strain 1996) and the increase in this analyte has already been reported in human obesity (Matsuura et al. 1998), in rodents (Tsushima et al. 2013) and in obese dogs, although in the latter species this increase was not enough to prevent systemic oxidative stress (Bosco et al. 2018). Thus, chronic feline obesity induces increased TAC by substrates other than uric acid and albumin.
In the obesity levels evaluated in this study, no evidence was found of change in TOC and lipid peroxidation. This may be explained, at least partially, by the increase in TAC, which could contribute to the inactivation of oxidizing compounds in feline obesity, preventing lipid peroxidation. A previous study demonstrated that obese dogs underwent oxidative stress as a result of increased TOC and lipid peroxidation, while TAC remained unchanged (Bosco et al. 2018). In studies on human obesity by Cătoi et al. (2013) and Pirgon et al. (2013), an increase detected in the oxidative stress index was attributed to increased TOC and reduced TAC. In rodents, a decrease in TAC was also observed among obese animals (Bełtowski et al. 2000; Furukawa et al. 2004). In addition to obesity, oxidative stress has been described in pathologies such as chronic kidney disease (CKD) in dogs (Almeida et al. 2013; Silva et al. 2013), in cats (Keegan and Webb 2010) and in humans (Rysz et al. 2004), and in feline infectious peritonitis (Pedersen 2014). In CKD in cats, an increase was detected in reduced glutathione: oxidized glutathione (GSH:GSSG) ratios. This suggests the activation of antioxidants to fight ROS, despite the significantly lower TAC of sick animals, precisely because they are unable to maintain a balance between oxidizing substances and antioxidants (Keegan and Webb 2010). Increased lipid peroxidation has already been described in human obesity (Konukoglu et al. 2006; Ozata et al. 2002) and in rodents (Bełtowski et al. 2000; Furukawa et al. 2004). In canine obesity, the increase in TBARS was not detected in animals on a short-term fattening diet (van de Velde et al. 2012). Thus, lipid peroxidation and TOC were not suitable markers for the evaluation of oxidative stress in natural feline obesity in this experiment.
The main limitations of the present study include the low number of animals used in each experimental group, the impossibility of standardizing the food of the selected cats and the innate feline characteristic of showing high excitability and not tolerating prolonged manipulation, which yielded a small sample. Furthermore, the influence that excitation can exert on oxidative stress parameters is not known in the feline species. It is essential for further research to evaluate the role of oxidative stress, inflammation and metabolic syndrome in feline obesity, given the paucity of studies with this species in the obese condition and the fact that most of the data found are extrapolated from other species. However, since cats have nutritional, metabolic, physiological and pathological characteristics that render many of these extrapolations meaningless, further investigations into this subject are needed to understand its clinical implications in feline obesity.
Conclusions
Natural obesity and overweight in cats alter their hematological and biochemical parameters, even if inside the reference interval of cats, which occurs together with alteration of oxidative stress parameters. Furthermore, the best way to assess oxidative stress in feline obesity is by determining the TAC, preferably using multiple methods.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aguiar DS, Torres MAO, Chaves DP et al (2018) Biochemical serum profile of obese domestic cats from São Luís city, Maranhão. Rev de Cienc Agrovet 17:254–259. https://doi.org/10.5965/223811711722018254
Alberti KGMM, Zimmet P, Shaw J (2006) Metabolic syndrome - a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 23:469–480. https://doi.org/10.1111/j.1464-5491.2006.01858.x
Almeida BFM, Narciso LG, Melo LM, Preve PP, Bosco AM, Lima VM, Ciarlini PC (2013) Leishmaniasis causes oxidative stress and alteration of oxidative metabolism and viability of neutrophils in dogs. Vet J 198:599–605. https://doi.org/10.1016/j.tvjl.2013.08.024
Almeida BFM, Amatti LZ, de Souza GG, Garcia LV, Montechiesi DF, Ignácio FS, de Oliveira PL, Costa LR, Floriano BP, Bosculo MRM, Joaquim JGF, Rubio CP (2021) Effect of uterine ozone therapy and anticoagulant sampling on oxidative stress parameters in mares. Res Vet Sci 136:503–511. https://doi.org/10.1016/J.RVSC.2021.04.002
Bailhache E, Nguyen P, Krempf M, Siliart B, Magot T, Ouguerram K (2003) Lipoproteins abnormalities in obese insulin-resistant dogs. Metabolism 52:559–564. https://doi.org/10.1053/META.2003.50110
Barrie J, Watson TDG, Stear MJ, Nash AS (1993) Plasma cholesterol and lipoprotein concentrations in the dog: The effects of age, breed, gender and endocrine disease. J Small Anim Pract 34:507–512. https://doi.org/10.1111/J.1748-5827.1993.TB03523.X
Bauer JE (1996) Comparative lipid and lipoprotein metabolism. Vet Clin Pathol 25:49–56. https://doi.org/10.1111/j.1939-165X.1996.tb00968.x
Bełtowski J, Wójcicka G, Górny D, Marciniak A (2000) The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J Physiol Pharmacol 51:883–896
Benzie I, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/ABIO.1996.0292
Biezus G, Casagrande RA, Baldissera MD, Bottari NB, Ferian PE, Morsch VM, Schetinger MRC, Withoeft JA, Pereira LHHS, Silva AS, Machado G (2017) Oxidative stress and changes on the adenosinergic system of cats infected by feline leukemia virus (FeLV). Acta Sci Vet 45:5. https://doi.org/10.22456/1679-9216.80760
Biourge V, Nelson RW, Feldman EC, Willits NH, Morris JG, Rogers QR (1997) Effect of weight gain and subsequent weight loss on glucose tolerance and insulin response in healthy cats. J Vet Intern Med 11:86–91. https://doi.org/10.1111/J.1939-1676.1997.TB00078.X
Blanchard G, Paragon BM, Sérougne C, Férézou J, Milliat F, Lutton C (2004) Plasma lipids, lipoprotein composition and profile during induction and treatment of hepatic lipidosis in cats and the metabolic effect of one daily meal in healthy cats. J Anim Physiol Anim Nutr (Berl) 88:73–87. https://doi.org/10.1111/J.1439-0396.2003.00462.X
Bonatto NCM, de Oliveira PL, Mancebo AM, Costa LR, Bosculo MRM, Bosco AM, Ciarlini PC, Floriano BP, de Barros LD, Almeida BFM (2021) Postprandial lipemia causes oxidative stress in dogs. Res Vet Sci 136:277–286. https://doi.org/10.1016/J.RVSC.2021.03.008
Bosco AM, Almeida BFM, Valadares TC, Baptistiolli L, Hoffmann DJ, Pereira AAF, Lima VMF, Ciarlini PC (2018) Preactivation of neutrophils and systemic oxidative stress in dogs with hyperleptinemia. Vet Immunol Immunopathol 202:18–24. https://doi.org/10.1016/J.VETIMM.2018.06.005
Burkholder W, Toll P (1998) Obesity. In: Hand MS, Thatcher CD, Remillard RLRP (eds) Small Animals Clinical Nutrition, 4th edn. Mark Morris, Marceline, pp 401–426
Butterwick R (2000) How Fat is That Cat? J Feline Med Surg 2:91–94. https://doi.org/10.1053/jfms.2000.0078
Cătoi AF, Pârvu A, Galea RF, Pop ID, Mureşan A, Cătoi C (2013) Nitric oxide, oxidant status and antioxidant response in morbidly obese patients: the impact of 1-year surgical weight loss. Obes Surg 23:1858–1863. https://doi.org/10.1007/S11695-013-0968-1
Cave N, Allan F, Schokkenbroek S, Metekohy CAM, Pfeiffer DU (2012) A cross-sectional study to compare changes in the prevalence and risk factors for feline obesity between 1993 and 2007 in New Zealand. Prev Vet Med 107:121–133. https://doi.org/10.1016/J.PREVETMED.2012.05.006
Chandler M, Cunningham S, Lund EM, Khanna C, Naramore R, Patel A, Day MJ (2017) Obesity and associated comorbidities in people and companion animals: a one health perspective. J Comp Pathol 156:296–309. https://doi.org/10.1016/J.JCPA.2017.03.006
Chandler ML (2016) Impact of obesity on cardiopulmonary disease. Vet Clin North Am Small Anim Pract 46:817–830. https://doi.org/10.1016/J.CVSM.2016.04.005
Chikamune T, Yasuaki S, Katamoto H, Ohashi F (1995) Serum lipid and lipoprotein concentrations in obese dogs. J Vet Med Sci 57:595–598. https://doi.org/10.1292/JVMS.57.595
Christian J (2010) Erythrokinetics and Erythrocyte Destruction. In: Weiss D, Wardrop K, Schalm O (eds) Schalm`s Veterinary Hematology. Wiley-Blackwell, Iowa, pp 136–143
Colliard L, Paragon B, Lemuet B, Bénet JJ, Blanchard G (2009) Prevalence and risk factors of obesity in an urban population of healthy cats. J Feline Med Surg 11:135–140. https://doi.org/10.1016/J.JFMS.2008.07.002
Coppack SW (2001) Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 60:349–356. https://doi.org/10.1079/PNS2001110
Costa LR, Silva NLT, Oliveira PL, Bonatto NCM, Vieira GC, Floriano BP, Barros LD, Bosculo MRM, Almeida BFM (2020) Diurnal variations in canine hematological parameters after commercial feed feeding. Semina: Ciênc Agrár 41:2219–2230. https://doi.org/10.5433/1679-0359.2020v41n5Supl1p2219
Courcier EA, O’Higgins R, Mellor DJ, Yam PS (2010) Prevalence and risk factors for feline obesity in a first opinion practice in Glasgow, Scotland. J Feline Med Surg 12:746–753. https://doi.org/10.1016/J.JFMS.2010.05.011
Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation the link between insulin resistance. Trends Immunol 25:4–7. https://doi.org/10.1016/J.IT.2003.10.013
Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R (2001) The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 86:355–362. https://doi.org/10.1210/jc.86.1.355
de Freitas VD, Castilho AR, da Conceição LAV, Sousa VRF, Mendonça AJ, Silva FG, Almeida ABPF (2017) Metabolic evaluation in overweight and obese cats and association with blood pressure. Ciênc Rural 48:e20170217. https://doi.org/10.1590/0103-8478CR20170217
Diez M, Nguyen P (2008) Obesity: epidemiology pathophysiology and management of the obese dog. In: Pibot P, Biourge V, Elliott D (eds) Encyclopedia of canine clinical nutrition. Royal Canin, France, pp 2–57
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285. https://doi.org/10.1016/j.clinbiochem.2003.11.015
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111. https://doi.org/10.1016/j.clinbiochem.2005.08.008
Farah R, Khamisy-Farah R (2015) Significance of MPV, RDW with the presence and severity of metabolic syndrome. Exp Clin Endocrinol Diabetes 123:567–570. https://doi.org/10.1055/S-0035-1564072
Fujita B, Strodthoff D, Fritzenwanger M, Pfeil A, Ferrari M, Goebel B, Figulla HR, Gerdes N, Jung C (2013) Altered red blood cell distribution width in overweight adolescents and its association with markers of inflammation. Pediatr Obes 8:385–391. https://doi.org/10.1111/J.2047-6310.2012.00111.X
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761. https://doi.org/10.1172/JCI21625
German A (2006) The growing problem of obesity in dogs and cats. J Nutr. 136: 1940S-1946S. https://doi.org/10.1093/JN/136.7.1940S
German A (2010) Obesity in companion animals. In Pract 32:42–50. https://doi.org/10.1136/inp.b5665
Hess RS, Kass PH, Winkle TJ (2003) Association between diabetes mellitus, hypothyroidism or hyperadrenocorticism, and atherosclerosis in dogs. J Vet Intern Med 17:489–494. https://doi.org/10.1111/j.1939-1676.2003.tb02469.x
Hoenig M (2006) The cat as a model for human nutrition and disease. Curr Opin Clin Nutr Metab Care 9:584–588. https://doi.org/10.1097/01.MCO.0000241668.30761.69
Hoenig M, Thomaseth K, Waldron M, Ferguson DC (2007) Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss. Am J Physiol Regul Integr Comp Physiol 292:R227–R234. https://doi.org/10.1152/ajpregu.00313.2006
Hoenig M, Wilkins C, Holson JC, Ferguson DC (2003) Effects of obesity on lipid profiles in neutered male and female cats. Am J Vet Res 64:299–303. https://doi.org/10.2460/AJVR.2003.64.299
Hunter MI, Nlemadim BC, Davidson DL (1985) Lipid peroxidation products and antioxidant proteins in plasma and cerebrospinal fluid from multiple sclerosis patients. Neurochem Res 10:1645–1652. https://doi.org/10.1007/BF00988606
Ishioka K, Omachi A, Sasaki N, Kimura K, Saito M (2009) Feline adiponectin: molecular structures and plasma concentrations in obese cats. J Vet Med Sci 71:189–194. https://doi.org/10.1292/jvms.71.189
Ishioka K, Soliman MM, Sagawa M, Nakadomo F, Shibata H, Honjoh T, Hashimoto A, Kitamura H, Kimura K, Saito M (2002) Experimental and clinical studies on plasma leptin in obese dogs. J Vet Med Sci 64:349–353. https://doi.org/10.1292/jvms.64.349
Jain NC (1986) Hematologic Techniques. In: Jain NC (ed) Schalm´s Veterinary Hematology, 4th edn. Lea & Febiger, Philadelphia, pp 20–86
Jeusette IC, Lhoest ET, Istasse LP, Diez MO (2005) Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am J Vet Res 66:81–86. https://doi.org/10.2460/AJVR.2005.66.81
Johnson MC (2005) Hyperlipidemia disorders in dogs. Compendium 27:361–370
Kaneko JJ, Harvey JW, Bruss ML (2008) Clinical biochemistry of domestic animals, 6th edn. Academic Press, London
Keegan RF, Webb CB (2010) Oxidative stress and neutrophil function in cats with chronic renal failure. J Vet Intern Med 24:514–519. https://doi.org/10.1111/j.1939-1676.2010.0498.x
Kil DY, Swanson KS (2010) Endocrinology of obesity. Vet Clin North Am Small Anim Pract 40:205–219
Konukoglu D, Serin O, Turhan MS (2006) Plasma leptin and its relationship with lipid peroxidation and nitric oxide in obese female patients with or without hypertension. Arch Med Res 37:602–606. https://doi.org/10.1016/J.ARCMED.2005.12.002
Korman R, Hetzel N, Knowles T, Harvey AM, Tasker S (2013) A retrospective study of 180 anaemic cats: features, aetiologies and survival data. J Feline Med Surg 15:81–90. https://doi.org/10.1177/1098612X12461008
Laflamme D (2012) Companion Animals Symposium: Obesity in dogs and cats: What is wrong with being fat? J Anim Sci. 90:1653–1662. https://doi.org/10.2527/JAS.2011-4571
Laflamme DP (2006) Understanding and managing obesity in dogs and cats. Vet Clin North Am Small Anim Pract 36:1283–1295. https://doi.org/10.1016/j.cvsm.2006.08.005
Laflamme DP (1997) Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 25:13–18
Lalor S, Gunn-Moore D, Cash R, Foot A, Reed N, Mellanby RJ (2014) Serum cardiac troponin I concentrations in cats with anaemia - A preliminary, single-centre observational study. J Small Anim Pract 55:320–322. https://doi.org/10.1111/JSAP.12210
Laufer Perl M, Havakuk O, Finkelstein A, Halkin A, Revivo M, Elbaz M, Herz I, Keren G, Banai S, Arbel Y (2015) High red blood cell distribution width is associated with the metabolic syndrome. Clin Hemorheol Microcirc 63:35–43. https://doi.org/10.3233/CH-151978
Lund EM, Armstrong PJ, Kirk CA, Klausner JS (2005) Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Int J Appl Res Vet Med 3:88–96
Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, Matsuzawa Y (1998) Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 47:929–933. https://doi.org/10.1016/S0026-0495(98)90346-8
Mori N, Kawasumi K, Suzuki T, Yamamoto I, Kobayashi M, Arai T (2012) Establishment of temporary criteria for metabolic syndrome (MS) diagnosis and assessment of the occurrence rate of MS in cats. J Anim Vet Adv 11:615–617. https://doi.org/10.3923/JAVAA.2012.615.617
Nemet D, Barkan S, Epstein Y, Friedland O, Kowen G, Eliakim A (2005) Short- and long-term beneficial effects of a combined dietary-behavioral- physical activity intervention for the treatment of childhood obesity. Pediatrics 115:e443–e449. https://doi.org/10.1542/peds.2004-2172
Öhlund M, Palmgren M, Holst BS (2018) Overweight in adult cats: A cross-sectional study. Acta Vet Scand 60:1–10. https://doi.org/10.1186/S13028-018-0359-7/FIGURES/4
Oliveira PL, Bonatto NCM, Bosculo MRM, Floriano BP, de Barros LD, Almeida BFM (2020) Effect of post-prandial lipemia on canine biochemical parameters. Comp Clin Path 29:763–775. https://doi.org/10.1007/s00580-020-03130-y
Osto M, Zini E, Reusch CE, Lutz TA (2013) Diabetes from humans to cats. Gen Comp Endocrinol 182:48–53. https://doi.org/10.1016/J.YGCEN.2012.11.019
Ozata M, Mergen M, Oktenli C, Aydin A, Sanisoglu SY, Bolu E, Yilmaz MI, Sayal A, Isimer A, Ozdemir IC (2002) Increased oxidative stress and hypozincemia in male obesity. Clin Biochem 35:627–631. https://doi.org/10.1016/S0009-9120(02)00363-6
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424. https://doi.org/10.1152/physrev.00029.2006
Pedersen NC (2014) An update on feline infectious peritonitis: diagnostics and therapeutics. Vet J 201:133–141. https://doi.org/10.1016/J.TVJL.2014.04.016
Pirgon Ö, Bilgin H, Çekmez F, Kurku H, Dündar BN (2013) Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol 5:33–39. https://doi.org/10.4274/JCRPE.825
Radakovich LB, Truelove MP, Pannone SC, Olver CS, Santangelo KS (2017) Clinically healthy overweight and obese dogs differ from lean controls in select CBC and serum biochemistry values. Vet Clin Pathol 46:221–226. https://doi.org/10.1111/vcp.12468
Rizzi T, Clinkenbeard K, Meinkoth J (2010) Normal hematology of the cat. In: Willey-Blackwell (ed) Schalm`s Veterinary Hematology, 6th edn. Philadelphia, pp 811–820
Robertson I (1999) The influence of diet and other factors on owner-perceived obesity in privately owned cats from metropolitan Perth, Western Australia. Prev Vet Med 40:75–85. https://doi.org/10.1016/S0167-5877(99)00024-0
Rowe E, Browne W, Casey R, Gruffydd-Jones TJ, Murray JK (2015) Risk factors identified for owner-reported feline obesity at around one year of age: Dry diet and indoor lifestyle. Prev Vet Med 121:273–281. https://doi.org/10.1016/J.PREVETMED.2015.07.011
Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Arnao MB, Ceron JJ (2016a) Validation of three automated assays for total antioxidant capacity determination in canine serum samples. J Vet Diagn Invest 28:693–698. https://doi.org/10.1177/1040638716664939
Rubio CP, Tvarijonaviciute A, Martinez-Subiela S, Hernández-Ruiz J, Ceron JJ (2016b) Validation of an automated assay for the measurement of cupric reducing antioxidant capacity in serum of dogs. BMC Vet Res 12:137. https://doi.org/10.1186/s12917-016-0760-2
Russell K, Sabin R, Holt S et al (2000) Influence of feeding regimen on body condition in the cat. J Small Anim Pract 41:12–18. https://doi.org/10.1111/J.1748-5827.2000.TB03129.X
Rysz J, Kasielski M, Apanasiewicz J, Bradley R, Harper EJ (2004) Increased hydrogen peroxide in the exhaled breath of uraemic patients unaffected by haemodialysis. Nephrol Dial Transplant 19:158–163. https://doi.org/10.1093/NDT/GFG499
Saely CH, Vonbank A, Rein P, Woess M, Beer S, Aczel S, Jankovic V, Boehnel C, Risch L, Drexel H (2008) Alanine aminotransferase and gamma-glutamyl transferase are associated with the metabolic syndrome but not with angiographically determined coronary atherosclerosis. Clin Chim Acta 397:82–86. https://doi.org/10.1016/J.CCA.2008.07.024
Sánchez-Chaparro MA, Calvo-Bonacho E, González-Quintela A, Cabrera M, Sáinz JC, Fernández-Labandera C, Aguado LQ, Meseguer AF, Valdivielso P, Román-García J, Ibermutuamur CArdiovascular RIsk Assessment Study Group (2010) Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care 33:e40. https://doi.org/10.2337/DC09-1707
Shacter E, Beecham EJ, Covey JM, Kohn KW, Potter M (1989) Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis 10:2297–22304. https://doi.org/10.1093/carcin/9.12.2297
Silva AC, de Almeida BF, Soeiro CS, Ferreira WL, de Lima VM, Ciarlini PC (2013) Oxidative stress, superoxide production, and apoptosis of neutrophils in dogs with chronic kidney disease. Can J Vet Res 77:136–141
Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF (2003) Adiponectin and protection against type 2 diabetes mellitus. Lancet 361:226–228. https://doi.org/10.1016/S0140-6736(03)12255-6
Tanner AE, Martin J, Saker KE (2007) Oxidative stress and inflammatory state induced by obesity in the healthy feline. J Anim Physiol Anim Nutr (Berl) 91:163–166. https://doi.org/10.1111/j.1439-0396.2007.00680_7.x
Tasker S (2012) Diagnostic approach to anaemia in cats. In Pract 34:370–381. https://doi.org/10.1136/INP.E4889
Thoresen S, Bredal W (1995) Determination of a reference range for fructosamine in feline serum samples. Vet Res Commun 19:353–361. https://doi.org/10.1007/BF01839317
Tsuda K, Kinoshita Y, Nishio I, Masuyama Y (2001) Hyperinsulinemia is a determinant of membrane fluidity of erythrocytes in essential hypertension. Am J Hypertens 14:419–423. https://doi.org/10.1016/S0895-7061(00)01247-4
Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I (2013) Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 288:27138–27149. https://doi.org/10.1074/JBC.M113.485094
Van de Velde H, Janssens GP, Stuyven E, Cox E, Buyse J, Hesta M (2012) Short-term increase of body weight triggers immunological variables in dogs. Vet Immunol Immunopathol 145:431–437. https://doi.org/10.1016/j.vetimm.2011.12.021
Vasan R (2003) Cardiac function and obesity. Heart 89:1127–1129. https://doi.org/10.1136/HEART.89.10.1127
Vayá A, Alis R, Suescún M, Rivera L, Murado J, Romagnoli M, Solá E, Hernandez-Mijares A (2015) Association of erythrocyte deformability with red blood cell distribution width in metabolic diseases and thalassemia trait. Clin Hemorheol Microcirc 61:407–415. https://doi.org/10.3233/CH-141859
Webb C, Lehman T, McCord K, Avery P, Dow S (2008) Oxidative stress during acute FIV infection in cats. Vet Immunol Immunopathol 122:16–24. https://doi.org/10.1016/j.vetimm.2007.11.004
Webb CB, Falkowski L (2009) Oxidative stress and innate immunity in feline patients with diabetes mellitus: the role of nutrition. J Feline Med Surg 11:271–276. https://doi.org/10.1016/j.jfms.2008.07.004
Yan Z, Fan Y, Meng Z, Huang C, Liu M, Zhang Q, Song K, Jia Q (2019) The relationship between red blood cell distribution width and metabolic syndrome in elderly Chinese: a cross-sectional study. Lipids Health Dis 18:34. https://doi.org/10.1186/S12944-019-0978-7
Zaldivar F, McMurray RG, Nemet D, Galassetti P, Mills PJ, Cooper DM (2006) Body fat and circulating leukocytes in children. Int J Obes (Lond) 30:906–911. https://doi.org/10.1038/sj.ijo.0803227
Zoran DL (2009) Feline obesity: clinical recognition and management. Compend Contin Educ Vet 31:E3–E3
Acknowledgements
To São Paulo Research Foundation (FAPESP, Proc. 2018/18220-0) and CAPES (AUXPE 0255/2021, n. 88881.638964/2021-01) for funding this research, and FAPESP for the scholarship granted (2018/19683-4).
Funding
This work was supported by São Paulo Research Foundation (FAPESP, Proc. 2018/18220-0) and CAPES (AUXPE 0255/2021, n. 88881.638964/2021-01). Author T.O.M. has received a research scholarship from FAPESP (2018/19683-4).
Author information
Authors and Affiliations
Contributions
The study conception and design were performed by Beatriz Perez Floriano, Marcel Gambin Marques and Breno Fernando Martins de Almeida. Animal selection, material preparation and sample collection were performed by Tainara de Oliveira Martins, Rebecca Cápera Ramos, Geovana Possidonio, Vinicius Aquiles Zamboni, Marcel Gambin Marques and Breno Fernando Martins de Almeida. Laboratory analysis were performed by Tainara de Oliveira Martins, Rebecca Cápera Ramos, Maria Rachel Melo Bosculo, Paula Lima Oliveira, Leticia Ramos Costa, Vinicius Aquiles Zamboni and Breno Fernando Martins de Almeida. The first draft of the manuscript was written by Tainara de Oliveira Martins and Rebecca Cápera Ramos and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The experiment was conducted according to the ethical principles of the Animal Research Ethics Committee of the University Center of the Integrated Faculties of Ourinhos (Protocol no. 007/2019).
Consent to participate
The participation of each feline was authorized by its owner, who signed a free and informed consent form.
Statement of Animal Ethics
The experiment was conducted according to the ethical principles of the Animal Research Ethics Committee of the University Center of the Integrated Faculties of Ourinhos (Protocol no. 007/2019). The participation of each feline was authorized by its owner, who signed a free and informed consent form.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martins, T.d., Ramos, R.C., Possidonio, G. et al. Feline obesity causes hematological and biochemical changes and oxidative stress – a pilot study. Vet Res Commun 47, 167–177 (2023). https://doi.org/10.1007/s11259-022-09940-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-022-09940-5