Abstract
The study aimed to assess plasma Myeloperoxidase enzyme (MPO) and Ferric-reducing antioxidant power (FRAP) in obese dogs and compare them with ideal body weight dogs. Thirty-two dogs were distributed into two groups according to a 9-point body condition score (BCS), as follows: Control group (n = 16), dogs with a BCS of 4 or 5; Obese group (n = 16), dogs with a BCS of 8 or 9. Plasma MPO and FRAP assays, neutrophil count, lipid profile (cholesterol and triglycerides), and systolic blood pressure (SBP) were evaluated in both groups. The duration of obesity was defined based on history. The obese group showed higher values for body weight, BCS, SBP, neutrophil count, triglycerides, and MPO than the Control group. A positive correlation was observed between MPO concentrations and BCS and body weight. FRAP concentrations showed a positive correlation with the duration of obesity. The results suggested that an inflammatory state caused by obesity may promote increased neutrophil count and MPO concentrations, besides the positive correlation between MPO with BCS and body weight. The obesity in dogs promoted slight active MPO elevation, influenced by body weight, BCS, and neutrophil count. The FRAP assay did not show the expected reduction and, therefore, needs further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a nutritional disorder (Montoya-Alonso et al. 2017) with a high prevalence in small animals (German 2006; Chandler et al. 2017; Kipperman and German 2018). In dogs, obesity represents a risk factor for several diseases, including reduced life expectancy (Montoya-Alonso et al. 2017). There are few reports of oxidative stress in obese dogs (Pasquini et al. 2013). The pathogenesis of canine obesity is not fully understood; however, there is a consensus that oxidative stress plays an important role (German 2006; Zoran 2010; Chandler et al. 2017; Kipperman and German 2018).
Oxidative stress promotes tissue damage caused by an imbalance between oxidant production and antioxidant defense (Laflamme 2012), which results in an increased production of reactive oxygen species (ROS) or decreased concentration of antioxidants (Hernández et al. 2018). The excessive production of these free radicals may cause oxidative damage in important biomolecules, thereby affecting cellular function (Barreiros et al. 2006).
Oxidative stress is an underlying mechanism of metabolic disorders presented in obese individuals (Pasquini et al. 2013). The mechanisms involved in the pathogenesis of canine obesity have not yet been fully elucidated, and oxidative stress plays an essential role (German 2006; Bach et al. 2007; Thengchaisri et al. 2014). Techniques such as myeloperoxidase (MPO) measurements quantify MPO enzyme released from neutrophil granules in situations of inflammation and necrosis (Loria et al. 2008).
Chronic low-grade inflammation contributes to obesity pathogenesis (Uribe-Querol and Rosales 2022). Neutrophils are early participants in inflammatory processes, and after stimulation, these cells release reactive oxygen and nitrogen species, which leads to degranulation and secretion of MPO and other enzymes (Sladoje et al. 2017). In human patients, circulating neutrophils are increased in obesity, with an association between neutrophil blood counts and higher body mass index (BMI) (Kim and Park 2008). Activation of MPO, a heme protein primarily expressed in granules of neutrophils, is associated with the development of obesity (Wang et al. 2014). The antibacterial activities of MPO involve the production of reactive oxygen and reactive nitrogen species (Khan et al.2018), and MPO is released from activated leukocytes at inflammatory sites, generating ROS (Victor et al. 2016).
The MPO enzyme is released into the extracellular fluid after oxidative stress and different inflammatory responses, and the pathogenesis of several other major chronic diseases such as cardiovascular diseases, liver diseases, diabetes, and cancer have been reported to be linked with MPO-derived oxidants (Khan et al.2018). Moreover, neutrophils in obese individuals present an activated phenotype as indicated by elevated plasma concentrations of MPO, a higher ROS generation, and enhanced release of proinflammatory cytokines (Uribe-Querol and Rosales 2022). Excess ROS can damage cell lipids, proteins, and DNA by oxidative action, which might result in loss of function and even cellular death (Lenquiste et al. 2015).
On the other hand, antioxidants serve as a protection for the body against the destructive effects of free radicals damage (Rubio et al. 2016). The total antioxidant capacity can be assessed by the ferric-reducing antioxidant power (FRAP) technique, based on the production of Fe2 + ion (ferrous form) from the reduction of Fe3 + ion (ferric form) present in 2,4,6-tripyridyl-s-triazine (TPTZ) complex, obtaining a global estimate of antioxidant molecules of the sample (Bitla et al. 2012). The end product (TPTZ) has blue color and the change in absorbance is related to the antioxidant capacity of the plasma (Rubio et al. 2016).
FRAP determination assessing the total antioxidant capacity was evaluated in overweight dogs submitted to a weight loss program (Bastien et al. 2015), and in a canine model of obesity (Van de Velde et al. 2012). The FRAP assay is quick and simple to perform and has some advantages, such as no need for highly specialized equipment or skills, inexpensive reagents, and sample pre-treatment is not required. However, it does not measure the antioxidants containing thiol groups and only measures the reducing capability based on the ferric ion, which some authors described as not relevant to antioxidant activity (Rubio et al. 2016). To the authors' knowledge, no studies were described determining the antioxidant capacity with the FRAP method in dogs with non-experimental obesity.
Few studies have focused on oxidative stress associated with canine obesity (Cline et al. 2009; Pasquini et al. 2013; Bosco et al. 2018). Therefore, this study aimed to evaluate the MPO and FRAP concentrations in lean and obese dogs with naturally occurring (non-experimental) obesity. The correlation between these markers and duration of obesity, body condition score (BCS), body weight, and neutrophil count were studied. The hypothesis was that obesity in dogs causes an increase in MPO while decreases the FRAP concentrations.
Materials and methods
The Institutional Ethics Committee approved this study for Animals Use (no. 239/2017). The dogs were recruited from hospital record archives between 2018 and 2019, according to BCS. The owners were contacted by telephone, and those interested in participating presented the dogs for clinical evaluation.
Forty-two client-owned dogs were distributed into two groups according to 9-point BCS: Control Group, dogs with a BCS of 5; Obese Group, dogs with a BCS of 8 or 9. An electronic scale was used to measure body weight.
Eligibility criteria included be healthy based on physical examination, with CBC (complete blood count), fasting blood glucose concentrations (12-h fast), urinalysis, and biochemical profile (creatinine and urea) within normal ranges. The exclusion criteria were patients with chronic diseases or clinical evidence of systemic illness. The duration of obesity was defined based on history. All dogs in the study were treated with premium food. The list of ingredients was checked to verify the inclusion of any antioxidants in the formulations. If confirmed, the dogs were excluded.
The length was estimated from the occipital base, with the measuring tape running through the spine, up to the back of the pelvic limbs, where they touched the ground. Waist measurement was performed in the umbilical area. Physical examination parameters included heart rate, respiratory rate, systolic blood pressure (SBP), examination of lymph nodes and mucous membranes, capillary refill time, hydration, temperature, cardiac and respiratory auscultation, and abdominal palpation.
Measurements of SBP were obtained according to Brown et al. (2007) in conscious dogs. Five consecutive measurements were obtained.
After fasting for around 12 h, blood samples were collected from the jugular vein for CBC (neutrophil counts), serum biochemistry (albumin, cholesterol, triglycerides, urea, and creatinine) determined by colorimetry in a semiautomatic analyser, MPO and FRAP concentrations. Urine samples were collected by cystocentesis for complete urinalysis. To the MPO and FRAP analyses, 4.5 mL of blood was placed in a tube containing ethylenediaminetetraacetic acid (EDTA) and centrifuged at 2.000 g for 3 min. The plasma samples were stored at -80 °C until the tests.
Plasma MPO concentration was measured spectrophotometrically by measuring tetramethylbenzidine (TMB) oxidation, based on the method described by Bradley et al. (1982), and validated in plasma assays by Rocha-Penha et al. (2017). For that, 30µL of plasma (1:100) were incubated with 20µL of phosphate buffer (pH 6.0) and 100µL of liquid substrate system, composed by TMB and hydrogen peroxide, at 37 °C for 10 min, protected from light. To determine whether the obtained activity was derived from myeloperoxidase, samples were incubated with 500 µmol/L of a specific and irreversible myeloperoxidase inhibitor (4-aminobenzoic acid hydrazide) at 37 °C for 30 min prior addition of substrate system. After incubation, 100µL of stop solution (sulfuric acid 2 N) was added and optical density determined in a spectrophotometer at 450 nm using a microplate reader. A standard curve was generated by incubation of horseradish peroxidase with the previous reagents.
The FRAP was evaluated using a standard protocol (Benzie and Strain 1996). Working FRAP reagent was prepared by mixing 25 ml acetate buffer, 2.5 ml 2,4,6-tris(2-pyridyl)- s-triazine (TPTZ) solution, and 2.5 mL FeCl3·6H2O solution. The reactive solution was prepared with 25 mL of 300 mmol/L acetate buffer, 2.5 mL of a 10 mmol/L TPTZ solution in 40 mmol/L HCl and 20 mmol/L FeCl3·6H2O in distilled water. Then, 300 µL of freshly prepared FRAP reagent was warmed to 37 °C and a reagent blank reading was taken at 593 nm. In a 96-well plate, 10 µL of plasma sample were combined with 30 µL of H2O and 300 µL of FRAP reagent. The mixture was subsequently incubated for 6 min at 37 oC. Following incubation, the samples were run in triplicate, analyzed at an absorbance of 593 nm in a spectrophotometer.
Statistical analysis
The Shapiro–Wilk test evaluated data normality. To compare the Control and Obese Groups, an unpaired Student's t-test was used for normally distributed data (age, body weight, neutrophil count, urea, creatinine, albumin, cholesterol, triglycerides, SAP, MPO, FRAP). Pearson's test was performed to establish the correlation of the MPO and FRAP with Body Weight, cholesterol, triglycerides, and duration of obesity (months), and the correlation of the MPO with a neutrophil count. The Spearman's test evaluates the correlation between MPO and FRAP with BCS. The analyses were performed with GraphPad Prism 8.4.2 (GraphPad Software, San Diego, CA). A significance level of 5% was considered.
Results
Thirty-two dogs met the inclusion criteria. The Control group (n = 16) was composed of 11 females (69%) and five males (31%), aged from 1.5 to 8 years (average of 4.2 ± 2.7), and the Obese group had 12 females (75%) and four males (25%), aged from 1.5 to 9 years (average of 5.9 ± 2.9). There was no significant difference between the two groups on age (p = 0.1). Ten dogs from the Control group (63%) and 12 from the Obese group (75%) were neutered.
The dogs of the Control group were Yorkshire (n = 1), Shetland Sheepdog (n = 1), French Bulldog (n = 1), Border Collie (n = 1), Dachshund (n = 2), and crossbreed (n = 10). The dogs of the Obese group were Border Collie (n = 1), Blue Heeler (n = 1), Pug (n = 1), Beagle (n = 2), Dachshund (n = 3), Labrador (n = 3), and crossbreed (n = 5). The length did not differ between groups. The waist of dogs in the Obese group was significantly higher than in the Control group (Table 1). The BCS medians were 5 and 9 for Control and Obese groups, respectively.
All dogs were fed commercial dry dog foods and none received antioxidant supplements. Regarding the duration of obesity, three dogs had been obese for less than a year (19%), three between 1 and 2 years (19%), three between 2 and 3 years (19%), two between 3 and 4 years (12%), and five dogs more than 4 years (31%).
The SBP of dogs in the Obese group was significantly higher than in the Control group (Table 2). Blood glucose concentrations showed no significant differences between groups (p = 0.5), and all dogs were normoglycemic. There were no significant differences between groups in the albumin, serum urea and creatinine. The triglycerides values and neutrophil count were highest in the Obese group, while cholesterol concentrations had no significant differences between groups (Table 2).
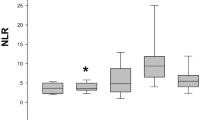
The mean MPO values were 6 ± 0.6 and 7 ± 1 ng/mL, respectively, in dogs of Control and Obese groups. MPO concentrations in dogs of Obese group were significantly higher than the Control group (p = 0.004). The mean FRAP values were 365 ± 59 and 364 ± 115 µmol/L, respectively, in dogs of Control and Obese groups. There was no significant difference between groups (p = 0.9). The MPO concentrations showed positive correlation with body weight (p = 0.04; r = 0.4), BCS (p = 0.001; r = 0.56) (Fig. 1A), and neutrophil count (p = 0.001; r = 0.6) (Fig. 1B). No correlation occurred between triglyceride concentrations, cholesterol concentrations, and duration of obesity. There was no correlation of the FRAP concentrations with body weight, BCS, triglyceride and cholesterol concentrations, but a weak correlation occurred with duration of obesity (p = 0.03; r = 0.31).
Discussion
The present study showed increase of MPO concentrations, but FRAP concentrations were not decreased in obese dogs. In both groups, there were higher proportions of females than males. It should be considered that female dogs have increased risk for obesity (Zoran 2010; Mao et al. 2013; Usui et al. 2016; Porsani et al. 2020). As expected, highest values of body weight were observed in Obese group. Dogs in Obese group showed higher SBP than those in Control group, being obesity a risk factor for hypertension. A study suggested that BCS may be a contributor for secondary hypertension in dogs (Montoya-Alonso et al. 2006).
Severe chronic obesity induces a significant increase in plasma cholesterol and triglyceride concentrations in dogs (Jeusette et al. 2005). Obese dogs showed a concurrent increase in cholesterol and triglyceride levels (Jeusette et al. 2005; Montoya-Alonso et al. 2017). However, in the present study, obese dogs presented higher triglyceride levels than lean dogs, while cholesterol levels did not show significant differences. Five dogs (31.3%) presented triglyceride values above the range for normal dogs. Therefore, attention to the lipid profile of obese dogs is essential.
The results of the present study indicated that chronic inflammation status in dogs of Obese group promoted the MPO enzyme elevation. Also, the oxidative metabolism of neutrophils evaluated by superoxide production showed that obese dogs with hyperleptinemia had high neutrophil superoxide production, suggesting that hyperleptinemia may be able to pre-active neutrophils in obese dogs and consequently induce systemic oxidative stress (Bosco et al. 2018). In oxidative stress, infiltration of adipose tissue by inflammatory cells and further production of excessive reactive oxygen species by these cells occurs, with resultant derangement of several adipose tissue-derived secretory factors, or adipokines, which may possibly contribute to the development of metabolic diseases through altered glucose and lipid homeostasis (Demerdash 2017).
On the other hand, FRAP analysis did not show a reduction in dogs of Obese group. Similarly, there was no difference in FRAP values in groups of non-obese healthy women (control), obese women, and hypertensive obese women (Roncoleta 2012). The decreased efficiency of antioxidant systems predisposes the oxidation of proteins, lipids, and DNA, which is a direct cause of oxidative stress (Choromańska et al. 2020). FRAP levels were decreased in humans with metabolic syndrome and can be a simple measure to assess the antioxidant status in obese patients (Bitla et al. 2012).
In the same way as for FRAP, there was no difference in serum albumin concentrations in obese and lean dogs in the present study. Otherwise, increased albumin was observed in dogs with obesity-related metabolic dysfunction (ORMD) plasma compared with dogs without ORMD (Tvarijonaviciute et al. 2016). Albumin is well known for its ability to bind molecules and represents the major and predominant antioxidant in plasma (Roche et al. 2008).
The neutrophil counts were higher in the Obese, compared with the Control group, despite being in the normal range. Similarly, Rafaj et al. (2016) observed a significant increase in the neutrophil counts in obese dogs concerning the control group. Also, Radakovich et al. (2017) evaluated hematological parameters in clinically healthy overweight/obese and lean dogs. The overweight/obese group had a higher total WBC count than the control group due to higher neutrophil counts, despite the normal ranges. The authors attributed this finding to a possible stress response or low-grade inflammation.
In humans, Zarkesh-Esfahani et al. (2004) concluded that leptin could influence the activation state of human peripheral blood neutrophils. Ryder et al. (2014) evaluated lean and obese individuals without metabolic syndrome and found that neutrophils correlated positively with increased visceral fat. Trellakis et al. (2012) observed the activation of inflammatory neutrophil function in healthy overweight and obese human subjects. The authors suggested that obesity may cause a chronic stimulation for the acute inflammatory response in the innate immune system and that peripheral blood neutrophils are involved in this obesity-related inflammation.
Neutrophil counts indicate the continuous activation of the immune system and chronic low-grade inflammation associated with canine overweight and obesity (Rafaj et al. 2016). In a parallel, MPO enzyme was released from neutrophil granules in situations of inflammation and necrosis (Loria et al. 2008), and obesity may cause this effect, as observed in the present study.
MPO concentration was positively correlated with body weight and BCS in the current study. In general, obese dogs present a state of inflammation related to pro-inflammatory cytokines released by adipose tissue (Hernández et al. 2018). Pongkan et al. (2020) concluded that obesity in dogs could induce increased plasma oxidative stress, impaired heart rate variability, and reduced cardiac systolic function compared to non-obese dogs. Similarly, serum MPO activity was positively correlated with waist: hip ratio and fat percentage in obese children and adolescents to elucidate if MPO was linked with cardiovascular risk parameters (El-Alameey et al. 2019). Furthermore, a significant positive correlation between MPO and BMI was observed in obese children in a study to assess whether MPO is an early indicator for insulin resistance and, consequently, a predictor for metabolic syndrome in these children (Helal et al. 2016). Both studies considered MPO a potential link between obesity and cardiovascular complications (El-Alameey et al. 2019; Helal et al. 2016). MPO was described as a potential factor in developing new therapeutic methods that would delay the development of cardiovascular complications in obese patients (Sladoje et al. 2017). Thus, increasing MPO activity in obese dogs may signal systemic changes that should be investigated, particularly cardiovascular disease.
FRAP concentrations showed weak correlation with duration of obesity, but no correlation was detected with body weight, BCS, cholesterol and triglycerides concentrations. FRAP assay allows to quantify the antioxidant power based on ferric reducing ability of plasma constituents, which include low molecular weight antioxidants, such as vitamins C, and E, serum uric acid and serum bilirubin (Bitla et al. 2012). Also, an increase in the serum concentration of FRAP was observed in obese human patients with metabolic syndrome, but it was not considered a reliable antioxidant defense, since this patients had also increase of acid uric that can chelate ions metals and interfere in the results (Chielle et al. 2018). Amirkhizi et al. (2007) described decreased antioxidant levels in obese women. The authors suggested that the discrepancy with some literature reports could be due to the duration of the obesity. They postulated that in early obesity patients, antioxidant enzyme activity would be stimulated, and when obesity persists for a long time, the sources of the antioxidant enzymes become depleted. Therefore, the association between FRAP and duration of obesity needs to be better understood.
On the other hand, a correlation between MPO and duration of obesity was not observed in the present study. In human patients, some studies showed oxidative stress associated with time in diseases characterized by chronic low-grade inflammation, such as diabetes (Gürler et al. 2000; Helmersson et al. 2004). The role of oxidative stress was investigated in the development of diabetic retinopathy, and there was a significant correlation between the serum lipid peroxidation concentrations and the disease duration (Gürler et al. 2000). Helmersson et al. (2004) evaluated the 8-iso-PGF2α, a free radical product of arachidonic acid and an indicator of oxidative stress, in older men with type 2 diabetes. Patients with diabetes ≥ 7 years ago had increased levels of urinary 8-iso-PGF2α, whereas those newly diagnosed with diabetes (< 7 years ago) had no alteration. Therefore, studies evaluating the association between obesity time and oxidative stress in dogs are warranted.
One of the main limitations of this study was not done a weight loss program for monitoring these markers. However, the results obtained can serve as a basis for further studies that evaluate FRAP and MPO assays in dogs with other comorbidities related to obesity, such as liver diseases, osteoarthritis, among others.
Conclusion
The obesity in dogs promoted MPO elevation, influenced by body weight and BCS, while FRAP assay did not show the expected reduction.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable requests.
References
Amirkhizi F, Siassi F, Minaie S, Djalali M, Rahimi A, Chamari M (2007) Is obesity associated with increased plasma lipid peroxidation and oxidative stress in women? ARYA Atheroscler 2:189–192
Bach JF, Rozanski EA, Bedenice D, Chan DL, Freeman LM, Lofgren JL, Oura TJ, Hoffman AM (2007) Association of expiratory airway dysfunction with marked obesity in healthy adult dogs. Am J Vet Res 68:670–675. https://doi.org/10.2460/ajvr.68.6.670
Barreiros ALBS, David JM, David JP (2006) Oxidative stress: relations between the formation of reactive species and the organism’s defense. Quím Nova 29:113–123. https://doi.org/10.1590/S0100-40422006000100021
Bastien BC, Patil A, Satyaraj E (2015) The impact of weight loss on circulating cytokines in Beagle dogs. Vet Immunol Immunopathol 163(3–4):174–182. https://doi.org/10.1016/j.vetimm.2014.12.003
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Bitla AR, Kumari NM, Reddy NS, Nagaraju KV, Sachan A, Kumar VP, Suchitra MM, Srinivasa-Rao PVLN (2012) Antioxidant status in patients with metabolic syndrome as measured by ferric reducing ability of plasma (FRAP) assay. J Clin Sci Res 3:114–120. https://doi.org/10.15380/2277-5706.JCSR.12.032
Bosco AM, Almeida BFM, Valadares TC, Baptistiolli L, Ho DJ, Pereira AAF, Lima VMF, Ciarlini PC (2018) Preactivation of neutrophils and systemic oxidative stress in dogs with hyperleptinemia. Vet Immunol Immunopathol 202:8–24. https://doi.org/10.1016/j.vetimm.2018.06.005
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermat 78:206–209. https://doi.org/10.1111/1523-1747.ep12506462
Brown S, Atkins C, Bagley R, Carr A, Cowgill L, Davidson M, Egner B, Elliott J, Henik R, Labato M, Littman M, Polzin D, Ross L, Snyder P, Stepien R (2007) Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 21:542–558. https://doi.org/10.1892/0891-6640(2007)21[542:gftiea]2.0.co;2
Chandler M, Cunningham S, Lund EM, Khanna C, Naramore R, Patel A, Day MJ (2017) Obesity and Associated Comorbidities in People and Companion Animals: A One Health Perspective. J Comp Pathol 156(4):296–309. https://doi.org/10.1016/j.jcpa.2017.03.006
Chielle EO, Gens F, Rossi EM (2018) Oxidative, inflammatory and cardiometabolic biomarkers of clinical relevance in patients with metabolic syndrome. J Bras Patol Med Lab 54:213–219. https://doi.org/10.5935/1676-2444.20180037
Choromańska B, Myśliwiec P, Łuba M et al (2020) Impact of Weight Loss on the Total Antioxidant/Oxidant Potential in Patients with Morbid Obesity-A Longitudinal Study. Antioxidants (Basel). 9(5):376. https://doi.org/10.3390/antiox9050376. (Published 2020 May 1)
Cline M, Lauten S, Cox S, Bartges J (2009) The relationship between obesity and markers of oxidative stress in dogs. J Anim Physiol Anim Nutr 93:141–142. https://doi.org/10.1111/j.1439-0396.2009.00921_2.x
Demerdash HM (2017) Role of Oxidative Stress and Associated Alteration in Enzyme Activities in Obesity Comorbidities. Obes Res Open J 4:32–43. https://doi.org/10.17140/OROJ-4-131
El-Alameey IR, Ahmed HH, Mahmoud RA, Kairy SA, Medany EA (2019) Significance of myeloperoxidase in the Onset of Cardiovascular Disease among Obese Children and Adolescents. Biomed Pharmacol J 12(4):1647–59. https://doi.org/10.13005/bpj/1795
German AJ (2006) The growing problem of obesity in dogs and cats. J Nutr 136:1940S-1946S. https://doi.org/10.1093/jn/136.7.1940S
Gürler B, Vural H, Yilmaz N, Oguz H, Satici A, Aksoy N (2000) The role of oxidative stress in diabetic retinopathy. Eye 14:730–735. https://doi.org/10.1038/eye.2000.193
Helal RG, Ahmed HH, Ali AA, Megawer AS, Kamel I, AbdelHamid ER (2016) Study of myeloperoxidase (MPO) as an early indicator of metabolic risks in obese children. IJPCR 8(8):1121–1126
Helmersson J, Vessby B, Larsson A, Basu S (2004) Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation 109(14):1729–1734. https://doi.org/10.1161/01.CIR.0000124718.99562.91
Hernández VGP, Gomes VR, Castro LTS, Borges NC, Fioravanti MCS (2018) Canine obesity: an inflammatory disease related to oxidative stress. Interventions Obes Diabetes 1:70–76. https://doi.org/10.31031/IOD.2018.01.000516
Jeusette IC, Lhoest ET, Istasse LP, Diez MO (2005) Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am J Vet Res 66(1):81–6. https://doi.org/10.2460/ajvr.2005.66.81
Khan AA, Alsahli MA, Rahmani AH (2018) Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med Sci 6(2):33. https://doi.org/10.3390/medsci6020033
Kim JA, Park HS (2008) White blood cell count and abdominal fat distribution in female obese adolescents. Metab Clin Exp 57:1375–1379. https://doi.org/10.1016/j.metabol.2008.05.005
Kipperman BS, German AJ (2018) The Responsibility of Veterinarians to Address Companion Animal Obesity. Animals 8(9):143. https://doi.org/10.3390/ani8090143
Laflamme DP (2012) Companion Animals Symposium: Obesity in dogs and cats: what is wrong with being fat? J Anim Sci 90:1653–1662. https://doi.org/10.2527/jas.2011-4571
Lenquiste SA, Marineli RS, Moraes ÉA, Dionísio AP, de Brito ES, Maróstica Junior MR (2015) Jaboticaba peel and jaboticaba peel aqueous extract shows in vitro and in vivo antioxidant properties in obesity model. Food Res Int 77(2):162–170. https://doi.org/10.1016/j.foodres.2015.07.023
Loria V, Dato I, Graziani F, Biasucci LM (2008) Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm 2008:135625. https://doi.org/10.1155/2008/135625
Mao J, Xia Z, Chen J, Yu J (2013) Prevalence and risk factors for canine obesity surveyed in veterinary practices in Beijing, China. Prev Vet Med 112:438–442. https://doi.org/10.1016/j.prevetmed.2013.08.012
Montoya-Alonso JA, Morris PJ, Bautista-Castaño I, Juste MC, Suarez L, Peña C, Hackett RM, Rawlings J (2006) Hypertension: a risk factor associated with weight status in dogs. J Nutr 136:2011S-2013S. https://doi.org/10.1093/jn/136.7.2011S
Montoya-Alonso JA, Bautista-Castaño I, Peña C, Hart LA, Park S (2017) Prevalence of canine obesity, obesity-related metabolic dysfunction, and relationship with owner obesity in an obesogenic region of Spain. Front Vet Sci 4:2–5. https://doi.org/10.3389/fvets.2017.00059
Pasquini A, Roberti S, Meucci V, Luchetti E, Canello S, Guidetti G, Biagi G (2013) Association between body condition and oxidative status in dogs. Food Nutr Sci 4:191–196. https://doi.org/10.4236/fns.2013.48A023
Pongkan W, Jitnapakarn W, Phetnoi W, Punyapornwithaya V, Boonyapakorn C (2020) Obesity-Induced Heart Rate Variability Impairment and Decreased Systolic Function in Obese Male Dogs. Animals 10(8):1383. https://doi.org/10.3390/ani10081383
Porsani MYH, Teixeira FA, Oliveira VV, Pedrinelli V, Dias RA, German AJ, Brunetto MA (2020) Prevalence of canine obesity in the city of São Paulo. Brazil. Sci Rep 10(1):14082. https://doi.org/10.1038/s41598-020-70937-8
Radakovich LB, Truelove MP, Pannone SC, Olver CS, Santangelo KS (2017) Clinically healthy overweight and obese dogs differ from lean controls in select CBC and serum biochemistry values. Vet Clin Pathol 46(2):221–226. https://doi.org/10.1111/vcp.12468
Rafaj RB, Kuleš J, Turković V, Rebselj B, Mrljak V, Kučer N (2016) Prospective hematologic and biochemical evaluation of spontaneously overweight and obese dogs. Vet Arh 86:383–394
Rocha-Penha L, Caldeira-Dias M, Tanus-Santos JE, de Carvalho CR, Sandrim VC (2017) Myeloperoxidase in hypertensive disorders of pregnancy and its relation with Nitric Oxide. Hypertension 69:1173–1180. https://doi.org/10.1161/HYPERTENSIONAHA.116.08854
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E (2008) The antioxidant properties of serum albumin. FEBS Lett 582(13):1783–1787. https://doi.org/10.1016/j.febslet.2008.04.057
Roncoleta F (2012) Oxidative and inflammatory status in obese women: relationship with anthropometric, lipid and clinical variables. Dissertation, Hospital Santa Casa de Misericórdia
Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ (2016) Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: an update. BMC Vet Res 12(1):166. https://doi.org/10.1186/s12917-016-0792-7
Ryder E, Diez-Ewald M, Mosquera J, Fernández E, Pedreañez A, Vargas R, Peña C, Fernández N (2014) Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab Syndr 8(4):197–204. https://doi.org/10.1016/j.dsx.2014.09.002
Sladoje DP, Kisić B, Mirić D (2017) The Monitoring of Protein Markers of Inflammation and Serum Lipid Concentration in Obese Subjects with Metabolic Syndrome. J Med Biochem 36(4):366–374. https://doi.org/10.1515/jomb-2017-0009
Thengchaisri N, Theerapun W, Kaewmokul S, Sastravaha A (2014) Abdominal obesity is associated with heart disease in dogs. BMC Vet Res 10:131. https://doi.org/10.1186/1746-6148-10-131
Trellakis S, Rydleuskaya A, Fischer C, Canbay A, Tagay S, Scherag A, Bruderek K, Schuler PJ, Brandau S (2012) Low adiponectin, high levels of apoptosis and increased peripheral blood neutrophil activity in healthy obese subjects. Obes Facts 5:305–318. https://doi.org/10.1159/000339452
Tvarijonaviciute A, Ceron JJ, de Torre C, Ljubić BB, Holden SL, Queau Y, Morris PJ, Pastor J, German AJ (2016) Obese dogs with and without obesity-related metabolic dysfunction - a proteomic approach. BMC Vet Res 20(12):211. https://doi.org/10.1186/s12917-016-0839-9
Uribe-Querol E, Rosales C (2022) Neutrophils Actively Contribute to Obesity-Associated Inflammation and Pathological Complications. Cells 11(12):1883. https://doi.org/10.3390/cells11121883
Usui S, Yasuda H, Koketsu Y (2016) Characteristics of obese or overweight dogs visiting private Japanese veterinary clinics. Asian Pac J Trop Biomed 6(4):338–343. https://doi.org/10.1016/j.apjtb.2016.01.011
Van de Velde H, Janssens GP, Stuyven E, Cox E, Buyse J, Hesta M (2012) Short-term increase of body weight triggers immunological variables in dogs. Vet Immunol Immunopathol 145(1–2):431–437. https://doi.org/10.1016/j.vetimm.2011.12.021
Victor VM, Rovira-Llopis S, Bañuls C, Diaz-Morales N, Martinez de Marañon A, Rios-Navarro C, Alvarez A, Gomez M, Rocha M, Hernández-Mijares A (2016) Insulin Resistance in PCOS Patients Enhances Oxidative Stress and Leukocyte Adhesion: Role of Myeloperoxidase. PLoS ONE 11(3):e0151960. https://doi.org/10.1371/journal.pone.0151960
Wang Q, Xie Z, Zhang W, Zhou J, Wu Y, Zhang M, Zhu H, Zou MH (2014) Myeloperoxidase deletion prevents high-fat diet-induced obesity and insulin resistance. Diabetes 63(12):4172–4185. https://doi.org/10.2337/db14-0026
Zarkesh-Esfahani H, Pockley AG, Wu Z, Hellewell PG, Weetman AP, Ross RJ (2004) Leptin indirectly activates human neutrophils via induction of TNF-alpha. J Immunol 172(3):1809–1814. https://doi.org/10.4049/jimmunol.172.3.1809
Zoran DL (2010) Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin North Am Small Anim Pract 40(2):221–39. https://doi.org/10.1016/j.cvsm.2009.10.009
Acknowledgements
The authors would like to thank Doctor Valeria Cristina Sandrim for the laboratory evaluation of oxidative stress.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The concept and design of the study, analysis and interpretation of the data, and writing review & editing were performed by Alessandra Melchert. Analysis and interpretation of the data, review & editing were performed by Priscylla Tatiana Chalfun Guimarães-Okamoto, Regina Kiomi Takahira, and Sheila Canevese Rahal. Data collection, investigation, and methodology were performed by Rodrigo Rodrigues Paulo, Victoria Elizabeth Galvão, Maira Beatriz Gandolfi Silva, Gustavo Gomes da Silva, Laura Pereira Porto, and Juliana Mayumi Tonossu. All authors drafted, revised, and approved the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee on Animal Use of School of Veterinary Medicine and Animal Science, São Paulo State University, Botucatu, Brazil (02.08.2018 / protocol number 239/2017 – CEUA).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Authors declare consent to publish the research data.
Competing interest
No conflict of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paulo, R.R., Galvão, V.E., da Silva, G.G. et al. Myeloperoxidase enzyme and Ferric-reducing antioxidant power concentrations in lean and obese dogs. Vet Res Commun 47, 1007–1013 (2023). https://doi.org/10.1007/s11259-022-10059-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-022-10059-w