Abstract
Background
The aims of this study were to evaluate nitric oxide (NO) metabolites (nitrite/nitrate NO x ) as proinflammatory parameter and total oxidant status (TOS) as well as total antioxidant response (TAR) as oxidative stress (OS) markers in morbidly obese (MO) patients in comparison with normal-weight healthy (NWH) subjects and to determine the post-bariatric surgery changes of NO x and OS indicators in relation with weight loss.
Methods
We examined serum NO x , TOS, and TAR in a bariatric group of MO patients and a NWH control group (n = 23 each group). In the NWH group, serum was examined once, while in the MO group, serum was examined before and at 3, 6, and 12 months after silastic ring vertical gastroplasty (SRVG).
Results
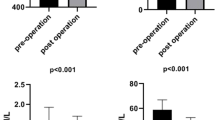
Serum NO x and TOS values were higher (p < 0.001), while TAR level was lower (p < 0.001) in MO patients as compared to the NWH group. No significant changes occurred at 12 months after surgery in the MO group as far as the NO x (p = 0.93), TOS (p = 0.11), and TAR (p = 0.15) levels were concerned as compared to baseline values. However, NO x increased at 6 months after surgery (p < 0.008) and then decreased by the 12th month after SRVG (p < 0.008), reaching almost baseline values.
Conclusions
At baseline, there was a high production of proinflammatory and OS markers in MO patients. SRVG surgical weight loss was not accompanied by significant changes of these parameters at 1 year after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a major public health problem worldwide [1]. It is associated with chronic inflammation and oxidative stress, which both play a crucial role in the development of insulin resistance, endothelial dysfunction, hypertension, type 2 diabetes, and, last but not least, atherosclerosis, leading to premature death [2–7].
Nitric oxide (NO) is a free radical gas known to be involved in the regulation of several physiological and pathophysiological processes such as vasodilatation, energy balance, and inflammation [8–10]. Nitric oxide synthesis is regulated by nitric oxide synthase (NOS). There are three NOS isoforms: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible/inflammatory NOS (iNOS) [8, 9]. eNOS isoform induces the production of small amounts of NO with vasodilatator and anti-atherosclerotic function. In morbidly obese (MO) patients, eNOS expression is down-regulated, promoting endothelial dysfunction. iNOS expression is up-regulated in MO patients by high levels of tumor necrosis factor-alpha (TNF-α) and induces damages on vascular and pancreatic beta cells, leading to obesity-induced metabolic dysfunction [8–10]. Nitric oxide can be considered as a useful marker of chronic inflammation and may play a diagnostic role in MO patients.
Oxidative stress is believed to be an important consequence of inflammation, which is modulated by the activity of several enzymes including cyclooxygenases, lipoxygenases, NOS, and peroxidases, all having the capacity to produce reactive oxygen species and reactive nitrogen species (ROS/RNS) [8, 11, 12]. Nitric oxide reacts with free radicals such as superoxide resulting to peroxynitrite, a non-radical species which inactivates NO and enhances oxidative stress damage. Inflammation and oxidative stress contribute to endothelial dysfunction and insulin resistance, while endothelial dysfunction and insulin resistance promote oxidative stress and inflammation [7].
Bariatric surgery has been proved to be the only efficient method of treatment for MO patients. It leads to significant and sustainable weight loss, amelioration, and/or remission of co-morbidities, as well as life quality improvement [13–15]. Silastic ring vertical gastroplasty (SRVG) is a restrictive, reversible surgical technique, is easy to perform, and does not imply many operative risks. Although SRVG has lost its popularity worldwide, in Romania, it has been successfully used due to good results in weight loss and lower costs as compared to other surgical techniques.
At present, NO production in MO patients and the impact of surgical weight loss on NO synthesis are still controversial. Some authors have reported no significant differences in NO metabolite (nitrite/nitrate NO x ) levels between obese and non-obese patients [16], while others have reported higher [17] or even lower [18] NO x levels in overweight and obese subjects as compared to normal-weight controls. Some authors have reported surgical weight loss to be associated with a reduction of serum NO x [16] after gastric partition, while others have reported increased values of serum NO concentration [19] after vertical-banded gastroplasty.
This study was designed to analyze the hypothesis that NO production is amplified (via iNOS up-regulation) and that it is associated with increased oxidative stress in MO patients as compared to normal-weight healthy (NWH) subjects. In addition, we hypothesized that via iNOS down-regulation, NO production decreases after SRVG weight loss and is accompanied by a reduction of oxidative stress, finally leading to a lower cardiovascular risk. In this respect, the aims of our study were twofold:
-
1.
to evaluate NO x and oxidative stress markers in MO patients as compared to NWH subjects
-
2.
to determine the post-operative changes of NO x and of oxidative stress in relation with SRVG weight loss
Materials and Methods
Study Design and Setting
In this prospective interventional controlled clinical study, the period of data collection was between January 2007 and February 2009. The study was conducted at the Second Surgical Clinic of “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania. The study protocol was reviewed and approved by the Ethics Committee of “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Subjects and Intervention
The first group consisted of 23 patients (20 women and three men) aged between 20 and 54 years who were submitted to SRVG, a purely restrictive procedure. This group of MO patients was examined at baseline, i.e., 2 days before surgery and at 3, 6, and 12 months, respectively, after SRVG. The inclusion criteria were morbid obesity with BMI of ≥40 or 35 kg/m2 to 39.9 kg/m2 with comorbidities, an indication for bariatric surgery, agreement to participate in the study, and a signed term of informed consent. The exclusion criteria were psychiatric disorders (schizophrenia), smoking, alcohol, or drug addiction; severe endocrine diseases other than diabetes; infectious and inflammatory diseases; cardiac, hepatic, or renal failure; and cancer or systemic diseases (systemic autoimmune diseases). None of the patients with diabetes was insulin dependent.
The second group consisted of 23 (20 women and three men) NWH subjects as controls. The age of the subjects included in this group ranged between 25 and 50 years and BMI was <25 kg/m2. The inclusion criteria were the same age and gender as in the case of the MO group and BMI between 18.5 and 24.9 kg/m2, while the exclusion criteria were diabetes or other endocrine diseases, glucose intolerance, lipid disorders, acute inflammation or infection, antibiotic and antiinflammatory treatment; cardiovascular, renal, hepatic, autoimmune, psychiatric diseases; cancer; alcohol and drug addiction; and smoking.
The study was conducted in accordance with the ethical principles of the World Medical Association Declaration of Helsinki [20]. All participants were informed about the aims and methods of the study, and they all provided their written informed consent.
Variables, Data Collection, and Laboratory Measurements
Anthropometric measurements were performed, and blood samples were drawn after overnight fast for glucose (Gluc), total cholesterol (CT), triglycerides (TG), HDL cholesterol (cHDL), NO x , total oxidant status (TOS), and total antioxidant response (TAR) assays before surgery and at 3, 6, and 12 months after SRVG .
Fasting venous blood samples were collected into tubes, and serum was separated after centrifugation at 1,500 g for 10 min. Samples were run immediately or stored at −80 °C until analysis for biochemical tests. The blood samples were analyzed at the Clinical Laboratory of the Second Surgical Clinic and at the Oxidative Stress Laboratory from the Physiology and Pathophysiology Department of “Iuliu Haţieganu” University of Medicine and Pharmacy Cluj-Napoca, Romania.
BMI was calculated by dividing the weight into kilograms by the square of the height in meters. Gluc, CT, and TG were determined using the standard enzymatic colorimetric method (kit Clini-Lab Diagnosticum Hungary). Serum cHDL was determined using the precipitation method (kit Clini-Lab Diagnosticum Hungary). Serum LDL cholesterol (cLDL) concentration was calculated by Friedewald formula [21]. The device used was Cobas Myra Plus Roche Switzerland.
Serum NO concentration was evaluated through its final stabile products, nitrite and nitrate (NO x ), using a colorimetric method. The principle of this assay is the reduction of nitrate by vanadium (III), combined with detection by the acidic Griess reaction. The Griess reaction was used as an indirect assay to determine the serum total nitrite (NO−2) and nitrate (NO−3) as a measure of NOS activity and of NO synthesis. Serum samples were passed through 10-kDa filters (Sartorius AG, Goettingen, Germany) and deproteinized by methanol/diethylether (3/1, v/v) (sample: methanol/diethylether, 1:9, v/v) [22]. In brief, 100 μL VCl3 (8 mg/mL) was added to 100 μL of the supernatant for reduction of nitrate to nitrite, followed by addition of the Griess reagents, 50 μL SULF (2 %), and 50 μL NEDD (0.1 %). After 30 min of incubation at 37 °C, absorbance was read at 540 nm. Serum NO x was expressed as nitrite μmol/l [23].
Oxidative stress was evaluated through TOS and TAR. Serum TOS was measured using a colorimetric method. The assay is based on the oxidation of ferrous ion to ferric ion in the presence of various oxidant species in acidic medium and the measurement of the ferric ion by xylenol orange. The assay is calibrated with hydrogen peroxide (H2O2), and the results are expressed in μmol H2O2 equiv./l [24]. Serum TAR was measured using a colorimetric method. In this method, the hydroxyl radical is produced by Fenton reaction, and the rate of the reactions is monitored by following the absorbance of colored dianisidyl radicals. Upon addition of a serum sample, the oxidative reactions initiated by the hydroxyl radicals present in the reaction medium are suppressed by the antioxidant components of the serum, preventing the color change and thereby providing an effective measure of the total antioxidant capacity of the serum. The assay is calibrated with Trolox, and the results are expressed as mmol trolox equiv./l [25]. Absorbance of the samples was measured in a Cecil 3000 spectrophotometer.
Statistical Analysis
The univariate normality of the data from the MO patients and NWH subjects, respectively, was analyzed using the Shapiro–Wilk test, graphical method (Q–Q plot), and evaluation of skewness and kurtosis together with their standard errors.
To analyze the average of the characteristics studied, we used the Student (t) test, and the results were presented as means ± standard deviation (SD), and the Friedman test for the data which were not normally distributed. We compared the data at different time points after surgery. We used the Wilcoxon test for the post-test analysis. The multiple-comparison correction of Bonferroni was used in order to keep the error rate (α) to the specified level of 0.05. The statistical analysis was performed with SPSS v.13.
Results
Age and gender were not different between the two study groups. Table 1 shows the descriptive characteristics of the NWH subjects and of the MO group. There were significant differences between the variables of the two groups. At baseline in MO patients, serum NO x and TOS values were higher (p < 0.001), while the TAR level was lower (p < 0.001) compared to the NWH group.
The changes in all parameters for the MO group at different time points after SRVG surgery are presented in Table 2. BMI values decreased significantly at 3, 6, and 12 months after surgery by 15.57 % (p < 0.0083), 26.12 % (p < 0.0083), and 33.89 % (p < 0.0083), respectively. At 12 months after surgery, the patients were obese (mean BMI = 30.03 kg/m2, median = 31.25 kg/m2). The decrease of the TG values was statistically significant (p < 0.0083), while no significant changes were noticed for Gluc, CT, cLDL, and cHDL at 12 months after surgery as compared to baseline.
The dynamics of NO x and oxidative stress markers (TAR and TOS) was different during the follow-up period. At 3 months after surgery, the NO x , TAR, and TOS values did not change significantly as compared to baseline. Our results demonstrated that the most important changes occurred at 6 months after surgery, when the increase of the NO x was statistically significant (p < 0.0083) and was accompanied by an increase of TOS (p = 0.064) and a decrease of TAR (p < 0.0083) as compared to the preoperative values. Between the 6th and the 12th month, there was a statistically significant (p < 0.0083) decrease of the NO x as well as of TOS values and an increase of TAR levels (p < 0.0083). No statistically significant changes of NO x levels and of oxidative stress parameters were noted at 1 year after surgery compared to baseline.
Discussion
In the present paper, we hypothesized that NO production and oxidative stress markers are increased in MO patients as compared to NWH subjects and that 1-year SRVG weight loss is associated with a decrease of NO synthesis, accompanied by a reduction of oxidative stress, leading to a decreased cardiovascular risk. Our results demonstrated that markers of chronic inflammation (NO x ) and oxidative stress were higher in MO patients as compared to NWH subjects. However, 1-year surgical weight loss was associated with no statistically significant changes in NO x , TOS, and TAR values. Significant changes in NO x were still observed at 6 months after surgery.
As mentioned before, chronic inflammation and oxidative stress are known to be important features of obesity and are characterized by strong interrelations [2, 3]. The up-regulation of the iNOS isoform leading to the production of NO at 1,000-fold more than eNOS is mediated by inflammatory cytokines that are increased in MO subjects [9, 17, 26]. This up-regulation of the iNOS might explain the increased marker of chronic inflammation (NO x ) in our group of MO patients who presented several co-morbidities, such as dyslipidemia, impaired fasting glucose, or hypertension. In accordance with our results, high production of NO was reported in obese and MO adults and children in comparison with controls by other authors as well [17, 26, 27]. Codoner-Franch et al. [26] demonstrated that nitrite plasma levels in severely obese children with different degrees of metabolic risk factors were higher when more than four metabolic risk factors were present, while Choi et al. [28] pointed out that NO production increases in obese otherwise healthy adolescents and that this increase starts from the time point when BMI is >25 kg/m2. NO metabolism is strongly correlated with oxidative stress reactions. Superoxide, a free radical induced by nicotinamide adenine dinucleotide phosphate oxidase, reacts with NO, producing peroxynitrite (OONO–) which at high concentrations induces oxidative damage with severe cell injury, leading to lipid peroxidation, foam cell formation, and finally atherosclerosis [8, 26]. On the other hand, some authors [29] demonstrated that the higher the production of ROS in obesity, the higher the expression of anti-oxidative enzymes such as superoxide dismutase (SOD) [29]. SOD is the most important antioxidant enzyme system, and there is a substrate competition between superoxide and nitric oxide. Altogether it is important to note that it becomes difficult to differentiate between the NO production per se and its inactivation as a result of the reaction with superoxide/SOD. In our study, due to the increased values of NO x and TOS and decreased level of TAR, we may say that in MO patients there is an important over-production of NO and oxidative stress. Lin et al. [16] observed no differences of NO x between MO patients and controls and explained the apparently normal NO production in MO patients as a result of over-expression of iNOS associated with inactivation of NO. On the other hand, it must be mentioned that the mean BMI in this case was 39 kg/m2, while in our group of patients BMI was 48.44 kg/m2. However, Olszanecka-Glinianowicz et al. [17] found no differences in the NO levels between the overweight group and the two obese groups, one with a BMI between 30 and 40 kg/m2 and the other with a BMI higher than 40 kg/m2. Sledzinski et al. [19] also reported no differences of the NO blood values between the MO patients and control subjects, while Pardina et al. [18] reported lower NO blood values in MO patients in comparison with the normal-weight group. Similarly, Maniscalco et al. [30] found that exhaled NO was reduced in severe obesity.
We expected 1-year surgical weight loss to be associated with a significant reduction of NO production, as well as of the oxidative stress markers. However, this was not the case. At 3 months after SRVG, there were no significant changes of NO x , TAR, and TOS, although the decrease of BMI was statistically significant. The most important changes were observed at 6 months after surgery and consisted in an increase of NO x and a decrease of TAR as compared to preoperative values, followed by a decrease of NO x and an increase of TAR at 1 year after surgery. There are several possible explanations. Firstly, these changes might be explained through the stress of weight reduction as the decrease of BMI was from 48.44 to 34.44 kg/m2 at 6 months after surgery. Some authors [19] reported an increase of serum NO concentration at 6 months after vertical-banded gastroplasty and interpreted it as a contributor to the beneficial effects of weight loss after bariatric surgery, mainly in the context of risk of atherosclerosis. Other authors [16] pointed out that at 3–6 months after gastric partition, weight loss was associated with a decrease in NO x levels, which could be explained by the down-regulation of NO production.
Secondly, the inflammatory processes which generate oxidative stress may still be ongoing throughout the first year after bariatric surgery, which might account for the lack of change in these parameters. Our results are in accordance with those of Van Dielen et al. [31], who demonstrated that inflammatory markers remain elevated for at least 3 months after gastric restrictive surgery, reaching nearly normal values just 2 years after the surgical treatment. Recently, Pardina et al. [32] pointed out that the expression of TNF-α does not vary in the liver and adipose tissue in case of gastric bypass, despite the weight loss and the amelioration of most of the parameters associated with co-morbidities of obesity, such as insulin resistance and dyslipidemia. It is worth underlining that at 1 year after SRVG, we observed a significant reduction of BMI from 47.27 to 31.25 kg/m2, which was also noted by other authors who reported their experience with Roux-en-Y gastric bypass (RYGB) [18, 27, 29]. As NO x , TAR, and TOS did not change in parallel with BMI reduction, we may assume that other parameters of chronic inflammation (TNF-α) might be involved in regulating these processes. Other authors such as Cabrera et al. [29] demonstrated that 1 year after RYGB, the oxidation indicators showed a significant reduction, although they remained higher in comparison with the control group, while Maniscalco et al. [30] reported that at 1 year after laparoscopic adjustable gastric banding, weight loss was accompanied by an increase, leading to restored levels of NO.
Thirdly, as NO inhibits lipolysis and as there is a continuous and increased level of lipolysis during the first year after surgery, the level of NO during this period of time might either decrease or remain unchanged, as in our study [18, 33]. In this respect, Pardina et al. [18] noted a similar NO dynamics, after RYGB, as we did, but with the difference that NO increase was observed 3 months after surgery, after which its level decreased and at 1 year after surgery reached almost the baseline values.
In conclusion, our study demonstrated that in MO patients, there is a high production of NO, as a marker of chronic inflammation, associated with an increased level of oxidative stress. Surgical weight loss is not associated with significant changes in NO levels and in oxidative stress markers at 1 year after SRVG, which suggests that other possible factors might be involved in modulating these pathogenetic mechanisms. Further studies are required in order to clarify the involvement and the dynamics of proinflammatory and oxidative stress markers in obesity and bariatric surgery.
References
Low S, Chew Chin M, Deurenberg-Yap M. Review on epidemic of obesity. Ann Acad Med Singapore. 2009;38:57–65.
Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;535918:1–17.
Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12:3117–32.
Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16.
Martínez JA. Mitochondrial oxidative stress and inflammation: a slalom to obesity and insulin resistance. J Physiol Biochem. 2006;62(4):303–6.
Keaney JF, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–9.
Kwang KK, Pyung CO, Quon MJ. Does reversal of oxidative stress and inflammation provide vascular protection? Cardiovasc Res. 2009;81:649–59.
Avogaro A, de Kreutzenberg SV. Mechanisms of endothelial dysfunction in obesity. Clinica Chimica Acta. 2005;360:9–26.
Noroha BT, Li JM, Wheatcroft SB, et al. Inducible nitric oxide synthase has divergent effects on vascular and metabolic function in obesity. Diabetes. 2005;54:1082–9.
Joost HG, Tschop MH. NO to obesity: does nitric oxide regulate fat oxidation and insulin sensitivity? Endocrinology. 2007;148(10):4545–7.
Iyer A, Fairlie DP, Prins JB, et al. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6:71–82.
Grattagliano I, Palmieri VO, Portincasa P, et al. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504.
Ward M, Prachand V. Surgical treatment of obesity. Gastrointest Endosc. 2009;70(5):985–90.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56.
Deitel M. The early effect of the bariatric operations on diabetes. Obes Surg. 2002;12:349.
Lin LY, Lee WJ, Shen HN, et al. Nitric oxide production is paradoxically decreased after weight reduction surgery in morbid obesity patients. Atherosclerosis. 2007;190:436–42.
Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Janowska J, et al. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-alpha and TNF soluble receptors in women with overweight and obesity. Metabolism. 2004;53(10):1268–73.
Pardina E, Ferrer R, Baena-Fustegueras JA, et al. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes Surg. 2010;20:623–32.
Sledzinski T, Sledzinski M, Smolenski RT, et al. Increased serum nitric oxide concentration after bariatric surgery—a potential mechanism for cardiovascular benefit. Obes Surg. 2010;20:204–10.
World Medical Association Declaration of Helsinki. Bull of the World Health Organization. 2001;79(4):373–4.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative centrifuge. Clin Chem. 1972;18:499–500.
Ghasemi A, Hedayati M, Biabani H. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess assay. J Med Sci Res. 2007;2:29–32.
Miranda K, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide: Biol and Chem. 2001;5(1):62–71.
Ozcan E. A new automated colorimetric method for measuring total oxidant status. Clinical Biochem. 2005;38(12):1103–11.
Ozcan E. A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical Biochem. 2004;37:112–9.
Codoner-Franch P, Tavárez-Alonso S, Murria-Estal R, et al. Nitric oxide production is increased in severely obese children and related to markers of oxidative stress and inflammation. Atherosclerosis. 2011;215:475–80.
Da Silva VRG, Moreira EAM, Wilhelm-Filho D et al. Proinflammatory and oxidative stress markers in patients submitted to Roux-en-Y gastric bypass after 1 year of follow-up. European Journal of Clinical Nutrition. 2012; 1–9
Choi WJ, Pai SH, Kim SK, et al. Increases in nitric oxide concentrations correlate strongly with body fat in obese humans. Clin Chem. 2001;47(6):1106–9.
Cabrera EJ, Valezi AC, Delfino VDA, et al. Reduction in plasma levels of inflammatory and oxidative stress indicators after Roux en-Y gastric bypass. Obes Surg. 2010;20:42–9.
Maniscalco M, de Laurentiis G, Zedda A, et al. Exhaled nitric oxide in severe obesity: effect of weight loss. Respir Physiol & Neurobiol. 2007;156:370–3.
van Dielen FMH, Buurman WA, Hadfoune M, et al. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89:4062–8.
Pardina E, Ferrer R, Baena-Fustegueras JA. Only C-reactive protein, but not TNF-α or IL6, reflects the improvement in inflammation after bariatric surgery. Obes Surg. 2012;22:131–9.
Pardina E, Lecube A, Llamas R, et al. Lipoprotein lipase but not hormone-sensitive lipase activities achieve normality after surgically induced weight loss in morbidly obese patients. Obes Surg. 2009;19:1150–8.
Acknowledgments
This work was supported by the research grant from the National Council for Scientific Research in Romanian Higher Education CNCSIS TD code 409 (80/1.10.2007). This study has been presented at the 16th World Congress of IFSO, Hamburg, Germany, Sept. 1–3, 2011. The authors express their great gratitude to Professor Mervyn Deitel, MD, and to Anna Maria Wolf, MD, for their suggestions on the manuscript.
Conflict of Interest
All authors, Adriana Florinela Cătoi, Alina Pârvu, Romeo Florin Galea, Ioana Delia Pop, Adriana Mureşan, and Cornel Cătoi, declare that they have no conflict of interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cătoi, A.F., Pârvu, A., Galea, R.F. et al. Nitric Oxide, Oxidant Status and Antioxidant Response in Morbidly Obese Patients: the Impact of 1-Year Surgical Weight Loss. OBES SURG 23, 1858–1863 (2013). https://doi.org/10.1007/s11695-013-0968-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-0968-1