Abstract
Arbuscular mycorrhizal fungi (AMF) and endophytic Epichloë are two important symbiotic microorganisms of perennial ryegrass (Lolium perenne). The present study evaluates the individual and combined effects of AMF and Epichloë on drought stress. Plant growth, phosphorus (P) uptake, chlorophyll content, net photosynthetic rate (Pn), soluble sugar concentration, malondialdehyde (MDA) concentration, and antioxidant enzyme activities were measured in a greenhouse experiment. Both AMF and Epichloë significantly increased Pn and total P content for each soil water content. In addition, the presence of AMF increased plant total dry weight, root activity, and soluble sugar concentration at each soil water content. Furthermore, catalase activity increased and MDA concentration decreased at 30% soil water content (SWC). Infection with Epichloë increased the polyphenol oxidase activity of plants for both non-AMF and AMF treatments at 30% SWC. AMF + Epichloë treatments increased plant P uptake, Pn, root activity, and soluble sugar concentration at each soil water content (SWC) (P < 0.001), and increased peroxidase (POD) activity at 30% SWC (P < 0.001). Plant-AMF-Epichloë symbiosis alleviated the damage caused by drought stress by promoting P uptake, photosynthesis, and the accumulation of osmoregulatory substances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In field conditions, plants are often faced with both biotic and abiotic stresses, such as disease, insect, drought, salt, heavy metal, and extreme temperature stresses that limit their growth and development. Drought stress severely affects plant growth and ultimately leads to yield loss (Gueta-Dahan et al. 1997; Wang et al. 2003). Drought is a recurring phenomenon, and among the most frequent natural disasters in many regions of the world (Catalin and Ionut 2013). Due to the impact of global warming, the intensity, scope, and frequency of extreme drought events are continuously increasing (Li and Li 2017).
During a drought, water deficiency adversely affects plant growth by influencing a host of biochemical and physiological processes, such as photosynthesis and nutrient uptake (Jaleel et al. 2008). A decrease of photosynthesis due to water deficiency has been attributed to limitations of both stomatal conductance and transpiration rate (Alam 1999; Shangguan et al. 1999); stomata closure is the first line of plant defense against drought stress (Yordanov et al. 2000). In addition, during drought stress, both the root activity and soluble sugar concentration (Zhang et al. 2016) as well as chlorophyll and carotenoid contents decrease (Yordanov et al. 2000). Drought stress also results in increases of reactive oxygen species (ROS) synthesis and membrane lipid peroxidation. Malonaldehyde (MDA) is one of the most important by-products of membrane lipid peroxidation and accumulates during stress exposure (Lacan and Baccou 1998); in response, plants require elevated levels of antioxidants to compensate for stress. Examples for enzymatic detoxification systems involved in such actions are catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) (Yordanov et al. 2000).
Perennial ryegrass (Lolium perenne) is an important forage and turfgrass throughout the world (Reed 1996) with strong sensitivity to low soil fertility and moisture (Reed 1996). One of the most severe problems in perennial ryegrass production is drought stress. Research on the grass-Epichloë endophytic association may lead to an improvement of water utilization and drought tolerance in perennial ryegrass (VanHeeswijck and McDonald 1992; Hume et al. 1993).
Endophytic Epichloë are ubiquitous in nature, and enter a very specific mutualistic association with most Poaceae plant species (Clay 2004). They can be found in intercellular spaces of aboveground vegetative tissues of grass (Craven 2012) and are mostly transmitted vertically by seed (Zhang et al. 2017). However, several species are transmitted horizontally by spores (Carroll 1988).
The symbiotic relationship between Epichloë endophytes and grasses has been defined as mutualistic (Müller and Krauss 2005). Epichloë live within the host and receives carbohydrates from the host grass (Müller and Krauss 2005). The presence of Epichloë increases aboveground biomass, tiller number, seed production, and nutrient uptake in host grasses (Malinowski and Belesky 1999; Schardl et al. 2004; Peng et al. 2013). In addition, the grass-Epichloë endophytic symbiosis can increase plant resistance to abiotic (Zhang et al. 2009; Xu et al. 2017) and biotic stresses (Wiewióra et al. 2015).
Strong field-based evidence indicates that infection with Epichloë coenophialum increases both growth and drought tolerance of the tall fescue (Festuca arundinacea) (West et al. 1993; West 1994). Moreover, studies on perennial ryegrass infected with E. festucae var. lolii to increase resistance to drought stress showed increased stomatal conductance (Gs), net photosynthetic rate (Pn), photorespiratory electron transport rate, and grass yield compared to plants without Epichloë infection (Ravel et al. 1995; Amalric et al. 1999). However, Hesse et al. (2005) reported that perennial ryegrass infected with Epichloë yielded less shoot dry weight than Epichloë-free control and flooding treatments. In the drought treatment, Epichloë infection significantly increased the seed yield. The various interactions between symbiont and its host may depend on host genetics as well as environmental conditions (Faeth and Sullivan 2003).
As one of the most important soil fungi, arbuscular mycorrhizal fungi (AMF) are widespread throughout the ecosystem (Renker et al. 2003), and colonize plant roots underground. AMF proliferate for the host plants and mobilize nutrients (especially P) (Atimanav and Adholeya 2002; Hohmann and Messmer 2017). In addition, the symbiotic association also exerts a wide range of effects on host plants such as improved growth and yield (Zaidi et al. 2003), as well as disease resistance (Lenoir et al. 2017) and increased tolerance of environmental stress (Heidari and Karami 2014).
Mycorrhizal association increases drought resistance in plants by inducing several defense mechanisms such as improving host nutrition (particularly P) (Gierczik et al. 2012), increasing water uptake by external hyphae (Gierczik et al. 2012), increasing stomatal sensitivity (Huang et al. 1985), increasing root hydraulic conductivity (Andersen et al. 1988), and decreasing osmotic adjustment (Wu and Xia 2006). These mechanisms are related to an increased activity of the most common antioxidant enzymes involved in detoxifying the ROS synthesized during stress (Wu et al. 2006; Khalafallah and Abo-Ghalia 2008; Roldan et al. 2008; Zhang et al. 2010). In addition, mycorrhizal associations improve the host water relationship, due to the indirect effect of hyphal nutrient uptake of e.g., nitrogen (N) (Frey and Schüepp 1993; Tobar et al. 1994) or in response to changes in plant hormone levels such as auxins, gibberellins, and cytokinins (Parvizi and Dashti 2015).
Plants may be hosts for both AMF and Epichloë in the field. However, few studies focused on the interactions of AMF and Epichloë under drought stress. Liu et al. (2017) tested the Epichloë and AMF associated drought resistance of Leymus chinensis in a greenhouse experiment and found that Epichloë infection significantly increased the drought resistance of the host grass. However, the beneficial effects were reduced by AMF inoculation, and physiological mechanisms both fungi use in their interaction potentially interact under changed environmental conditions. This requires further exploration. A previous study by our group showed that perennial ryegrass inoculated with Claroideoglomus etunicatum had an increased P uptake in both Epichloë infected and uninfected plants under normal soil water conditions (Li et al. 2018). The interaction among the perennial ryegrass-AMF-Epichloë endophytic relationship under drought stress remains unclear. This study investigated the role of the AMF species Claroideoglomus etunicatum and the endophyte Epichloë festucae var. lolii, as well as their interaction effects on the growth and drought stress of perennial ryegrass. Biomass, P uptake, photosynthetic parameters, enzyme activities, and osmotic adjustment of perennial ryegrass substances were determined to investigate the physiological mechanism of their interaction. This study hypothesized that both C. etunicatum and E. festucae var. lolii will improve plant growth and P uptake as well as alleviate stress-induced damage. This positive effect is further enhanced by AMF + Epichloë.
2 Materials and methods
2.1 Plants, fungi, and experimental design
Seeds of the ‘Fairway’ cultivar of perennial ryegrass were provided by the College of Pastoral Agriculture Science and Technology, Lanzhou University, China. Seeds either with (E+) or without (E-) endophytic Epichloë were collected from previously studied plants with verified Epichloë infection status (Nan 1996). Prior to the experiment, 50 seeds of E+ and E- each were randomly selected, and microscopic examination of the aleurone layer was conducted via staining with aniline blue solution (Nan 1996).
The AMF Claroideoglomus etunicatum was provided by the Bank of Glomeromycota in China (BGC), accessible via the Institute of Plant and Environmental Resources of the Chinese Academy of Agriculture, Beijing, China (http://www.yzs.baafs.net.cn/Default.aspx). C. etunicatum was maintained in pot cultures of white clover (Trifolium repens) and the pots were stored at room temperature. The pots were allowed to dry when the total spore density of AMF reached ~100 spores per g dry soil. Then, substrate and a small amount of T. repens root fragments (30 g/pot) were mixed with soil-sand mixture (AMF). Control plants (NM) received the same quantity (30 g/pot) of dead inoculum as the inoculated plants.
This study was designed as a fully-crossed three factorial experiment: AMF (2 levels) × Epichloë (2 levels) × soil water content (3 levels) = 12 treatments. Each treatment was replicated four times. Well-filled and healthy-looking E+ and E- seeds were selected, surface sterilized with 75% ethanol for 30 s, and 1% NaClO for 3 min, followed by rinsing with sterile water three times. The disinfection efficacy was tested by placing sterilized seeds onto potato dextrose agar (PDA) for 4 days. Then, the sterilized seeds were placed in a 25 °C incubator for germination. For each pot, six germinated Epichloë infected and uninfected seeds were transplanted to related treatments. The hyphae of E. festucae var. lolii of each plant were examined via leaf sheath staining with aniline blue-lactic acid-glycerol solution and optically investigated at 100–400x magnification (Florea et al. 2015) after 2 weeks of growth. Three plants with similar size were retained in each pot after microscopic examination. Then, each pot was not watered anymore to reduce the water-holding capacity to 30% soil water content (SWC). Three different soil water-holding capacities were established: 30%, 50%, and 70% SWC. Plants were maintained in a greenhouse for a further 6 weeks. The plants were weighted and watered at 5:30–6:30 pm to maintain soil water contents at 30%, 50%, and 70% SWC according to treatment. During those 6 weeks, the height and tiller number of plants were measured weekly.
2.2 Potting medium
The soil used for the experiments was collected from the Xinglong Mountain, Lanzhou, China. The mixture of soil and sand (1:1) was sieved through a 2-mm sieve, and then autoclaved twice at 121 °C for 1 h each, with an interval of 3 days between sterilizations. The mixture had 15.87 mg kg−1 plant-available P, with a pH of 6.58. The pots had a size of 6 cm × 22 cm × 15 cm (bottom width × height × top width) and each contained 1.5 kg of potting mix.
2.3 Plant growth and harvesting
Plants were grown from May to August in a greenhouse, located at the Yuzhong campus of Lanzhou University, China. The plants were assigned a fully random position and were watered with a modified Long Ashton nutrient solution (-P) (Duan et al. 2011) every other day. The greenhouse had average temperatures of 23–28/20–25 °C (day/night). During the growth period, the photosynthetic photon flux density of the greenhouse was 180–850 μmol m−2 s−1. In addition, plants received three supplemental lights in each greenhouse, resulting in light levels near the pots of approximately 1200 μmol m−2 s−1. Six weeks later, the photosynthesis rates of the top leaf of three plants of each pot were measured from 9:00 to 11:00 am using a Li-6400 portable photosynthesis system (LI-COR Inc., Lincoln, NE, USA). The chamber was equipped with a red/blue LED light source set at 1200 μmol m−2 s−1, and the air carbon dioxide concentration was 410 ± 10 μmol CO2 mol−1. The plants of each pot were harvested. The samples of three plants of each pot were mixed, and divided into two subsamples. One sample of approximately 1.2 g was used to determine all analyses with the published methods including chlorophyll concentration (Li et al. 2000), CAT (Beers and Sizer 1952), POD (Li et al. 2000), polyphenol oxidase (PPO) (Kumar and Khan 1982), as well as MDA, and soluble sugar concentration (Li et al. 2000). Moreover, root activity was determined using the triphenyl tetrazolium chloride (TTC) method (Li et al. 2000). The remainder of each sample was oven dried to determine the shoot dry weight, and the shoot P concentration using the method of Hanson (1950).
The roots of each pot were washed and divided into two sub-samples. One sample of approximately 0.1 g was used to determine the AMF colonization percentage using the gridline intersect method of Giovannetti and Mosse (1980). After clearing in 10% KOH and staining in 1% trypan blue (Phillips and Hayman 1970), ~0.1 g of 1-cm root segments were selected at random and observed using a dissecting microscope at × 40 magnification. Presence of vesicles, arbuscules, and intraradical hyphae was recorded. The remainder of the sample was used to determine root dry weight and root P concentration using the method of Hanson (1950).
2.4 Statistical analysis
Data points in the figures represent mean values (± standard error of the mean, SEM) based on four replicates of each treatment. The homogeneity of variance was tested using Levene’s test, and the normality of residual variance was tested using linear-regression analysis. The effects of AMF and Epichloë on the weekly shoot height and tiller number were evaluated via repeated-measures ANOVA, and the result of the AMF colonization rate was analyzed using a generalized linear model with normal distribution and identity link function. The effects of AMF, Epichloë, and soil water content on plant dry weight, P uptake, and other physiological and biochemical parameters were analyzed via three-factor ANOVA with SPSS 19.0 (SPSS Inc., Chicago, USA). Comparisons between means were tested using Tukey’s Honestly Significant Difference (HSD) at the P ≤ 0.05 level.
3 Results
3.1 Percentage of AMF colonization
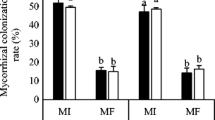
AMF structures were not observed in the roots of non-AMF treatments, while plants inoculated with AMF showed 40.8–59.6% root colonization. Compared to non Epichloë treatments, plants infected with Epichloë showed a much lower percentage of AMF colonization rate at 70% SWC (13.9%) (P = 0.001). The AMF colonization of Epichloë infected treatment showed a significant decline with decreasing soil water content, and the values were 13.2% lower at 50% SWC (P = 0.001), and 30.9% lower at 30% SWC (P < 0.001), compared to 70% SWC (Fig. 1). Infection with Epichloë showed similar percentages of AMF colonization at 70% and 50% SWC.
Percentage of AMF colonization of perennial ryegrass infected with (E+) and without (E-) Epichloë and colonized either with (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC at harvest. Means ± SEM of four replicates are shown. Bars with the same lowercase letter do not differ significant for Epichloë × soil water content interaction at P ≤ 0.05 by Tukey’s HSD. See Table 1 for ANOVA results
3.2 Chlorophyll content and net photosynthetic rate
The tested AMF, Epichloë, and soil water contents imposed significant main effects on the net photosynthetic rate, while the combination of AMF and soil water affected the plant chlorophyll content (Table 1).
The presence of AMF increased the chlorophyll content by 20.74% at 70% (P = 0.003), and by 25.36% at 30% SWC (P < 0.001), compared to non-AMF treatments (Fig. 2a). AMF + Epichloë treatments significantly increased the plant net photosynthetic rate at each soil water content, and the values of AMF + Epichloë increased by more than 172.5% at 50% and 30% SWC (P < 0.001) compared to the control treatment (Fig. 2b).
Chlorophyll content (a) and net photosynthetic rate (b) of perennial ryegrass infected with (E+) and without (E-) Epichloë and colonized either with (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC at harvest. Means ± SEM of four replicates are shown. Bars with the same lowercase letter do not differ significant for AMF × soil water content interaction (a) and AMF × Epichloë × soil water content interaction (b) at P ≤ 0.05 by Tukey’s HSD, respectively. See Table 1 for ANOVA results
3.3 Plant growth
Infection with AMF and Epichloë demonstrated an improvement of host plant growth. Repeated-measures ANOVA indicated that differences in the tiller number of both AMF and Epichloë treatments (either alone or combined), as well as in the shoot heights of both AMF alone and AMF+ Epichloë treatments markedly changed over time for the three various water contents (Supplementary Fig. S1; Supplementary Table S1).
The tested AMF, Epichloë, and soil water contents exerted significant main effects on the total dry weight of perennial ryegrass, and an AMF × soil water content interaction was found for the total dry weight (Table 1). Compared to no AMF treatments, the presence of AMF significantly increased the total dry weight at each soil water content (Fig. 3), and the root dry weight at 30% SWC (Supplementary Fig. S2b). The presence of Epichloë increased the total dry weight by 5.8% at 50% SWC (P = 0.048), and by 6.7% at 30% SWC (P = 0.007) compared to non Epichloë plants (Fig. 3). The shoot dry weight largely followed a similar trend than total dry weight (Supplementary Fig. S2a).
Total dry weight of perennial ryegrass infected with (E+) and without (E-) Epichloë and colonized either with (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC at harvest. Means ± SEM of four replicates are shown. Bars with the same lowercase letter do not differ significant for AMF × soil water content interaction at P ≤ 0.05 by Tukey’s HSD. See Table 1 for ANOVA results
3.4 Phosphorous uptake
The plant total P content was significantly affected by the main effects and interactions of soil water content, Epichloë, and AMF (Table 1). Compared to non-AMF treatment, the presence of AMF significantly increased the total P content for plants both Epichloë infected and uninfected at each soil water content (Fig. 4). For both non AMF and AMF treatments, plants infected with Epichloë had much higher total P contents at 50% and 30% SWC, as well as for AMF treatment at 70% SWC. Compared to control treatment, AMF + Epichloë treatments increased the total P content by 90.9%, 149.2%, and 148.5% at 70%, 50%, and 30% SWC (P < 0.001) (Fig. 4). Shoot P content and root P content followed the same trend with regard to total dry weight, where the values in AMF + Epichloë treatments significantly increased at each soil water content compared to the control treatment (Supplementary Fig. S3a, b).
Total P content of perennial ryegrass infected with (E+) and without (E-) Epichloë and colonized either with (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC at harvest. Means ± SEM of four replicates are shown. Bars with the same lowercase letter do not differ significant for AMF × Epichloë × soil water content interaction at P ≤ 0.05 by Tukey’s HSD. See Table 1 for ANOVA results
3.5 Enzyme activity
The POD and PPO activity of plants were significantly affected by interactions of AMF × Epichloë × soil water content (Table 1). Compared to control treatment, AMF + Epichloë treatment increased POD activity by 48.81% at 30% SWC (P < 0.001) (Fig. 5a). Compared to the non-AMF treatment, the presence of AMF significantly increased CAT activity by 65.39% at 30% SWC (P < 0.001) (Fig. 5b). The PPO value largely followed the same pattern as POD, and the values significantly increased in plants infected with Epichloë for both non-AMF and AMF treatments by 30% SWC, compared to plants uninfected with Epichloë (Fig. 5c).
POD (a), CAT (b) and PPO (c) enzyme activity of perennial ryegrass infected with (E+) and without (E-) Epichloë and colonized either with (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC at harvest. Means ± SEM of four replicates are shown. Bars with the same lowercase letter do not differ significant for AMF × Epichloë × soil water content interaction (a, c) and AMF × soil water content interaction (b) at P ≤ 0.05 by Tukey’s HSD. See Table 1 for ANOVA results
3.6 Root activity, soluble sugar concentration, and MDA concentration
MDA and soluble sugar concentration were significantly affected by the interactions of AMF× Epichloë, and soil water content, while the root activity was significantly affected by the soil water content and both AMF and Epichloë inoculation (Table 1). Compared to non-AMF treatment, the colonization of AMF significantly increased root activity and soluble sugar concentration for both Epichloë infected and non-infected plants at each SWC (Fig. 6a, b), and decreased the MDA concentration by 21.5% at 30% SWC (Fig. 6c) (P < 0.001). AMF + Epichloë treatments showed much higher soluble sugar concentrations at each soil water content than AMF alone plants (Fig. 6b). Compared to control treatment, AMF + Epichloë treatments increased root activity by 97.9–108.4% (P < 0.001), and increased the soluble sugar concentration by 62.2–148.2% for all three soil water contents (P < 0.001) (Fig. 6a, b).
Root activity (a), soluble sugar concentration (b) and MDA concentration (c) of perennial ryegrass infected with (E+) and without (E-) Epichloë and colonized either with (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC at harvest. Means ± SEM of four replicates are shown. Bars with the same lowercase letter do not differ significant for AMF × Epichloë × soil water content interaction (a, b), and AMF × soil water content interaction (c) at P ≤ 0.05 by Tukey’s HSD. See Table 1 for ANOVA results
4 Discussion
The present study addressed the interactions between aboveground microorganisms and belowground microorganisms with their host plants during drought stress exposure. These effects can be identified via differences in chlorophyll content, photosynthetic rate, enzyme activities, soluble sugar concentration, root activity, MDA concentration, and plant biomass. The presence of the two symbiotic microorganisms AMF and Epichloë provides a complementary boost of P uptake, and leads to physiological changes of host plants that improve drought tolerance. The AMF + Epichloë treatments had a greater effect on perennial ryegrass. Thus, the initial hypothesis that the positive effect will be enhanced by AMF + Epichloë was supported.
In the present experiment, mycorrhizal plants, regardless of whether they are exposed to drought stress, achieved higher net photosynthesis, total dry weight, and total P content at each water content. This could be possibly associated with the fact that the presence of AMF has a wide range of effects on host plants including improved P uptake (Smith and Read 2008), photosynthesis (Wu and Xia 2006), and increased growth and yield (Zaidi et al. 2003). This indicated that the presence of AMF was beneficial and improved both growth and health of plants (Avis et al. 2008; Heidari and Karami 2014). A change in P uptake and photosynthesis has also been reported as one of the mechanisms with which AMF-colonized plants increase their drought resistance (Ruizlozano et al. 1995). In addition, an earlier study reported that MDA decreased while antioxidant enzymes increased in response to the colonization of AMF under pathogen stress (Li et al. 2018). The present study indicates that AMF-colonized plants had significantly higher PPO and lower MDA concentrations under drought stress. This indicates that AMF inoculated plants developed mechanisms to reduce oxidative damage caused by drought stress (Wu et al. 2013). The mechanisms that underlie the reduction of oxidative damage caused by AMF may be associated with the fact that AMF themselves can accumulate ROS (Fester and Hause 2005), and may affect the expression of AMF antioxidant enzyme genes (Corradi et al. 2009). Thus, the positive strategy for perennial ryegrass to cope with environmental stress is establish an AM symbiosis with appropriate AMF (Azcón and Barea 1997).
The results also show that the presence of Epichloë significantly improved the drought resistance of perennial ryegrass, which agrees with previous studies on grass-Epichloë symbiosis (Ren et al. 2014; Liu et al. 2017). This agrees with the significantly increasing plant net photosynthetic rate and total dry weight in response to Epichloë infection at 50% and 30% SWC. The presence of Epichloë enhanced the resistance to drought stress by increasing P uptake, photosynthesis, and plant growth (Malinowski and Belesky 1999; Peng et al. 2013; Liu et al. 2017). Epichloë also significantly increased PPO activity and decreased MDA concentration of plants at 30% SWC. It has been reported that plants with strong drought resistance also had higher root activity than plants with weak drought resistance (Liu et al. 2002). These results suggest that Epichloë might decrease plant cell membrane damage resulting from drought stress, and contribute to the drought resistance of the host by enhancing its antioxidant capacity (White and Torres 2010; Zhang and Nan 2010; Yang et al. 2014; Liu et al. 2017). However, Cheplick et al. (2000) and Briggs et al. (2013) reported that Epichloë do not exert major effects on the water stress physiology of perennial ryegrass. This difference indicated that Epichloë may exert synergistic or antagonistic effects on their hosts, which depend on host genotype-Epichloë and environmental conditions to promote the drought tolerance of grasses for degraded land restoration or forage production (Rahman et al. 2015).
Both AMF and Epichloë metabolize carbohydrates from host plants. When AMF and Epichloë simultaneously interact with a host, previous studies provide contradictory results, reporting that the presence of the Epichloë both significantly decreased (Omacini et al. 2006; Liu et al. 2011) or increased (Novas et al. 2005) the AMF root colonization rate. The current study showed that the AMF colonization percentage was only reduced by Epichloë at 70% SWC, indicating that the interactions between AMF and Epichloë were regulated by soil water conditions. Under the well-watered condition, Epichloë shows a competitive relationship with AMF, even though, Epichloë did not inhibit the positive effects AMF exert on plant, e.g., growth and enzyme activities. However, when plants are exposed to drought stress, such an antagonistic interaction disappeared, Epichloë changed its strategy from competition to cooperation with AMF, e.g., the highest Pn, chlorophyll content, P uptake, growth, and enzyme activities in the AMF + Epichloë treatment. This change may be an important symbiotic strategy of plant-AMF-Epichloë symbiosis in field conditions. These discrepancies among studies may also be due to the identity and genotype of both the host and symbiont species (Klironomos 2003; Liu et al. 2011; Larimer et al. 2012), nutrient resource levels (Zhou et al. (2016), abiotic environmental conditions (Tintjer et al. 2008), and the duration of drought stress. Studies with potted plants typically use a relatively short drought duration, while chronic drought in field situations promoted extensive AMF colonization to resist stress (Augé 2001).
Although, no significant water × AMF × Epichloë interactions were found with plant biomass (shoot, root, and total dry weight), infection of perennial ryegrass by AMF + Epichloë significantly enhanced the benefits of each with regard to plant P uptake and physiological index of plants under drought stress. These results are similar to a previous report by Li et al. (2016), which reported that shoot height, tiller number, and both blade width and length of perennial ryegrass were significantly increased by infection with Epichloë under drought stress. This may be one of the regulatory mechanisms of ryegrass to drought that is mediated by Epichloë. The present study shows that under well-watered conditions, the presence of Epichloë promotes root growth, while, under drought stress, the root length of plants was significantly increased by AMF infection or by the combination of both symbiotic microorganisms. In addition, the beneficial effect on shoot height and tiller number of AMF + Epichloë infected plants was additive under drought stress. These synergistic or addictive effects are very important for plants under field drought conditions, and this result is consistent with AMF colonization. This indicates that plants with both types of symbiotic microorganisms not only have an advantage with regard to obtaining soil water and nutrients, but also change their strategy in response to drought stress both morphologically and physiologically. A previous study by Larimer et al. (2012) reported that the effects of AMF + Epichloë on plant growth were additive. Increased plant performance was only found for antagonistic Epichloë, while their negative effects were alleviated when associated with AMF, and the impact of beneficial Epichloë was not altered by infection with AMF. This may be because the plant-AMF-Epichloë symbiosis is a dynamic process, where each of the components adjusted their strategy to adapt to stressful environmental conditions.
The current study assessed the effects of two mutualistic fungal symbionts on their host grass under various soil water conditions, and indicates the mechanisms underlying the interaction of Epichloë infected perennial ryegrass with the AMF colonized root system under various soil water conditions in the field. The promoted nutrient uptake and accumulation of substances for osmotic adjustment by AMF and Epichloë under drought stress are helpful for an understanding of the physiological mechanism of the plant-microorganism interactions under drought stress under field conditions and long term drought resistance. Further work should study the ecological implications of the combinatory effects of endophytes and AMF under field conditions.
References
Alam SM (1999) Nutrient uptake by plants under stress conditions. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 285–314
Amalric C, Sallanon H, Monnet F, Hitmi A, Coudret A (1999) Gas exchange and chlorophyll fluorescence in symbiotic and non-symbiotic ryegrass under water stress. Photosynthetica 37:107–112
Andersen CP, Markhart AHIII, Dixon RK, Sucoff EI (1988) Root hydraulic conductivity of vesicular-arbuscular mycorrhizal green ash seedlings. New Phytol 109:465–471
Atimanav G, Adholeya A (2002) AM inoculations of five tropical fodder crops and inoculum production in marginal soil amended with organic matter. Biol Fertil Soils 35:214–218
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Avis TJ, Gravel V, Antoun H, Tweddell RJ (2008) Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol Biochem 40:1733–1740
Azcón R, Barea J (1997) Mycorrhizal dependency of a representative plant species in mediterranean shrublands (Lavandula spica) as a key factor to its use for revegetation strategies in desertification-threatened areas. Appl Soil Ecol 7:83–92
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Briggs L, Crush J, Ouyang L, Sprosen J (2013) Neotyphodium endophyte strain and superoxide dismutase activity in perennial ryegrass plants under water deficit. Acta Physiol Plant 35:1513–1520
Carroll G (1988) Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecol 69:2–9
Catalin SI, Ionut M (2013) Drought phenomena and groundwater scarcity in eastern Romania (Siret-Prut region). EGU General Assembly 15:6997
Cheplick GP, Perera A, Koulouris K (2000) Effect of drought on the growth of Lolium perenne genotypes with and without fungal endophytes. Funct Ecol 14:657–667
Clay K (2004) Fungi and the food of the gods. Nature 427:401–402
Corradi N, Buffner B, Croll D, Colard A, Horak A, Sanders IR (2009) High-level molecular diversity of copper-zinc superoxide dismutase genes amongand within species of arbuscular mycorrhizal fungi. Appl Environ Microbiol 75:1970–1978
Craven KD (2012) Population studies of native grass-endophyte symbioses provide clues for the roles of host jumps and hybridization in driving their evolution. Mol Ecol 21:2562–2564
Duan T, Facelli E, Smith SE, Smith FA, Nan Z (2011) Differential effects of soil disturbance and plant residue retention on function of arbuscular mycorrhizal (AM) symbiosis are not reflected in colonization of roots or hyphal development in soil. Soil Biol Biochem 43:571–578
Faeth SH, Sullivan TJ (2003) Mutualistic asexual endophytes in a native grass are usually parasitic. Am Nat 161:310–325
Fester T, Hause T (2005) Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15:373–379
Florea S, Schardl CL, Hollin W (2015) Detection and isolation of Epichloë species, fungal endophytes of grasses. Curr Protoc Microbiol 38:19A.1.1–19A.1.24
Frey B, Schüepp H (1993) Acquisition of nitrogen by external hyphae of arbuscular mycorrhizal fungi associated with Zea mays. New Phytol 124(2):221–230
Gierczik K, Sasvári Z, Posta K (2012) Effects of pre and post inoculation by mycorrhizal fungi on growth and production of spice pepper. J Landscape Ecol 10:385–391
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gueta-Dahan Y, Yaniv Z, Zilinskas A, Ben Hayyim G (1997) Salt and oxidative stress; similar and specific responses and their relation to salt tolerance in Citrus. Planta 203:460–469
Hanson WC (1950) The photometric determination of phosphorus in fertilizers using the phosphovanado-molybdate complex. J Sci Food Agric 1:172–173
Heidari M, Karami V (2014) Effects of different mycorrhiza species on grain yield, nutrient uptake and oil content of sunflower under water stress. J Saudi Soc Agric Sci 13:9–13
Hesse U, Schoberlein W, Wittenmayer L, Forster K, Warnstorff K, Diepenbrock W, Merbach W (2005) Influence of water supply and endophyte infection (Neotyphodium) on vegetative and reproductive growth of two Lolium perenne genotypes. Eur J Agron 22:45–54
Hohmann P, Messmer MM (2017) Breeding for mycorrhizal symbiosis: focus on disease resistance. Euphytica 213:113
Huang RS, Smith WK, Yost RS (1985) Influence of vesicular arbuscular mycorrhiza on growth, water relations and leaf orientation in Leucaena leucocephala. New Phytol 99:229–243
Hume DE, Popay AJ, Barker DJ (1993) Effects of Acremonium endophyte on growth of ryegrass and tall fescue under varying levels of soil moisture and argentine stem weevil attack. Proceedings of the 2nd international symposium on acremonium/grass interactions, AgResearch, Palmerston North, New Zealand, 161–164
Jaleel CA, Gopi R, Sankar B, Gomathinayagam M, Panneerselvam R (2008) Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. Comp Rend Biol 331:42–47
Khalafallah AA, Abo-Ghalia HH (2008) Effect of arbuscular mycorrhizal fungi on the metabolic products and activity of antioxidant system in wheat plants subjected to short-term water stress, followed by recovery at different growth stages. J Appl Sci Res 4:559–569
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Kumar KB, Khan P (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana) leaves during senescence. Indian J Exp Biol 20:412–416
Lacan D, Baccou JC (1998) High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 204:377–382
Larimer AL, Bever JD, Clay K (2012) Consequences of simultaneous interactions of fungal endophytes and arbuscular mycorrhizal fungi with a shared host grass. Oikos 121:2090–2096
Lenoir I, Fontaine J, Tisserant B, Laruelle F, Lounèshadj SA (2017) Beneficial contribution of the arbuscular mycorrhizal fungus, Rhizophagus irregularis, in the protection of Medicago truncatula roots against benzo[a]pyrene toxicity. Mycorrhiza 27:465–476
Li YP, Li YH (2017) Advances in adaptability of meteorological drought indices in China. J Arid Meteorol 35:709–723
Li HS, Sun Q, Zhao SJ, Zhang WH (2000) Principle and techniques of botanic, chemical and physiological experiments, 1st edn. Senior Education Press, Beijing
Li HQ, Wang JJ, Zhang GM, Lin WH, Tian P (2016) Effects of fungal endophytes on the growth of perennial ryegrass under drought condition. Pratacul Sci 33:599–607
Li F, Guo YE, Christensen MJ, Gao P, Li YZ, Duan TY (2018) An arbuscular mycorrhizal fungus and Epichloë festucae var. lolii reduce Bipolaris sorokiniana disease incidence and improve perennial ryegrass growth. Mycorrhiza 28:159–169
Liu H, Zheng G, Guan J, Li G (2002) Changes of root activity and membrane permeability under drought stress in maize. Acta Agr B Sin 17:20–22
Liu Q, Parsons AJ, Xue H, Fraser K, Ryan GD, Newman JA, Rasmussen S (2011) Competition between foliar Neotyphodium lolii endophytes and mycorrhizal Glomus spp. fungi in Lolium perenne depends on resource supply and host carbohydrate content. Funct Ecol 25:910–920
Liu H, Chen W, Wu M, Wu RH, Zhou Y, Gao YB, Ren AZ (2017) Arbuscular mycorrhizal fungus inoculation reduces the drought-resistance advantage of endophyte-infected versus endophyte-free Leymus chinensis. Mycorrhiza 27:1–9
Malinowski DP, Belesky DP (1999) Neotyphodium coenophialum-endophyte infection affects the ability of tall fescue to use sparingly available phosphorus. J Plant Nutr 22:835–853
Müller CB, Krauss J (2005) Symbiosis between grasses and asexual fungal endophytes. Curr Opin Plant Biol 8:450–456
Nan ZB (1996) Incidence and distribution of endophytic fungi in seeds of some native and introduced grasses in China. Acta Pratacul Sin 5:1–8 (In Chinese with English Abstract)
Novas MV, Cabral D, Godeas AM (2005) Interaction between grass endophytes and mycorrhizas in Bromus setifolius from Patagonia, Argentina. Symbiosis 40:23–30
Omacini M, Eggers T, Bonkowski M, Gang AC, Jones TH (2006) Leaf endophytes affect mycorrhizal status and growth of co-infected and neighbouring plants. Funct Ecol 20:226–232
Parvizi K, Dashti F (2015) The effect of in vitro mycorrhization on growth characteristics, changes in endogenous hormones and performance of microplants in potato (Solanum tuberosum). J Cent Eur Agric 16:445–462
Peng QQ, Li CJ, Song ML, Nan ZB (2013) Effects of seed hydropriming on growth of Festuca sinensis, infected with Neotyphodium endophyte. Fungal Ecol 6:83–91
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. T British Mycol Soc 55:158–IN118
Rahman MH, Simpson WR, Matthew C, Sabreen S (2015) Response of diploid perennial ryegrass to fungal endophyte AR29 infections under water stress. Commun Soil Sci Plan 46:845–860
Ravel C, Charmet G, Balfourier F (1995) Influence of the fungal endophyte Acremonium lolii on agronomic traits of perennial ryegrass in France. Grass For Sci 50:75–80
Reed KFM (1996) Improving the adaptation of perennial ryegrass, tall fescue, phalaris, and cocksfoot for Australia. New Zeal J Agr Res 39:457–464
Ren AZ, Wei MY, Yin LJ, Wu LJ, Zhou Y, Li X, Gao YB (2014) Benefits of a fungal endophyte in Leymus chinensis depend more on water than on nutrient availability. Environ Exp Bot 108:71–78
Renker C, Heinrichs J, Kaldorf M (2003) Combining nested PCR and restriction digest of the internal transcribed spacer region to characterize arbuscular mycorrhizal fungi on roots from the field. Mycorrhiza 13:191–198
Roldan A, Diaz-Vivancos P, Hernandez JA, Carrosco L, Caravaca F (2008) Superoxide dismutase and total peroxidase activities in relation to drought recovery performance of mycorrhizal shrub seedlings grown in an amended semiarid soil. J Plant Physiol 165:715–722
Ruizlozano JM, Azcon R, Gomez M (1995) Effects of arbuscular-mycorrhizal glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ Microbiol 61:456–460
Schardl CL, Leuchtmann A, Spiering MJ (2004) Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55:315–340
Shangguan Z, Shao M, Dyckmans J (1999) Interaction of osmotic adjustment and photosynthesis in winter wheat under soil drought. J Plant Physiol 154:753–758
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Tintjer T, Leuchtmann A, Clay K (2008) Variation in horizontal and vertical transmission of the endophyte Epichloe elymi infecting the grass Elymus hystrix. New Phytol 179:236–246
Tobar R, Azcon R, Barea JM (1994) Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol 126:119–122
VanHeeswijck R, McDonald G (1992) Acremonium endophytes in perennial ryegrass and other pasture grasses in Australia and New Zealand. Aust J Agric Res 43:1683–1709
Wang W, Vinour B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
West CP (1994) Physiology and drought tolerance of endophyte-infected grasses. In: Bacon CW, White JF Jr (eds) Biotechnology of endophytic fungi of grasses. CRC Press, Boca Raton, pp 87–99
West CP, Izekor E, Turner KE, Elmi AA (1993) Endophyte effects on growth and persistence of tall fescue along a water-supply gradient. Agron J 85:264–270
White JF, Torres MS (2010) Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol Plant 138:440–446
Wiewióra B, Żurek G, Żurek M (2015) Endophyte-mediated disease resistance in wild populations of perennial ryegrass (Lolium perenne). Fungal Ecol 15:1–8
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Wu QS, Zou YN, Xia RX (2006) Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine) roots. Eur J Soil Biol 42:166–172
Wu QS, Srivastava AK, Zou YN (2013) AMF-induced tolerance to drought stress in citrus: a review. Sci Hortic 164:77–87
Xu L, Li X, Han L, Li D, Song G (2017) Epichloë endophyte infection improved drought and heat tolerance of tall fescue through altered antioxidant enzyme activity. Eur J Hortic Sci 82:90–97
Yang T, Ma S, Dai CC (2014) Drought degree constrains the beneficial effects of a fungal endophyte on Atractylodes lancea. J Appl Microbiol 117:1435–1449
Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 38:171–186
Zaidi A, Khan MS, Amil M (2003) Interactive effect of rhizotrophic microorganisms on yield and nutrient uptake of chickpea (Cicer arietinum). Eur J Agron 19:15–21
Zhang YP, Nan ZB (2010) Growth and anti-oxidative systems changes in Elymus dahuricus is affected by Neotyphodium endophyte under contrasting water availability. J Agron Crop Sci 193:377–386
Zhang XX, Li CJ, Nan ZB (2009) Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J Hazard Mater 175:703–709
Zhang XX, Fan XM, Li CJ, Nan ZB (2010) Effects of cadmium stress on seed germination, seedling growth and antioxidative enzymes in Achnatherum inebrians plants infected with a Neotyphodium endophyte. Plant Growth Regul 60:91–97
Zhang SS, Kang HM, Yang WZ, Xiang ZY (2016) Effects of arbuscular mycorrhizal fungi on growth and photosynthetic characteristics of Nyssa yunnanensis seedlings under drought stress. Acta Ecol Sin 36:6850–6862
Zhang W, Card SD, Mace WJ, Christensen MJ, Mcgill CR, Matthew C (2017) Defining the pathways of symbiotic Epichloë colonization in grass embryos with confocal microscopy. Mycologia 109:153–161
Zhou Y, Li X, Qin J, Liu H, Chen W, Niu Y, Ren AZ, Gao YB (2016) Effects of simultaneous infections of endophytic fungi and arbuscular mycorrhizal fungi on the growth of their shared host grass Achnatherum sibiricum under varying N and P supply. Fungal Ecol 20:56–65
Acknowledgements
This research was supported by the China Agriculture Research System (CARS-22 Green Manure).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig S1
Shoot height (a) and tiller number (b) of perennial ryegrass with (E+) and without (E-) Epichloë and colonized either with (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC before harvested. Means ± SEM of four replicates are shown. (DOCX 102 kb)
Supplementary Fig S2
Shoot dry weight (a) and root dry weight (b) of perennial ryegrass with (E+) and without (E-) Epichloë and colonized with either (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC at harvest. Means ± SEM of four replicates are shown. Bars with the same lowercase letter do not differ significant for AMF × soil water content interaction at P ≤ 0.05 by Tukey’s HSD. (DOCX 98 kb)
Supplementary Fig S3
Shoot P content (a) and root P content (b) of perennial ryegrass with (E+) and without (E-) Epichloë and colonized either with (AMF) and without (NM) AMF at 70%, 50%, and 30% SWC at harvest. Means ± SEM of four replicates are shown. Bars with the same lowercase letter do not differ significant for AMF × Epichloë × soil water content interaction at P ≤ 0.05 by Tukey’s HSD. (DOCX 94 kb)
Table S1
(DOC 26 kb)
Rights and permissions
About this article
Cite this article
Li, F., Deng, J., Nzabanita, C. et al. Growth and physiological responses of perennial ryegrass to an AMF and an Epichloë endophyte under different soil water contents. Symbiosis 79, 151–161 (2019). https://doi.org/10.1007/s13199-019-00633-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-019-00633-3