Abstract
Purpose
Although dyslipidemia can cause kidney damage, whether it independently contributes to the progression of chronic kidney disease (CKD) remains controversial. The research aims to evaluate the predictive value of serum lipids and their ratios in the progression of CKD.
Methods
The retrospective, case–control study included 380 adult subjects with CKD stage 3–4 (G3-4) at baseline. The end point of follow-up was the progression of CKD, defined as a composite of renal function rapid decline [an annual estimated glomerular filtration rate (eGFR) decline > 5 mL/min/1.73 m2] or the new-onset end-stage renal disease (ESRD) [eGFR < 15 mL/min/1.73 m2]. Logistic regression analysis was performed to examine the association between CKD progression and lipid parameters. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive power of lipid parameters in the progression of CKD.
Results
Over a median follow-up of 3.0 years, 96 participants (25.3%) developed CKD progression. In multivariable logistic regression analysis, logarithm-transformed urinary albumin-to-creatinine ratio (log ACR) [odds ratio (OR) 1.834;95% confidence interval (CI) 1.253–2.685; P = 0.002] and total cholesterol to high-density lipoprotein cholesterol ratio (TC/HDL-C) [OR 1.345; 95% CI 1.079–1.677; P = 0.008] were independently associated with CKD progression. The ROC curve showed the combined predictor of ACR and TC/HDL-C ratio was acceptable for CKD progression diagnosis (area under the ROC curve [AUC] = 0.716, sensitivity 50.0%, specificity 84.2%), and the cut-off value was − 0.98.
Conclusions
The combination of TC/HDL-C ratio and ACR had predictive value in the progression of CKD, and may help identify the high-risk population with CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyslipidemia is known to be an important risk factor for cardiovascular disease [1]. Since glomerulosclerosis and atherosclerosis have similar pathophysiological mechanisms, dyslipidemia can undoubtedly damage the kidneys [2]. In 1982, Moorhead et al. [3] investigated the association between hyperlipidemia and renal function impairment, and since then, various new evidences have supported this hypothesis. Experimental evidence shows that hyperlipidemia can damage podocytes [4], mesangial cells and tubulointerstitial [5] through mechanisms such as inflammation [6], oxidative stress [7], endothelial dysfunction [8], and culminate in glomerulosclerosis. In animal models, hyperlipidemic diets induce renal injury [9], and lipid-lowering strategies ameliorate renal injury [10]. Interestingly, epidemiological studies on the relationship between serum lipids and chronic kidney disease (CKD) progression remain inconclusive. In a large prospective cohort of 3939 CKD patients aged 21–74 years, total cholesterol (TC), triglycerides (TG), triglyceride very-low-density lipoprotein cholesterol (VLDL-C), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A-I (apoA-I), apolipoprotein B (apoB) and lipoprotein(a) [Lp(a)] were not independently associated with progression of kidney disease. The kidney disease outcomes were composite end point of end-stage renal disease (ESRD) or 50% decline in estimated glomerular filtration rate (eGFR) from baseline [11]. Ki Heon Nam and colleagues reported a U-shaped association was observed between serum HDL-C levels and adverse renal outcomes in the Korean cohort study of 2168 participants with CKD. Both low and high serum HDL-C levels may be detrimental to patients with non-dialysis CKD. The primary outcome was a composite of a 50% decline in eGFR from the baseline value or the onset of ESRD during the follow-up period [12]. In a cohort of 1,943,682 male US veterans followed up for a median of 9 years, Benjamin Bowe et al. reported that a significant association exists between low HDL-C levels and risks of incident CKD and CKD progression (doubling of serum creatinine, eGFR decline of 30% or more) [13].
Previous studies have shown that although the lipid ratio is a more accurate predictor of the risk of atherosclerosis and cardiovascular disease than each lipid component alone [14, 15], conclusions on the association between lipid ratio and the progression of CKD are divergent and conflicting. In the kidney sub-study of the Chinese Stroke Primary Prevention Trial (CSPPT), a total of 12,549 hypertensive patients with eGFR ≥ 60 mL/min/1.73 m2 were included in the analysis. The results showed that a higher ratio of TG and TG/HDL-C ratio was an independent risk factor for the rapid decline of renal function in hypertensive adults with normal renal function [16]. A prospective cohort study among 4483 initially healthy men participating in the Physicians’ Health Study showed a high ratio of TC/HDL-C ratio was significantly associated with an increased risk of developing renal dysfunction in men with an initial creatinine < 1.5 mg/dL [17]. Another study of 1891 Chinese adults with normal or near-normal kidney function at baseline also found that Non-HDL-C/HDL-C ratio was an independent risk factor for the development of CKD [18]. However, a population-based, cross-sectional survey conducted in a city located in Southern China showed that serum TG was the only suitable predictor for CKD in men, and none of serum lipids and lipid ratio could be used as a predictor for CKD in women [19].
CKD is a major public health problem that threatens human health [20]. China has the largest number of CKD patients worldwide (approximately 119.5 million) [21], and the prevalence of dyslipidemia is currently greater than 41.9% [22]. However, the degree, if any, of the causative role of dyslipidemia and the possible mechanism remain unclear in the Chinese CKD population. Therefore, we investigated the data of 380 patients with CKD stage 3–4 (G3-4) from a tertiary university-based hospital in Wuhan to evaluate the value of serum lipids and their ratio in predicting CKD progression.
Materials and methods
Participants
From January 1, 2012 to July 15, 2018, patients aged 18 years or older with CKD stage 3–4 (G3-4) who were regularly followed for more than 12 months in Wuhan Fourth Hospital, Tongji Medical College, Huazhong University of Science and Technology were included in the study and were followed up until July 15, 2019.
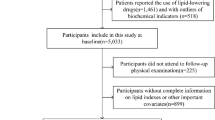
Of the 621 potential participants, we excluded the following participants: (1) 187 participants treated with drugs such as hypolipidemics, corticosteroids and immunosuppressants (systemic diseases); (2) 34 participants with missing data; (3) 14 participants with liver and thyroid gland diseases; and (4) 6 participants undergoing renal replacement therapy, resulting in a final sample size of 380. The flow of the participants is presented in Fig. 1.
Ethical considerations
This study was performed in accordance with the ethical principles of the Declaration of Helsinki and was approved by the ethics Committee of Wuhan Fourth Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (KY2021-0021-01).
Data sources
The demographic characteristics, medication history and laboratory data were extracted from electronic medical record system. Laboratory data consisted of complete blood count, blood biochemistry and serum lipid. The data were reviewed by a trained team of physicians.
Definitions
The diagnosis of CKD is based on the criteria of the 2012 kidney disease: Improving Global Outcomes, that is, a single measurement showing eGFR < 60 mL/min/1.73 m2 and/or proteinuria [albumin-to-creatinine ratio (ACR) ≥ 30 mg/g] for more than 3 months [23]. ACR was calculated as urinary albumin divided by urinary creatinine. TC/HDL-C ratio was calculated as TC level (mmol/L) divided by HDL-C level (mmol/L), and the rest by the same amount. Non-HDL-C was calculated as TC minus HDL-C. The end point of follow-up was the progression of CKD, defined as a composite of renal function rapid decline (an annual eGFR decline > 5 mL/min/1.73 m2) [24] or the new-onset ESRD (eGFR < 15 mL/min/1.73 m2). Participants were divided into two groups—progressors and non-progressors according to whether CKD was progressing. Annual change in eGFR, estimated as (eGFR at baseline—eGFR at exit)/t, where t was the time length in years from baseline to the exit visit. The eGFR was calculated using the equation from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [25]. For women with Scr (in mg/dL) ≤ 0.7, eGFR = 144 × (Scr/0.7)−0.329 × 0.993Age, and for women with Scr > 0.7, eGFR = 144 × (Scr/0.7)−1.209 × 0.993Age. For men with Scr ≤ 0.9, eGFR = 141 × (Scr/0.9)−0.411 × 0.993Age, and for men with Scr > 0.9, eGFR = 141 × (Scr/0.9)−1.209 × 0.993Age. The Youden index was calculated as sensitivity plus specificity minus 1. The variable value corresponding to the maximum Youden index was the cut-off value.
Statistical analyses
Continuous data were tested for normal distribution with the Kolmogorov–Smirnov test and presented as means ± standard deviation or median (interquartile range, IQR), as appropriate, and analyzed using the t-test or the Mann–Whitney U test. Categorical data were presented as frequencies or percentages and analyzed using the chi-square test. Logistic regression models were used to calculate the odds ratio (OR) and 95% confidence interval (CI) for the associations of serum lipids and their ratios with the progression of CKD. CKD progression (yes or no) was the dependent variable. The variables with P < 0.05 in univariate logistic regression were included as covariates into multiple logistic regression for further analysis. The receiver operating characteristic (ROC) curve was used to assess the ability of variables with P < 0.05 in multiple logistic regression and their combined predictors to distinguish CKD progression cases. Two-sided P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, IL) and MedCalc 19.0.4 (http://www.medcalc.org).
Results
Baseline characteristics of participants
Among 380 subjects, the mean age was 68.72 years, 242 (63.7%) subjects were men. The median duration of follow-up was 3.0 years (interquartile range 1.92–4.65 years), during which 96 patients (25.26%) developed CKD progression. Table 1 compared the demographic and biochemical characteristics of participants by the non-progression group and progression group.
Association of lipid parameters and CKD progression
In univariable logistic regression analysis, hemoglobin (HGB), albumin (ALB), serum uric acid (UA), logarithm-transformed urinary ACR (log ACR) were significantly associated with the progression of CKD. After adjustment for HGB, ALB, UA and log ACR in multivariate logistic regression analysis, log ACR [odds ratio (OR) 1.834;95% confidence interval (CI) 1.253–2.685; P = 0.002] and TC/HDL-C ratio (OR 1.345; 95% CI 1.079–1.677; P = 0.008) were independently associated with the progression of CKD (Table 2).
Evaluate the predictors of CKD progression
The ROC curve in Fig. 2 compared the ability of three variables (ACR, TC/HDL-C ratio and combined predictor of ACR and TC/HDL-C ratio) to predict the progression of CKD in the study population. The area under the ROC curve (AUC), 95% CI, optimal threshold, sensitivity, specificity and Youden index of each variable are shown in Table 3.
Discussion
Dyslipidemia is a common complication of progressive kidney disease. Patients with kidney disease are a very heterogeneous population with a wide range of aetiologies of renal damage, levels of renal function and proteinuria, comorbidities (especially concurrent diabetes), renal replacement modalities and treatments. All these factors can affect the levels, composition and quality of circulating lipids, resulting in specific dyslipidemia, which in favor of a more atherogenic profile [26, 27]. Patients with advanced CKD usually have worse lipid metabolism [28]. In addition, in order to avoid the influence of dialysis on serum lipid, we finally chose patients with non-dialysis-dependent CKD stage 3–4 as the research object.
Patients with non-dialysis-dependent CKD stage 3 or higher tend to show high TG and low HDL-C levels, normal or reduced LDL-C and TC levels [26, 29]. It is basically in line with the characteristics of blood lipid changes in patients with CKD progression in our study. Progressors had higher TG and TC, slightly higher LDL-C and lower HDL-C compared with non-progressors, but there was no statistical difference. Multivariable logistic regression analysis showed that both TC/HDL-C ratio and ACR were independently associated with the progression of CKD. Although our findings are similar to those of the Physicians’ Health Study (PHS) by Tobias Kurth et al. [17], there are still many differences. The main outcome measures in PHS were elevated creatinine, defined as ≥ 1.5 mg/dL (133 μmol/L), and reduced estimated creatinine clearance, defined as ≤ 55 ml/min. The outcome of our study was the progression of CKD, defined as a composite of renal function rapid decline (an annual eGFR decline > 5 mL/min/1.73 m2) or the new-onset ESRD (eGFR < 15 mL/min/1.73 m2), which was much stricter than their standard. The PHS was composed of apparently healthy, mostly white men with mild or no renal insufficiency (creatinine < 1.5 mg/dL) at baseline. Participants in our study all had moderate renal insufficiency. Unlike the general population, the circulating lipid composition and quality of CKD patients undergo profound changes characterized by accumulation of residual particles, increased proportion of small and dense LDL particles, impaired HDL maturation, and defective HDL antioxidant, anti-inflammatory and reverse cholesterol transport (RCT) capacities [28, 30,31,32]. These changes may mask the effect of lipid quantity on the progression of CKD. In addition, data on proteinuria at baseline that would have provided information regarding possible glomerular damage was not analyzed in PHS.
The ROC curve showed that only ACR higher than 146.15 mg/g rather than TC/HDL-C ratio could differentiate patients with disease progression because the AUC was statistically significant. However, relatively low sensitivities (73.85%) or specificities (56.73%) do not warrant its clinical use as a marker of CKD progression. In order to improve the predictive ability of ACR, a combined predictor was introduced. We found that the combined predictor of ACR and TC/HDL-C ratio was acceptable for CKD progression diagnosis (AUC = 0.716, sensitivity 50.0%, specificity 84.18%), and the optimal cut-off was − 0.98. Regretfully, the combined predictor had an only slightly higher predictive ability for CKD progression than ACR (P = 0.1751). These results suggest that lipid parameters alone cannot differentiate the progression of CKD, but if combined with other biomarkers that have an important effect on CKD, such as ACR, the predictive power of lipid parameters can be significantly improved. In addition, urine protein rather than conventional lipid parameters, namely lipid levels and lipid ratio, remains a major risk factor for the progression of CKD. Thus, we strongly advise health care providers to increase their efforts to prevent and/or reverse albuminuria in their patients.
Limitations of our present study should be considered when interpreting these results. First, selection bias is an important limitation to this study and may affect the external validity of our results. Most of patients who attend Wuhan Fourth Hospital are local residents, which may not represent the entire Chinese population in terms of income, educational level, lifestyle, and health profile. Second, it is important to consider some confounding variables, such as information on past medical history (e.g., blood pressure [33] or diabetes), tobacco or alcohol use, diet, education, physical activity and other possible unmeasured confounders (e.g., body-mass index, waist circumference [23, 33] and apolipoprotein) have not been collected in this study, which may affect the assessment of clinical risk. Thirdly, measurements of proteinuria and eGFR show a high degree of intraindividual variability, requiring multiple measurements to obtain more accurate and reliable data. In this study, only the data at the time of enrollment and the end of follow-up were used. Fourthly, the important features of renal dyslipidemia, profound changes in lipid composition and quality, were not included in this study. Given the previous evidence that changes in lipid composition and quality might be associated with CKD progression [28, 30,31,32], they should be considered in future studies. Finally, due to the retrospective case–control design of the present study, we should be cautious regarding the interpretation of whether dyslipidemia is a causal or a consequence of CKD. Strengths of this study include the requirement of sustained impaired eGFR (an annual eGFR decline > 5 mL/min/1.73 m2) when defining outcomes helped improve the specificity, because a decrease in eGFR may represent transient changes in kidney perfusion or function that are not necessarily related to the casual pathway that is driving the development of CKD. Furthermore, the equation for estimating the GFR in this study was designed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). Studies showed that compared with the measured GFR using urinary or plasma clearance of exogenous filtration markers, the bias was significantly reduced in almost all subjects with different eGFR levels by using the CKD-EPI equation than the Modification of Diet in Renal Disease (MDRD) [25, 34].Finally, we adjusted for many potential covariates, including age, ACR, HGB, eGFR, blood urea nitrogen (BUN), serum creatinine (Scr), UA, aspartate transaminase (AST), alanine transaminase (ALT) and follow-up duration, which thus made our results more reliable.
In summary, both TC/HDL-C ratio and ACR are independent risk factors for CKD progression. The combination of TC/HDL-C ratio and ACR may be superior to ACR in predicting the progression of CKD. Further prospective studies are required to support our results. Focusing on lipidomics research may help to further reveal the relationship between dyslipidemia and CKD progression, and provide new therapeutic targets for future interventions in this population.
Data availability
All data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aleksandra Pikula ASB, Wang J (2015) Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham Study. Neurology 84(5):472–479
Diamond JR (1991) Analogous pathobiologic mechanisms in glomerulosclerosis and atherosclerosis. Kidney Int Suppl 31:S29-34
Moorhead JF, Chan MK, El-Nahas M (1982) Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2(8311):1309–1311
Merscher S, Pedigo CE, Mendez AJ (2014) Metabolism, energetics, and lipid biology in the podocyte—cellular cholesterol-mediated glomerular injury. Front Endocrinol (Lausanne) 5:169
Johnson AC, Stahl A, Zager RA (2005) Triglyceride accumulation in injured renal tubular cells: alterations in both synthetic and catabolic pathways. Kidney Int 67(6):2196–2209
Trevisan R, Dodesini AR, Lepore G (2006) Lipids and renal disease. J Am Soc Nephrol 17(4 Suppl 2):S145-147
Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J (2008) Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl 111:S4-9
Rajendran P, Rengarajan T, Thangavel J et al (2013) The vascular endothelium and human diseases. Int J Biol Sci 9(10):1057–1069
Joles JA, Kunter U, Janssen U et al (2000) Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J Am Soc Nephrol 11(4):669–683
Blanco S, Vaquero M, Gomez-Guerrero C, Lopez D, Egido J, Romero R (2005) Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens 18(4 Pt 1):557–565
Rahman M, Yang W, Akkina S et al (2014) Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin J Am Soc Nephrol 9(7):1190–1198
Nam KH, Chang TI, Joo YS et al (2019) Association between serum high-density lipoprotein cholesterol levels and progression of chronic kidney disease: results from the KNOW-CKD. J Am Heart Assoc 8(6):e011162
Bowe B, Xie Y, Xian H, Balasubramanian S, Al-Aly Z (2016) Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int 89(4):886–896
Sanchez-Inigo L, Navarro-Gonzalez D, Pastrana-Delgado J, Fernandez-Montero A, Martinez JA (2016) Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens 34(7):1257–1265
Edwards MK, Blaha MJ, Loprinzi PD (2017) Atherogenic index of plasma and triglyceride/high-density lipoprotein cholesterol ratio predict mortality risk better than individual cholesterol risk factors, among an older adult population. Mayo Clin Proc 92(4):680–681
Zhang X, Wang B, Yang J et al (2019) Serum lipids and risk of rapid renal function decline in treated hypertensive adults with normal renal function. Am J Hypertens 32(4):393–401
Schaeffner ES, Kurth T, Curhan GC et al (2003) Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol 14(8):2084–2091
Zuo PY, Chen XL, Liu YW, Zhang R, He XX, Liu CY (2015) Non-HDL-cholesterol to HDL-cholesterol ratio as an independent risk factor for the development of chronic kidney disease. Nutr Metab Cardiovasc Dis 25(6):582–587
Zhang L, Yuan Z, Chen W et al (2014) Serum lipid profiles, lipid ratios and chronic kidney disease in a Chinese population. Int J Environ Res Public Health 11(8):7622–7635
Bikbov B, Purcell CA, Levey AS et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733
Zhang L, Wang F, Wang L et al (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379(9818):815–822
Huang Y, Gao L, Xie X, Tan SC (2014) Epidemiology of dyslipidemia in Chinese adults: meta-analysis of prevalence, awareness, treatment, and control. Popul Health Metr 12(1):28
Inker LA, Astor BC, Fox CH et al (2014) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63(5):713–735
Levin A, Stevens PE (2014) Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85(1):49–61
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Visconti L, Benvenga S, Lacquaniti A et al (2016) Lipid disorders in patients with renal failure: role in cardiovascular events and progression of chronic kidney disease. J Clin Transl Endocrinol 6:8–14
Kwan BC, Kronenberg F, Beddhu S, Cheung AK (2007) Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 18(4):1246–1261
Bulbul MC, Dagel T, Afsar B et al (2018) Disorders of lipid metabolism in chronic kidney disease. Blood Purif 46(2):144–152
Mikolasevic I, Zutelija M, Mavrinac V, Orlic L (2017) Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovasc Dis 10:35–45
Vaziri ND (2006) Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 290(2):F262-272
Kronenberg F (2018) HDL in CKD-the devil is in the detail. J Am Soc Nephrol 29(5):1356–1371
Rysz J, Gluba-Brzozka A, Rysz-Gorzynska M, Franczyk B (2020) The role and function of HDL in patients with chronic kidney disease and the risk of cardiovascular disease. Int J Mol Sci 21(2):601
Ji B, Zhang S, Gong L et al (2013) The risk factors of mild decline in estimated glomerular filtration rate in a community-based population. Clin Biochem 46(9):750–754
Stevens LA, Schmid CH, Greene T et al (2010) Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56(3):486–495
Funding
This work was financially supported by Wuhan Health and Family Planning Commission research projects (No. WX18Q31).
Author information
Authors and Affiliations
Contributions
HW and JD conceived and designed the study. WL screened the abstract and full text, assessed studies and drafted the manuscript. ZD performed statistical analyses. HW revised the manuscript. All authors read the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declared no competing interests.
Ethical approval
This study was performed in accordance with the ethical principles of the Declaration of Helsinki and was approved by the ethics Committee of Wuhan Fourth Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (KY2021-0021-01). All participants have written informed consent before the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W., Du, Z., Wei, H. et al. Total cholesterol to high-density lipoprotein cholesterol ratio is independently associated with CKD progression. Int Urol Nephrol 54, 2057–2063 (2022). https://doi.org/10.1007/s11255-021-03099-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-03099-9