Abstract
Purpose
To investigate whether non-high-density lipoprotein cholesterol (Non-HDL-C), remnant cholesterol (RC), and the ratios of lipid indexes are more closely associated with early progression of kidney dysfunction than traditional lipid indexes; and to explore the association between changes in serum lipids during follow-up and annual decline rate in estimated glomerular filtration rate (eGFR).
Methods
In this prospective cohort study, 3909 participants with normal eGFR and age≥40 years at baseline were followed for 3.3 years. Progression of kidney dysfunction was assessed as annual decline rate in eGFR. Spearman correlation analysis, linear correlation models, and multiple logistic regression were used to assess the associations between lipid indexes at baseline/both baseline and follow-up and the annual decline rate in eGFR.
Results
Compared with ΔLDL-C (β = 0.412), other lipid indexes such as ΔLDL-C/HDL-C (β = 0.565), ΔTC/HDL-C (β = 0.448), and ΔNon-HDL-C/HDL-C (β = 0.448) were more closely associated with annual decline rate in eGFR. High TG/HDL-C (OR = 1.699(1.177–2.454)) and TC/HDL-C (OR = 1.567(1.095–2.243)) at baseline, as well as high TC/HDL-C (OR = 1.478 (1.003–2.177)) and TG/HDL-C (OR = 1.53(1.044–2.244)) at both baseline and follow-up were associated with the annual decline rate in eGFR <0.5. High Non-HDL-C (OR = 1.633(1.025–2.602)) and LCI (OR = 1.631(1.010–2.416)) at both baseline and follow-up resulted in a 63% increase in risk of annual decline rate in eGFR >1.
Conclusion
High Non-HDL-C, RC and the ratios of lipid indexes were more closely associated with early progression of kidney injury than the increase of traditional lipid indexes. These lipid indexes should be monitored, even in participants with normal traditional serum lipid levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is associated with high mortality [1], although the process of tracking this slowly progressing disease is difficult until kidney failure has occurred. It is therefore reasonable and practical to assess the early progression of kidney dysfunction via changes in estimated glomerular filtration rate (eGFR) over time [2, 3].
Dyslipidemia is associated with the pathogenesis of kidney insufficiency and occurs commonly in CKD [4]. Studies suggested that patients with normal serum cholesterol levels or those aggressively using statins to reduce low-density lipoprotein cholesterol (LDL-C), can still progress to CKD or end-stage renal disease (ESRD) [5, 6]. When traditional lipid indexes including total cholesterol (TC), triglycerides (TG), LDL-C, and high-density lipoprotein cholesterol (HDL-C) are used as control targets, high residual risk in kidney injury may go undetected. Compared with traditional lipid indexes, non-high-density lipoprotein cholesterol (Non-HDL-C) and remnant cholesterol (RC) produce lipid-laden macrophages, known as foam cells, at a faster rate, with ensuing plaque formation that leads to atherosclerosis resulting in a more severe atherosclerotic disease, and are likely to capture more information on pro-atherogenic lipoproteins [7, 8]. Ratios of lipid indexes such as TC/HDL-C, TG/HDL-C, Non-HDL-C/HDL-C, and lipid comprehensive index (LCI) can better predict the risks of atherosclerosis and related diseases compared with the traditional lipid indexes. Therefore, this study hypothesized that Non-HDL-C, RC, and ratios of lipid indexes are more closely associated with progression of kidney dysfunction than the traditional lipid indexes.
According to several previous studies [9, 10], serum lipid stratification is associated with lower eGFR and higher proteinuria, which can in part be attributed to lipid deposition that possibly damages the arteriole mesangial cells and glomerular basement membrane [11]. It, therefore, appears reasonable that deterioration in serum lipid levels may be associated with progression of kidney injury. However, there is a lack of studies on the association between changes in serum lipids and progression of kidney dysfunction in adults. In this study, annual decline rate in eGFR was used as an indicator to determine the association between lipid indexes and early progression of kidney dysfunction in the middle-aged and older populations with normal eGFR. This study also investigated whether changes in serum lipids during follow-up were associated with the annual decline rate in eGFR.
Methods
Study participants
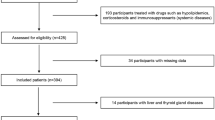
This study was part of the baseline and follow-up survey based on the cancer risk assessment of Chinese diabetic patients (REACTION) study [12]. The baseline survey was conducted between 2011 and 2012, and recruited 10,216 adults aged ≥ 40 years from the Pingguoyuan community in Beijing, China, a single center of the REACTION study. A follow-up was conducted 3 years later between April and October, 2015. The exclusion criteria were as follows: (1) previous kidney disease or eGFR < 90 mL/min/1.73 m2 or hyperfiltration; (2) use of lipid-lowering drugs; (3) missing data on lipid indexes, other important covariates, or recorded outliers; and (4) loss to follow-up. Ultimately, 3909 participants were included as shown in Fig. 1
The study was approved by the Medical Ethics Committee of Ruijin Hospital of Shanghai Jiaotong University. Written informed consent was provided at baseline in accordance with the Declaration of Helsinki.
Outcomes
Progression of kidney dysfunction is expressed as annual decline rate in eGFR in units of mL/min/1.73 m2 per year. eGFR shows a downward trend with age and generally changes with an early compensatory increase followed by a decline. Controlling the annual decline rate in eGFR between 0.5 and 1 over 2–3 years can gain more benefit to delay the progression of early kidney injury. Annual decline rate in eGFR > 1 indicates a decline in kidney function, while <0.5 indicates an increase in eGFR [2, 13].
In this prospective cohort study with median follow-up period of 3.3 years, the participants with normal baseline eGFR experienced early changes in kidney function. Therefore, they were divided into three groups according to annual decline rate in eGFR <0.5, 0.5–1, or >1.
Data collection
Standard questionnaires were used to collect baseline information on all participants, such as behavioral information, medications prescribed, and medical history. After a 5-min rest, blood pressure (BP) was measured three times using mercury sphygmomanometer in accordance with standard protocols. The mean value of the three readings was recorded as systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Blood samples (10 mL) were obtained from all participants following a fast of minimum 10 h to assess fasting plasma glucose (FPG), serum lipids, and serum creatinine (Scr) at baseline and follow-up. Serum lipids and Scr were measured using enzymatic methods with an autoanalyzer (c16000 system, ARCHITECT ci16200 analyzer; Abbott Laboratories, Chicago, IL, USA).
Definitions
Dysglycemia was defined according to the American Diabetes Association guidelines or based on diagnosis of diabetes mellitus (DM) or taking antidiabetic drugs. Hypertension was diagnosed according to antihypertensive medication use and/or SBP ≥ 140 and/or DBP ≥ 90 mm Hg, or based on a previous diagnosis. Dyslipidemia was defined as TC ≥ 6.22 and/or TG ≥ 2.26 and/or LDL-C ≥ 4.14 and/or HDL-C < 1.04 (mmol/L). Moreover, TC (<5.18, 5.18–6.19, 6.22≤), TG (<1.70, 1.70–2.25, 2.26≤) and LDL-C (<3.37, 3.37–4.12, 4.14≤) were divided into three categories (normal, marginally increased and increased, respectively) in mmol/L; HDL-C was divided into normal (1.04≤) and decreased (<1.04) in mmol/L [14]. Non-HDL-C, RC, and ratios of lipid indexes were classified according to tertiles, and the third quantile was considered to be an increase. Hyperfiltration was defined according to the 90th percentile of age (10-year intervals) and sex categories [15, 16] at baseline. Current smokers were those who regularly smoked cigarettes (one per day or seven per week) and current drinkers were defined as regularly drinking (once per week) over the past 6 months.
During follow-up, poor control of plasma glucose, lipids, BP, or body mass index (BMI) was defined as lack of dysglycemia, dyslipidemia, hypertension, or overweight/obesity, respectively at baseline, but the corresponding condition was diagnosed at follow-up. According to the World Health Organization, overweight and obesity were defined as BMI of 25.0–29.9 and 30.0≤ kg/m2, respectively.
Calculation
(1) eGFR was calculated according to the Modification of Diet in Renal Disease (MDRD) study equation:
eGFR=175 × (Scr)−1.234 × (age)−0.179 × [0.79 if the patient is female]and CKD Epidemiology Collaboration (CKD-EPI) equation:
In female: with Scr ≤ 0.7 mg/dL: eGFR = 144 × (Scr/0.7)−0.329 × (0.993)age;
with Scr > 0.7 mg/dL: eGFR = 144 × (Scr/0.7)−1.209 × (0.993)age.
In male: with Scr ≤ 0.9 mg/dL: eGFR = 141 × (Scr/0.9)−0.411 × (0.993)age;
with Scr > 0.9 mg/dL: eGFR = 141 × (Scr/0.9)−1.209 × (0.993)age.
(2) Annual decline rate in eGFR = \(\left( {1 - \root {{{{\mathrm{t}}}}} \of {{\frac{{{{{\mathrm{eGFR}}}}\,{{{\mathrm{at}}}}\,{{{\mathrm{follow}}}} - {{{\mathrm{up}}}}}}{{{{{\mathrm{eGFR}}}}\,{{{\mathrm{at}}}}\,{{{\mathrm{baseline}}}}}}}}} \right) \times 100\%\). t was the number of years of follow-up.
(3) Weight and height were measured according to the standard protocols. BMI = weight/height squared (k/m2).
(4) Non-HDL-C = TC − HDL-C; RC = TC − (HDL-C + LDL-C); LCI = TC × TG × LDL-C/HDL-C.
(5) ΔCharacteristics = follow-up characteristics − baseline characteristics.
Statistical analysis
The variables were subjected to the Kolmogorov–Smirnov test. Normal and non-normal variables are presented as mean (±standard deviation) and median (inter-quartile range), respectively. Categorical variables are presented as percentages. Differences between different annual decline rates in eGFR groups were analyzed using the Kruskal–Wallis test (skewed variables) or χ2 test (categorical variables). Pairwise comparisons were performed using the post-test of ANOVA. The Bonferroni test was used when groups had homogeneity of variance; The Tamhane test was used when groups had no homogeneity of variance, and P < 0.017 (0.05/3 = 0.017) was considered statistically significant according to the number of comparisons of the three groups.
Spearman correlation analysis and linear correlation models were used to explore the association between each Δlipid index and annual decline rate in eGFR. Scatter plots and standardized residual plots were used to test linear assumptions, and the covariates were then adjusted in linear correlation models. Next, baseline lipid indexes and annual decline rate in eGFR were treated as categorical variables, and the associations were analyzed using multiple logistic regression. Finally, the participants were divided into B0H0, B1H0, B0H1, and B1H1 groups according to each lipid index (e.g., B0/B1: baseline normal TC/baseline high TC, F0/F1: follow-up normal TC/follow-up high TC), and the associations between these different groups and annual decline rate in eGFR groups were explored in multiple logistic regression models. In the sensitivity analysis, CKD Epidemiology Collaboration (CKD-EPI) was used to calculate eGFR. P < 0.05 was set as statistically significant. All analyses were two-sided and performed using SPSS software V.23.0 for Windows (SPSS, Chicago, IL, USA).
Results
Participants’ characteristics
Table 1 shows baseline characteristics and changes during follow-up of participants in the three groups. The group with annual decline rate in eGFR between 0.5 and 1 had lower levels of TG, TG/HDL-C, and LCI, and higher HDL-C at baseline, and minimal changes in the levels of LDL-C, TC/HDL-C, and Non-HDL-C/HDL-C during follow-up, and with low percentage of current drinkers. TG, LDL-C, and TG/HDL-C levels increased the most in the group with annual decline rate in eGFR >1. This group also had higher levels of TC, LDL-C, and Non-HDL-C at follow-up.
Spearman correlation analysis of Δcharacteristics and annual decline rate in eGFR
As shown in Table 2, in addition to RC and HDL-C, increases in lipid indexes were associated with increase in annual decline rate in eGFR. Among them, r (correlation coefficient) of ΔLDL-C/HDL-C (0.095), ΔTC/HDL-C (0.085), ΔNon-HDL-C/HDL-C (0.085), ΔNon-HDL-C (0.082), ΔLDL-C (0.081), and ΔLCI (0.069) were higher than ΔSBP (0.068). These results suggested that controlling the increases in serum lipids can slow the decline of eGFR.
Linear correlation analysis of Δlipid indexes and annual decline rate in eGFR
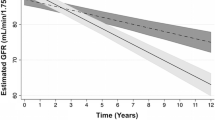
Further adjusted the covariates and corresponding baseline lipid indexes in linear correlation analysis to explore which changes in lipid indexes were most closely associated with the annual decline rate in eGFR, as shown in Fig. 2 ΔLDL-C/HDL-C (β = 0.565), ΔTC/HDL-C (β = 0.448), ΔNon-HDL-C/HDL-C (β = 0.448) and ΔLDL-C (β = 0.412) were associated with the annual decline rate in eGFR, with all P values < 0.001. Compared with traditional lipid indexes, the increase of ratios of lipid indexes were found to be more closely associated with annual decline rate in eGFR.
β-coefficient of Δlipid indexes for eGFR annual decline rate from linear correlation analysis. Adjusted sex, baseline age, smoking and drinking status, ΔBMI, ΔFPG, ΔSBP, corresponding baseline lipid index and plasma glucose, BP, lipid, BMI control when analyzed each Δlipid index. Abbreviations : TC total cholesterol; TG triglyceride; HDL-C high-density lipoprotein cholesterol; LDL-C low-density lipoprotein cholesterol; Non-HDL-C non-high-density lipoprotein cholesterol; LCI, lipid comprehensive index. *P < 0.001
Multiple logistic regression between baseline lipid indexes stratification and the different groups of annual decline rate in eGFR
As shown in Fig. 3 (Online Resource Table 1), in the fully adjusted models, the third quantile of TG/HDL-C (OR = 1.699(1.177–2.454)), TC/HDL-C (OR = 1.567(1.095–2.243)), Non-HDL-C/HDL-C (OR = 1.478 (1.033–2.115)), and LCI (OR = 1.459(1.026–2.073)) were associated with an annual decline rate in eGFR <0.5, P for trend were all <0.05. These results suggested that participants with higher ratios of lipid indexes at baseline may have a higher risk of entering the early compensatory period of kidney injury during follow-up, which manifested as an increase in eGFR.
Forest plot of multiple logistic regression between baseline lipid indexes and different eGFR annual decline rate groups. The OR (odds ratio) and 95%CI (confidence interval) of increased or the third quantile of each lipid index and eGFR annual decline rate groups were shown in the figure. Regarded the eGFR annual decline rate0.5~1 in the normal or first quantile of each lipid index as reference. Adjusted sex, baseline age, smoking and drinking status, BMI, FPG, SBP, and plasma glucose, BP, lipid, BMI control when analyzed each lipid index. Abbreviations: TC total cholesterol; TG triglyceride; HDL-C high-density lipoprotein cholesterol; LDL-C low-density lipoprotein cholesterol; Non-HDL-C non-high-density lipoprotein cholesterol; LCI lipid comprehensive index. *P of OR and trend of the lipid index for eGFR annual decline rate < 0.5 were less than 0.05
Multiple logistic regression between lipid changes and the different groups of annual decline rate in eGFR
The participants were divided into B0H0, B1H0, B0H1, and B1H1 groups according to each lipid index. After adjusting for covariates as shown in Fig. 4 (Online Resource Table 2), compared with the B0H0 group, TC/HDL-C (OR = 1.478 (1.003–2.177)) and TG/HDL-C (OR = 1.53(1.044–2.244)) of the B1H1 group were associated with annual decline rate in eGFR <0.5, resulting in a 50% increase in risk. Non-HDL-C (OR = 1.633(1.025–2.602)) and LCI (OR = 1.631(1.010–2.416)) of B1H1 group were associated with annual decline rate in eGFR >1. High Non-HDL-C and ratios of lipid indexes at both baseline and follow-up increased the risk of increased annual decline rate in eGFR by 63%. P of trend of TG, TG/HDL/C and LCI for annual decline rate in eGFR <0.5 and LCI for annual decline rate in eGFR >1 were less than 0.05.
Forest plot of multiple logistic regression between lipid changes and different eGFR annual decline rate groups. From top to bottom corresponds to the OR (odds ratio) and 95%CI (confidence interval) of the B1H0, B0H1, and B1H1 groups of each blood lipid index. Groups were divided by each lipid index, respectively. e.g., B0/B1: baseline normal TC/baseline high TC, F0/F1: follow-up normal TC/follow-up high TC. Regarded the eGFR annual decline rate0.5~1 in the B0H0 of each lipid index as reference. Adjusted sex, baseline age, smoking and drinking status, ΔBMI, ΔFPG, ΔSBP, and plasma glucose, BP, BMI control when analyzed each lipid index. Abbreviations : TC total cholesterol; TG triglyceride; HDL-C high-density lipoprotein cholesterol; LDL-C low-density lipoprotein cholesterol; Non-HDL-C non-high-density lipoprotein cholesterol; LCI, lipid comprehensive index. P of trend of TG TG/HDL/C and LCI for eGFR annual decline rate < 0.5 and LCI for eGFR annual decline rate > 0.5 were less than 0.05. *P < 0.05 of the OR for eGFR annual decline rate < 0.5. #P < 0.05 of the OR for eGFR annual decline rate > 1
Sensitivity analysis
The CKD-EPI equation was used to calculate eGFR. As shown in Online Resource Table 3, ΔLDL-C/HDL-C (r = 0.082), ΔTC/HDL-C (r = 0.073), ΔNon-HDL-C/HDL-C (r = 0.073), ΔNon-HDL-C (r = 0.07), ΔLDL-C (r = 0.069), and ΔLCI (r = 0.063) were correlated with eGFR annual decline rate. In Online Resource Fig. 1, ΔRemnant cholesterol (β = 0.339), ΔLDL-C/HDL-C (β = 0.236), ΔTC/HDL-C (β = 0.196), ΔNon-HDL-C/HDL-C (β = 0.196), ΔNon-HDL-C (β = 0.144) and ΔLDL-C (β = 0.14) were associated with the annual decline rate in eGFR. Compared with traditional lipid indexes, the increase of ratios of lipid indexes were more closely associated with annual decline rate in eGFR.
As shown in Online Resource Table 4, the third quantile of LDL-C was associated with annual decline rate in eGFR <0.5 (OR = 1.52(1.084,2.13)) or >1 (OR = 1.812(1.328,2.473)). The ratios of lipid indexes increased approximately 40% risk of annual decline rate in eGFR <0.5, and Remnant cholesterol increased 26% risk of annual decline rate in eGFR >1, P for trend were all <0.05. As shown in Online Resource Table 5, compared with the B0H0 group, HDL-C (OR = 1.92(1.04,3.545)), Non-HDL-C (OR = 1.397(1.016,1.922)) and LCI (OR = 1.31(1.009,1.7)) of the B1H1 group were associated with annual decline rate in eGFR <0.5, resulting in 30–90% increase in risk; TC、LDL-C、Non-HDL-C and LCI of the B1H1 group increased the risk of annual decline rate in eGFR >1 by 40–117%.
Discussion
In participants with normal baseline eGFR, the increase of the ratios of lipid indexes was more closely associated with annual decline rate in eGFR than the increase of traditional lipid indexes. High ratios of lipid indexes at baseline or at both baseline and follow-up resulted in 50%–70% or 50% increases, respectively, in the risk of annual decline rate in eGFR <0.5 over 3 years. Compared with participants with normal lipids at baseline and follow-up, high Non-HDL-C and LCI at baseline and follow-up caused a 63% increase in risk of annual decline rate in eGFR > 1.
From a pathophysiological perspective, dyslipidemia leads to kidney injury by damaging glomerular epithelial cells and mesangial cells and causing renal atherosclerosis [11, 17]. In contrast, CKD can further aggravate dyslipidemia, usually at the stages of severe CKD and ESRD [18]. In this prospective cohort study, participants had normal baseline eGFR and experienced only early kidney impairment over a mean period of 3.3 years. Therefore, the present results were interpreted within the context of the effect of lipids on the progression of kidney dysfunction. Dyslipidemia caused by kidney injury should be further investigated in follow-up CKD patients.
Some cohort studies [19,20,21] showed that Non-HDL-C, RC, and ratios of lipid indexes were associated with kidney injury as reflected by CKD and eGFR decline. However, these studies were restricted to specific populations such as all men [19] or CKD patients [20, 21], and did not examine multiple lipid indexes. Other cohort studies [22, 23] suggested that among the lipid indexes, logTG/HDL or TC/HDL-C is most significantly associated with CKD. However, these studies did not include measurements of LDL-C, RC, or LCI, and also did not adjust for control of plasma glucose, BP, lipids, and BMI during follow-up, which can influence the incidence of CKD [24, 25]. This prospective cohort study included multiple lipid indexes and adjusted for control of plasma glucose, BP, lipids, and BMI during follow-up, suggesting that the third quantile of TC/HDL-C, TG/HDL-C, Non-HDL-C/HDL-C, and LCI at baseline were associated with annual decline rate in eGFR <0.5 over the 3 years, P for trend were <0.05. Higher serum lipid stratification was related to higher risk of early kidney injury. These participants experienced increases in eGFR within 3 years, which may be an adaptive mechanism of early kidney injury (that is, glomerular hyperfiltration), and can cause subsequent decline in kidney function [26]. Therefore, even in middle-aged and older individuals with normal eGFR, elevation in the levels of serum lipids should be controlled to reduce early kidney injury.
Studies on the association between changes in serum lipids and decreased kidney function are limited in adults. The SHARP trial [27] included participants with moderate-to-severe kidney disease, and found that although CKD progressed, LDL-C in the placebo group was almost unchanged over 4 years. Another study conducted in new-onset type 2 DM patients [28] found that increased TG was a risk factor for decreased eGFR, although it only included participants with decreased eGFR, and kidney function was assessed according to the absolute difference between baseline and follow-up eGFR. In this study, participants with normal eGFR at baseline were followed up and progressed to increased eGFR, decreased eGFR, or change within an appropriate range. Results in this study suggested that increases in Non-HDL-C, RC, and ratios of lipid indexes were associated with increase in annual decline rate in eGFR. Increased serum lipids can cause lipid deposition, which can lead to kidney injury [11]. This study also found that high Non-HDL-C, RC, and ratios of lipid indexes at both baseline and follow-up were risk factors for kidney injury. High levels of TC/HDL-C and TG/HDL-C or high Non-HDL-C and LCI at both baseline and follow-up resulted in a 50% and 63% increase in risk of annual decline rate in eGFR <0.5 and >1, respectively. Among the serum lipids that were statistically significant for annual decline rate in eGFR <0.5, P for trend of TG, TG/HDL-C and LCI were <0.05. For annual decline rate in eGFR >1, P for trend of LCI were <0.05. This indicated that compared with participants with normal serum lipid levels at both baseline and follow-up, baseline high lipid levels, follow-up high lipid levels, baseline and follow-up high lipid level had the trend of increased risk of kidney injury. Thus, the deterioration of serum lipid levels can lead to early progression of kidney dysfunction. Therefore, sustained control of serum lipids should be performed to reduce kidney function damage.
Non-HDL-C, RC, and ratios of lipid indexes may be better markers for risk assessment than traditional lipid indexes. A prior study suggested that TG/HDL-C correlates well with highly atherogenic particles and can comprehensively reflect the balance between atherogenic and anti-atherosclerotic factors [29]. Non-HDL-C/HDL-C can better reflect interactions between factors involved in lipoprotein metabolism. It can therefore better predict atherosclerosis compared with any single lipid index [30]. RC represents the amount of TC transported in intermediate-density lipoproteins (IDL), very low-density lipoprotein (VLDL), and chylomicron residues [31]. LCI amplifies various subtle abnormalities of atherosclerosis and anti-atherosclerotic lipoproteins [32]. Previous studies showed that even in participants with good control of LDL-C, BP and blood glucose, high TG and low HDL-C were independent risk factors for CKD [33, 34]; and because dyslipidemia is generally of mixed types [35], it may be more comprehensive to simultaneously assess multiple lipid indexes to accurately determine the risk of kidney injury. The present study also suggested that these indexes are more closely associated with annual decline rate in eGFR than traditional lipid indexes. Therefore, in addition to LDL-C, additional measurements of Non-HDL-C, RC, and the ratios of lipid indexes should be used to identify kidney injury and benefit the population at high residual risk of kidney disease.
The novelty of this study was that the trend of compensatory increase followed by decline in eGFR, rather than only eGFR decline, was considered when analyzing early kidney injury. This should serve as a reminder to help prevent the early progression of kidney dysfunction caused by neglect of adequate clinical managements of serum lipid even in a relatively short periods of time. This study investigated multiple serum lipid indexes, and emphasized that control of both baseline and long-term elevation of serum lipids should be sustained to reduce early kidney injury in individuals with normal eGFR.
Study strength and limitations
The major strength of this cohort study was that it was performed prospectively to demonstrate early kidney injury in participants with normal eGFR at baseline within a relatively short period of time. The study investigated the association between lipid indexes/lipid changes and the annual decline rate in eGFR and emphasized the role of Non-HDL-C, RC, and ratios of lipid indexes in the prevention of early kidney injury. Moreover, using an analytic model, the study adjusted for new-onset DM, hypertension, dyslipidemia, and overweight/obesity during follow-up, which enhanced the reliability of these results.
This study also had several limitations. First, data on proteinuria and HbA1c at follow-up were not available. Therefore, this study could not accurately define the progression of early kidney injury due to the lack of information on proteinuria nor could it analyze ΔHbA1c as a covariate. However, the annual decline rate in eGFR is a more effective surrogate for kidney injury than changes in proteinuria [2] and also genuinely reflects the progression of kidney dysfunction. FPG and ΔFPG were used as a covariate to adjust the effect of plasma glucose in this study. Second, there was some selection bias. This study only included participants ≥40 years old with normal eGFR at baseline, which highlighted the significance of prevention before occurrence of kidney injury. The number of men, current smokers, and drinkers was low in the group with annual decline rate in eGFR between 0.5–1. Although these factors were adjusted as covariates, they may have still caused selection bias. Third, this study only excluded users of lipid-lowering drugs and did not consider other drugs that may have affected serum lipids.
Conclusion
In this prospective cohort study, we found that increased serum lipids during follow-up increased the risk of decline in eGFR. High Non-HDL-C, RC and the ratios of lipid indexes at baseline or at both baseline and follow-up were associated with early progression of kidney injury. These observations suggested that the levels of multiple lipid indexes should be therapeutically controlled to prevent and slow the early progression of kidney dysfunction in participants with normal eGFR. Even in individuals with normal serum lipid levels, relatively high TC, TG, or LDL-C and relatively low HDL-C may lead to relatively high Non-HDL-C and ratios of lipid indexes. It is straightforward to calculate Non-HDL-C, RC, and ratios of lipid indexes following routine blood examination. Therefore, individuals with abnormal serum lipid combinations should be monitored to prevent early kidney injury. The results of this study may prompt participants to pay increased attention to protection of kidney function and provide public health recommendations for the prevention of CKD.
Data availability
The datasets generated and/or analyzed during the current study are obtained from a third party and are not publicly available but are available from these contact details: Phone: 86-010-5549-9402. Email: jingtaodou@163.com on reasonable request.
Abbreviations
- BMI:
-
body mass index
- BP:
-
blood pressure
- CKD:
-
Chronic kidney disease
- DBP:
-
diastolic blood pressure
- DM:
-
diabetes mellitus
- eGFR:
-
estimated glomerular filtration rate
- ESRD:
-
end-stage renal disease
- FPG:
-
fasting plasma glucose
- HbA1c:
-
glycated hemoglobin A1c
- HDL-C:
-
high-density lipoprotein cholesterol
- LCI:
-
lipid comprehensive index
- LDL-C:
-
low-density lipoprotein cholesterol
- OGTT:
-
oral glucose tolerance test
- RC:
-
remnant cholesterol
- SBP:
-
Systolic blood pressure
- Scr:
-
serum creatinine
- TC:
-
total cholesterol
- TG:
-
triglycerides.
References
R. Lozano, M. Naghavi, K. Foreman, S. Lim, K. Shibuya, V. Aboyans, J. Abraham, T. Adair, R. Aggarwal, S.Y. Ahn et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012). https://doi.org/10.1016/S0140-6736(12)61728-0
A.S. Levey, R.T. Gansevoort, J. Coresh, L.A. Inker, H.L. Heerspink, M.E. Grams, T. Greene, H. Tighiouart, K. Matsushita, S.H. Ballew et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am. J. Kidney Dis. 75, 84–104 (2020). https://doi.org/10.1053/j.ajkd.2019.06.009
A.S. Levey, L.A. Inker, K. Matsushita, T. Greene, K. Willis, E. Lewis, D. de Zeeuw, A.K. Cheung, J. Coresh, GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 64, 821–835 (2014). https://doi.org/10.1053/j.ajkd.2014.07.030
M. Rahman, W. Yang, S. Akkina, A. Alper, A.H. Anderson, L.J. Appel, J. He, D.S. Raj, J. Schelling, L. Strauss, et al. Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin. J. Am. Soc. Nephrol 9, 1190–1198 (2014).
M.E. Molitch, R.A. DeFronzo, M.J. Franz, W.F. Keane, C.E. Mogensen, H.H. Parving, Diabetic nephropathy. Diabetes Care 26(Suppl 1), S94–S98 (2003). https://doi.org/10.2337/diacare.26.2007.s94
D. Lloyd-Jones, R.J. Adams, T.M. Brown, M. Carnethon, S. Dai, G. De Simone, T.B. Ferguson, E. Ford, K. Furie, C. Gillespie et al. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121, 948–954 (2010). https://doi.org/10.1161/circulationaha.109.192666
M.F. Piepoli, A.W. Hoes, S. Agewall, C. Albus, C. Brotons, A.L. Catapano, M.-T. Cooney, U. Corrà, B. Cosyns, C. Deaton, et al. European Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol 23, (2016)
A. Saeed, E.V. Feofanova, B. Yu, W. Sun, S.S. Virani, V. Nambi, J. Coresh, C.S. Guild, E. Boerwinkle, C.M. Ballantyne, R.C. Hoogeveen, Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J. Am. Coll. Cardiol. 72, 156–169 (2018). https://doi.org/10.1016/j.jacc.2018.04.050
B.L. Kasiske, Hyperlipidemia in patients with chronic renal disease. Am. J. Kidney Dis. 32, S142–S156 (1998). https://doi.org/10.1053/ajkd.1998.v32.pm9820472
M.R. Hager, A.D. Narla, L.R. Tannock, Dyslipidemia in patients with chronic kidney disease. Rev. Endocr. Metab. Disord. 18, 29–40 (2017). https://doi.org/10.1007/s11154-016-9402-z
J. F. Moorhead, M. K. Chan, M. El-Nahas, Z. Varghese Lipid Nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 2, (1982) 1309-1311. https://doi.org/10.1016/s0140-6736(82)91513-6
Y. Bi, J. Lu, W. Wang, Y. Mu, J. Zhao, C. Liu, L. Chen, L. Shi, Q. Li, Q. Wan et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J. Diabetes 6, 147–157 (2014). https://doi.org/10.1111/1753-0407.12108
W.F. Clark, J.M. Sontrop, J.J. Macnab, R.S. Suri, L. Moist, M. Salvadori, A.X. Garg, Urine volume and change in estimated GFR in a community-based cohort study. Clin. J. Am. Soc. Nephrol. 6, 2634–2641 (2011). https://doi.org/10.2215/cjn.01990211
Joint Committee for Developing Chinese guidelines on P, Treatment of Dyslipidemia in A, Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing. Za Zhi 35, 390–419 (2007)
H.I. Lim, S.J. Jun, S.W. Lee, Glomerular hyperfiltration may be a novel risk factor of restrictive spirometry pattern: analysis of the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2015. PLOS ONE 14, e0223050 (2019)
T. Melsom, U.D. Mathisen, O.C. Ingebretsen, T.G. Jenssen, I. Njølstad, M.D. Solbu, I. Toft, B.O. Eriksen, Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care 34, 1546–1551 (2011). https://doi.org/10.2337/dc11-0235
W.F. Keane, B.L. Kasiske, M.P. O'Donnell, Lipids and progressive glomerulosclerosis. A model analogous to atherosclerosis. Am. J. Nephrol 8, 261–271 (1988)
M. Tonelli, N. Wiebe, B. Culleton, A. House, C. Rabbat, M. Fok, F. McAlister, A.X. Garg, Chronic kidney disease and mortality risk: a systematic review. J. Am. Soc. Nephrol. 17, 2034–2047 (2006). https://doi.org/10.1681/asn.2005101085
E.S. Schaeffner, T. Kurth, G.C. Curhan, R.J. Glynn, K.M. Rexrode, C. Baigent, J.E. Buring, J.M. Gaziano, Cholesterol and the risk of renal dysfunction in apparently healthy men. J. Am. Soc. Nephrol. 14 (2003)
K. Tsuruya, H. Yoshida, M. Nagata, T. Kitazono, K. Iseki, C. Iseki, S. Fujimoto, T. Konta, T. Moriyama, K. Yamagata et al. Impact of the triglycerides to high-density lipoprotein cholesterol ratio on the incidence and progression of CKD: a longitudinal study in a large Japanese population. Am. J. Kidney Dis. 66, 972–983 (2015). https://doi.org/10.1053/j.ajkd.2015.05.011
PY. Zuo, XL. Chen, YW. Liu, R. Zhang, XX. He, CY. Liu, Non-HDL-cholesterol to HDL-cholesterol ratio as an independent risk factor for the development of chronic kidney disease. Nutr., Metab. Cardiovasc. Dis 25, 582–587 (2015)
X. Wang, H. Chen, X. Shao, C. Xiong, G. Hong, J. Chen, X. Li, X. You, P. Gao, Y. Chen et al. Association of lipid parameters with the risk of chronic kidney disease: a longitudinal study based on populations in Southern China. Diabetes Metab. Syndr. Obes. 13, 663–670 (2020). https://doi.org/10.2147/DMSO.S229362
Z. Yan-Bo, S. Li-Ting, W. Wei, G. Huan, Y. Handong, M. Xinwen, G. Kunquan, Y. Kun, Z. Xiaomin, H. Meian, et al., Association of blood lipid profile with incident chronic kidney disease: a Mendelian randomization study. Atherosclerosis 300, 19–25 (2020)
J.H. Huh, D. Yadav, J.S. Kim, J.W. Son, E. Choi, S.H. Kim, C. Shin, K.C. Sung, J.Y. Kim, An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism 67, 54–61 (2017). https://doi.org/10.1016/j.metabol.2016.11.003
H.R. Yun, H. Kim, J.T. Park, T.I. Chang, T.H. Yoo, S.W. Kang, K.H. Choi, S. Sung, S.W. Kim, J. Lee et al. Obesity, metabolic abnormality, and progression of CKD. Am. J. Kidney Dis. 72, 400–410 (2018). https://doi.org/10.1053/j.ajkd.2018.02.362
C. Zoccali, F. Mallamaci, he overdriven glomerulus as a cardiovascular risk factor. Kidney Int 93, 13–15 (2018). https://doi.org/10.1016/j.kint.2017.08.035
C. Baigent, M.J. Landray, C. Reith, J. Emberson, D.C. Wheeler, C. Tomson, C. Wanner, V. Krane, A. Cass, J. Craig et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011). https://doi.org/10.1016/S0140-6736(11)60739-3
C. Wang, L. Wang, K. Liang, F. Yan, X. Hou, F. Liu, L. Chen, Poor control of plasma triglycerides is associated with early decline of estimated glomerular filtration rates in new-onset type 2 diabetes in china: results from a 3-year follow-up study.J. Diabetes Res. 2020, 3613041 (2020). https://doi.org/10.1155/2020/3613041
C. Maruyama, K. Imamura, T. Teramoto, Assessment of LDL particle size by triglyceride/HDL-cholesterol ratio in non-diabetic, healthy subjects without prominent hyperlipidemia. J. Atheroscler. Thromb. 10, 186–191 (2003). https://doi.org/10.5551/jat.10.186
A.L. Catapano, I. Graham, G. De Backer, O. Wiklund, M.J. Chapman, H. Drexel, A.W. Hoes, C.S. Jennings, U. Landmesser, T.R. Pedersen et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 253, 281–344 (2016). https://doi.org/10.1016/j.atherosclerosis.2016.08.018
M.J. Chapman, H.N. Ginsberg, P. Amarenco, F. Andreotti, J. Borén, A.L. Catapano, O.S. Descamps, E. Fisher, P.T. Kovanen, J.A. Kuivenhoven, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 32, 1345–1361 (2011)
D. Wadhwa, V.K. Mahajan, K.S. Mehta, P.S. Chauhan, R.S. Yadav, S. Bhushan, V. Sharma, A. Sharma, A. Sharma, Malondialdehyde, lipoprotein-a, lipoprotein ratios, comprehensive lipid tetrad index and atherogenic index as surrogate markers for cardiovascular disease in patients with psoriasis: a case-control study. Arch. Dermatol. Res 311, 287–297 (2019)
F.M. Sacks, M.P. Hermans, P. Fioretto, P. Valensi, T. Davis, E. Horton, C. Wanner, K. Al-Rubeaan, R. Aronson, I. Barzon et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation 129, 999–1008 (2014). https://doi.org/10.1161/circulationaha.113.002529
G.T. Russo, S. De Cosmo, F. Viazzi, A. Pacilli, A. Ceriello, S. Genovese, P. Guida, C. Giorda, D. Cucinotta, R. Pontremoli, P. Fioretto, Plasma triglycerides and HDL-C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes: the AMD Annals Initiative. Diabetes Care 39, 2278–2287 (2016). https://doi.org/10.2337/dc16-1246
C.R. Harper, T.A. Jacobson, Managing dyslipidemia in chronic kidney disease. J. Am. Coll. Cardiol. 51, 2375–2384 (2008). https://doi.org/10.1016/j.jacc.2008.03.025
Acknowledgements
All authors appreciate the efforts of all participants who contributed to sample measurements and data collection.
Funding
This work was supported by National Natural Science Foundation of China [grant number: 82073648 and 31672375].
Author information
Authors and Affiliations
Contributions
Conception and design of the study: Q.Z., J.D., Y.M., Y.H. Collection and assembly of data: Q.Z., Y.Z., X.S., J.D., Y.M. Data analysis and interpretation: J.W., M.W., Q.Z., Y. Zu., Y. Zh., X.S. Manuscript writing: Q.Z., J.D. Literature search: Y.Z. Editing: H.G. Review of this manuscript: Y.M., Y.H. Q.Z. and J.D. contributed equally to this study and share first authorship. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Ruijin Hospital of Shanghai Jiaotong University and informed consent was taken from all individual participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhai, Q., Dou, J., Wen, J. et al. Association between changes in lipid indexes and early progression of kidney dysfunction in participants with normal estimated glomerular filtration rate: a prospective cohort study. Endocrine 76, 312–323 (2022). https://doi.org/10.1007/s12020-022-03012-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03012-z