Abstract
1,25-Dihydroxyvitamin D (1,25(OH)2D3), the active form of vitamin D, modulates both innate and adaptive immune responses. Emerging epidemiological data has also demonstrated disease-modifying and immunomodulatory effects of vitamin D in a wide range of human autoimmune diseases, including rheumatoid arthritis (RA). To evaluate in vitro effects of 1,25(OH) 2D3 in primary cultures of peripheral blood monocyte-derived macrophages of RA patients, monocyte/macrophages, isolated from peripheral blood mononuclear cells of RA patients and healthy subjects by exploiting their ability to adhere to plastic, were treated with increasing concentrations of 1,25(OH)2D3 for 48 h. TNF-α, IL-1 α, IL-1β, IL-6 and RANKL production was determined by ELISA and nitric oxide (NO) release using the Griess method. Immunocytochemistry analysis was also performed to evaluate alterations in transmembrane TNF-α expression after 1,25(OH) 2D3 treatment. A significant dose-dependent decrease in TNF-α and RANKL production by cultured RA macrophages after 1,25(OH)2D3 treatment was found, whereas a significant reduction in normal cells was observed only at higher concentrations. IL-1 α, IL-1β and IL-6 levels were reduced by 1,25(OH) 2D3 at higher concentrations in all cell populations. TNF-α immunostaining was less intense in treated cells compared with untreated. 1,25(OH) 2D3 significantly reduced NO levels regardless of the concentration used. Vitamin D downregulated proinflammatory mediators in monocyte-derived macrophages, and RA cells appeared more sensitive than normal cells. These effects further provide a rationale for the therapeutic value of vitamin D supplementation in the treatment for RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By binding its nuclear receptor (VDR), the steroid hormone 1,25-dihydroxyvitamin D [1,25(OH)2D3], the active form of vitamin D, regulates the expression of target genes that encode proteins involved in calcium homoeostasis and bone metabolism.
Besides this classical role, it has been demonstrated that vitamin D is able to maintain tolerance and promote protective immunity. Almost all immune cells, both those of the innate response and those of the adaptive response, express receptors for active vitamin D (VDR) and are also capable of synthesizing the active vitamin D metabolite [1]. Vitamin D affects innate immune responses by enhancing the development of monocytes into macrophages and by influencing their chemotaxis and cytokine expression; at the same time, vitamin D suppresses immunoglobulin production and B-cell proliferation and differentiation and directly affects T-cell response by inhibiting the release of Th1 and Th17 cytokines and by inducing Th2 cytokine production. Additionally, epidemiological and clinical evidence suggested a significant association between vitamin D deficiency and an increased incidence of autoimmune diseases, including rheumatoid arthritis (RA), as well as studies in RA animal models, which showed the effectiveness of vitamin D treatment in the suppression of disease development and disease severity [1].
Rheumatoid arthritis is an autoimmune disease characterized by chronic inflammation of synovial joints and destruction of cartilage and bone, as well as by systemic extra-articular inflammation [2]. Although the cause of RA is still unknown, macrophages are likely to contribute in several ways to the pathogenesis of RA [3]. First, their numbers increase massively in RA synovial membrane [4], and they show clear signs of activation and orchestrate the development and perpetuation of RA chronic inflammation by releasing proinflammatory cytokines and reactive oxygen species. Second, RA macrophages directly contribute to the degradation of articular cartilage and subchondral bone [5], [6]. Furthermore, the activation of RA monocyte–macrophages is not locally restricted at the synovial level, but involves also those of the peripheral circulation [7], [8]. Thus, even if macrophages are not recognized as initiators of the pathogenic cascade in RA, their role as amplifiers of local and systemic inflammation is well accepted, with a direct contribution to joint degradation. Most effective drugs for the treatment for RA act to decrease the production of cytokines produced primarily by macrophages [9].
The aim of this study is to evaluate vitamin D responsiveness by primary cultures of human monocyte-derived macrophages, isolated from whole blood of patients with RA, in terms of proinflammatory cytokines (TNF-alpha, IL-1α, IL-1β, IL-6), osteoclastogenic cytokine (RANKL) and nitric oxide (NO) production.
Patients and methods
Patients
Whole blood was obtained from eleven healthy donors (2 men, 9 women) aged 48.3 ± 6.2 years (mean ± SD, range 41–61) and 15 untreated patients (5 men, 10 women) aged 55.2 ± 7.32 years (mean ± SD, range 42–65), with RA previously diagnosed, according to the classification criteria of the American College of Rheumatology [10]. The enrolled patients had suffered from RA for <1 year and had moderate disease activity (3.2 < DAS 28 < 5.1). Donors gave their informed consent, and the study was approved by the Institutional Ethics Committee.
Monocytes isolation and macrophages differentiation
Human macrophages were obtained from monocytes isolated from heparinated whole blood samples collected by venipuncture, by exploiting their ability to adhere to plastic. Peripheral blood mononuclear cells (PBMC), consisting of lymphocytes and monocytes, were separated from erythrocytes by density centrifugation on a Ficoll (PAA; Austria) gradient. After washing, human monocytes were purified from PBMC by positive selection with anti-CD14 magnetic beads, as recommended by the manufacturer (Miltenyi Biotec), and were resuspended in culture media (2 × 106 cells/well) consisting of RPMI 1640 (Sigma) supplemented with antibiotics (penicillin 100 IU/ml and streptomycin 100 mg/ml; PAA; Austria) and with 10 % foetal calf serum (FCS; PAA; Austria) and incubated at 37 °C in a water-saturated atmosphere with 5 % CO2.
After 24 h, the media and non-adherent cells, consisting mainly of lymphocytes, were removed and adherent cells were incubated with complete media (RPMI, 10 %FCS, penicillin and streptomycin) for 14 days. Media were replaced every 2 days.
Cells were >95 % CD14+, as determined by FACS analysis (data not shown) prior to culture.
Macrophages stimulation
On day 15, the media were removed, and cells were washed with phosphate-buffered saline (PBS; PAA; Austria) and then exposed to 1 μg/mL of lipopolysaccharide (LPS; Sigma, St. Louis, MO, USA) for 6 h. Cells were then treated with increasing concentrations (10−10–10−7 M) of 1,25(OH)2D3 (vitamin D3, Roche) for 48 h. Treatment conditions previously used for RA PBMC were adopted [11].
At the end of the vitamin D3 exposure, the cells were used for different test assays. All experiments were performed in triplicate for each sample.
Untreated cells served as control cultures.
Vitamin D receptor m-RNA expression and protein levels
Total RNA was isolated from untreated and treated cells using the RNeasy mini-RNA isolation Kit (Qiagen, Hilden), according to the manufacturer’s recommendations for cultured cells. To generate a cDNA template for the following real-time PCR, 1 μg of total RNA was reverse transcripted with the Transcriptor First Strand cDNA Synthesis Kit (Roche, Mannheim). Real-time PCR (qPCR) was carried out with the Applied Biosystems 7300 Real-Time PCR System. A total of 20-μL reactions were prepared with the TaqMan Universal PCR Master Mix, VDR TaqMan Gene Expression Assay (Hs00172113_m1; Applied Biosystems, Foster City, CA, USA), cDNA template (1 μl) and RNase-free water. The Ct (cycle threshold fluorescence values) was automatically given by SDS 2.1 software (Applied Biosystems), and the relative expression levels of VEGF mRNA were calculated using the comparative ΔΔCt method [12] after normalization with the endogenous gene β-actin (Hs99999903_m1; Applied Biosystems, Foster City, CA, USA). The relative quantitative value was expressed as 2−ΔΔCt.
Total VDR protein levels in cell lysates were measured by the specific enzyme-linked immunosorbent assay (Cusabio; China). The VDR concentrations were calculated over the standard curve, and the results were shown as pg/mg proteins.
TNF-α, IL-1α, IL-1β, IL-6 release measurement
After vitamin D3 exposure, the cell culture supernatants were collected and stored at −80 °C until further use. Levels of TNF-α (Thermo Scientific), IL-1α, IL-1β, IL-6 (R&D Systems, Minneapolis, MN) and RANKL (Biovendor) were determined using the quantitative sandwich enzyme immunoassay technique (ELISA), with commercial kits, according to the instructions of the manufacturers.
The sample cytokine concentrations were obtained by interpolation of absorbance values from the standard curve, prepared for each analysed cytokine, and were normalized per mg/ml intracellular proteins. The protein content of each well was determined by the Bradford method (Bio-Rad protein assay, Bio-Rad Laboratories, Richmond, CA, USA).
TNF-α levels: inhibition assay
For the inhibition experiments, the cells were preincubated for 30 min, at 37 °C, in the presence of monoclonal antibody (diluted to 1:100; Millipore), which detects the vitamin D receptor, and were then treated with vitamin D3, as previously described. The specificity of the vitamin D effect was determined by a negative control, replacing the specific primary antibody with non-immune immunoglobulin of the same isotype (R&D Systems, Minneapolis, MN, USA).
After the vitamin D3 exposure, the levels of TNF-α in culture medium were detected by ELISA (Thermo Scientific), with the commercial kit according to the instructions of the manufacturers. The results were expressed as pg/mL and normalized per mg/ml intracellular proteins.
TNF-α expression: immunocytochemistry
For immunocytochemical staining of TNF-α, cells were seeded onto LabTek Chamber slides (Nunc, Wiesbaden, Germany), treated with increased concentration of vitamin D for 48 h and then fixed with 4 % paraformaldehyde at 4 °C for 20 min. Blocking of endogenous peroxidase activity and non-specific binding was achieved by incubation with Perox Abolish (BioCare, USA) and albumin serum bovine 0.1 % (Sigma, St. Louis, MO, USA), respectively. Cells were then incubated with the specific primary antibody, monoclonal anti-human TNF-α/TNFSF1A (R&D Systems, Minneapolis, MN, USA) for 45 min. Immunostaining was performed using a biotinylated secondary antibody and followed by incubation with streptavidin–horseradish peroxidase (Goat-on-Rodent HRP-Polymer, Biocare, USA). Adding diaminobenzidine as a substrate developed the peroxidase reaction.
Cell nuclei were counterstained with haematoxylin.
Nitric oxide measurement
Nitric oxide (NO) levels in the culture supernatants of treated macrophages were detected by the Griess colorimetric test. A total of 150 μl of Griess reagent (Sigma) was added to the equal volume of the sample in 96-well plates, and after 15 min, the absorbance was read at 540 nm and compared with the absorbance curves of serial dilutions of sodium nitrate in complete culture medium.
Statistical analysis
Data were obtained from experiments carried out in triplicate and are expressed as the mean ± standard deviations (SD). The paired t test and one-way randomised ANOVA were applied to evaluate the differences between treatment groups, and the level of significance was set at p < 0.05.
Results
Effect of vitamin D on m-RNA VDR expression and protein levels
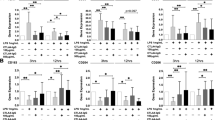
According to previous studies [1], [3], human macrophages express and produce VDR both in normal and in pathological conditions. There was no statistically significant difference between normal and RA cells. The mRNA expression and similarly VDR protein levels were significantly greater in treated cells compared with untreated cells only when exposed to higher concentrations of vitamin D (10−8 and 10−7) (Fig. 1).
Effects of 1,25(OH)2D3 on VDR m-RNA expression (a) and protein production (b) in monocytes/macrophages derived from whole blood of RA patients and healthy controls. VDR m-RNA expression and protein levels were determined by the Qualitative Real-Time PCR and the ELISA, respectively. The data represent the mean of results obtained from cultures of treated macrophages from 15 RA patients and 11 healthy subjects. The asterisks and section sign, respectively, indicate p value < 0.05 and 0.01, with respect to untreated cells
Effect of vitamin D on proinflammatory cytokine production
TNF-α levels were detected in supernatants of normal and pathological cells after treatment with vitamin D and compared to those of untreated cells (controls). First of all, RA macrophages produced significantly more TNF-α than macrophages from healthy volunteers (p < 0.01), and vitamin D reduced TNF-α levels in all cell populations. RA monocyte-derived macrophages treated with vitamin D showed a significantly lower production of TNF-α compared with untreated cells. Vitamin D reduced TNF-α levels regardless of the concentration used and in a dose-dependent manner; also at lower levels of concentration, the drug induced a dramatic decrease in TNF-α levels (p < 0.01). Unlike RA cells, TNF-α levels were significantly reduced in normal cells only when treated with higher concentrations of vitamin D (p < 0.05); lower concentrations of vitamin D (10−9 and 10−10 M) produced no appreciable effects (Fig. 2a). Preincubation of cells with a monoclonal antibody that detects the vitamin D receptor abolished 1,25(OH) 2D3-mediated changes in TNF-α levels. Figure 3 shows TNF-α levels in normal and RA macrophage cultures treated with variable vitamin D concentrations, in the presence of a non-specific antibody and an anti-vitamin D receptor antibody.
Effects of 1,25(OH)2D3 on proinflammatory cytokines TNF-α (a), IL-1 α (b), IL-1ß (c) and IL-6 (d) production in monocytes/macrophages derived from whole blood of RA patients and healthy controls. Culture supernatants were collected 48 h after 1,25(OH)2D3 stimulation, and cytokines concentrations were determined by the ELISA. The data represent the mean of assay results from cultures of treated macrophages from 15 RA patients and 11 healthy subjects. The error bars represent the standard deviation. The asterisks and section sign, respectively, indicate p value < 0.05 and 0.01, with respect to untreated cells. The double asterisks indicate p value < 0.05 with respect to healthy controls
Effects of 1,25(OH)2D3 on TNF-α cytokine production in monocytes/macrophages derived from whole blood of RA patients and healthy controls, in the presence of a monoclonal antibody that detects the vitamin D receptor (a) and non-immune immunoglobulin of the same isotype (b). The data represent the mean of enzyme-linked immunosorbent assay results from cultures of treated macrophages from 15 RA patients and 11 healthy subjects. The error bars represent the standard deviation. The asterisks and section sign, respectively, indicate p value < 0.05 and 0.01, with respect to untreated cells. The double asterisks indicate p value < 0.05 with respect to healthy controls
The IL-1α, IL-1β and IL-6 synthesis was significantly reduced only at higher concentrations of vitamin D. In all cell populations, no significant differences were observed in cells treated with vitamin D 10−10 and 10−9 M, when compared to untreated cells. Interestingly, RA and normal cells produced similar levels of IL-1α, IL-1β and IL-6 when treated with a higher concentration of vitamin D (10-7 M). Figure 2b–d) shows IL-1α, IL-1β and IL-6 levels in RA and normal monocyte-derived macrophages treated with variable vitamin D concentrations.
Effect of vitamin D on TNF-α expression
The effect of vitamin D on the expression of TNF-α was evaluated by immunocytochemistry analysis. Diffuse immunostaining for TNF-α was present at the membrane level of the majority of untreated cells. After vitamin D treatment for 48 h, the intensity of this staining seemed to be reduced and this effect appeared to be similar in normal and RA cells. Figure 4 shows the effect of different concentrations of vitamin D on TNF-α expression in monocyte-derived macrophages from healthy and RA patients.
Effects of 1,25(OH)2D3 on TNF-α expression in monocyte-derived macrophages obtained from whole blood of RA patients and healthy controls (magnification ×40). TNF-α expression in RA monocyte-derived macrophages in basal conditions and after treatment with increasing concentrations of 1,25(OH)2D3 has been detected by immunocytochemistry. 1,25(OH)2D3 treatment induced a decrease in TNF-α expression at all levels of concentration, in all cell populations
Effect of vitamin D on pro-osteoclastogenic cytokine production
RA macrophages produced significantly more RANKL than control cells. Vitamin D-treated RA macrophages showed a significant reduction in RANKL production when compared with untreated cells. The maximum effect was observed when cells were treated with the highest concentrations of vitamin D (10−8 and 10−7 M) suggesting a dose-dependent effect (p < 0.01). The production of RANKL by control cells was significantly reduced by vitamin D only at higher concentrations (10−8 and 10−7). Figure 5 shows the effect of different concentrations of vitamin D on RANKL synthesis in monocyte-derived macrophages from healthy and RA patients.
Effects of 1,25(OH)2D3 on RANKL production in monocytes/macrophages derived from whole blood of RA patients and healthy controls. RANKL levels in cell culture medium after treatment with 1,25(OH)2D3 concentrations ranging from 10−10 to 10−7 M for 48 h have been detected by the ELISA assay. The data represent the mean of enzyme-linked immunosorbent assay results from cultures of treated macrophages from 15 RA patients and 11 healthy subjects. The error bars represent the standard deviation. The asterisks and section sign, respectively, indicate p value < 0.05 and 0.01, with respect to untreated cells. The double asterisks indicate p value < 0.01 with respect to healthy controls
Effect of vitamin D on nitric oxide production
In the culture supernatants, NO synthesis was determined by the Griess reaction. As shown in Fig. 6, treatment of normal and RA macrophage cultures with vitamin D resulted in downregulation of NO production. RA vitamin D-treated macrophages showed a significant reduction in NO production when compared with untreated cells, at all levels of concentrations used. Also at a lower level of concentration, the drug induced a dramatic decrease in NO synthesis in RA cells (p < 0.01). However, normal cells showed reduced NO production only when treated with higher concentrations of vitamin D.
Effects of 1,25(OH)2D3 on nitric oxide (NO) production in monocytes/macrophages derived from whole blood of RA patients and healthy controls. NO levels were detected by the Griess method, after treatment with 1,25(OH)2D3 ranging from 10−10 to 10−7 M for 48 h. The data represent the mean of results obtained from cultures of treated macrophages from 15 RA patients and 11 healthy subjects. The error bars represent the standard deviation. The asterisks and section sign, respectively, indicate p value < 0.05 and 0.01, with respect to untreated cells. The double asterisks indicate p value < 0.01 with respect to healthy controls
Conclusions
Although first observations linking vitamin D with the immune system were reported in the early 1980s, only the emerging epidemiological data of the last few years, which have evidenced the increasing global prevalence of vitamin D insufficiency and the correlation between vitamin D status and the incidence of autoimmune diseases, have driven investigators to once again turn their attention to the immunological action of vitamin D. The first autoimmune disorder to be investigated was multiple sclerosis [13], since then an anti-inflammatory and immunomodulatory role of vitamin D has been demonstrated via various immunological mechanisms in a wide range of autoimmune human diseases, including diabetes mellitus, inflammatory bowel disease, systemic lupus erythematosus and RA [1]. Significantly lower 25(OH)D serum levels were observed in RA patients from North versus South Europe with a circannual rhythm in winter and summer time, and a significant negative correlation has been documented between 25(OH)D values and RA severity [14]. A 3-month open-label trial on 19 RA patients showed a positive effect of the alphacalcidol, a vitamin D analogue, on disease activity in 89 % of patients [15]. Similarly, disease-modifying and immunomodulatory effects of 1,25-dihydroxycholecalciferol and/or its analogues have been revealed in arthritis mouse models [16].
To date, the pathophysiological role of vitamin D in RA remains to be clarified. Pioneering works have focused on the influence of vitamin D in RA synovial fibroblasts and cells of adaptive immunity, but much less is known concerning the effects of vitamin D in innate immunity cells. In this study, we have evaluated the impact of 1,25(OH)2D3 in peripheral blood monocyte-derived macrophages isolated from RA patients. Treatment of RA macrophages with increasing concentrations of 1,25(OH)2D3, ranging from 10−10 to 10−7 M induced a significant dose-dependent decrease in protein production for TNF-α-soluble form, a well-known key molecule in early inflammatory responses, which acts at sites remote from the TNF-α producing cells. Preincubation of cells with a monoclonal antibody that detects the vitamin D receptor abolished 1,25(OH) 2D3-mediated changes in TNF-α levels. Interestingly, we observed a significant reduction in normal cells only after treatment with higher concentrations. TNF-α is a pleiotropic cytokine, which coordinates the inflammatory response through the induction of other cytokines such as IL-1 and IL-6, and by the recruitment of immune and inflammatory cells and up-regulation of adhesion molecules. TNF-α also represents the major therapeutic target for RA: anti-TNFα drugs, which consist of TNFα-neutralizing antibodies and fusion proteins, have been demonstrated to ameliorate clinical manifestations, reduce joint damage and radiographic progression, and induce remissions [17], [18]. Evidence exists that not only soluble TNF-α, but also its precursor form, transmembrane TNF-α, binds to TNF receptors and mediates proinflammatory and proarthritic effects [19]. Transmembrane TNF-α has been shown to be sufficient to induce arthritis with synovial hyperplasia and inflammation in transgenic mice only expressing membrane-bound TNF-α [20], [21]. The immunocytochemistry analysis showed the diffuse immunostaining for TNF-α at the membrane level of the majority of untreated cells and the reduced intensity of this staining after 1,25(OH)2D3 treatment in all cell populations. Thus, the 1,25(OH)2D3 appears to be able to impair the TNF-α functions in macrophages by reducing the transmembrane form, which acts in a cell-to-cell contact fashion, as well as the soluble form, which acts at sites remote from the TNF-α-producing cells. It is interesting that RA macrophages were more sensitive to vitamin D treatment than normal cells, even if no change in phenotype during vitamin D treatment was observed, as demonstrated by the analysis of VDR m-RNA expression and protein levels.
Unlike TNF-α, synthesis of IL-1α, IL-1β and IL-6 was significantly downregulated by 1,25(OH)2D3 treatment only at higher concentrations of 10−8 and 10−7 M in all cell populations. This different modulation could depend on the fact that interleukins synthesis is regulated by a variety of factors that macrophages produce themselves, such as the IL-15, macrophage migration inhibitory factor, nitric oxide, TGF-β [22–25]. Additionally, the different influence of vitamin D on cytokines production by monocyte–macrophages could be related to the complex network of pathways that regulate the cytokines gene expression and to the intracellular signalling pathways engaged by vitamin D. It has been recently demonstrated that vitamin D mediates anti-inflammatory effects in activated monocyte-macrophages by regulating the mitogen-activated protein kinase 1 (MAPK-1), the critical regulator of cytokines production, including TNF-α, IL-1 and IL-6 [26]. Nevertheless, IL-6 production in activated macrophages, unlike TNF-α, is also positively regulated by two distinct Src homology domain 2-containing tyrosine phosphatase-1 (SHP-1) [27], [28].
The dose-dependent inhibition of all tested cytokines in macrophages activated by stimulation with lipopolysaccharide has previously been demonstrated. Lipopolysaccharide is the major component of the outer membrane of Gram-negative bacteria, considered to be the most potent activator of the macrophage secretory response [26]. The reasons for these varying data could depend not only on differences in cell types, but also on differences in the duration of treatment and the concentration of drug used.
In addition to TNF-α, IL-1 and IL-6, another proinflammatory mediator produced by RA macrophages is NO, a well-known free radical implicated in cytotoxic tissue injury in a variety of diseases and whose overproduction has been linked to lymphocyte dysfunction characteristic of RA [29]. We showed that 1,25(OH)2D3 significantly reduced NO levels in culture medium at any level of concentration used. It has been demonstrated that NO by RA macrophages induces synovial cells to produce TNF-α [30], favouring inflammation and bone destruction, and NO production is in turn stimulated by TNF-α [31]. Thus, the decrease in NO production observed in treated cells could also be induced by the decrease in TNF-α.
Parallel with the reduced synthesis of proinflammatory cytokines, a strong inhibition was seen in RANKL production by RA macrophages. RANKL, a member of the TNF superfamily, exists as a transmembrane cell-bound and as a soluble form and is a component of the trimolecular control factor complex (OPG/RANK/RANKL). RANKL binds to the RANK receptor on the surface of osteoclast precursors and mature osteoclasts, resulting in differentiation and activation of osteoclasts; on the contrary, the binding to the decoy receptor osteoprotegerin (OPG) inhibits osteoclastic bone resorption [32]. RANKL expression has been documented at the sites of active RA bone erosions [33] as well as in multinucleated cells expressing osteoclast markers [34], demonstrating that the bone erosion, the hallmark of RA, is the result of osteoclastic bone resorption at the sites of synovitis. Additionally, an arthritis RANKL-deficient mouse, obtained by using a transfer of arthritogenic serum, developed inflammation similar to that seen in the control mice, but the dramatically reduced degree of bone erosion [35]. Similarly, RANKL blockade in adjuvant-induced arthritis, TNF-mediated arthritis and collagen-induced arthritis experimental models, even if did not inhibit inflammation, preserved from bone erosions [36–38]. We found that RANKL levels in the medium of RA-treated cells decreased at any level of concentrations used, when compared with those of untreated cells. Interestingly, vitamin D treatment reduced RANKL production in healthy and RA macrophages with an IC50 of 172 and 58.71 nM, respectively, suggesting the more potent antiresorptive effect under pathological conditions. The possible explanation for the high vitamin D concentration that give the half-maximal response in RANKL production by normal macrophages might be related to the classical role of vitamin D on mineral homoeostasis and maintenance of skeletal architecture. In physiological conditions, in order to regulate calcium levels, vitamin D increases the efficiency of intestinal calcium absorption and optimizes the activity of bone cells, by regulating metabolic activity of osteoblasts and osteoclasts differentiation and resorption. A number of papers reported that vitamin D significantly increased RANKL levels in normal primary human [39] and murine osteoblasts [40] suggesting its osteoclastogenic potential, whereas under pathological conditions characterized by excessive bone resorption, its action was to suppress osteoclasts formation and activity [41].
Taking into account the ability of TNF-α to induce osteoclastogenesis in vitro [42], and to activate osteoclasts through a direct action independent of RANKL [43], we hypothesized that vitamin D could indirectly reduce RA osteoclastogenesis and consequently bone erosion, also by downregulating TNF-α synthesis. Of noteworthy, we observed the much more potent effect of vitamin D on inhibition of TNF-α: the IC50 values in normal and RA macrophages were 73.35 and 2 nM, respectively. In line with these data, it has been recently described the different impact of 1,25(OH)2D3 at various concentrations on the production of RANKL and the secretion of TNF-α in healthy and RA PBMCs stimulated with anti-CD3 and anti-CD-28 [44].
In conclusion, the preliminary data presented in this report, even if more complete and exhaustive studies are required, demonstrated for the first time the immunomodulatory activity of vitamin D under these experimental conditions and pointed out its inhibitor potential in peripheral RA macrophages mediators, including the proinflammatory cytokine network, NO and RANKL. Our data, together with those previously obtained in RA synovial cells and those obtained in preclinical studies, further support the interest for vitamin D and urge additional investigations to evaluate the possible use of this secosteroid as therapeutic intervention in RA. Nevertheless, taking into account the toxic effects of supra-physiological doses of vitamin D, it would be better to evaluate the use of structural analogues that exert similar immunoregulatory activity without causing hypercalcemia.
References
Maruotti N, Cantatore FP. (2010) Vitamin D and the immune system. J Rheumatol 37(3):491–495 [Epub 2010 Jan 15]
Choy EH (2006) Joint inflammation and cytokine inhibition in rheumatoid arthritis. Clin Exp Med 6:13–19
Maruotti N, Cantatore FP, Crivellato E et al (2007) Macrophages in rheumatoid arthritis. Histol Histopathol 22:581–586
Mulherin D, Fitzgerald O, Bresnihan B (1996) Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 39:115–124
Hummel KM, Petrow PK, Franz JK et al (1998) Cysteine proteinase cathepsin K mRNA is expressed in synovium of patients with rheumatoid arthritis and is detected at sites of synovial bone destruction. J Rheumatol 25:1887–1894
Hou WS, Li W, Keyszer G et al (2002) Comparison of cathepsins K and S expression within the rheumatoid and osteoarthritic synovium. Arthritis Rheum 46:663–674
Schulze-Koops H, Davis LS, Kavanaugh AF et al (1997) Elevated cytokine messenger RNA levels in the peripheral blood of patients with rheumatoid arthritis suggest different degrees of myeloid cell activation. Arthritis Rheum 40:639–647
Lioté F, Boval-Boizard B, Weill D et al (1996) Blood monocyte activation in rheumatoid arthritis: increased monocyte adhesiveness, integrin expression, and cytokine release. Clin Exp Immunol 106:13–19
Lavagno L, Gunella G, Bardelli C et al (2004) Anti-inflammatory drugs and tumor necrosis factor-alpha production from monocytes: role of transcription factor NF-kappa B and implication for rheumatoid arthritis therapy. Eur J Pharmacol 501:199–208
Aletaha D, Neogi T, Silman AJ et al (2010) Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
Rausch-Fan X, Leutmezer F, Willheim M et al (2002) Regulation of cytokine production in human peripheral blood mononuclear cells and allergen-specific th cell clones by 1alpha,25-dihydroxyvitamin D3. Int Arch Allergy Immunol 128:33–41
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Cantorna MT, Hayes CE, DeLuca HF (1996) 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA 93:7861–7864
Cutolo M, Otsa K, Laas K et al (2006) Circannual Vitamin D serum levels and disease activity in rheumatoid arthritis: northern versus Southern Europe. Clin Exp Rheumatol 24:702–704
Andjelkovic Z, Vojinovic J, Pejnovic N et al (1999) Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol 17:453–456
Cantorna MT, Hayes CE, DeLuca HF (1998) 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr 128:68–72
Caporali R, Sarzi-Puttini P, Atzeni F et al (2010) Switching TNF-alpha antagonists in rheumatoid arthritis: the experience of the LORHEN registry. Autoimmun Rev 9:465–469
Atzeni F, Sarzi-Puttini P, Gorla R, Marchesoni A, Caporali R (2011) Switching rheumatoid arthritis treatments: an update. Autoimmun Rev 10:397–403
Alsalameh S, Winter K, Al-Ward R, Wendler J, Kalden JR, Kinne RW (1999) Distribution of TNF-alpha, TNF-R55 and TNF-R75 in the rheumatoid synovial membrane: TNF receptors are localized preferentially in the lining layer; TNF-alpha is distributed mainly in the vicinity of TNF receptors in the deeper layers. Scand J Immunol 49:278–285
Georgopoulos S, Plows D, Kollias G (1996) Transmembrane TNF is sufficient to induce localized tissue toxicity and chronic inflammatory arthritis in transgenic mice. J Inflamm 46:86–97
Alexopoulou L, Pasparakis M, Kollias G (1997) A murine transmembrane tumor necrosis factor (TNF) transgene induces arthritis by cooperative p55/p75 TNF receptor signaling. Eur J Immunol 27:2588–2592
Kinne RW, Bräuer R, Stuhlmüller B et al (2000) Macrophages in rheumatoid arthritis. Arthritis Res 2:189–202
McInnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7:429–442
Obermeier F, Gross V, Schölmerich J (1999) Interleukin-1 production by mouse macrophages is regulated in a feedback fashion by nitric oxide. J Leukoc Biol 66:829–836
Baugh JA, Donnelly SC (2003) Macrophage migration inhibitory factor: a neuroendocrine modulator of chronic inflammation. J Endocrinol 179:15–23
Zhang Y, Leung DY, Richers BN et al (2012) Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188:2127–2135
Rego D, Kumar A, Nilchi L, Wright K, Huang S, Kozlowski M (2011) IL-6 production is positively regulated by two distinct Src homology domain 2-containing tyrosine phosphatase-1 (SHP-1)-dependent CCAAT/enhancer-binding protein β and NF-κB pathways and an SHP-1-independent NF-κB pathway in lipopolysaccharide-stimulated bone marrow-derived macrophages. J Immunol 186:5443–5456
Geng Y, Zhang B, Lotz M (1993) Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J Immunol 151:6692–6700
Nagy G, Koncz A, Fernandez D et al (2007) Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radic Biol Med 42:1625–1631
Chae HJ, Park RK, Chung HT et al (1997) Nitric oxide is a regulator of bone remodelling. J Pharm Pharmacol 49:897–902
Miesel R, Murphy MP, Kröger H (1996) Enhanced mitochondrial radical production in patients with rheumatoid arthritis correlates with elevated levels of tumor necrosis factor alpha in plasma. Free Radic Res 25:161–169
Neve A, Corrado A, Cantatore FP (2011) Osteoblast physiology in normal and pathological conditions. Cell Tissue Res 343:289–302
Pettit AR, Walsh NC, Manning C et al (2006) RANKL protein is expressed at the pannus-bone interface at sites of articular bone erosion in rheumatoid arthritis. Rheumatology (Oxford) 45:1068–1076
Gravallese EM, Harada Y, Wang JT et al (1998) Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 152:943–951
Pettit AR, Ji H, von Stechow D et al (2001) TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol 159:1689–1699
Kong YY, Feige U, Sarosi I et al (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304–309
Redlich K, Hayer S, Maier A et al (2002) Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum 46:785–792
Lubberts E, van den Bersselaar L, Oppers-Walgreen B et al (2003) IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol 170:2655–2662
Corrado A, Neve A, Macchiarola A, Gaudio A, Marucci A, Cantatore FP (2013) RANKL/OPG ratio and DKK-1 expression in primary osteoblastic cultures from osteoarthritic and osteoporotic subjects. J Rheumatol 40:684–694
Thomas GP, Baker SU, Eisman JA, Gardiner EM (2001) Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol 170:451–460
Takasu H (2008) Anti-osteoclastogenic action of active vitamin D. Nutr Rev 66(10 Suppl 2):S113–S115
Maruotti N, Grano M, Colucci S et al (2011) Osteoclastogenesis and arthritis. Clin Exp Med 11:137–145
Fuller K, Murphy C, Firstein B, Fox SW, Chambers TJ (2002) TNFalpha potently activates osteoclasts, though a direct action independent and strongly synergistic with RANKL. Endocrinology 143:1108–1118
Luo J, Wen H, Guo H, Cai Q, Li S, Li X (2013) 1,25-Dihydroxyvitamin D3 inhibits the RANKL pathway and impacts on the production of pathway-associated cytokines in early rheumatoid arthritis. Biomed Res Int 2013:101805. doi:10.1155/2013/101805 [Epub 2013 Apr 22]
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neve, A., Corrado, A. & Cantatore, F.P. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med 14, 275–283 (2014). https://doi.org/10.1007/s10238-013-0249-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-013-0249-2