Abstract
Purpose
We evaluated a systematic review on the therapeutic efficacy of topical steroids in children with phimosis to provide data for the clinical options of pediatric phimosis.
Methods
We searched the related original studies on topical steroid therapy in pediatric phimosis before August 2014. Two reviewers independently performed the study selection, data extraction, risk of bias and reporting quality assessment with confirmation by cross-checking. The quality of eligible studies was appraised with the ‘Cochrane handbook.’ The meta-analysis was performed by REVMAN 5.2 software.
Results
Eleven studies were included with 1669 patients among which 1093 received topical steroids and 576 cases treated with placebo or only manual reduction. Significant difference of the treatment efficacy was detected among the three methods [OR 7.46, 95 % CI (4.42, 12.58), p < 0.00001]. In subgroup analysis, significant difference of the treatment efficacy was also detected whether with placebo or manual reduction only [respectively, OR 5.04, 95 % CI (3.19, 7.95), p < 0.00001; OR 16.28, 95 % CI (6.06, 43.69), p < 0.00001].
Conclusions
Compared to the placebo or manual reduction method, the topical steroid therapy is more effective in the treatment of phimosis in children. Although there is still controversy in the different type and dosage of steroid, this could be used against phimosis before circumcision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phimosis is defined as a condition with a complete or partial failure to retract the foreskin which may due to either a narrowness of the opening of the prepuce, or congenital adhesion between the glans and prepuce, or both [1]. By 3 years of age, 90 % of foreskins can be retracted, less than 1 % of males have phimosis by 17 years of age [2]. The persistent unretractable prepuce could limit penis’ development, cause metal stenosis and dysuresia. Phimosis also predisposes to inflammatory and melanodermia; in most penis carcinoma patients, phimosis or redundant prepuce was found.
Traditionally, the most common treatment of nonretractable foreskin has been circumcisied. The procedure although fundamentally cure phimosis, but as an invasive surgical treatment, there is a certain risk of complications, such as bleeding, infection, preputial edema and pain.
Although many recent studies tend to recommend topical steroid treatment of phimosis as first-line therapy, it is just at the very beginning stage with controversial. The effectiveness remains unproven. The present study evaluates the therapeutic efficacy of topical steroids in children with phimosis using a meta-analysis in order to provide reference evidence for clinical decision making.
Materials and methods
Study selection
A systematic literature research was performed using EMBASE (1974–August 2014), PubMed (1966–August 2014), Cochrane Controlled Trials Register databases, MEDLINE, Pascal, Blackwell Science, Google, Google scholar, SUDOC, international register of trials and congress abstract databases which studies on topical steroid therapy in pediatric phimosis. The following Mesh search headings were used: ‘topical steroid,’ ‘stretching method,’ ‘unretractable foreskin,’ ‘case–control study’ and ‘Pediatric phimosis.’ Searches were also performed using the terms ‘children phimosis’ and ‘phimosis in childhood,’ and citations scanned were reviewed.
Inclusion criteria
-
RCT literature or well-designed nonrandomized comparative study literatures;
-
The research cases should under adolescent and meet the diagnostic criteria of phimosis;
-
Intervention measure: the experimental group using topical steroids with or without joint reduction, the control group using a placebo and (or) joint joint manual reduction;
-
Primary outcome: all documents required to provide statistical results of clinical efficacy.
Exclusion criteria
-
Duplicate publication or data unavailable literature;
-
Experimental studies, case reports, lessons learned, discuss theory, review, summary and other types of research literature;
-
Noncontemporary comparison study of literature with larger time span.
Data extraction
The following information was extracted from each trial: date, design of study, average age, intervention, drugs and dosage, treatment time, follow-up period, assessment of therapeutic effects. Two independent researchers are responsible for the data extraction.
Quality evaluation
The meta-analysis was performed based on the recommendations from the Cochrane Collaboration and the Quality of Reporting of Meta-Analyses (QUORUM) guidelines. Jadad scale was performed to evaluate the quality of evidence of included studies [3]. Studies achieved a score of 3B or higher levels indicated to be a higher quality. The quality evaluation was conducted independently by two investigators under the criterion. Disagreements were resolved by a consensus; when this failed, a third author adjudicated.
Statistical analysis
Statistical analyses were performed with REVMAN 5.2.
A fixed effect model was adopted unless there was evidence of unexplained heterogeneity (I 2 ≥ 25 %) [4], in which case, a random effects model was used. Heterogeneity was assessed as the proportion of variation, and heterogeneity was assessed by the statistic, with values up to 25, 50 % and above 50 % indicating low, moderate and high levels of heterogeneity, respectively. Odds ratio (OR) was calculated for each study, both with 95 % confidence interval (CI). The p values for overall effect were calculated with the Z test, and significance was set at p < 0.05. Publication bias was assessed by funnel plot.
Results
Characteristics of the individual study
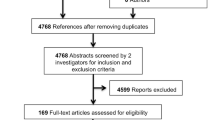
Primary searches retrieved 75 relevant literatures in English. After screening titles and abstracts, 61 articles were excluded for not met the inclusion criteria. Then, we found three articles were not RCT or well-designed comparative articles and were excluded after reading full text. Finally, we retrieved 11 [1, 5–14] comparative studies about topical steroid therapy in pediatric phimosis (Fig. 1).
These 11 studies enrolled 1671 patients, 8 of them are placebo controlled and 3 of them are manual reduction controlled. The characteristics of individual studies are shown (Table 1).
Quality of the individual study
Among the 11 studies, no high risk of bias was found. Quality of the individual study is shown (Table 2). However, heterogeneity was found in one study after assessing qualitative estimation of publication bias of the studies from the funnel plot.
Efficacy outcomes
The 11 studies represented 1669 participants (1093 in the steroid therapy group and 576 in the control group). We found significant heterogeneity in our primary analysis (I 2 = 61 %). A random model was selected. Besides, a subgroup analysis was carried out to detect the heterogeneity more detailedly.
The result showed a significantly statistical difference between the treatment group and the control group (random model), [OR 7.46, 95 % CI (4.42, 12.58), p < 0.00001] (Fig. 2). In subgroup analysis, we evaluated the clinical efficacy of steroid therapy compared to placebo and manual reduction, respectively (random model). We also chose the fix model to analysis a subgroup in which all Asian studies were excluded. All of the results showed statistical difference between the treatment group and the control group [respectively, OR 5.04, 95 % CI (3.19, 7.95), p < 0.00001 (Fig. 3); OR 16.28, 95 % CI (6.06, 43.69), p < 0.00001 (Fig. 4); OR 5.44, 95 % CI (3.69, 8.01), p < 0.00001 (Fig. 5)]. The subgroup analysis that only studies using betamethasone were included did not change the significant efficacy [OR 6.96, 95 % CI (4.91, 9.87), p < 0.00001 (Fig. 6)].
Discussion
Our study showed that topical steroid treatment of phimosis had advantages in clinical efficacy to other noninvasive treatment, regardless of whether compared to placebo or to manual reduction.
Heterogeneity was detected in our study (I 2 = 61 %). According to the subgroup analysis, it came from two factors mainly: patients’ race involved in the study and treatment duration of the study. After we excluded all Asian articles and articles in which the drug was not given twice a day for 4 weeks, the heterogeneity decreased to a fine level (I 2 = 18 %). But we do not have enough data for more detailed subgroup analysis, for example the race and treatment duration. We must point out that the different treatment duration that caused heterogeneity occurred mainly in the articles that were manual controlled. Therefore, the heterogeneity was extremely in a high level in the manual group (I 2 = 73 %).
Traditionally, circumcision is the most common treatment in many areas for pediatric phimosis. But it has been pointed out that between the age of 3 and 6 years—the ‘phallic period’ of childhood development—circumcision may affect the psychological status of the child and eventually cause psychological and behavioral disturbance. Castration anxiety, despite its controversial nature, may develop during the phallic period. Yilmaz et al. [15] evaluated patients using the Diagnostic and Statistical Manual-III-Revised (DSMIII-R) test with the aim of eliminating castration anxiety of circumcision in the phallic period. One hundred and forty-nine children with phimosis who required circumcision were included in the study. The average age of the children was 4.47 years. DSM-III-R test results showed a significant shift to anxiety in the circumcision group. Therefore, elective circumcision was generally avoided during this period. The conclusion showed necessity of topical steroid therapy before circumcision in children.
The total cure response rate (moderate to no phimosis in last follow-up) in patients involved in our study was 84.26 %. Zavras et al. [16] studied the treatment of phimosis with fluticasone used in 1185 boys, which showed a cure rate in 88.3 %. Reddy [17] has evaluated a long-term prospective study and reached a cure rate in 76.9 %. Both of these studies are used as evidences for EUA guideline on steroids therapy. Their results support our conclusion that topical steroid is an effective method for treating primary pediatric phimosis. We must point out that all of the studies showed a high response rate in the first month of treatment (90 %). So the difference of total cure rate, though it is unobvious, may be related to the difference of the management in the follow-up time. Zampieri et al. [11] studied about the efficacy (response rate) of topical steroids in treating phimosis at different age stages and found that most successful treatment was in patients aged between 4 and 8 years, suggesting the efficacy of an early beginning treatment.
Our study also showed significant difference between topical steroid and placebo or manual reduction. The advantages mainly come from the anti-inflammatory and immuno-suppressive effect of topical steroid. Topical steroids can make some interactions with some specific receptors, producing anti-inflammatory substance, and inhibit pro-inflammatory substance, including the reduction in type I and type III collagen synthesis in many cell types [18, 19]. Besides, Chu and Monsour found that steroids could cause thinning of the skin and improve the elasticity of the foreskin by decreasing the synthesis of hyaluronic acid, which had an anti-proliferative effect on the epidermis [13, 20] which might be the mechanism of topical steroid treatment in phimosis. However, some histological studies showed opponent results. Borges et al. collected the foreskin of 40 patients for 2 years, and these samples were divided into groups with or without previous topical corticosteroid. They carried out histochemical hematoxylin and eosin and Picrosirius analyses of the foreskin and found that fibrosis was higher in patients who used topical corticosteroid [21]. And Favorito et al. [22] observed an increase in the collagen type III of the patients submitted to topical treatment. In other words, the mechanism still remains unclear.
Yang et al. [23] studied the effects of highly potent and moderately potent topical steroids in treating pediatric phimosis. Both steroids were effective in all age groups. Although there was plenty of evidence regarding the effectiveness of topical steroid therapy, most studies focused on comparison between steroid therapy and placebo rather than on different steroid therapies. Thus, based on current data, what we could conclude was that steroid therapies were effective over placebo in the treatment of phimosis. However, more prospective studies that directly compared the efficacy among different steroid therapies are needed to draw a firm conclusion.
It is also important to compare steroid therapy with other noninvasive or minimally invasive alternatives in a systematic review. Unfortunately, although there are huge amount of articles on the steroid therapy of phimosis, only limited controlled studies are available, especially for the comparison between steroid therapy and other noninvasive or minimally invasive alternatives. We have compared steroid therapy with placebo and manual stretching in our study. Although limited controlled articles were available in minimally invasive alternatives, we could also make a description on such procedures. For example, a French group indicated preputioplasty with circumcision was very effective (0 % recurrence rate) in treatment of 90 children with a phimosis [24]. A similar study was also conducted by a British group which indicated 70 % patient results were good or very good [25]. To directly compare steroid therapy with preputioplasty with circumcision, more prospective case-controlled studies were needed.
Last but not least, the cost-effective analysis is important but difficult to conduct because the medical cost ranges a lot in different countries. Thus, only qualitative conclusion can be drawn in current database. After searching all the articles on steroid therapy again, we found that there were limited articles on cost-effective analysis. All of them concluded that steroid therapy was the most cost-effective strategy. For example, a cost-effective analysis conducted by a French group indicated that steroid therapy only cost 360 French francs ($352.38) per patient, while circumcisions costs 3330 ($3233.01) per patient. [26] Another cost analysis in USA drew the same conclusion: steroid therapy was the most cost-effective strategy which only cost between $758 and $800 per patient [27].
The major limitations were: there were not enough articles met the inclusion criteria so that we could not evaluate the efficacy of different steroids in pediatric phimosis or the different efficacy of the same steroid in different races to reduce the heterogeneity in our study. The ideal model for this study is to include articles with the same steroids, dosage, patient race and control group in one subgroup, which needs a huge amount of articles with high quality in the future.
Besides, we did not get enough data of cure rate in each month in treatment duration. In other words, this study could not show the efficacy of steroids in treating phimosis in each point-in-time during the whole treatment duration.
In summary, current evidence suggests that the topical steroid therapy is more effective in the treatment of phimosis in children compared to the placebo or manual reduction method. Compared with surgical treatment, topical steroid treatment has advantages of noninvasive, fewer complications, significant reduction in health spending and less impact in children’s psychological development. Although there is still controversy in the different type and dosage of steroid, topical steroid therapy can be used as conventional treatment methods before circumcision. And it is a more powerful evidence for the EUA guideline recommendation that steroids can be used as a first-line therapy for primary phimosis.
References
Letendre J, Barrieras D, Franc-Guimond J, Abdo A, Houle AM (2009) Topical triamcinolone for persistent phimosis. J Urol 182(4 Suppl):1759–1763. doi:10.1016/j.juro.2009.03.016
Oster J (1968) Further fate of the foreskin. Incidence of preputial adhesions, phimosis, and smegma among Danish schoolboys. Arch Dis Child 43(228):200–203
Phillips B (2004) GRADE: levels of evidence and grades of recommendation. Arch Dis Child 89:489
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 327(7414):557–560. doi:10.1136/bmj.327.7414.557
Golubovic Z, Milanovic D, Vukadinovic V, Rakic I, Perovic S (1996) The conservative treatment of phimosis in boys. Br J Urol 78(5):786–788
Lindhagen T (1996) Topical clobetasol propionate compared with placebo in the treatment of unretractable foreskin. Eur J Surg 162(12):969–972
Lund L, Wai KH, Mui LM, Yeung CK (2005) An 18-month follow-up study after randomized treatment of phimosis in boys with topical steroid versus placebo. Scand J Urol Nephrol 39(1):78–81. doi:10.1080/00365590410002519
Lee JW, Cho SJ, Park EA, Lee SJ (2006) Topical hydrocortisone and physiotherapy for nonretractile physiologic phimosis in infants. Pediatr Nephrol (Berlin, Germany) 21(8):1127–1130. doi:10.1007/s00467-006-0104-8
Pileggi Fde O, Vicente YA (2007) Phimotic ring topical corticoid cream (0.1% mometasone furoate) treatment in children. J Pediatr Surg 42(10):1749–1752. doi:10.1016/j.jpedsurg.2007.05.035
Esposito C, Centonze A, Alicchio F, Savanelli A, Settimi A (2008) Topical steroid application versus circumcision in pediatric patients with phimosis: a prospective randomized placebo controlled clinical trial. World J Urol 26(2):187–190. doi:10.1007/s00345-007-0231-2
Zampieri N, Corroppolo M, Zuin V, Bianchi S, Camoglio FS (2007) Phimosis and topical steroids: new clinical findings. Pediatr Surg Int 23(4):331–335. doi:10.1007/s00383-007-1878-x
Nascimento FJ, Pereira RF, Silva JL 2nd, Tavares A, Pompeo AC (2011) Topical betamethasone and hyaluronidase in the treatment of phimosis in boys: a double-blind, randomized, placebo-controlled trial. Int Braz J Urol 37(3):314–319
Chu CC, Chen KC, Diau GY (1999) Topical steroid treatment of phimosis in boys. J Urol 162(3 Pt 1):861–863
Zampieri N, Corroppolo M, Camoglio FS, Giacomello L, Ottolenghi A (2005) Phimosis: stretching methods with or without application of topical steroids? J Pediatr 147(5):705–706. doi:10.1016/j.jpeds.2005.07.017
Yilmaz E, Batislam E, Basar MM, Basar H (2003) Psychological trauma of circumcision in the phallic period could be avoided by using topical steroids. Int J Urol 10(12):651–656
Zavras N, Christianakis E, Mpourikas D, Ereikat K (2009) Conservative treatment of phimosis with fluticasone proprionate 0.05 %: a clinical study in 1185 boys. J Pediatr Urol 5(3):181–185. doi:10.1016/j.jpurol.2008.11.006
Reddy S, Jain V, Dubey M, Deshpande P, Singal AK (2012) Local steroid therapy as the first-line treatment for boys with symptomatic phimosis: a long-term prospective study. Acta Paediatr (Oslo, Norway : 1992) 101(3):e-130–e-133. doi:10.1111/j.1651-2227.2011.02534.x
Rao LS, Long WS, Kaneko T, Blumenberg M (2003) Regulation of transcription factor activity by extracellular signals in epidermal keratinocytes. Acta Dermatovenerol 12:3–14
Radoja N, Komine M, Jho SH, Blumenberg M, Tomic-Canic M (2000) Novel mechanism of steroid action in skin through glucocorticoid receptor monomers. Mol Cell Biol 20(12):4328–4339
Monsour MA, Rabinovitch HH, Dean GE (1999) Medical management of phimosis in children: our experience with topical steroids. J Urol 162(3 Pt 2):1162–1164
Sabino Borges LG, Perez-Boscollo AC, Rocha LP, Silva RC, Guimaraes CS, Castro EC, Correa RR (2012) Foreskin analysis of circumcised boys with and without previous topical corticosteroid. Fetal Pediatr Pathol 31(5):265–272. doi:10.3109/15513815.2012.659381
Favorito LA, Balassiano CM, Rosado JP, Cardoso LE, Costa WS, Sampaio FJ (2012) Structural analysis of the phimotic prepuce in patients with failed topical treatment compared with untreated phimosis. Int Braz J Urol 38(6):802–808
Yang SS, Tsai YC, Wu CC, Liu SP, Wang CC (2005) Highly potent and moderately potent topical steroids are effective in treating phimosis: a prospective randomized study. J Urol 173(4):1361–1363. doi:10.1097/01.ju.0000156556.11235.3f
Binet A, François-Fiquet C, Bouche-Pillon MA (2015) Review of clinical experience for a new preputioplasty technique as circumcision alternative. Ann Chir Plast Esthet. doi:10.1016/j.anplas.2015.01.003
Munro NP, Khan H, Shaikh NA, Appleyard I, Koenig P (2008) Y-V preputioplasty for adult phimosis: a review of 89 cases. Urolgoy 72(4):918–920
Berdeu D, Sauze L, Ha-Vinh P, Blum-Boisgard C (2001) Cost-effectiveness analysis of treatments for phimosis: a comparison of surgical and medicinal approaches and their economic effect. BJU Int 87(3):239–244
Van Howe RS (1998) Cost-effective treatment of phimosis. Pediatrics 102(4):43
Author contributions
JML, JY and YTC drafted the manuscript. YTC, SHC, CX and TD performed the study selection, data extraction, risk of bias and reporting quality assessment. YTC performed the statistical work. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Jiaming Liu, Jin Yang and Yuntian Chen have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Liu, J., Yang, J., Chen, Y. et al. Is steroids therapy effective in treating phimosis? A meta-analysis. Int Urol Nephrol 48, 335–342 (2016). https://doi.org/10.1007/s11255-015-1184-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1184-9