Abstract
Purpose
We tested whether short-term vitamin D supplementation improves insulin resistance in patients with kidney disease, a condition with little intrinsic vitamin D activity.
Methods
PubMed, EMBASE and CENTRAL were searched for relevant observational studies and randomized clinical trials (RCTs). Random-effects models were employed for meta-analysis, and effect sizes were summarized as standardized mean difference (SMD) with 95 % confidence intervals. Separate analyses were done for RCTs and non-randomized intervention studies (NRIS).
Results
Seventeen studies (5 RCTs and 12 NRIS) were included. The meta-analysis population (n = 131) was mostly middle aged (40–50 years), male and non-diabetic, and on hemodialysis. The duration (4–12 weeks) and type of supplementation varied between studies. Among RCTs, compared to placebo, vitamin D supplementation was associated with significant decrease in fasting glucose [SMD −1.13, (−2.11 to −0.11)] and PTH levels [SMD −1.50, (−2.95 to −0.04)] but no difference in fasting insulin levels [SMD 1.32, (−0.15 to 2.79)]. Among NRIS, there was only a significant decrease in PTH levels [SMD −1.68, (−2.55 to −0.82)] between pre- and post-vitamin D treatment levels.
Conclusions
Short-term (4–12 weeks) supplementation with vitamin D is associated with lower fasting glucose levels in ESRD with no change in fasting insulin levels. However, the findings from this study are limited by the studies that were used in the meta-analysis, which were mostly small, used multiple different vitamin D compounds and dosing regimens, and had large heterogeneity, and funnel plots showed that there was a dearth of studies with null or negative finding. Therefore, larger RCTs need to be performed to answer this important clinical question.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance is described as a reduced biological effect at a given level of serum insulin and consequently results in hyperinsulinemia to maintain glucose homeostasis [1]. Altered glucose metabolism and insulin resistance are recognized at all stages of chronic kidney disease (CKD) and end-stage renal disease (ESRD) [2, 3]. In ESRD, insulin resistance is an independent non-traditional risk factor for cardiovascular mortality and is associated with protein energy wasting and malnutrition [4]. While the exact mechanism remains unclear, a post-receptor defect in the insulin receptor signaling pathway in skeletal muscle is the likely primary abnormality. Other suggested contributors include adipose tissue dysregulation, inflammation, anemia, metabolic acidosis, uremic toxins and vitamin D deficiency [4]. Of these, vitamin D requires particular attention because of the kidney’s intricate role in vitamin D metabolism, the high prevalence of vitamin D deficiency in CKD, the availability of safe vitamin D and its analogs and the potential pleiotropic effects of vitamin D which may include CKD-related insulin resistance [5, 6].
Animal studies have demonstrated improvement in insulin resistance with administration of vitamin D with both increased insulin sensitivity and insulin secretion being affected [7, 8]. The presence of vitamin D receptors on pancreatic beta cells leading to increased intracellular free calcium and thereby insulin secretion, and immune modulation that prevents beta-cell apoptosis are proposed non-classical mechanisms by which vitamin D may improve insulin resistance [9, 10].
Observational studies and small randomized controlled trials (RCTs) have evaluated the link between vitamin D deficiency and insulin resistance in CKD. These studies have shown both an improvement in glucose metabolism with vitamin D supplementation in ESRD patients [11–13] and no improvement [14]. To clarify this uncertainty, we conducted a systematic review and meta-analysis to obtain a summary understanding and effect size of the impact of vitamin D supplementation (both nutritional and active) on glucose metabolism in dialysis patients. To our knowledge, this is the first meta-analysis of its kind.
Subjects, materials and methods
Our search strategy was developed with the help of a medical librarian and included a search of PubMed, EMBASE and The Cochrane Library CENTRAL Register of Controlled Trials through Aug 31, 2013. Limits were preset to studies conducted in adult humans (18 years and older) and manuscripts published in the English language. The search strategy for all databases was built on MeSH terms for “vitamin D” and “renal dialysis” and “insulin resistance” or “blood glucose,” with related keywords in the Title/Abstract added to the search.
Two authors (H. S. and V. P.) conducted the search and reviewed all abstracts independently. Manuscripts of potential relevance were retained for a review of the full text. Additional publications were identified from citations of manuscripts, review articles and personal reference lists. Only original manuscripts available in full text were included. Authors were contacted when full texts were needed. Disagreements regarding final inclusion of a study were resolved by consensus or by a third author (M. L. M.).
Data were abstracted in accordance with eligibility criteria set a priori. Studies were included if (1) any vitamin D analog or derivative was administered as an intervention, by any route and for any duration, (2) study participants receiving the intervention were on hemodialysis (HD) or peritoneal dialysis (PD), (3) primary or secondary outcomes involved the measurement of fasting blood glucose or fasting serum insulin as surrogate measures of insulin resistance at baseline and the end of study. We accepted the following study designs: prospective or retrospective, RCTs or non-randomized intervention studies (NRIS) or observational studies. Additional data extraction using standardized abstraction forms included age, sex, weight/BMI, diabetes mellitus status, type of vitamin D, route, dose and duration, type and average duration (months) of dialysis, serum vitamin D, PTH, calcium and phosphate levels as well as randomization, blinding procedures and loss to follow-up.
Statistical analyses
We analyzed the pooled sample of RCTs separately from that of NRIS [15]. Meta-analyses were performed to quantify the change in mean levels of fasting serum glucose, fasting serum insulin and PTH. Most of the NRIS were reported as single-group studies with pre- and post-intervention results. Pre-effect size and post-effect size estimations require knowledge of pre–post correlations (ρ 12) that were not reported in the primary studies. Therefore, we analyzed these paired comparisons in sensitivity analyses under assumptions of low (ρ 12 = 0.2), moderate (ρ 12 = 0.5) and high (ρ 12 = 0.8) correlations.
A priori random-effects models were employed, and standardized mean differences (SMDs) with 95 % confidence intervals (CI) were generated for continuous outcomes using the Dersimonian–Laird model. The SMD is the difference in means between the two groups divided by study-specific standard deviation [16, 19]. The SMD value should be interpreted as the number of standard deviations between the means being compared and is independent of measurement scale [16, 19]. A negative SMD indicates lower levels, whereas a positive SMD indicates higher levels of outcome. Cohen’s rule of thumb guides interpretation of magnitude of effect size, SMD 0.2: small, SMD 0.5: moderate, SMD > 0.8: large [17].
Heterogeneity across studies was assessed by the Cochran Q statistic and I 2 statistic of measured inconsistency (the percentage of total variance across studies attributable to real differences between studies than by chance). The magnitude of heterogeneity was categorized as I 2 = 25 %: low, I 2 = 50 %: moderate and I 2 = 75 %: high [18]. Heterogeneity was expected given the wide variation in study design. Strategies to address heterogeneity included use of random-effects modeling that assumes both within-study and between-study variance, and sensitivity analyses excluding 1–2 studies with outlying effect sizes [19]. Funnel plots of effect size against study-level standard error were constructed using the Begg–Mazumdar method to evaluate publication bias. Risk of bias in RCTs was assessed by the tool provided by Cochrane Back Review Group [20]. Statistical significance was set at two-sided p value of 0.05 for all analyses. Statistical analyses were performed with Comprehensive Meta-Analysis software version 2.
Results
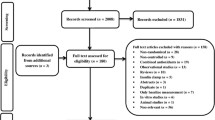
Figure 1 provides a summary of the search and manuscript retrieval for this review. The initial literature search yielded a total of 223 articles from PubMed and EMBASE; no new studies were identified from Cochrane CENTRAL. Of note, one paper suggested by personal reference was added to this review. This study was not retrieved by any database search [14]. The final systematic review was performed on 17 studies (Fig. 1) [11–14, 21–33].
Study methodology
Tables 1 and 2 provide a summary of the reviewed studies. Most of the studies included in this review were small. Of the 17 studies, four were RCTs [14, 23, 28, 31]. While Mak [13] did not report a randomization procedure, HD patients were divided into treatment and placebo groups, and therefore, the study was included as an RCT. The remaining 12 studies were NRIS that also reported a control group of healthy volunteers who served as comparison for demonstrating improvement from baseline values in the HD group after vitamin D treatment [11, 12, 21, 22, 24–27, 29, 30, 32, 33].
Intervention
Vitamin D formulations varied widely, with the majority of the older studies employing calcitriol (Tables 1, 2). The duration and dose of vitamin D were also variable; most studies evaluated vitamin D effects after 4–12 weeks, though this ranged widely from the shortest duration being 2 h after intravenous calcitriol [11, 12], to the longest duration of 24 weeks (6 months) [30]. In the NRIS, healthy controls did not receive any intervention. In the RCTs, control groups received dihydrotachysterol [13], cinacalcet [14] or placebo [23, 28, 31].
Demographic characteristics
Baseline patient characteristics were not well reported especially in the older NRIS (Table 1). Age diversity was notable, but the mean age typically ranged from 40 to 50 years across most studies. While the eligibility criterion only included adult participants, we included two 1992 studies by Mak that specified the age range as 16–22 years. Sex was reported in seven studies, and the majority were male (>50 %). Diabetic patients were included in three studies [14, 30, 32]. The mean BMI reported in four studies was between 20.6 and 34.6 kg/m2 [14, 27, 29, 33]. Body weight was reported as within 115–120 % of ideal weight for height [11, 12, 24, 25]. Ibrahim et al. [32] studied the impact of cholecalciferol on outcomes in HCV seropositive and seronegative HD patients; for this review, we only used data from HCV seronegative patients for generalizability. Hung et al. [14] studied a cohort of African–American HD patients; race was not reported in the other studies.
ESRD and renal dialyses
Ulutas et al. [33] was the only study done in PD patients. The mean duration of HD, if reported, ranged from 23 to 47 months. The etiology of ESRD was variable; dietary restrictions were variably reported and typically were for fluids, sodium, potassium and phosphate but not protein. Most studies excluded patients if they had a history of acute or chronic inflammatory conditions, malignancy, were scheduled for or received a renal transplant, had abnormally elevated serum phosphate and calcium levels or took medications affecting liver function or glucose metabolism (except studies that included diabetics). Participants in most studies were on phosphate binders (calcium carbonate or aluminum hydroxide).
Baseline secondary hyperparathyroidism and vitamin D status
The severity of secondary hyperparathyroidism varied across the studies, with baseline mean intact PTH (iPTH) levels ranging from mildly (<400 pg/ml) to severely elevated (>1,000 pg/ml) (Tables 3, 4). Exposure to medications to treat secondary hyperparathyroidism was variable and reported only in the Blair et al. [30] (cinacalcet) and Hung et al. [14] (paricalcitol, cinacalcet) studies. Participants in Ulutas et al. [33] study continued to take 1-α-calciferol for secondary hyperparathyroidism along with the intervention [33]. Other studies had protocols, where participants stopped taking vitamin D supplements 2–4 weeks prior to the start of the study [11–13, 26, 32], or were never on vitamin D supplementation [22, 31]. Of note, in the two Mak studies [11, 12], participants discontinued oral calcitriol only 3 days prior to study intervention. Baseline vitamin D levels were variably reported (Tables 1, 2).
Outcomes and meta-analyses
Short-term vitamin D supplementation was associated with decreased fasting serum glucose levels in three of five RCTs, whereas NRIS demonstrated non-significant decrements; fasting serum insulin outcomes were variable (Tables 3, 4). Fasting plasma glucose values were fairly normal in most studies, especially in the NRIS (Table 3, 4). Improved hemoglobin A1C (HbA1C %) values were also observed (Tables 3, 4) [21, 23, 31, 32]. There was greater evidence for the improvement of glucose-stimulated insulin secretion based on hyperglycemic clamp testing or glucose tolerance testing (Table 4) [11, 12, 23–26]. Similar results were obtained with testing for HOMA-IR, except in the Hung et al. [14] study (Tables 3, 4) [14, 31–33]. Changes in serum PTH and calcium levels were associated with changes in the primary outcome and also noted independently.
For the meta-analyses, we excluded the Blair et al. study to avoid bias from the large attrition between the pre- and post-treatment groups (Table 4) [30]. Meta-analyses of RCTs showed significantly decreased SMD of post-treatment fasting serum glucose levels [SMD −1.13, 95 % CI (−2.11 to −0.11); p value 0.03; heterogeneity: I 2 = 83.03], non-significant increase in fasting serum insulin values [SMD 1.32, 95 % CI (−0.15 to 2.79); p value 0.08; heterogeneity: I 2 = 87.47] and significant decrease in PTH levels [SMD −1.50, 95 % CI (−2.95 to −0.04); p value 0.04; heterogeneity: I 2 = 90.03] when compared to the placebo group (Table 3; Fig. 2a, b, c). Meta-analyses of pooled NRIS showed significant improvement only in serum PTH levels after vitamin D treatment: fasting serum glucose levels [SMD −0.22, 95 % CI (−0.51 to 0.07) p value 0.14; heterogeneity: I 2 = 57.80], fasting serum insulin levels [SMD 0.45, 95 % CI (−0.17 to 1.08) p value 0.16; heterogeneity: I 2 = 88.23] and serum PTH levels [SMD −1.68, 95 % CI (−2.55 to −0.82) p value <0.001; heterogeneity: I 2 = 88.52] (Table 4; Fig. 3a, b, c). Sensitivity analyses at different levels of correlation coefficient (0.2, 0.8) showed similar results (data not shown). In RCTs and NRIS that demonstrated improvement in glucose levels, baseline fasting glucose levels were elevated compared to studies that did not show significant change (Tables 3, 4).
Heterogeneity and sensitivity analyses
The Q values and I 2 values revealed significant heterogeneity as expected (Table 5). There were too few RCTs for sensitivity analyses. For NRIS, after removing three studies with outlying effect sizes [12, 26], with a very different study methodology [11, 12] or with additional exclusion of the trial of PD patients [33], heterogeneity was reduced significantly but the summary effect size for serum glucose levels did not change overall (SMD −0.18, 95 % CI (−0.40 to 0.04) p value 0.11, heterogeneity I 2 = 13.4); similar sensitivity analyses did not affect summary effect sizes for serum insulin levels (SMD 0.58, 95 % CI (−0.21 to 1.38) p value 0.15; heterogeneity I 2 = 89.00) or serum PTH levels (SMD −1.53, 95 % CI (−1.96 to −1.07) p value <0.001; heterogeneity I 2 = 90.08).
Risk of bias assessment
Most of the RCTs were single-blinded studies with insufficient reporting on randomization, allocation concealment and blinding. There was no selective reporting or loss to follow-up in most studies; however, methods to deal with attrition were not reported. The Blair et al. [30] study used as-treated analysis to deal with attrition. Only Hung et al. [14] had a low risk of bias based on the Cochrane Back Review Group tool [20]. Funnel plots demonstrated a deficiency of studies with null or negative results suggesting a high grade of publication bias.
Discussion
To our knowledge, this is the first systematic review and meta-analysis that evaluates the impact of short-term supplementation with vitamin D or its analogs on glucose metabolism in dialysis patients. A large meta-analysis of observational studies had demonstrated a significant reduction in mortality in CKD and ESRD patients who received calcitriol or synthetic vitamin D analogs over 3–5 years [34]. Prior to this, a meta-analysis of RCTs failed to demonstrate a protective effect of vitamin D for mortality; however, mortality was not the primary outcome of those primary trials [35]. We found reasonably strong evidence, largely driven by five RCTs, of short-term (up to 12 weeks) vitamin D supplementation associated improvement in glucose metabolism in dialysis patients. Based on Cohen’s rule of thumb, the meta-analysis of RCTs demonstrated a significantly large improvement in fasting glucose levels; this effect was not significant among NRIS. A significant decrement in serum PTH was seen in meta-analyses of both RCTs and NRIS. Long-term glycemic outcomes (HbA1C %) also showed an improvement with associated decreases in PTH, though these results could not be meta-analyzed.
Fasting insulin levels are an accepted marker for insulin resistance in normoglycemic subjects; the meta-analysis population was mostly non-diabetic [1]. Review of studies that tested glucose-responsive insulin sensitivity and insulin secretion revealed an increase in these measures and thereby improvement in insulin resistance after vitamin D supplementation (Table 4) [11, 12, 22, 24–26]. Marked variation in testing of HOMA-IR precluded an interpretable quantification of summary effect size.
There was a large degree of heterogeneity in the meta-analysis. However, meta-regression to explain these differences was not done due to risk of serious bias given few RCTs [16, 36]. Unexplained heterogeneity could be secondary to residual confounding and/or bias, especially in NRIS [15].
Paricalcitol, a vitamin D analog, was found to have a null impact in the Hung et al. [14] study. It also had a null effect on short-term glucose metabolism among non-diabetic, non-dialysis stage 3–4 CKD patients (deBoer et al., 8 weeks) and on left ventricular mass (PRIMO trial, 48 weeks) [37, 38]. Paricalcitol has been found to suppress 25(OH)D levels, raising the possibility that vitamin D analogs may function differently from compounds that raise 25(OH)D levels [37]. As non-classical effects of vitamin D are likely dependent on circulating levels of 25(OH)D and local levels of extra-renal 1-α-hydroxylase enzyme, further clinical trials are needed that study compounds which raise 25(OH)D levels [5].
Autier et al. [39] examined the association of vitamin D with various health outcomes in extensive meta-analyses of prospective cohort studies and RCTs but did not find compelling evidence of a beneficial effect of vitamin D supplementation on glucose metabolism outcomes. However, these trials involved non-ESRD patients who had higher baseline levels of vitamin D and physiological renal activity enabling vitamin D activation, a significant difference from patients in our studies. Moreover, an interesting observation in our meta-analysis was that vitamin D-associated improvement in fasting glucose levels was seen in participants with impaired glucose tolerance at baseline, a common feature of the ESRD population. Also, fasting insulin levels were notably lower or borderline in most of these HD study populations than would be expected for insulin resistance in the general population (variably defined to be >12–>17 mU/ml) [40]. This could be explained by the fact that insulin is removed by HD, again attesting to the special status of this population [41].
It remains uncertain whether correction of hyperparathyroidism is a mediator for vitamin D-associated changes in glucose metabolism or whether these changes in PTH levels are merely expected side effects of supplementation. In the study demonstrating protective effect of vitamin D supplementation on mortality in ESRD patients, meta-regression demonstrated greater risk reduction among those with higher baseline PTH levels [34]. There is evidence to suggest an independent association of PTH and insulin resistance [42]. It is beyond the scope of this meta-analysis to establish the mechanisms of the observed differences.
Our study has its limitations. Firstly, outcomes varied by definition and measurement, only a few studies measured outcomes of interest such as HOMA-IR [14, 31, 32]. Hyperglycemia and glucose intolerance only manifest at later stages of insulin resistance when it cannot be overcome by insulin secretion [4]. Hyperinsulinemic euglycemic clamp studies and intravenous glucose tolerance tests are cumbersome and were infrequently conducted, though they are the gold standard test for insulin resistance [11–14]. Secondly, the design and quality of studies varied extensively. Despite this, RCTs demonstrated a significant summary effect of vitamin D supplementation on fasting serum glucose levels. Thirdly, most of the studies had small sample sizes and were focused on short-term outcomes (4–12 weeks) that are not long enough to adequately study relevant clinical outcomes.
In conclusion, improved fasting glucose levels are observed after short-term vitamin D supplementation in dialysis patients with associated decreases in serum PTH levels. However, well-designed larger clinical trials are needed to focus on long-term clinical outcomes of insulin resistance and related cardiovascular outcomes in ESRD.
References
Singh B, Saxena A (2010) Surrogate markers of insulin resistance: a review. World J Diabetes 1(2):36–47
de Boer IH (2008) Vitamin D and glucose metabolism in chronic kidney disease. Curr Opin Nephrol Hypertens 17(6):566–572
Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, Ritz E (1998) Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int 53(5):1343–1347
Hung AM, Ikizler TA (2011) Factors determining insulin resistance in chronic hemodialysis patients. Contrib Nephrol 171:127–134
Jones G (2007) Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3). Semin Dial 20(4):316–324
Holick MF (2007) Vitamin D deficiency. New Engl J Med 357(3):266–281
Maestro B, Campion J, Davila N, Calle C (2000) Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 47(4):383–391
Norman AW, Frankel JB, Heldt AM, Grodsky GM (1980) Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 209(4458):823–825
Dusso AS, Brown AJ, Slatopolsky E (2005) Vitamin D. Am J Physiol Ren Physiol 289(1):F8–F28
Sergeev IN, Rhoten WB (1995) 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology 136(7):2852–2861
Mak RH (1992) Intravenous 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int 41(4):1049–1054
Mak RH (1992) Amelioration of hypertension and insulin resistance by 1,25-dihydroxycholecalciferol in hemodialysis patients. Pediatr Nephrol 6(4):345–348
Mak RH (1998) 1,25-Dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney Int 53(5):1353–1357
Hung AM, Sundell MB, Plotnikova NE, Bian A, Shintani A, Ellis CD, Siew ED, Ikizler TA (2013) A pilot study of active vitamin D administration and insulin resistance in African American patients undergoing chronic hemodialysis. J Ren Nutr 23(3):185–193
Reeves BC, Deeks JJ, Higgins JPT, Wells GA (2008) Chapter 13: including non-randomized studies. In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions. Version 5.0.1 [updated September 2008]. The cochrane collaboration. www.cochrane-handbook.org
Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA (2014) Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol 14:30. doi:10.2286/1471-2288-14-30
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Erlbaum, Hillsdale
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Deeks JJ, Higgins JPT, Altman DG (2008) Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (eds) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008]. The cochrane collaboration. www.cochrane-handbook.org
Furlan AD, Pennick V, Bombardier C, van Tulder M (2009) Editorial Board CBRG: 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 34(18):1929–1941
Lind L, Lithell H, Wengle B, Wrege U, Ljunghall S (1988) A pilot study of metabolic effects of intravenously given alpha-calcidol in patients with chronic renal failure. Scand J Urol Nephrol 22(3):219–222
Quesada JM, Martin-Malo A, Santiago J, Hervas F, Martinez ME, Castillo D, Barrio V, Aljama P (1990) Effect of calcitriol on insulin secretion in uraemia. Nephrol Dial Transpl 5(12):1013–1017
Turk S, Yeksan M, Tamer N, Gurbilek M, Erdogan Y, Erkul I (1992) Effect of 1,25 (OH)2D3 treatment on glucose intolerance in uraemia. Nephrol Dialysis Transpl 7(12):1207–1212
Lin SH, Lin YF, Lu KC, Diang LK, Chyr SH, Liao WK, Shieh SD (1994) Effects of intravenous calcitriol on lipid profiles and glucose tolerance in uraemic patients with secondary hyperparathyroidism. Clin Sci (Lond) 87(5):533–538
Lu KC, Shieh SD, Lin SH, Chyr SH, Lin YF, Diang LK, Li BL, Sheu WH, Ding YA (1994) Hyperparathyroidism, glucose tolerance and platelet intracellular free calcium in chronic renal failure. Q J Med 87(6):359–365
Kautzky-Willer A, Pacini G, Barnas U, Ludvik B, Streli C, Graf H, Prager R (1995) Intravenous calcitriol normalizes insulin sensitivity in uremic patients. Kidney Int 47(1):200–206
Gunal AI, Celiker H, Celebi H, Ustundag B, Gunal SY (1997) Intravenous alfacalcidol improves insulin resistance in hemodialysis patients. Clin Nephrol 48(2):109–113
Khajehdehi P, Taheri S (2003) Effect of oral calcitriol pulse therapy on the lipid, calcium, and glucose homeostasis of hemodialysis-patients: its safety in a combination with oral calcium carbonate. J Ren Nutr 13(2):78–83
Strozecki P, Kretowicz M, Odrowaz-Sypniewska G, Manitius J (2004) The influence of intravenous 1,25(OH)2D3 therapy on glucose metabolism in hemodialyzed patients with secondary hyperparathyroidism. Ren Fail 26(4):345–348
Blair D, Byham-Gray L, Lewis E, McCaffrey S (2008) Prevalence of vitamin D [25(OH)D] deficiency and effects of supplementation with ergocalciferol (vitamin D2) in stage 5 chronic kidney disease patients. J Ren Nutr 18(4):375–382
Bonakdaran S, Ayatollahi H, Mojahedi MJ, Sharifipoor F, Shakeri M (2008) Impact of treatment with oral calcitriol on glucose intolerance and dyslipidemia(s) in hemodialysis patients. Saudi J Kidney Dis Transpl 19(6):942–947
Ibrahim MA, Sany D, El Shahawy Y, Awdallah A (2012) Effect of activated vitamin D on glucoparameters in HCV seropositive and seronegative patients on chronic hemodialysis. Ren Fail 34(10):1188–1194
Ulutas O, Taskapan H, Taskapan MC, Temel I (2013) Vitamin D deficiency, insulin resistance, serum adipokine, and leptin levels in peritoneal dialysis patients. Int Urol Nephrol 45:879–884
Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daures JP, Argiles A (2013) Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol 37(3):239–248
Palmer SC, Gregor DO, Makaskill P, Craig JC, Elder GJ, Strippoli GFM (2007) Meta-analysis: Vitamin D compounds in chronic kidney disease. Ann Intern Med 147(12):840–853
Thompson SG, Higgins JPT (2002) How should meta-regression analyses be undertaken and interpreted? Stat Med 21(11):1559–1574
De Boer IH, Sachs M, Hoofnagle AN, Utzschneider KM, Kahn SE, Kestenbaum B, Himmelfarb J (2013) Paricalcitol does not improve glucose metabolism in patients with stage 3–4 chronic kidney disease. Kidney Int 83(2):323–330
Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C et al (2012) Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA J Am Med Assoc 307(7):674–684
Autier P, Boniol M, Pizot C, Mullie P (2014) Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol 2(1):76–89
Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R (2003) Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 26(12):3320–3325
Abe M, Kaizu K, Matsumoto K (2007) Plasma insulin is removed by hemodialysis: evaluation of the relation between plasma insulin and glucose by using a dialysate with or without glucose. Ther Apher Dial 11(4):280–287
Alemzadeh R, Kichler J (2012) Parathyroid hormone is associated with biomarkers of insulin resistance and inflammation, independent of vitamin D status, in obese adolescents. Metab Syndr Relat Disord 10(6):422–429
Acknowledgments
Dr. Melamed and Dr. Abramowitz are supported by Grant DK 087783 from the National Institute of Diabetes, Digestive and Kidney Diseases and by Gottschalk Awards from the American Society of Nephrology (ASN Foundation for Kidney Research). Dr. Melamed was also supported by Grant DK 078774.
Conflict of interest
The results presented in this paper have not been published previously. None of the authors have any conflicts of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarathy, H., Pramanik, V., Kahn, J. et al. The effects of short-term vitamin D supplementation on glucose metabolism in dialysis patients: a systematic review and meta-analysis. Int Urol Nephrol 47, 537–549 (2015). https://doi.org/10.1007/s11255-015-0909-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-0909-0