Abstract
In order to investigate the effects of nitrate and ammonium on the amphibians in a pasture zone of the Catalonian Pyrenees, larvae of Rana temporaria from several ponds were exposed to different concentrations of nitrate (0–500 mg/L) and ammonium (0–1.2 mg/L). High concentrations of nitrate in the water caused mortality and reduced larval size of R. temporaria, whereas no effects on larvae were observed in ammonium conditions. The results suggest that, if the levels of nitrate reach about 100 mg/L, the possibility of survival of R. temporaria larvae may be reduced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In the last few decades, intensification of agricultural activities and farm practices, intensive fertilization, and inappropriate application of chemical or organic fertilizers have led to deterioration in water quality in Europe. Human activities have more than doubled the nitrogen input to the land (Vitousek et al. 1997). Moreover, intensive farming produces ammonia-rich residues. Therefore, the addition of fertilizers to the land and the excrements from farms and pastures are increasing nitrogen levels in surface and in aquatic ecosystems. Pollution of water is widespread (Carpenter et al. 1998; Holland et al. 2005) and is increased due to technological advances in agricultural practices (Tilman et al. 2001; Galloway et al. 2003). Excess nitrogen causes several environmental stresses, especially to aquatic organisms and particularly to amphibians that have permeable skin and a biphasic life history (Blaustein et al. 1994).
Amphibians are vulnerable to water quality because they are in contact with water as embryos and larvae, and frequently as adults. The negative effects of nitrogen compounds in amphibians have already been demonstrated (Xu and Oldham 1997; Marco et al. 1999; Ortiz et al. 2004), but can vary among species (Hecnar 1995; Schuytema and Nebeker 1999; Egea-Serrano et al. 2008) and among populations of the same species (Johansson et al. 2001). Reduced feeding, size loss, low activities, and corporal abnormalities are some reported effects of nitrogen toxicity (Hecnar 1995; Xu and Oldham 1997; Marco et al. 1999; Ortiz et al. 2004). In the pastures of the Catalonian Pyrenees (Iberian Peninsula) the levels of nitrogen are typically high and the streams, which come from higher altitude, leach nitrogen that is carried into the water of some amphibians’ breeding ponds. We tested the effects of nitrate and ammonium on Rana temporaria from the Pyrenees (1,500 m.a.s.l.) in the laboratory in order to determine the contribution of nitrogen to mortality, malformation rate, growth rate, and development of this species.
Materials and Methods
We collected three spawns of Rana temporaria from several ponds in the Catalonian Pyrenees (Iberian Peninsula) at embryonic stage (0–3 stage of Gosner 1960). The study zone was situated in a pasture area of the Vall d’Aneu near the village of Son (42°37′8”, 1°4′55”; 1,500 a.s.l.) crossed by a stream (Riera del Tinter). At the moment of egg collection, the concentration of the water field was 6.89 mg/L nitrate, <0.05 mg/L nitrite, and 0.53 mg/L of ammonium, with pH 7.1. It should be noted that nitrate levels have temporal variation due to the high nitrate solubility.
The embryos of each spawn were kept in a recipient (5 l drinking water) until they reached stage 25. The experiment was carried out in a closed room with 24.5°C temperature and natural light. Groups of five tadpoles at stage 25 from the same spawn were exposed to different concentrations of nitrate and ammonium. Concentrations were 10, 50, 100, 200, and 500 mg/L NO −3 using NaNO3 for the nitrate treatments and 0.05, 0.15, 0.3, 0.6, and 1.2 mg/L NH +4 using NO3NH4 for the ammonium treatments. NO3NH4 is the most used chemical fertilizer in the world and had been used for several toxicology tests. So, they were used for comparison of our results with other studies which had been carried out in agricultural lands. We also used control treatments. For each sample, we used rectangular recipients (17.5 × 12 × 8.5 cm) with capacity of 1 L. We replicated the treatments three times for each spawn. Treatment solutions were prepared with reconstituted water (48 mg/L NaHCO3, 30 mg/L CaSO4, 30 mg/L MgSO4, and 2 mg/L KCl) for good quality of water, using distilled water. In the ammonium treatments we needed to use TRIS buffer to control pH, which was stabilized at 7.4 in all the solutions. Water solutions were changed every 3 days in order to be sure that water conditions did not change during the experiment. pH was checked at the moment of water exchange and did not present variation from the initial pH of 7.4. Previous studies conducted with a similar methodology did not show differences from the original nitrate in a period of 7 days (Marco et al. 1999). Tadpoles were fed with fish food ad libitum and excess food was removed every day.
Larval mortality and the presence of corporal malformations (e.g., tail flexure, oedemas, and asymmetric bodies) were monitored every 24 h, and the accumulated data were collected 15 days after the beginning of the experiment. Total length (from snout to tail tip) and body length (total length without tail length) of each larvae in the different treatments were also measured at 7 and 15 days using an OLYMPUS 3.2 digital camera and a millimetre scale. We obtained the measures of length with the Corel Draw 7.0 program. For statistical analysis, we calculated the growth increments (size at last measurement minus previous measurement) as the length variable because there were differences in size between eggs of different spawns at the beginning of the experiment (analysis of variance, ANOVA; F = 16.39, P < 0.0001).
To determine the effects of ammonium and nitrate on larval survival, malformation rate, and length (total length and body length), we used a linear model ANOVA implemented in PROC GLM of SAS for each variable. We normalized the survival and malformations variables by arcsine transformation and the growth variables by log transformation. We also analyzed the effect of spawn in each variable. We used a post hoc test (Tukey honestly significant difference, HSD) to determine the different effects of each concentration.
Results and Discussion
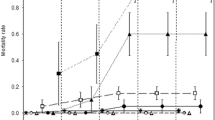
A high concentration of nitrate in the water causes mortality and reduces larval size in Rana temporaria from the Catalonian Pyrenees. Larva survival was significantly affected by nitrate treatment (F = 32.53, P < 0.0001) at the end of the experiment. The effect on spawn showed no significant difference among concentrations (F = 2.70, P < 0.082), and neither did the interaction between treatment and spawn (F = 1.69, P < 0.123). No corporal malformation was observed. These results agree with those of other authors who have found effects in other species, with different tolerances (Baker and Waights 1993; Marco et al. 1999; Johansson et al. 2001; Smith et al. 2006). We also observed that toxicity of nitrate increased with exposure time. Effects began to be observed 7 days after the beginning of the experiment (Fig. 1), and after 15 days more than 50% of the larvae had died from the 100 mg/L NO −3 treatment. In a review of effects of nitrate on aquatic animals (Camargo et al. 2005), this question was analyzed and it was suggested that lethal effects may need an exposure time at the levels we tested (0–500 mg/L), as was the case in our study.
Nitrate treatment affected growth at 7 and 15 days, and total length and body length began to decrease in the 100 mg/L treatment (Table 1; Fig. 2). The spawn did not affect larval size and the interaction between spawn and length was not significant in either of the length variables (Table 1). There is evidence that the effect of nitrate on larval growth may be due to the direct effects of the anions (Ireland 1991) or to the conversion of oxygen-carrying pigments to forms incapable of carrying oxygen, such as methemoglobin (Eckart et al. 1988). When nitrate is reduced to nitrite in the digestive tract, it causes methemoglobinemia, which prevents the carrying of oxygen in the blood.
Effects of nitrate concentration on the larval size of Rana temporaria larvae. For each treatment, the same letters at the bottom of the column are not significantly different at P ≤ 0.05 determined by Tukey’s test. a Effects of nitrate concentration on the length of R. temporaria larvae at 7 days. b Effects of nitrate concentration on the length of R. temporaria larvae at 15 days
Some studies show that the concentration of nitrate that causes effects in anuran tadpoles ranges from 5.0 to 40 mg/L depending on the species and the compounds used (reviewed in Rouse et al. 1999; Camargo et al. 2005). The comparison of the results reviewed suggests there may be substantial variation in nitrate tolerance among Ranids (Smith et al. 2006). Other studies demonstrated that northern frogs are more sensitive to nitrate than are southern frogs in Europe, probably due to the different nitrate levels to which frogs are exposed, which may represent an adaptation (Johansson et al. 2001). It would thus be interesting to study the populations in the south of the study area, with more agricultural and farm activity, in order to test this phenomenon. Another important fact is that, when nitrate occurs simultaneously with other stressor factors in the environment such as perchlorate (Ortiz-Santaliestra and Sparling 2007) or ultraviolet (UV)-B radiation (Macias et al. 2007), the effect may be greater than with nitrate alone.
We did not find effects of ammonium on larval survival (F = 1.76, P < 0.147) or growth (total length: F = 1.14, P < 0.432; body length: F = 1.57, P < 0.170) of R. temporaria larvae. The spawn had a significant effect on survival (F = 6.79, P < 0.003) but no significant interaction between spawn and treatment was found (F = 1.74, P < 0.111). Growth was not different between spawns (total length: F = 0.59, P < 0.553; body length: F = 2.49, P < 0.085) and no corporal malformations were found. These results contrast with others results found in different species (Camargo et al. 2005; Griffis-Kyle and Ritchie 2007). Ammonium nitrate toxicity could be caused mainly by the ammonia ion (Baker and Waights 1993; Adams and Bearling 1994). Unionized ammonia is considered the most toxic form of nitrogen, and this toxicity increases with pH and temperature (Adams and Bearling 1994). Although the laboratory conditions during the experiment were pH 7.4 and 24.5°C temperature, we did not found effects of ammonium or unionized ammonia toxicity in our experiment. On the other hand, the levels of nitrate in the ammonium nitrate treatments used were low (maximum concentration <7 mg/L) and we decided not to consider the amount of nitrate in the ammonium treatments.
In conclusion, we found effects of nitrate on Rana temporaria larvae at levels of 100 mg/L in the Catalonian Pyrenees, whereas no effects on larvae were observed in any ammonium conditions. The study area is a pasture zone and the levels of nitrate can vary at different periods of the year and even on different days, depending on the pasture time, cattle concentration, and other factors such as temperature and precipitation. The results suggest that the mortality observed in the field may be due to nitrogen compounds if the levels of nitrate reached about 100 mg/L.
References
Adams N, Bearling D (1994) Organic pollution: biochemical oxygen and ammonia. In: Calow P (ed) Handbook of ecotoxicology. Blackwell Scientific Publications, Oxford
Baker J, Waights V (1993) The effects of sodium nitrate on the growth and survival of toad tadpoles (Bufo bufo) in the laboratory. Herpetol J 3:147–148
Blaustein AR, Wake DB, Sousa WP (1994) Amphibian declines: judging stability, persistence, and susceptibility of population to local and global extinctions. Conserv Biol 8:60–70. doi:10.1046/j.1523-1739.1994.08010060.x
Camargo JA, Alonso A, Salamanca A (2005) Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates. Chemosphere 58:1255–1267. doi:10.1016/j.chemosphere.2004.10.044
Carpenter S, Caraco NF, Correll DL, Howarth EW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568. doi:10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2
Eckart R, Randall D, Augustine G (1988) Animal physiology, mechanisms and adaptations, 3rd edn. W H Freeman and company, New York
Egea-Serrano A, Tejedo M, Torralva M (2008) Analysis of the avoidance of nitrogen fertilizers in the water column by juvenile Iberian water frog, Pelophylax perezi (Seoane, 1885), in laboratory conditions. Bull Environ Contam Toxicol 80:178–180. doi:10.1007/s00128-007-9341-1
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB et al (2003) The nitrogen cascade. Bioscience 53:404–420. doi:10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Griffis-Kyle KL, Ritchie ME (2007) Amphibian survival, growth and development in response to mineral nitrogen exposure and predator cues in the field: an experimental approach. Oecologia 152:633–642. doi:10.1007/s00442-007-0686-2
Hecnar SJ (1995) Acute and chronic toxicity of ammonium nitrate fertilizer to amphibians from southern Ontario. Environ Toxicol Chem 14:2131–2137. doi:10.1897/1552-8618(1995)14[2131:AACTOA]2.0.CO;2
Holland EA, Braswell BH, Sulzman J, Lamarque J-F (2005) Nitrogen deposition onto the United States and Western Europe: synthesis of observations and models. Ecol Appl 15:38–57. doi:10.1890/03-5162
Ireland PH (1991) Separate effects of acid-derived anions and cations on growth of larval salamanders of Ambystoma maculatum. Copeia 1991:132–137. doi:10.2307/1446255
Johansson M, Räsänen K, Merilä J (2001) Comparison of nitrate tolerance between different populations of the common frog Rana temporaria. Aquatic Toxicol 54:1–14. doi:10.1016/S0166-445X(00)00182-X
Macias G, Marco A, Blaustein AR (2007) Combined exposure to ambient UVB radiation and nitrite negatively affects survival of amphibian early life stages. Sci Total Environ 385:55–65. doi:10.1016/j.scitotenv.2007.06.016
Marco A, Quilchano C, Blaustein AR (1999) Sensitivity to nitrate and nitrite in pond-breeding amphibians from the Pacific Northwest, USA. Environ Toxicol Chem 18:2836–2839. doi:10.1897/1551-5028(1999)018<2836:STNANI>2.3.CO;2
Ortiz ME, Marco A, Saiz N, Lizana M (2004) Impact of ammonium nitrate on growth and survival of six European amphibians. Arch Environ Contam Toxicol 47:234–239. doi:10.1007/s00244-004-2296-x
Ortiz-Santaliestra ME, Sparling DW (2007) Alteration of larval development and metamorphosis by nitrate and perchlorate in southern leopard frogs (Rana sphenocephala). Arch Environ Contam Toxicol 53:639–646. doi:10.1007/s00244-006-0277-y
Rouse JD, Bishop CA, Struger J (1999) Nitrogen pollution: an assessment of its threat to amphibian survival. Environ Health Perpect 107:799–803. doi:10.2307/3454576
Schuytema GS, Nebeker AV (1999) Comparative effects of ammonium and nitrate compounds on Pacific treefrog and African clawed frog embryos. Arch Environ Contam Toxicol 36:200–206. doi:10.1007/s002449900461
Smith GR, Temple KG, Dingfelder HA, Vaala DA (2006) Effects of nitrate on the interactions of the tadpoles of two ranids (Rana clamitans and Rana catesbeiana). Aquat Ecol 40:125–130. doi:10.1007/s10452-005-9015-1
Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R et al (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284. doi:10.1126/science.1057544
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Xu Q, Oldham RS (1997) Lethal and sublethal effects of nitrogen fertilizer ammonium nitrate in common toad (Bufo bufo) tadpoles. Arch Environ Contam Toxicol 32:298–303. doi:10.1007/s002449900188
Acknowledgements
We are grateful to Pepe Guillen of the Centre for Nature and Sustainable Development in the Pyrenees, Fundacio Territori i Paisatge (Planes de Son) for help in fieldwork and to Rosa Teira of the Department of Environmental and Soil Sciences (University of Lleida) for help in the protocol used. We also thank Maximo Blanco of AQUALIA for the water analysis and his comments, and the Fundacio Territori i Paisatge (La Caixa de Catalunya) for funding this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oromí, N., Sanuy, D. & Vilches, M. Effects of Nitrate and Ammonium on Larvae of Rana temporaria from the Pyrenees. Bull Environ Contam Toxicol 82, 534–537 (2009). https://doi.org/10.1007/s00128-009-9667-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9667-y