Abstract
The effects of urban growth are multidimensional and therefore they cannot be assessed using only one indicator. The objective of this study was to assess the environmental quality of urbanized rivers through the evaluation of multiple indicators, including water quality, habitat condition, macroinvertebrate assemblages, and anuran amphibians as bioindicators. Twelve sites along three rivers were characterized regarding water quality and habitat condition using a Habitat Model Affinity score, during high-flow and low-flow seasons between 2009 and 2013. Fourteen water quality parameters were assessed at each site. A general Water Quality Index was applied to the physical, chemical, and bacteriological data. Macroinvertebrate taxa richness, Plecoptera–Ephemeroptera–Trichoptera richness, and the Biotic Index of San Luis Sierras were the metrics used to analyze macroinvertebrate assemblage shifts. Species richness and relative abundance of amphibians were estimated from calling and visual encounter surveys, in parallel with all aforementioned parameters. Principal Component Analysis identified that macroinvertebrate-based metrics were superior in reflecting the water quality and habitat condition impairments, whereas amphibian metrics showed a differential sensitivity to specific water quality parameters such as nutrient enrichment. This study contributes to achieve a more comprehensive understanding of the complexity of the chemical and biological processes experienced by urban affected environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The unprecedented growth rate of towns and cities around the world has created an urgent need to work toward the understanding of the impacts of urbanization on natural ecosystems and the services they provide (Lafortezza & Sanesi, 2019). The establishment of new urban areas involves complex environmental changes including modifications in the physical, chemical, and environmental conditions of an area, which can be reflected in new biotic assemblages (Hamer & McDonnell, 2008). Urbanization causes profound impacts on aquatic ecosystems. Particularly, urban rivers experience multidimensional changes, affecting hydrology (frequency and magnitude of high-flow events), geomorphology (channelization, dredging and bed/bank stabilization works), habitat heterogeneity (increase of sediment input), and water quality (increase of pollutants concentration) (Walsh et al., 2005; Booth et al., 2016; Wilson et al., 2021).
Traditionally, rivers water quality used to be monitored via physical, chemical, and bacteriological determinations, however, these type of analyses present some limitations such as (i) any interpretation of water quality is restricted to the measured parameters and (ii) the parameters reflect the state of the water body at the time of sampling, making it difficult to detect changes in quality between sampling seasons (Abbasi & Abbasi, 2012). Biomonitoring meaning the assessment and monitoring of water quality based on the organisms that inhabit an aquatic ecosystem present certain advantages over the physical–chemical approach (Prat & Munné, 2014). The main advantage of biomonitoring is that bioindicators can reflect cumulative effects of several environmental changes in a temporal dimension (Parmar et al., 2016; Stancheva & Sheath, 2016; Fierro et al., 2017; Mekonen et al., 2017; Rakshit et al., 2017). In that sense, physical–chemical quality of water is able to recover shortly after a disturbance, whereas the biological component can take from weeks to months or years to recuperate, depending on the organisms monitored and the size and durability of the disturbance.
Numerous studies highlight the importance of using multiple indicators to estimate ecological integrity, and the inclusion of biological indices to estimate water quality and ecological status (Shibata et al., 2004; Zampella et al., 2006; Walker et al., 2009; Pandey et al., 2018; Mao et al., 2019). A multi-indicator approach (including both, biotic and environmental factors) will allow to compare the status of degraded rivers and will provide a better understanding of the overall integrity of the evaluated systems. In this study, we use benthic macroinvertebrates, amphibians, and habitat condition as indicators of environmental health as they are well known to respond to different impacts resulted from urbanization.
Within an urban context, benthic macroinvertebrate assemblages show shifts in community structure as there are able to reflect overall stream degradation (Wang et al., 2020; Ji et al., 2022). Numerous studies have demonstrated the negative influence of increased urbanization on the macroinvertebrate community taxa richness, abundance, EPT taxa richness, Shannon–Wiener diversity, and number of intolerant taxa (Wang et al., 2012; Sterling et al., 2016; Martins et al., 2017; Luo et al., 2018).
On the other hand, anuran amphibians have experienced unprecedented declines and even local extinctions worldwide (Wake & Vredenburg, 2008; Blaustein et al., 2011). Habitat degradation and alteration (Cushman, 2006), diseases and pathogens (Rollins-Smith, 2009), global warming (Pounds et al., 2006), physiological effects due to increased concentrations of pollutants in water (Sowers et al., 2009; Dodd, 2010; Babini et al., 2018; Calderon et al., 2019), alterations in the hydrology of aquatic environments, and decline in water quality are known to be factors related to the decrease of amphibians (McKibbin et al., 2008; Sparling, 2010; Bounas et al., 2020). For these reasons, this group of vertebrates could be a suitable indicator of aquatic ecosystems degradation.
Finally, variations in substrate characteristics, flow, breeding site availability, and habitat heterogeneity within the channel lead to a decrease in the habitat quality of the sites and will be reflected in the structure of aquatic biota. Therefore, a changing or unstable habitat is considered one of the main stressors in aquatic systems (Karr et al., 1986). This is why, it is important to evaluate the quality of the local habitat in order to distinguish its effect from the influence of water contaminants.
Developing countries have experienced significant population and urban growth (García-Ayllón, 2016) and the province of San Luis (Argentina) has not been excluded from this phenomenon. Therefore, in order to implement prioritized conservation strategies, it is urgently needed to increase the knowledge of ecological status, biodiversity, and health assessment of fluvial ecosystems, in different ecoregions (Rocha et al., 2020). The objectives of this study were (i) to examine effects of urbanization on habitat degradation and water quality, (ii) to assess the effect of habitat degradation and water quality characteristics on macroinvertebrate assemblages and amphibian richness and abundance as bioindicators, and (iii) to conclude which bioindicator is the most suitable to monitor environmental quality of these human-impacted rivers. Our primary hypothesis is that urbanization will have a negative effect on the water characteristics and environmental quality of these high-gradient rivers. It is expected that as a result of water quality degradation and impaired habitat conditions, amphibians and macroinvertebrate metrics will decrease along the longitudinal profile of the rivers. Furthermore, we expect that macroinvertebrate and amphibians will show a different response to environmental quality degradation of these rivers as they have different environmental requirements and uses of these water bodies. The information gathered in this study will contribute to design monitoring protocols and enhance the inventory of freshwater ecosystem health condition.
Material and methods
Study area and sampling design
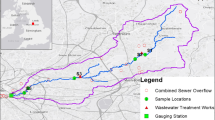
This study was performed in the province of San Luis, Argentina. The province is located between the 31° and 36° parallels of South latitude and the 64° and 67° meridians of West longitude. From 1991 to 2010, three of the most touristic towns, Potrero de los Funes, El Trapiche, and El Volcán, have at least doubled their legal local populations. Potrero de los Funes suffered an increase in population from 410 to 1698 people, El Trapiche from 541 to 1200, and El Volcán 769 from to 1775 people (Instituto Nacional de Estadística y Censos, 2010). Moreover, the aforementioned towns are characterized by a high influx of tourists year round, with the highest concentrations during summer season, due to the presence of relatively high-gradient rivers where several recreational activities are done, such as swimming, relaxing, walking, and jogging. Four sampling sites were set along these three urban-impacted rivers: Trapiche River (TR), Volcán River (VR), and Potrero de los Funes River (PFR) (Fig. 1). TR, VR, and PFR are second-order watercourses (Strahler, 1954) and the sections sampled presented an average width and maximum depth of 5.57 ± 1.32 m and 0.19 ± 0.04 m, respectively. Rivers are located in different parts of the San Luis Central Sierras System, which belongs to the Eastern Sierras Pampeanas in central Argentina. This morphostructural unit is defined by a morphological criterion of variable heights and similar geological features cut transversally by rivers, with a steep west slope where these three rivers are located (Peña Zubiate et al., 2006). Soils are poor developed and vegetation is dominated by xerophytic deciduous shrub (Morello et al., 2018). In areas near the rivers, different species of trees and shrubs can be found, such as molle de beber (Lithraea molleoides [Vell.] Engl.), tala (Celtis tala Gillies ex Planch), molle (Schinus fasciculata [Griseb] I.M. Johnst.), chañar (Geoffroea decorticans [Gillies ex Hook. & Arn.] Burkart), sombra de toro (Jodina rhombifolia [Hook. & Arn.] Reissek rhombifolia), piquillin (Condalia microphylla Cav.), among others (Montenegro et al., 2007). Climate is mesothermic and semi-arid with an average annual rainfall of 600 mm (Nievas et al., 2019) mostly concentrated during late spring and summer season. Google Earth free and public high-resolution remote sensing images showed that the land use/cover upstream rivers can be classified as rangeland according to the classification system presented by Anderson (1976). The urbanization gradient was established at each river as a percent of urban land use (e.g., housing, roads, bridges, recreational infrastructure, others) in a radius of 150 m around each sampling plot (Calderon et al., 2017) using QGIS Geographic Information System (QGIS Development Team, 2020) (Fig. 1). Two sampling events were carried out during high‐flow season (December–March) and repeated again during low‐flow season (May–August) between 2009 and 2013.
Assessment of stream corridor characteristics
A habitat assessment of the stream corridor condition was conducted at each site. The method used follows the United States Environmental Protection Agency (EPA) Rapid Habitat Assessment protocol outlined by Barbour et al. (1999) for high-gradient streams. Ten different habitat characteristics (i.e., epifaunal substrate/available cover, embeddedness, velocity/depth regime, sediment deposition, channel flow status, channel alteration, frequency of riffles, bank stability, vegetative protection, and riparian vegetative zone width) were assessed at each site and given a score on a scale of 0–20; 0 being poor and 20 being optimal. Therefore, high scores (above 80) indicate that stream corridor is practically undisturbed. Interpretation of habitat assessment results was conducted through the calculation of Habitat Model Affinity (HMA) for high-gradient streams (New York State Department of Environmental Conservation, 2019; Calderon et al., 2019). The percentage of similarity between the HMA scores for a reference condition and the HMA scores for each site classified the reaches by the level of disturbance: natural (80–100%), altered (70–79%), moderate (60–69%), and severe (< 60%).
Physical, chemical, and bacteriological sampling, analytical methods, and water quality
The samples for the physical and chemical analysis were collected in glass containers of 2 l capacity, and for the analysis of dissolved oxygen, samples were taken separately in a Winkler flask. Samples were collected at an equidistant depth between the bottom and the surface at the center of the river channel. For bacteriological analysis, each water sample was taken aseptically with a sterilized 1 l glass bottles with screw cap, at a depth of approximately 15–30 cm from the surface. The preservation, transportation, and analysis were performed following the standard protocols of the Standard Methods for the Examination of Water and Wastewater (APHA, 2005) at the Instituto de Química San Luis (INQUISAL) lab of the Universidad Nacional de San Luis. The data set contained 13 parameters: electrical conductivity S.M. 2510-B (EC), dissolved oxygen S.M 4500-O-C (DO), turbidity S.M. 2130-B (Tbd), total hardness S.M. 2340-C (TH), biochemical oxygen demand S.M. 5210-B (BOD), alkalinity S.M. 2320-B (Alc), chemical oxygen demand S.M. 5220-B (COD), chloride S.M. 4500-Cl (Cl−), phosphate S.M.-4500-PO4 3−-E (PO43−), sodium S.M. 3500-Na-B (Na+), nitrate S.M.-4500-NO3-E (NO3−), total suspended solids S.M. 2540-C (TSS), and total coliforms S.M. 9221-B (TC). EC and pH were measured in situ using a portable waterproof meter, Oakton WD-35431-03. All the water quality parameters were expressed in milligram per liter, except pH, EC (μS cm−1), turbidity (Nephelometric Turbidity Units, NTU), and TC (MPN/100 mL).
The Water Quality Index (WQI) (Mingo Magro, 1981) was used to estimate the water quality of the sites. The WQI was calculated using twelve parameters for water quality characterization: DO, TSS, pH, EC, COD, BOD, Cl−, Na+, TH, TC, PO43−, and NO3− (for more information regarding WQI calculation see Calderon et al. [2019]). The WQI quantifies water quality as follows: excellent (100–90), good (89–80), intermediate (79–70), acceptable (69–60), and unacceptable (59–0).
Biological indicators
Benthic macroinvertebrates were collected at the same time that water sampling was performed (four sampling events in total). Multihabitat samples were collected at each site using a 0.09 m2 area and 300 µm meshed Surber net. The substrate within the quadrant was vigorously disturbed for 3 min as the current swept macroinvertebrate fauna into the net. The selected microhabitats were one riffle and one pool at every site, and the two samples were combined for comparison between the different sites. All macroinvertebrate collections were preserved in 70% ethanol and returned to the laboratory for sorting and identification. The specimens were identified in the laboratory, down to the lowest taxonomic level required by the Biotic Index of San Luis Sierras (BISLS), which means family level for some orders (i.e., Coleoptera, Diptera) and genus-level for others (i.e., Ephemeroptera, Trichoptera) (Vallania et al., 1996). BISLS, based on the Trent index developed by Woodiwiss (1964), combines taxonomic richness and the sensitivity of taxa to estimate the biological water quality of the site. This index ranges from 1 for extremely contaminated environments to 12 for environments with no contamination (Calderon et al., 2014). Other metrics used to analyze macroinvertebrate assemblages were taxa richness and Ephemeroptera, Plecoptera, and Trichoptera richness (EPT). These macroinvertebrate metrics were selected taking into account time, expertise, and human resources limitations as they are qualitative metrics that do not require high-resolution taxa identification while providing valuable information for biomonitoring (Marshall et al., 2006; Heino & Soininen, 2007).
Species richness and relative abundance of amphibians were estimated from calling surveys; the presence of the species detected was supported by visual encounter surveys. All sites were visited 6 to 12 times throughout the spring and summer seasons, which corresponds to the months of maximum amphibian activity and rainfall. Anuran calls were recorded for five minutes on each site. Two visual encounter surveys plots (100 × 5 m) were installed one in each side of the channel (Lips et al., 2001). After the calling registration and recording, one observer searched each plot while walking at a standard pace using 15 min to examine each area thoroughly. In accordance with North American Amphibian Monitoring Program protocol, surveys were conducted at least 0.5 h after dusk and completed by 01:00 (Weir & Mossman, 2005).
Data analysis
The Shapiro–Wilk normality test was applied to assess normality. The majority of variables did not follow normal distribution, and it could not be remedied by data transformation. Therefore, non-parametric statistical procedures were applied. Principal Component Analysis (PCA) was applied to mean values of the fourteen physical–chemical parameters and WQI scores, and it was used to identify variation among sampling sites to further reduce the contribution of variables with minor significance. The significance of differences in amphibians and macroinvertebrate assemblage metrics among sites was analyzed using non-parametric Kruskal–Wallis test of differences in means as well as Dunn’s pairwise comparison test, using a significance level of α = 0.05 (Harguinteguy et al., 2013; Szczepocka et al., 2014). Although, sample locations within a stream may not meet the assumptions of being independent, each site was considered as an independent sample, since along the course they receive successive impacts (bridges, canalizations, effluents) that could change water and biota in short distances. Furthermore, it has been reported that fast self-purification occurs in those sierra rivers (González et al., 2014).
Relationships among relevant physical–chemical variables previously detected by PCA, HMA scores, and biological metrics (macroinvertebrate richness, EPT, BISLS, amphibian richness, and abundance) were quantified using Spearman’s rank correlation coefficient (rho) in order to account for the possible presence of non-normal distribution (Tran et al., 2010). PCA was used to visualize the grouping of sites according to the environmental variables (water quality and habitat) and biological metrics (Ludwig & Reynolds, 1988). All procedures were carried out using the statistical software R version 3.5.1 (R Core Team, 2018). Kruskal–Wallis and Dunn’s pairwise comparison tests were conducted using the package agricolae (De Mendiburu, 2014) and PCA was performed using the FactoMineR and ggplot2 packages (Lê et al., 2008; Wickham et al., 2016).
Results
Stream corridor habitat characterization
HMA values varied among sites from natural to severely disturbed (Fig. 2). Site TR1 was the most natural in terms of the evaluated characteristics, with a low sediment deposition and an optimal vegetation cover and width of the riparian vegetation. As the river flows through the urban area, a decrease in vegetation cover and width of the riparian area was observed. Furthermore, the decrease of streambank vegetation was reflected in the instability of the banks, with evidence of erosive processes. TR3 was characterized by high sediment deposition, practically non-existent riparian vegetation and a high degree of channel alteration. TR4 showed an improved quality of habitat with an increase in the width of the riparian vegetation; however, high sediment deposition was observed.
The main factors affecting habitat within Volcán River (VR) sites were the natural high concentration of suspended and deposited sediments in the channel. VR1 and VR2 showed an optimal vegetation cover and width, while they were decreased in VR3. Potrero de los Funes River (PFR) also exhibited a variation in the physical conditions of the habitat among sites. PFR1 showed a low degree of degradation in terms of vegetation and obtained an optimal score for HMA. PFR2 was very similar to PFR1, except for the riparian vegetation characteristics. The sites with the lowest habitat quality were PFR3 and PFR4, with high embeddedness and sediment deposition in the riverbed, a high degree of channel modification, low vegetation cover and vegetation width, and low bank stability, especially in PFR4.
Physical, chemical, and bacteriological water quality
WQI values for TR and PFR varied from good to excellent quality, whereas for VR, WQI varied from intermediate to good quality of water. PCA resulted in the selection of two principal components (PC) that explained 78.73% of the total variability of the analyzed data matrix according to the eigenvalue-one criterion (variances greater than 1). PC1, which explained 52.55% of the variability of the original data, was positively correlated with Tbd, SO4, EC, Cl−, Na+, K+, TH, and Alc, and negatively correlated with WQI. PC2 explained 24.18% of the variance, and it was positively determined by NO3−, PO43−, and TC, while DO was negatively loaded on the axis (Fig. 3). Therefore, PC1 represented the mineral component, whereas PC2 corresponded to the organic component. The sites in VR distributed to the right of PC1, mainly characterized by waters with high mineral loads. Sites TR1, TR2, and TR3, together with PFR1 were grouped to the left of Component 1, characterized by superior overall quality. On the other hand, TR4, PFR2, PFR3, and PFR4 sites were located on Component 2, as determined by a high nutrients load and TC.
Biological indicators
A total of 39 taxa were identified in the benthic macroinvertebrate samples. No specimens of the Order Plecoptera were recorded. The most common families across sites (present in over 90% of sites) were Physidae (Basommatophora), Elmidae (Coleoptera), Chironomidae (Diptera), Simuliidae (Diptera), Baetidae (Ephemeroptera), Leptohyphidae (Ephemeroptera), Hydroptilidae (Trichoptera), and Naididae (Haplotaxida) (Table 1).
Significant differences were found in mean macroinvertebrate taxa richness when all the studied sites were compared (H(11) = 29.83; P = 0.001). Taxa richness of TR1, TR2, VR1, VR2, VR3, PFR1, PFR2, and PFR3 was significantly higher than TR4, VR4 and PFR4. No significant differences were found between TR4-VR4 (Z = − 0.37; P = 0.86), VR4-PFR4 (Z = 0.61; P = 0.77), and TR4-PFR4 (Z = 1.10; P = 0.48). TR showed a decrease in the mean taxa richness from 16.8 (± 0.4) for TR0 to 11.2 (± 0.8) for TR4. VR showed a similar trend with a decrease from 16.3 (± 0.3) for VR1 to 11.7 (± 1.9) for VR4, and PFR suffered a decrease from 16.7 (± 0.2) for PFR0 to 13.7 (± 0.6) for PFR4 (Fig. 4).
Regarding the EPT richness mean values, TR1, VR1, VR2, VR3, PFR1, PFR2, and PFR3 were significantly higher than TR2, TR3, TR4, VR4, and PFR4 (H(11) = 35.83; P = 0.0002). No significant differences were found between TR2-TR3 (Z = 1.45; P = 0.33), TR3-TR4 (Z = 0.69; P = 0.63), TR4-VR4 (Z = − 0.94; P = 0.49), and VR4-PFR4 (Z = 0.24; P = 0.91) (Fig. 5).
BISLS values ranged from 10, not-contaminated (at TR1 and PFR1) to low-contaminated (rest of the sites). The BISLS values for TR1 and PFR1 were significantly higher when compared with TR4, VR4, and PFR4 (H(11) = 28.37; P = 0.003). No significant differences were detected between TR2-TR3 (Z = 0.53; P = 0.73), TR3-TR4 (Z = 1.09; P = 0.55), TR4-VR1 (Z = − 1.72; P = 0.29), and the rest of the pairwise comparisons between VR sites (P > 0.05). Finally, no significant differences were found between VR4-PFR2 (Z = 2.17; P = 0.17), PFR2-PFR3 (Z = 0.09; P = 0.95), and PFR3-PFR4 (Z = 0.87; P = 0.51) (Fig. 6).
Five species of anuran amphibians were detected in the urbanized rivers studied: Rhinella arenarum (Hensel, 1867), Odontophrynus occidentalis (Berg, 1896), Leptodactylus mystacinus (Burmeister, 1861), Boana cordobae (Barrio, 1965), and Boana pulchella (Duméril & Bibron, 1841). Significant differences in amphibian species richness (H(11) = 31.58; P = 0.001) and relative abundance (H(11) = 30.64; P = 0.001) were found when all sites were compared.
Relationships between physical and chemical water quality, HMA and bioindicators metrics
The results of the Spearman’s rank correlation coefficient analysis (Table 2) indicated that HMA was significantly correlated with WQI, DO, PO43−, and TC. Macroinvertebrate taxa richness showed a significant relationship with decreases in PO43− and TC. EPT was also inversely correlated with PO43− and TC, and positively correlated with DO. IBSSL showed a significant positive correlation with WQI and it was inversely correlated with PO43− and TC. Correlation analysis found that PO43−, NO3−, TC, DO, Tbd, and EC were the significant variables influencing amphibian community metrics (Table 2).
In addition, HMA positively correlated with macroinvertebrate taxa richness (ρ = 0.589; P < 0.01), EPT (ρ = 0.680; P < 0.01), BISLS (ρ = 0.636; P < 0.01), and amphibian richness (ρ = 0.382; P < 0.05).
Multiple indicators analysis
A Principal Component Analysis (PCA) was performed on the multiple indicators used to demonstrate the effect of urbanization: WQI, HMA, macroinvertebrate taxa richness, EPT, BISLS, and total abundance of amphibians. PCA resulted in the selection of two principal components (PC) that retained 86.62% of the total variability of the analyzed data according to the eigenvalue-one criterion (variances greater than 1) (Table 3). PC1 explained 56.79% of the variance, and the variables that contributed the most to it were HMA and macroinvertebrate assemblage metrics. PC2 explained 29.83% of the variance, and it was positively correlated with amphibian richness and abundance, and negatively correlated with WQI (Fig. 6). Biplot shows that studied sites located in VR (except for VR4) were those that presented a better biological quality indicated by HMA, amphibian, and macroinvertebrate metrics. However, the physical–chemical quality of the VR sites, indicated though WQI, was not the best. TR1 and PFR1 were located in the area of good physical–chemical quality and biological quality in terms of macroinvertebrates, but this was not the case for amphibian metrics. At the other extreme of PC2, we found the sites on TR3, TR4, VR 4, PFR3, and PFR4, which had the lowest value of WQI and bioindicators metrics (Fig. 7).
Discussion
Most of the sites showed some degree of degradation in habitat conditions and riparian zones. Through the analysis of the data, it was possible to determine that HMA was negatively associated with indicators of poor water quality, and it was positively associated with indicators of good water quality. These identified relationships could indicate that habitat assessment can be used as a potential predictor of how physical changes in habitat may translate into changes in water quality. HMA showed a significant correlation with macroinvertebrate metrics, which was not entirely unexpected since all the aspects evaluated by the HMA were selected by taking into account the physical requirements for the resident aquatic assemblages (Barbour et al., 1999). Moreover, HMA also showed some degree of association with amphibian richness, and this could be related to the fact that HMA considers habitat metrics important for amphibian colonization and reproduction (Calderon et al., 2019). Urban development not only affects the water quality of a stream, it also affects its physical characteristics (Walsh et al., 2005). Therefore, habitat assessment can add an important dimension to monitoring data, providing a more comprehensive evaluation of human-induced impacts on water resources at a low cost (Rogers et al., 2002; Ridley et al., 2005; Tran et al., 2010; Duffy, 2011). Furthermore, and since aquatic biological assembles not only respond to water quality changes, this rapid habitat assessment could provide a tool to discern between the effects of water contaminants from potential physical stress due to habitat degradation.
In this study, WQI is an index that determines the quality of the water based on a series of mandatory and optional parameters. Thus, the operator is able to select in advance some of the optional parameters considered relevant for the study. In this study, three complementary parameters that amphibian and macroinvertebrate assemblages are showed to be sensitive were selected: TH, Cl−, and Na+ (Hart et al., 1991; Ultsch et al., 1999; Laposata & Dunson, 2000; Kefford et al., 2003; Gómez-Mestre et al., 2004; Dunlop et al., 2008). PCA assisted in the identification of two major components in water quality; the mineral component represented by parameters such as EC, TH, Alc, and several ions and the organic component represented by DO, and nutrient and bacterial loads. Therefore, this analysis allowed to (i) identify the relationships among parameters and (ii) select those representatives of each component in order to evaluate their effect on the biological component. A decrease in WQI from upstream sites (TR1, VR1, PFR1) to the sites located at the end of the urban area (TR4, VR4, PFR4) could be observed in the biplot. VR showed significantly higher values in most of the mineral components and this resulted in lower values of the WQI. However, this decrease was mostly related to the type of soil on which the river flows rather than the levels of pollutants in the water. Furthermore, VR was characterized by highly oxygenated waters, with comparatively lower levels of nitrates, phosphates, and bacterial load. These observations agree with the fact that water quality indices can often incur in loss of information (Almeida et al., 2012), making necessary to also analyze the individual behavior of the physical and chemical parameters to better understand water quality variations.
Macroinvertebrate taxa richness varied among sites, fewer taxa were observed at sites located in the middle or downstream of the urbanization. In general, some taxa of Ephemeroptera, Trichoptera, Hemiptera, Coleoptera, and Diptera orders disappeared in urbanized sites. Previous studies demonstrated the negative effects of urbanization on macroinvertebrate community structure, and taxa richness reduction has been observed in a wide range of previously published papers (Chadwick et al., 2006; Dewson et al., 2007; Merrit et al., 2008; Violin et al., 2011; Wang et al., 2012; Madera et al., 2016; Edegbene et al., 2019; Luo et al., 2018). In general, taxa richness decreases with increased urbanization due to the exclusion of sensitive taxa and some authors highlight the importance of richness-based metrics to measure the effect of urbanization as they show strong associations with urban intensity (Cuffney et al., 2010; Martins et al. 2017). In our study, the decrease taxa richness was directly related to changes in the physical–chemical quality of water and the degradation of physical habitat. Increase in surface runoff, erosive processes, sediment deposition, and changes in channel morphology are some of the alterations experienced by urban rivers (Roy et al., 2003), and these changes were reflected as shifts in macroinvertebrate assemblages. However, further macroinvertebrate assemblage composition and structure analyses are needed to identify other useful metrics.
The Ephemeroptera, Plecoptera, and Trichoptera (EPT) orders are known to have a low tolerance to water contamination and they are usually the first groups among macroinvertebrates to reflect changes in their environments (Lenat & Penrose, 1996; Weijters et al., 2009). Moreover, numerous investigations and monitoring programs use the EPT index to assess the degree of degradation of water bodies, with a decrease in the number of taxa in places with a higher level of disturbance (Hazelton, 2003; Wang & Kanehl, 2003; Bonada et al., 2006; Miserendino et al., 2008; Munné & Prat, 2009; Davies et al., 2010; White, 2017; Hamid & Rawi, 2017; Luo et al., 2018). In this study, EPT taxa richness decreased across sites in a very similar way to taxa richness. Genera such as Camelobaetidius and Farrodes disappeared in degraded sites, whereas Americabaetis and Caenis, known for their resistance and broad distributions (Domínguez & Fernández, 2009; Amaral et al., 2015), persisted in every site. Order Trichoptera showed a similar behavior with sensitive families like Polycentropodidae, Glossosomatidae, and Philopotamidae specimens (Alba-Tercedor, 1996) present in less disturbed sites, whereas Hydroptilidae was recorded in every site, disturbed and undisturbed. EPT showed a negative relationship with the concentration of PO43−, TC and a positive relationship with DO, and HMA. Other authors have reported similar results between EPT taxa and PO43− (Chambers et al., 2012), bacterial load, DO, and habitat condition (Tran et al., 2010). In all cases, the decrease in sensitive taxa due to the increase in urbanization is coupled with the decrease of available habitat for colonization (assessed through HMA), as well as the decrease in DO. It is necessary to point out that Plecoptera is an order biographically absent in the Province of San Luis rivers and it was not recorded in any of the samples.
BISLS showed a slight decrease at those sites located below the urbanized area. This index is based on two basic characteristics of macroinvertebrate assemblages, taxa richness, and taxa sensitivity. BISLS does not consider relative abundance of taxa, which makes the processing of the samples relatively faster. However, those characteristics make BISLS also likely to result in loss of information. This index has been used in previously published investigations (Calderon et al., 2014, 2017; González et al., 2014) and the authors concluded that BISLS is more sensitive when applied to highly degraded systems. Although a stronger correlation was expected between WQI and BISLS, which was the case for TR and PFR, this was not observed in VR, with lower WQI and similar BISLS values when compared to the other two studied rivers. However, the low values of WQI in VR were primarily related to the mineral characteristics of the soil than to the level of anthropic impact. This fact reinforces the notion of the complementarity between traditional physical–chemical approaches and biomonitoring.
No significant correlation was found between WQI and amphibian metrics. This lack of relationship was not completely unexpected, since WQI was not intended to respond to water quality requirements for amphibians. WQI values for VR were on average lower than in the other rivers; however, VR showed a higher anuran amphibian reproductive activity. Thus, the analysis of the effects of individual physical and chemical parameters was necessary to understand the behavior of amphibians as bioindicators within urban settings. Amphibian abundance and richness were negatively affected by the increased concentrations of NO3−, PO43−, Tbd, TC, and higher level of habitat degradation caused by urbanization (Calderon et al., 2019).
The final PCA analysis allowed for the observation of the distribution of the sites based on the multiple indicators evaluated during this study. The sites located upstream of urban areas (TR1, PFR1) were associated with higher values of WQI, HMA, and biological metrics. But, as the rivers flow through the urban area they suffer a remarkable degradation mainly due to the lack of riparian vegetation and nutrient enrichment. On the other hand, VR sites were, on average, more preserved sites with higher HMA values and biological metrics; however, this was not accompanied by the physical–chemical quality of the water (naturally enriched by minerals). As was observed in VR, several authors highlight the importance of riparian vegetation in the conservation of channel morphology (Millar, 2000), control of surface runoff, recharge of the water cycle, maintenance of water quality, and in the provision of habitat for biodiversity (Clausen et al., 2000; Dosskey et al., 2010). Riparian vegetation acts as a buffer in the terrestrial-aquatic interface, protecting the water bodies, reducing the speed that surface runoff reaches the channel, and acting as a filter for nutrients, pollutants, and sediments from the surroundings (Tabacchi et al., 2000). Finally, PCA analysis allowed to identify that macroinvertebrate-based metrics better reflected the overall physical–chemical quality and habitat condition of the sites, whereas amphibian metrics were mainly driven by habitat characteristics and nutrient enrichment. However, this differentiation in terms of responses, and the sensitivity of organisms to different stressors, is necessary in order to evaluate the effect of the multiple impacts suffered by natural environments during the urbanization process. The use of multiple indicators, especially in environments affected by non-point sources of pollution, such as urbanization and/or agriculture, contributes to the comprehensive understanding of the complexity of the chemical and biological processes involved (Tran et al., 2010).
Conclusion
The urbanized rivers studied in this research displayed some degree of impairment in their environmental quality, either due to their physical–chemical water quality or habitat degradation level, or a combination of both. Furthermore, these changes were represented by shifts in the assessed biological components. Macroinvertebrate assemblages were more suitable bioindicators of water quality, whereas amphibians showed an interesting response to nutrient loadings. Clearly, the combination of the physical–chemical water quality, habitat condition, and bioindicator metrics analysis as a whole allows a more comprehensive ecological assessment than could be achieved through separated analysis. Therefore, the incorporation of multiple indicators to identify a variety of causal mechanisms of river degradation could be a useful tool for determining environmental restoration endpoints, regulatory decision making, and ecological risk assessments. The results obtained in this study can assist in the outlining of appropriate monitoring, management, and restoration programs of this and other areas experiencing urban expansion.
Data availability
Not applicable.
References
Abbasi, T. & S. A. Abbasi, 2012. Water Quality Indices, Elsevier, Amsterdam
Alba-Tercedor, J., 1996. Macroinvertebrados acuáticos y calidad de las aguas de los ríos. In IV Simposio del agua en Andalucía (SIAGA), Vol. 2. 203–213.
Almeida, C., S. O. González, M. Mallea & P. González, 2012. A recreational water quality index using chemical, physical and microbiological parameters. Environmental Science and Pollution Research 19: 3400–3411. https://doi.org/10.1007/s11356-012-0865-5.
Amaral, P. H. M. D., L. S. D. Silveira, B. F. J. V. Rosa, V. C. D. Oliveira & R. D. G. Alves, 2015. Influence of habitat and land use on the assemblages of Ephemeroptera, Plecoptera, and Trichoptera in neotropical streams. Journal of Insect Science 15: 60. https://doi.org/10.1093/jisesa/iev042.
American Public Health Association—APHA, 2005. Standard Methods for the Examination of Water and Wastewater, 21st ed. American Public Health Association, Washington DC:
Anderson, J. R., 1976. A Land Use and Land Cover Classification System for Use with Remote Sensor Data, US Government Printing Office, Washington DC:
Babini, M. S., C. L. Bionda, Z. A. Salinas, N. E. Salas & A. L. Martino, 2018. Reproductive endpoints of Rhinella arenarum (Anura, Bufonidae): populations that persist in agroecosystems and their use for the environmental health assessment. Ecotoxicology and Environmental Safety 154: 294–301. https://doi.org/10.1016/j.ecoenv.2018.02.050.
Barbour, M. T., J. Gerritsen, B. D. Snyder & J. B. Stribling, 1999. Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish: US Environmental Protection Agency, Office of Water Washington, DC. https://www.epa.gov/sites/production/files/2019-02/documents/rapid-bioassessment-streams-rivers-1999.pdf.
Blaustein, A. R., B. A. Han, R. A. Relyea, P. T. Johnson, J. C. Buck, S. S. Gervasi & L. B. Kats, 2011. The complexity of amphibian population declines: understanding the role of cofactors in driving amphibian losses. Annals of the New York Academy of Sciences 1223: 108–119. https://doi.org/10.1111/j.1749-6632.2010.05909.x.
Bonada, N., M. Rieradevall, N. Prat & V. H. Resh, 2006. Benthic macroinvertebrate assemblages and macrohabitat connectivity in Mediterranean-climate streams of northern California. Journal of the North American Benthological Society 25: 32–43. https://doi.org/10.1899/0887-3593(2006)25[32:BMAAMC]2.0.CO;2.
Booth, D. B., A. H. Roy, B. Smith & K. A. Capps, 2016. Global perspectives on the urban stream syndrome. Freshwater Science 35: 412–420. https://doi.org/10.1086/684940.
Bounas, A., M. Keroglidou, E. A. Toli, I. Chousidis, D. Tsaparis, I. Leonardos & K. Sotiropoulos, 2020. Constrained by aliens, shifting landscape, or poor water quality? Factors affecting the persistence of amphibians in an urban pond network. Aquatic Conservation: Marine and Freshwater Ecosystems 30: 1037–1049. https://doi.org/10.1002/aqc.3309.
Calderon, M. R., P. González, M. Moglia, S. O. González & M. B. Jofré, 2014. Use of multiple indicators to assess the environmental quality of urbanized aquatic environments in San Luis, Argentina. Environmental Monitoring and Assessment 186: 4411–4422. https://doi.org/10.1007/s10661-014-3707-8.
Calderon, M. R., M. M. Moglia, R. P. Nievas, P. L. Colombetti, S. P. González & M. B. Jofré, 2017. Assessment of the environmental quality of two urbanized lotic systems using multiple indicators. River Research and Applications 33: 1119–1129. https://doi.org/10.1002/rra.3160.
Calderon, M. R., C. A. Almeida, P. González & M. B. Jofré, 2019. Influence of water quality and habitat conditions on amphibian community metrics in rivers affected by urban activity. Urban Ecosystems 22: 743–755. https://doi.org/10.1007/s11252-019-00862-w.
Chadwick, M. A., D. R. Dobberfuhl, A. C. Benke, A. D. Huryn, K. Suberkropp & J. E. Thiele, 2006. Urbanization affects stream ecosystem function by altering hydrology, chemistry, and biotic richness. Ecological Applications 16: 17961807. https://doi.org/10.1890/1051-0761(2006)016[1796:UASEFB]2.0.CO;2.
Chambers, P., D. McGoldrick, R. Brua, C. Vis, J. Culp & G. Benoy, 2012. Development of environmental thresholds for nitrogen and phosphorus in Streams. Journal of Environmental Quality 41: 7–20. https://doi.org/10.2134/jeq2010.0273.
Clausen, J. C., K. Guillard, C. M. Sigmund & K. M. Dors, 2000. Water quality changes from riparian buffer restoration in Connecticut. Journal of Environmental Quality 29: 1751–1761. https://doi.org/10.2134/jeq2000.00472425002900060004x.
Cuffney, T., R. Brightbill, J. May & R. Waite, 2010. Responses of benthic macroinvertebrates to environmental changes associated with urbanization in nine metropolitan areas. Ecological Applications 20: 1384–1401. https://doi.org/10.1890/08-1311.1.
Cushman, S. A., 2006. Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biological Conservation 128: 231–240. https://doi.org/10.1016/j.biocon.2005.09.031.
Davies, P. J., I. A. Wright, S. J. Findlay, O. J. Jonasson & S. Burgin, 2010. Impact of urban development on aquatic macroinvertebrates in south eastern Australia: degradation of in-stream habitats and comparison with non-urban streams. Aquatic Ecology 44: 685–700. https://doi.org/10.1007/s10452-009-9307-y.
De Mendiburu, F., 2014. Agricolae: statistical procedures for agricultural research. R package version 1: 1–4.
Dewson, Z. S., A. B. James & R. G. Death, 2007. A review of the consequences of decreased flow for instream habitat and macroinvertebrates. Journal of the North American Benthological Society 26: 401–415. https://doi.org/10.1899/06-110.1.
Dodd, C. K., 2010. Amphibian Ecology and Conservation: A Handbook of Techniques, Oxford University Press:
Domínguez, E. & H. R. Fernández, 2009. Macroinvertebrados Bentónicos Sudamericanos. Sistemática y Biología, Fundación Miguel Lillo, Tucumán, Argentina:
Dosskey, M. G., P. Vidon, N. P. Gurwick, C. J. Allan, T. P. Duval & R. Lowrance, 2010. The role of riparian vegetation in protecting and improving chemical water quality in streams. JAWRA Journal of the American Water Resources Association 46: 261–277. https://doi.org/10.1111/j.1752-1688.2010.00419.x.
Duffy, B. T., 2011. Relationship of substrate, silt and algal cover indices to stream nutrients. Dissertation, 35th Annual Meeting of the New England Association of Environmental Biologists.
Dunlop, J. E., N. Horrigan, G. McGregor, B. J. Kefford, S. Choy & R. Prasad, 2008. Effect of spatial variation on salinity tolerance of macroinvertebrates in Eastern Australia and implications for ecosystem protection trigger values. Environmental Pollution 151: 621–630. https://doi.org/10.1016/j.envpol.2007.03.020.
Edegbene, A. O., F. O. Arimoro & O. N. Odume, 2019. Developing and applying a macroinvertebrate-based multimetric index for urban rivers in the Niger Delta, Nigeria. Ecology and Evolution 9: 12869–12885. https://doi.org/10.1002/ece3.5769.
Fierro, P., C. Valdovinos, L. Vargas-Chacoff, C. Bertrán & I. Arismendi, 2017. Macroinvertebrates and fishes as bioindicators of stream water pollution. In Tutu, H. & B. P. Grover (eds), Water Quality InTech, Rijeka: 23–38.
García-Ayllón, S., 2016. Rapid development as a factor of imbalance in urban growth of cities in Latin America: a perspective based on territorial indicators. Habitat International 58: 127–142. https://doi.org/10.1016/j.habitatint.2016.10.005.
Gómez-Mestre, I., M. Tejedo, E. Ramayo & J. Estepa, 2004. Developmental alterations and osmoregulatory physiology of a larval anuran under osmotic stress. Physiological and Biochemical Zoology 77: 267–274. https://doi.org/10.1086/378143.
González, S. O., C. A. Almeida, M. Calderon, M. A. Mallea & P. González, 2014. Assessment of the water self-purification capacity on a river affected by organic pollution: application of chemometrics in spatial and temporal variations. Environmental Science and Pollution Research 21: 10583–10593. https://doi.org/10.1007/s11356-014-3098-y.
Hamer, A. J. & M. J. McDonnell, 2008. Amphibian ecology and conservation in the urbanising world: a review. Biological Conservation 141: 2432–2449. https://doi.org/10.1016/j.biocon.2008.07.020.
Hamid, S. & C. S. Rawi, 2017. Application of aquatic insects (Ephemeroptera, Plecoptera and Trichoptera) in water quality assessment of Malaysian headwater. Tropical Life Sciences Research 28: 143–162. https://doi.org/10.21315/tlsr2017.28.2.11.
Harguinteguy, C. A., R. Schreiber & M. L. Pignata, 2013. Myriophyllum aquaticum as a biomonitor of water heavy metal input related to agricultural activities in the Xanaes River (Córdoba, Argentina). Ecological Indicators 27: 8–16. https://doi.org/10.1016/j.ecolind.2012.11.018.
Hart, B. T., P. Bailey, R. Edwards, K. Hortle, K. James, A. McMahon, C. Meredith & K. Swadling, 1991. A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia 210: 105–144. https://doi.org/10.1007/BF00014327.
Hazelton, P. 2003. Analysis of Ephemeroptera, Plecoptera and Trichoptera (EPT) richness and diversity of Guilford Creek, Guilford, NY. State University of New York at Oneonta. http://www.oneonta.edu/ACADEMICS/biofld/PUBS/ANNUAL/2003/(200)%20Plecoptera%20and%20trichoptera.pdf.
Heino, J. & J. Soininen, 2007. Are higher taxa adequate surrogates for species-level assemblage patterns and species richness in stream organisms? Biological Conservation 137: 78–89. https://doi.org/10.1016/j.biocon.2007.01.017.
Instituto Nacional de Estadística y Censos (INDEC), 2010. Censo Nacional de Población, Hogares y Viviendas 2010. Censo del Bicentenario. https://www.indec.gob.ar/ftp/cuadros/poblacion/censo2010_tomo1.pdf.
Ji, F., D. Han, L. Yan, S. Yan, J. Zha & J. Shen, 2022. Assessment of benthic invertebrate diversity and river ecological status along an urbanized gradient using environmental DNA metabarcoding and a traditional survey method. Science of the Total Environment 806: 150587. https://doi.org/10.1016/j.scitotenv.2021.150587.
Karr, J. R., K. D. Fausch, P. L. Angermeier, P. R. Yant & J. Schlosser, 1986. Assessing biological integrity in running waters: a method and its rationale. Special Publication 5. Illinois Natural History Survey. https://semspub.epa.gov/work/01/554353.pdf.
Kefford, B. J., P. J. Papas & D. Nugegoda, 2003. Relative salinity tolerance of macroinvertebrates from the Barwon River, Victoria, Australia. Marine and Freshwater Research 54: 755–765. https://doi.org/10.1071/MF02081.
Lafortezza, R. & G. Sanesi, 2019. Nature-based solutions: settling the issue of sustainable urbanization. Environmental Research 172: 394–398. https://doi.org/10.1016/j.envres.2018.12.063.
Laposata, M. M. & W. A. Dunson, 2000. Effects of spray-irrigated wastewater effluent on temporary pond-breeding amphibians. Ecotoxicology and Environmental Safety 46: 192–201. https://doi.org/10.1006/eesa.1999.1895.
Lê, S., J. Josse & F. Husson, 2008. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25: 1–18.
Lenat, D. & D. Penrose, 1996. History of EPT taxa richness metric. Bulletin of the North American Benthological Society 13: 305–307.
Lips, K. R., J. K. Reaser, B. E. Young & R. Ibanez, 2001. Amphibian monitoring in Latin America: a protocol manual (No. 30). Society for the Study of Amphibians and Reptiles.
Ludwig, J. A. & J. Reynolds, 1988. Statistical Ecology, Wiley, New York.
Luo, K., X. Hu, Q. He, Z. Wu, H. Cheng, Z. Hu & A. Mazumder, 2018. Impacts of rapid urbanization on the water quality and macroinvertebrate communities of streams: a case study in Liangjiang New Area, China. Science of the Total Environment 621: 1601–1614. https://doi.org/10.1016/j.scitotenv.2017.10.068.
Madera, L. C., L. C. Angulo, L. C. Díaz & R. Rojano, 2016. Evaluación de la Calidad del Agua en algunos puntos afluentes del Río Cesar (Colombia) utilizando macroinvertebrados acuáticos como bioindicadores de contaminación. Información Tecnológica 27: 103–110. https://doi.org/10.4067/S0718-07642016000400011.
Mao, F., X. Zhao, P. Ma, S. Chi, K. Richards, J. Clark, D. Hannah & S. Krause, 2019. Developing composite indicators for ecological water quality assessment based on network interactions and expert judgment. Environmental Modelling & Software 115: 51–62. https://doi.org/10.1016/j.envsoft.2019.01.011.
Marshall, J. C., A. L. Steward & B. D. Harch, 2006. Taxonomic resolution and quantification of freshwater macroinvertebrate samples from an Australian dryland river: the benefits and costs of using species abundance data. Hydrobiologia 572: 171–194.
Martins, R. T., S. R. M. Couceiro, A. S. Melo, M. P. Moreira & N. Hamada, 2017. Effects of urbanization on stream benthic invertebrate communities in Central Amazon. Ecological Indicators 73: 480–491. https://doi.org/10.1016/j.ecolind.2016.10.013.
McKibbin, R., W. T. Dushenko & C. A. Bishop, 2008. The influence of water quality on the embryonic survivorship of the Oregon spotted frog (Rana pretiosa) in British Columbia, Canada. Science of the Total Environment 395: 28–40. https://doi.org/10.1016/j.scitotenv.2008.01.050.
Mekonen, S., I. Petros & M. Hailemariam, 2017. The role of nematodes in the processes of soil ecology and their use as bioindicators. Agricultural and Biology Journal of North America 8: 132–140. https://doi.org/10.5251/abjna.2017.8.4.132.140.
Merrit, R. W., K. W. Cummins & M. B. Berg, 2008. An Introduction to the Aquatic Insects of North America, 4th ed. Kendall Hunt, Dubuque.
Millar, R. G., 2000. Influence of bank vegetation on alluvial channel patterns. Water Resources Research 36: 1109–1118. https://doi.org/10.1029/1999WR900346.
Mingo Magro, J., 1981. La Vigilancia de la Contaminación Fluvial, MOPU, Madrid:
Miserendino, M. L., C. Brand & C. Y. Di Prinzio, 2008. Assessing urban impacts on water quality, benthic communities and fish in streams of the Andes Mountains, Patagonia (Argentina). Water, Air, and Soil Pollution 194: 91–110. https://doi.org/10.1007/s11270-008-9701-4.
Montenegro C., M. Strada, G. Parmuchi, J. Bono, M. Stamati, E. Manghi, M. Brouver, F. Bertolami & E. Wabo, 2007. Mapa forestal Provincia de San Luis, actualización año 2002. Unidad de Manejo del Sistema de Evaluación Forestal. Dirección de Bosques, Secretaría de Ambiente y Desarrollo Sustentable. https://www.argentina.gob.ar/ambiente/bosques/umsef.
Morello, J., S. Matteucci, A. Rodríguez & M. Silva, 2018. Ecorregiones y Complejos Ecosistémicos Argentinos, GEPAMA & Orientación Gráfica Editora, Buenos Aires:
Munné, A. & N. Prat, 2009. Use of macroinvertebrate-based multimetric indices for water quality evaluation in Spanish Mediterranean rivers: an intercalibration approach with the IBMWP index. Hydrobiologia 628: 203. https://doi.org/10.1007/s10750-009-9757-1.
New York State Department of Environmental Conservation, 2019. Standard operating procedure: Biological monitoring of surface waters in New York State. New York State Department of Environmental Conservation, Division of Water. https://www.dec.ny.gov/docs/water_pdf/sop20819biomonitoring.pdf.
Nievas, R. P., M. R. Calderon & M. M. Moglia, 2019. Environmental factors affecting the success of exotic plant invasion in a wildland-urban ecotone in temperate South America. Neotropical Biology and Conservation 14: 257. https://doi.org/10.3897/neotropical.14.e37633.
Pandey, L. K., I. Lavoie, S. Morin, J. Park, J. Lyu, S. Choi, H. Lee & T. Han, 2018. River water quality assessment based on a multi-descriptor approach including chemistry, diatom assemblage structure, and non-taxonomical diatom metrics. Ecological Indicators 84: 140–151. https://doi.org/10.1016/j.ecolind.2017.07.043.
Parmar, T. K., D. Rawtani & Y. K. Agrawal, 2016. Bioindicators: the natural indicator of environmental pollution. Frontiers in Life Science 9: 110–118. https://doi.org/10.1080/21553769.2016.1162753.
Peña Zubiate, C. A., A. d’Hiriart, E. R. Aguirre, M. A. Demmi, S. M. García, A. A. Marchi & A. P. Pascuarelli, 2006. Carta de suelos de la República Argentina Hoja San Luis, Provincia de San Luis. Instituto Nacional de Tecnología Agropecuaria y Gobierno de la Provincia de San Luis, Argentina. https://inta.gob.ar/sites/default/files/script-tmp-carta_de_suelos_hoja_san_luis.pdf.
Pounds, J. A., M. R. Bustamante, L. A. Coloma, J. A. Consuegra, M. P. Fogden, P. N. Foster, E. La Marca, K. L. Masters, A. Merino-Viteri, R. Puschendorf, S. R. Ron, G. A. Sanchez-Azofeifa, C. J. Still & B. E. Young, 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439: 161–167. https://doi.org/10.1038/nature04246.
Prat, N. & A. Munné, 2014. Biomonitoreo de la calidad del agua en los ríos ibéricos: lecciones aprendidas. Limnetica 33: 47–64. https://doi.org/10.23818/limn.33.05.
QGIS Development Team, 2020. QGIS geographic information system. QGIS Association. https://www.qgis.org.
R Core Team, 2018. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/.
Rakshit, D., G. Sahu, A. K. Mohanty, K. K. Satpathy, M. P. Jonathan, K. Murugan & S. K. Sarkar, 2017. Bioindicator role of tintinnid (Protozoa: Ciliophora) for water quality monitoring in Kalpakkam, Tamil Nadu, south east coast of India. Marine Pollution Bulletin 114: 134–143. https://doi.org/10.1016/j.marpolbul.2016.08.058.
Ridley, S. P. D., G. T. Busteed, L. B. Kats, T. L. Vandergon, L. F. Lee, R. G. Dagit, J. L. Kerby, R. N. Fisher & R. M. Sauvajot, 2005. Effects of urbanization on the distribution and abundance of amphibians and invasive species in southern California streams. Conservation Biology 19: 1894–1907. https://doi.org/10.1111/j.1523-1739.2005.00295.x.
Rocha, L., C. Feijoó, C. Hegoburu, E. Navarro, A. Torremorell & H. R. Fernández, 2020. Use of ecosystem health indicators for assessing anthropogenic impacts on freshwaters in Argentina: a review. Environmental Monitoring and Assessment 192: 3–26. https://doi.org/10.1007/s10661-020-08559-w.
Rogers, C. E., D. J. Brabander, M. T. Barbour & H. F. Hemond, 2002. Use of physical, chemical, and biological indices to assess impacts of contaminants and physical habitat alteration in urban streams. Environmental Toxicology and Chemistry: an International Journal 21: 1156–1167. https://doi.org/10.1002/etc.5620210607.
Rollins-Smith, L. A., 2009. The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochimica Et Biophysica Acta Biomembranes 1788: 1593–1599. https://doi.org/10.1016/j.bbamem.2009.03.008.
Roy, A. H., A. D. Rosemond, M. J. Paul, D. S. Leigh & J. B. Wallace, 2003. Stream macroinvertebrate response to catchment urbanisation (Georgia, USA). Freshwater Biology 48: 329–346. https://doi.org/10.1046/j.1365-2427.2003.00979.x.
Shibata, T., H. M. Solo-Gabriele, L. E. Fleming & S. Elmir, 2004. Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Research 38: 3119–3131. https://doi.org/10.1016/j.watres.2004.04.044.
Sowers, A. D., M. A. Mills & S. J. Klaine, 2009. The developmental effects of a municipal wastewater effluent on the northern leopard frog, Rana pipiens. Aquatic Toxicology 94: 145–152. https://doi.org/10.1016/j.aquatox.2009.06.013.
Sparling, D. W., 2010. Water-quality criteria for amphibians. Amphibian Ecology and Conservation: a Handbook of Techniques Part 2: 105–120.
Stancheva, R. & R. G. Sheath, 2016. Benthic soft-bodied algae as bioindicators of stream water quality. Knowledge and Management of Aquatic Ecosystems 417: 15. https://doi.org/10.1051/kmae/2016002.
Strahler, A. N., 1954. Quantitative geomorphology of erosional landscapes. In 19th International Geological Congress, Vol. 13. 341–354.
Sterling, J. L., A. D. Rosemond & S. J. Wenger, 2016. Watershed urbanization affects macroinvertebrate community structure and reduces biomass through similar pathways in Piedmont streams, Georgia, USA. Freshwater Science 35: 676–688. https://doi.org/10.1086/686614.
Szczepocka, E., B. Szulc, K. Szulc, B. Rakowska & J. Żelazna-Wieczorek, 2014. Diatom indices in the biological assessment of the water quality based on the example of a small lowland river. Oceanological and Hydrobiological Studies 43: 265–273. https://doi.org/10.2478/s13545-014-0141-z.
Tabacchi, E., L. Lambs, H. Guilloy, A. M. Planty-Tabacchi, E. Muller & H. Decamps, 2000. Impacts of riparian vegetation on hydrological processes. Hydrological Processes 14: 2959–2976. https://doi.org/10.1002/1099-1085(200011/12)14:16/17%3c2959::AID-HYP129%3e3.0.CO;2-B.
Tran, C. P., R. W. Bode, A. J. Smith & G. S. Kleppel, 2010. Land-use proximity as a basis for assessing stream water quality in New York State (USA). Ecological Indicators 10: 727–733. https://doi.org/10.1016/j.ecolind.2009.12.002.
Ultsch, G. R., D. F. Bradford & J. Freda, 1999. Physiology: coping with the environment. In McDiarmid, R. W. & R. Altig (eds), Tadpoles: The Biology of Anuran Larvae University of Chicago Press, Chicago: 189–214.
Vallania, E. A., P. A. Garelis, E. S. Trípole & M. A. Gil, 1996. Un índice biótico para las Sierras de San Luis (Argentina). Rev UNRC 16: 129–136.
Violin, C. R., P. Cada, E. B. Sudduth, B. A. Hassett, D. L. Penrose & E. S. Bernhardt, 2011. Effects of urbanization and urban stream restoration on the physical and biological structure of stream ecosystems. Ecological Applications 21: 1932–1949. https://doi.org/10.1890/10-1551.1.
Walker, T., P. Crittenden, V. Dauvalter, V. Jones, P. Kuhry, O. Loskutova, K. Mikkola, A. Nikula, E. Patova, V. Ponomarev, T. Pystina, O. Ratti, N. Solovieva, A. Stenina, T. Virtanen & D. Scott, 2009. Multiple indicators of human impacts on the environment in the Pechora Basin, north-eastern European Russia. Ecological Indicators 9: 765–779. https://doi.org/10.1016/j.ecolind.2008.09.008.
Wake, D. B. & V. T. Vredenburg, 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proceedings of the National Academy of Sciences 105: 11466–11473. https://doi.org/10.1073/pnas.0801921105.
Walsh, C. J., A. H. Roy, J. W. Feminella, P. D. Cottingham, P. M. Groffman & R. P. Morgan, 2005. The urban stream syndrome: current knowledge and the search for a cure. Journal of the North American Benthological Society 24: 706–723. https://doi.org/10.1899/04-028.1.
Wang, L. & P. Kanehl, 2003. Influences of watershed urbanization and instream habitat on macroinvertebrates in cold water streams. Journal of the American Water Resources Association 39: 1181–1196. https://doi.org/10.1111/j.1752-1688.2003.tb03701.x.
Wang, B., D. Liu, S. Liu, Y. Zhang, D. Lu & L. Wang, 2012. Impacts of urbanization on stream habitats and macroinvertebrate communities in the tributaries of Qiangtang River, China. Hydrobiologia 680: 39–51. https://doi.org/10.1007/s10750-011-0899-6.
Wang, L., H. Li, J. Dang, Y. Zhao, Y. E. Zhu & P. Qiao, 2020. Effects of urbanization on water quality and the macrobenthos community structure in the Fenhe River, Shanxi Province, China. Journal of Chemistry. https://doi.org/10.1155/2020/8653486.
Weijters, M. J., J. H. Janse, R. Alkemade & J. T. A. Verhoeven, 2009. Quantifying the effect of catchment land use and water nutrient concentrations on freshwater river and stream biodiversity. Aquatic Conservation: Marine and Freshwater Ecosystems 19: 104–112. https://doi.org/10.1002/aqc.989.
Weir, L. A. & M. J. Mossman, 2005. North American Amphibian Monitoring Program (NAAMP). In Lannoo, M. J. (ed), Amphibian Declines: Conservation Status of United States Species University of California Press, Berkeley, California: 307–313.
White, J. Y. 2017. The effects of urban land use, riparian forest cover, and instream habitat complexity on macroinvertebrate assemblages. Dissertation, University of Melbourne.
Wickham, H., W. Chang & M. H. Wickham, 2016. Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version 2: 1–189.
Wilson, H. L., M. F. Johnson, P. J. Wood, C. R. Thorne & M. P. Eichhorn, 2021. Anthropogenic litter is a novel habitat for aquatic macroinvertebrates in urban rivers. Freshwater Biology 66: 524–534. https://doi.org/10.1111/fwb.13657.
Woodiwiss, F., 1964. The biological system of stream classification used by the Trent River Board. Chemistry & Industry 11: 443–447.
Zampella, R. A., J. F. Bunnell, K. J. Laidig & N. A. Procopio, 2006. Using multiple indicators to evaluate the ecological integrity of a coastal plain stream system. Ecological Indicators 6: 644–663. https://doi.org/10.1016/j.ecolind.2005.08.027.
Acknowledgements
Authors gratefully acknowledge Instituto de Química de San Luis “Dr. Roberto Olsina”- Consejo Nacional de Investigaciones Científicas y Tecnológicas (INQUISAL-CONICET) and Universidad Nacional de San Luis (Project PROICO 2-2418) for financial support. We thank MSc. Angela Stires for the valuable language and grammar revision of the manuscript. Finally, we would like to thank two anonymous reviewers for their comments that helped to improve the quality of this manuscript.
Funding
This work was supported by Universidad Nacional de San Luis through a Grant Project PROICO 02-2418 and a Postdoctoral Grant for Dr. Mirian Calderon (RESOL-2020-134-APN-DIR#CONICET) from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design of the results provided in this manuscript. Material preparation, data collection, and analysis were performed by MRC, SPG, JMPI, and MBJ. The first draft of the manuscript was written by MRC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Research involving human and animal participants
Not applicable.
Additional information
Handling Editor: Marcelo S. Moretti
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calderon, M.R., González, S.P., Pérez-Iglesias, J.M. et al. Anthropogenic impacts on rivers: use of multiple indicators to assess environmental quality status. Hydrobiologia 850, 469–487 (2023). https://doi.org/10.1007/s10750-022-05090-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05090-6