Abstract
Urbanization poses threats to earth’s biota, and retention of remnant native habitat in protected areas within expanding urban boundaries may help alleviate threats to wildlife. However, it is unclear for nearly all nonsynanthropic (i.e., not benefiting from an association with humans) species whether vital rates in urban habitats can sustain populations or if populations persist only through immigration from outside the urban boundary. We conducted a three-year study of spotted towhees (Aves: Pipilo maculatus) breeding in four undeveloped parks in Portland, OR, USA, to measure park-specific seasonal reproductive output (F) and annual adult survival (SA). We developed a stochastic model that combined F and SA with an estimate of first-year survival to measure population growth rate (λ) in all parks assumed to be closed to immigration. F differed among parks but SA did not. Relatively high F was possible because many pairs raised >1 brood/season. When combined with empirical estimates of survival through the 30-day period of post-fledging parental care (SD = 0.645), only 2 of 4 parks were self-sustaining (i.e., λ > 1.0). However, SD reflected substantial loss of fledglings to domestic cats (Felis catus). Assuming no loss to cats and either partial compensatory or additive mortality of fledglings substantially improved prospects of population persistence for declining (sink) populations. Moreover, allowing low levels of immigration to sinks reversed population declines in most parks even when vital rates were insufficient to maintain populations. Our results suggest that nonsynanthropic bird species can persist in urban landscapes, but also that offspring mortality in the post-fledging period may be a critical determinant of population viability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current rates of urbanization are unmatched in human history and continue to accelerate above that of human population growth (Seto et al. 2012, Aronson et al. 2014). In the United States, the main pattern of expansion is of the spread of single-family dwellings that radiate outward from the city center along transportation corridors (Ramalho and Hobbs 2012). The spider web-like growth fragments, degrades, and reduces the area of natural and semi-natural habitat available for wildlife. Urbanization is a major contributor to global loss of biodiversity (McDonald et al. 2008, Seto et al. 2012), but it is also possible for native wildlife to persist in urban areas (e.g., Hedblom and Söderström 2010). However, success at maintaining urban populations of native wildlife is challenging because geographic differences in economies, climate and biotic diversity often prohibit universal management strategies (Seto et al. 2011).
Responses of native species to urbanization and habitat loss also vary (McKinney 2008, Fischer et al. 2012). While few species remain at the upper end of the urbanization gradient where little habitat exists (Blair and Launer 1997, Germaine and Wakeling 2001, Chace and Walsh 2006, McKinney 2008, Sims et al. 2008), others appear well adapted to urban spaces (Partecke et al. 2004, 2006, Guénard et al. 2015), and in some urban landscapes species richness may be independent of position along the urban to peri-urban/rural gradient (e.g., Hedblom and Söderström 2010) or even peak at intermediate levels of urbanization (McKinney 2008, Sims et al. 2008). Local biota has greater capacity to persist with increases in the number and size of protected greenspaces in the landscape (Crooks et al. 2004, McKinney 2008), and with the exception of forest specialists (e.g., Hedblom and Söderström 2010), abundances of individual species can be higher in urbanized than in peri-urban/rural settings (Shochat 2004, Leston and Rodewald 2006, Fischer et al. 2012).
An important caveat, however, is that even where networks of greenspaces and other seemingly suitable habitats exist, it is unclear for most species whether populations in human dominated landscapes are self-sustaining, or if they persist only because of enrichment from outside sources (e.g., van Heezik et al. 2010, Schaub et al. 2010, 2013). Indeed, Snep et al. (2006) and Hedblom and Söderström (2010) suggest that peri-urban habitat surrounding cities might serve as a source of individuals for urban habitats, and urban environments certainly provide potential benefits that might draw individuals in from surrounding areas. For birds, cities in seasonal northern latitudes provide a thermally favorable environment that advances spring phenology (Neil and Wu 2006), which along with food supplementation by humans (Robb et al. 2008), may lead to higher overwinter survival (Horak and Lebreton 1998, Evans et al. 2009, Stracey and Robinson 2012) and earlier start to breeding (Fleischer et al. 2003, Partecke et al. 2004, Stracey and Robinson 2012; reviewed by Chamberlain et al. 2009). Lengthy breeding seasons may enhance seasonal production of young, but whether annual production of young by urban breeders is lower (Chamberlain et al. 2009) or higher (Fischer et al. 2012, Stracey and Robinson 2012) than conspecifics breeding in nearby rural areas remains an open question.
Predators are the main causes of nest failure for most birds (Ricklefs 1969), and snakes contribute heavily to nest loss in many systems (Patten and Bolger 2003, Weatherhead and Blouin-Demers 2004, Cox et al. 2013). Low abundance of snakes in urbanized environments (Patten and Bolger 2003) may thus improve prospects of success for ground and shrub nesting birds. By contrast, the absence of large predators from cities often allows smaller synanthropic (i.e., benefiting from their association with humans) predator populations to grow (Crook and Soulé 1999) such that densities of urban predators may exceed that found in surrounding less urbanized lands (reviewed by Fischer et al. 2012). However, as suggested by the higher survival rates of adult birds in urban habitats (see above), predator abundance and predation rates on adults, young, and nests may be poorly linked because of resource supplementation to omnivorous predators or predator specialization on exotic species in urban environments (the “predator paradox”; Shochat 2004, Rodewald et al. 2011, Fischer et al. 2012). On the other hand, predation of birds by domestic cats (Felis catus) may compromise persistence of native species (Lepczyk et al. 2003, Baker et al. 2008, Sims et al. 2008, van Heezik et al. 2010, Balogh et al. 2011), while indirect effects of cats expressed through alteration of parental behavior may have even greater negative influence on avian populations than that caused by direct mortality (Beckerman et al. 2007, Bonnington et al. 2013). Physical effects of the urban environment, including potential exposure to contaminants (Roux and Marra 2007), excessive noise and light pollution (Fuller et al. 2007, Kempenaers et al. 2010), and death from vehicular or building collisions (Klem et al. 2009, Loss et al. 2014a, b) have the potential to create poor environments for breeding birds. Given the dearth of detailed avian demographic studies in urban environments (but see Leston and Rodewald 2006, Mannan et al. 2008, Stracey and Robinson 2012), much remains to be learned of the balance between the costs and benefits of breeding in urban landscapes for birds and other vertebrates.
To that end, we conducted demographic studies of spotted towhees (Pipilo maculatus; henceforth towhee) in four parks in the Southwest Hills of Portland, Oregon, USA, to determine if towhee populations in Portland are self-sustaining, have the potential to function as an internal system of sources and sinks (sensu Pulliam 1988), or are dependent on outside (peri-urban/rural) sources for persistence. We do so by using park-specific estimates of vital rates from our field studies to develop a stochastic population model to estimate population growth rate (λ) under different scenarios of first-year survival and immigration. Our different scenarios of first-year survival arise as a consequence of modeling different combinations of post-fledging mortality driven by domestic cat predation of dependent fledglings, and survival of independent first-year birds to their first year of reproduction.
Methods
Study area and species
Portland is a medium-sized city with a population of 619,000; ~2,500,000 residents live in the greater metropolitan area (2014; U.S. Census Bureau). Portland’s park system is well-developed and the four parks included in our study are located in the residential landscape of the Southwest Hills in southern Multnomah and northern Clackamas counties. Lesser Park (LSSR; 9 ha, perimeter =2056 m), Maricara Park (MARC; 9.8 ha, perimeter =1417 m), and West Portland Park (WPDX; 16.2 ha, perimeter =2946 m) all have a 15–25 m tall canopy of mainly Douglas-fir (Pseudotsuga canadensis) and big-leafed maple (Acer macrophyllum) that is ~60 year old. Canopy height at Springbrook Park (SPBK; 24.1 ha, perimeter =3566 m) is slightly lower (15–20 m) and there is a more equitable distribution of trees among conifers and angiosperms with red alder (Alnus rubra) and paper birch (Betula papyrifera) also being common. All parks are undeveloped except for trails, with understories including native sword fern (Polystichum munitum), salal (Gaultheria shallon), Indian-plum (Oemleria cerasiformis), dull Oregon-grape (Mahonia nervosa), beaked hazelnut (Corylus cornuta), and thimbleberry (Rubus parviflorus), as well as non-native Himalayan blackberry (Rubus armeniacus), English ivy (Hedera helix), and English holly (Ilex aquifolium).
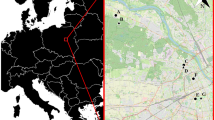
The landscape around all four parks is similar (Fig. 1); all are surrounded almost entirely by private homes and forest cover dominates. Tree canopy cover approaches 100 % at the park edges and remains high (~60 %) beyond park borders because both undeveloped canopy (no impermeable surfaces below trees) and developed canopy (impermeable surface beneath trees) remain relatively constant at between 10 % to 20 %, and 30 % to 50 % of land area, respectively, out to 500 m beyond park edges. Although relatively close to one another (Fig. 1), we treated the parks as separate units for several reasons. First, while floristically similar, structural differences existed (M. T. Murphy, unpubl. Data). Second, trail development and human use was much lower at WPDX and LSSR than at MARC and SPBK, and third, we never recorded any adult dispersal between parks. The latter, and previous work showing that towhees at each park had relatively unique cloacal bacterial signatures (Klomp et al. 2008), suggested the potential for independent demographic processes.
Image of the Southwest Hills in Portland, OR, USA, with the four study parks where L, M, S, and W refer to Lesser, Maricara, Springbrook, and West Portland parks. Red lines represent major highways dissecting the landscape, while light green areas represent parks supported by Portland Parks and Recreation
Towhees can serve as a model for a suite of ground-foraging bird species found in P&GS in Portland and the Pacific Northwest that feed their young invertebrates during the breeding season (i.e., Song Sparrows [Melospiza melodia], Dark-eyed Juncos [Junco hyemalis], Pacific Wren [Troglodytes pacificus]). Like the others, towhees are year-round residents, socially monogamous, and multi-brooded, and like all except the Pacific Wren, lay 3 to 4 eggs per clutch in open-cup nests that are placed on the ground (Bartos Smith et al. 2012, Shipley et al. 2013). We also chose to study towhees because, although widely distributed in nonurban habitats across the western United States (Bartos Smith and Greenlaw 2015), they are common in Portland’s parks and greenspaces (P&GSs) (M. T. Murphy unpubl. Data) and offered an opportunity to study a species not a priori adapted to urban environments. As a ground-nester, towhees also belong to an ecological group for which few data on reproductive success exist in cities (Chamberlain et al. 2009). Lastly, because they are resident, mortality is driven by local phenomena.
Field methods
We located nests and collected reproductive data for color-banded pairs from the 2004 through 2006 breeding seasons in all parks except WPDX (reproductive data for 2005 and 2006). Nest searching began in March of each year and continued until the end of the breeding season (early August). Nests were checked every 2–3 days to record number of eggs, number to hatch, and number of young to fledge. Towhees sometimes desert nests if disturbed during egg-laying; we therefore often waited until incubation to count eggs. If laying date of eggs was not observed directly we recorded hatch date, and from this we obtained laying date by subtracting one day for each egg laid and 12 days for incubation (S. Bartos Smith, unpubl. data). Laying date was converted to Julian date. Young were banded just before fledging (day 8–10 days) with a metal federal and three unique color bands. We then monitored activity of pairs that either failed or fledged young to locate new nest attempts. Identical data were collected from these nests.
Adults were captured and individually marked during the breeding seasons of 2004 through 2006 at all four parks (Table 1). We captured males in mist nets using a conspecific taxidermic mount along with song playback, whereas females were captured by mist nets placed near nests during the nestling period. We monitored the seasonal reproductive output of a subset of individually marked females (n = 138) by documenting the outcome of all nests using methods described above. Adult return rate was established by resighting color-banded birds during surveys (2005–2007) in which parks were exhaustively searched for towhees several times per week in April and early May, or by recapture at targeted nests.
Survival analyses
We used Program MARK (White and Burnham 1999) to calculate apparent adult annual survival (SA) for bird in all parks by considering all possible combinations of sex and time (year) on both survival (φ(.), φ(sex), φ(time), φ(sex*time)) and detectability (p(.), p(sex), p(time), p(sex*time)). Goodness of fit tests were run on the fully time-dependent model using program RELEASE (within MARK) to verify that our data adequately fit the assumptions of equal survival and detection across years. The “median ĉ” function was used to estimate ĉ (the variance inflation factor), and we adjusted all models by this value. Survival estimates (φ) were based on the top model(s), which were those in which the difference (∆) in AIC was ≤2.0 (Burnham and Anderson 2002).
Population model and simulations
We calculated growth rate as the finite rate of increase (λ) using empirically derived estimates of apparent annual adult survival (SA) and seasonal production of young by individually marked females (F: reduced by 50 % to represent females [F/2]), and an estimated survival rate of young to age one year (i.e., the first year of breeding [SYr1]; Pulliam 1988):
We had to estimate SYr1 because, although a portion of the fledged towhees remain in their natal park (e.g., 3 of 14 radio transmittered young from 2008 of Shipley et al.’s study (2013) recruited into the SPBK population in 2009), most seem to disperse through the well-vegetated landscape (Fig. 1) to settle in other parks, greenspaces, or residential backyards. Reports of banded juveniles have come from bird feeders in residential backyards up to 4 km from nest sites. We thus assume that emigration and immigration cancel so that, functionally, the simulation of each parks’ population dynamics could be seen as driven by in situ birth and death rates. This allowed us to evaluate whether each park’s vital rates were sufficient to sustain it without immigration.
SYr1 was separated into the immediate post-fledging period when young are still dependent on parents for care (SD), and the period of independent life that follows and ends with the beginning of the next breeding season (SI); SYr1 = SD*SI. We applied Shipley et al.’s (2013) measure of SD over the 30-day post-fledging period of towhees at SPBK to all parks. We view this as justified as a first approximation of SD given that SA was very similar at all parks (see below). We then incorporated a range of values for SI estimated as a proportion of SA that varied as either 0.7*SA, 0.8*SA, 0.9*SA, or 1.0*SA. The four proportions were chosen because they included the range of ratios of hatch-year to adult survival recorded by Siriwardena et al. (1998) from young banded as independent individuals in the summer of their hatch and adults banded at the same time. We then used STELLA (v. Stella 10.0.3) to simulate a decade of population change in which, at each annual iteration, a new value of F, SA, and SD was selected randomly from within ±2 standard errors (SE) of the park-specific mean of F and SA or ±20 % of SD (± 0.129) to calculate λ. SI was estimated for each year of the decade at the designated proportion of the randomly selected SA. We chose to simulate a decade of change because the vital rates that we used reflected conditions over a relatively brief period (3 years) that, while likely to persist into the near future (a decade), may not persist for longer given the possibility of habitat change due to increased densification from infill, natural succession, or climate change.
We capped population size at a park-specific carrying capacity (K) that was based on the highest estimated count of breeding pairs in each park over the period of study (LSSR =20, MARC =24, WPDX =24, and SPBK =35). All observed values were higher than what would be expected given the area of each park and the average territory size (1.02 ha) reported from three studies of the towhee’s sister species of identical size (P. erythrophthalmus; Greenlaw 2015), suggesting that all four populations were at or near full capacity over the period of study. To start the simulations all populations were seeded with an initial population size (N0) of 0.25*K. The value of 0.25 was arbitrary and irrelevant for generating estimates of λ because the model did not incorporate density-dependence. However, it was essential to have all populations begin at the same size in relation to K so that it would be possible to compare final population size (NFinal) after a decade of change. Mean λ, averaged over the decade long simulation, was our measure of population viability for each simulation, while NFinal equaled population size in the 10th year. When run as a deterministic model (i.e., all vital rates held constant), the STELLA model produced estimates of λ equal to that generated by a two-stage (juvenile and adult) Leslie matrix for a postbreeding census of a birth pulse population (see Dinsmore et al. 2010 for the matrix formulation and Appendix 1 for more information on the STELLA model).
We also modified the model to explore ramifications of variation in SD and immigration on population viability. Whittaker and Marzluff’s (2009) estimate of SD (0.835) for towhees from Seattle, Washington, where losses of fledglings to domestic cats was not documented (K. A. Whittaker, pers. comm.), was higher than that reported by Shipley et al. (2013; SD = 0.645) from SPBK where cat predation accounted for over a third of fledgling mortality. Our baseline model of post-fledging survival incorporated losses to cats (SD = 0.645), but we also performed simulations in which we assumed no losses to cats, and that fledgling mortality was either entirely additive (i.e., fledglings formerly lost to cats were not killed by other predators; SD = 0.808), or that fledgling mortality exhibited partial compensation such that half the fledglings lost to cats survived but the other half were killed by other predators (SD = [0.645 + 0.808]/2 = 0.726). We applied the same error estimate for SD in the baseline model (0.129) to the other two. In a separate set of simulations run with baseline conditions we relaxed the assumption of a closed population to test for the capacity of low level immigration to rescue declining populations. Immigration was allowed to occur randomly to parks with sink populations at a rate equal to one female every other year.
We ran 500 simulations of the baseline model (SD = 0.645) for all parks, but used only every 5th simulation to calculate grand mean λ (n = 100) to minimize the possibility that randomly chosen values were serially correlated. Further analyses of the baseline data showed that only 50 simulations were required before a stable grand mean λ was obtained, and therefore evaluation of population viability when either SD exceeded 0.645 or immigration was allowed were based on 50 decade-long simulations (i.e., every 5th simulation of 250 used to calculate grand mean λ).
Statistical analyses
Resident, multibrooded species often exhibit a midseason peak in egg number (e.g., Dhondt et al. 2002). To account for this potential source of variation, we tested for differences in number of eggs/nest and fledglings/nest using analysis of covariance (ANCOVA) with park and year as fixed effects and laying date and its quadratic as covariates. Total seasonal productivity of individually marked females was analyzed using analysis of variance. Assessment of the significance of differences in SA between sexes and among parks was based on overlap of 95 % confidence intervals (CI) calculated within MARK. Use of 95 % confidence intervals to establish statistical significance using α = 0.05 can lead to overly conservative conclusions (Payton et al. 2003), but with only one possible exception that was ultimately consistent with our conclusions, our results appear robust to the potential flaw of using confidence intervals for this purpose.
Different scenarios created by combining the various levels of post-fledging mortality (SD = 0.645, 0.726, and 0.808) and 10-month estimate of survival living as independent juveniles (SI = 0.7, 0.8, 0.9, or 1.0*SA) generated 12 λs for each park. Evaluation of differences in λ within parks across different combinations of SD and SI, and among parks for the same combination of SD and SI, were made by examining whether 95 % CI surrounding grand mean estimates of λ overlapped estimates of λ from other simulations. Assessment of whether populations were sustainable were made by evaluating whether the 95 % CI of grand mean λ for each park at each combination of SD and SI included 1.0; λ of a sustainable but non-growing population included 1.0 in its 95 % CI, whereas 95 % CI of declining and increasing populations fell below and above 1.0, respectively. To assess the impact of immigration on population persistence, we identified parks with declining populations under conditions specified by our baseline model (i.e., lowest post-fledging survival of 0.645), and then reran the simulations with the possibility of randomly adding a female immigrant, on average, every other year. Average vital rates did not change and therefore λ was predicted to be unchanged between models with and without immigration. However, NFinal was predicted to be greater with limited immigration, and if rescue was possible, then NFinal had to also either equal or exceed N0. We tested the latter prediction by assessing whether the 95 % CI of relative final population size (NFinal/K) exceeded starting population size (= 0.25*K).
All analyses were done in Statistix version 9.0 (Analytical Software, Tallahassee, Florida, USA) with significance accepted at P ≤ 0.05. Statistics are reported as mean ± SE, and n.
Results
Reproduction
Number of eggs in nests (“apparent clutch size”) ranged from 2 to 5 (mean = 3.25 ± 0.035 eggs, mode = 3 eggs, N = 338; excluding 8 nests with 1 egg). Results of the analysis of covariance showed that apparent clutch size exhibited a midseason peak, but did not vary among either years or parks (Table 1). By contrast, a similar analysis showed that no relationship existed between of number of fledglings per nesting attempt and either laying date or year, but a significant park effect existed due to the fewer number of young produced per nest at SPBK compared to WPDX (Table 1). Limiting the analysis of number of fledglings per attempt to just successful nests (i.e., fledged ≥1 young) again failed to detect any temporal effects (results not shown), but that successful nests at SPBK produced fewer young (2.07 ± 0.111, n = 69) than successful nests at both WPDX (2.62 ± 0.131, n = 50) and MARC (2.63 ± 0.126, n = 54), while nests at LSSR were intermediate (2.35 ± 0.128, n = 52) and not different from nests at other parks (analysis of variance: F = 5.00, df = 3, 221, P = 0.002, and post hoc comparisons made with Tukey’s test).
Number of successful nests/year for individually marked females ranged from 0 (10.1 % of females) to 3 (6.5 % of females), with a mean of 1.4 (± 0.06; 48.6 % and 34.8 % of females had 1 and 2 successful nests, respectively). Chance of complete failure or fledging of young from 1, 2 or 3 nests did not differ among parks (X 2 = 6.11, df = 9, P = 0.729). For individually marked females, total number of young produced per season (i.e., seasonal productivity) followed the same ranking of parks as for nest productivity; highest seasonal productivity was at WPDX and LSSR, followed by MARC and then SPBK (Table 2). However, seasonal productivity for individually marked females did not differ among parks (F = 1.61, df = 3, 134, P = 0.191) despite mean seasonal productivity of both LSSR and WPDX falling outside the 95 % CI of seasonal productivity at SPBK (and vice versa; Table 2).
Adult survival
None of the 265 adult towhees that contributed to our survival analyses (2004 through 2007; Table 2) went undetected in one year and reappeared later, and therefore detectability (p) for all of our top MARK models was 1.00. Estimates of c-hat for each park based on the fully time-dependent model were all less than 1.5 (LSSR =1.03, MARC =1.33, SPBK =1.46, WPDX =1.24), indicating a good fit between the survival models and our data. Nonetheless, we adjusted all models by the park-specific c-hat values to maximize accuracy. For each park, the most strongly supported model was based on time- and sex-independent survival and detectability (φ(.)p(.)), followed by the model with sex-dependent survival (φ(sex)p(.); Table 3). At MARC, the sex-dependent survival model was not well supported as its ∆QAICc was >2.00, and at LSSR and WPDX, the sex-dependent model was less than half as likely as the simplest sex-independent model. SPBK was the only park where the sex-dependent survival model was nearly as well-supported as the simplest model (Table 3). However, the confidence intervals for SA of males (φ(males) = 0.574, SE = 0.073, CI = 0.429–0.706) and females (φ(females) = 0.431, SE = 0.084, CI = 0.280–0.597) overlapped at SPBK. Because of this, and the generally strong support for the simplest model at all four parks, we did not differentiate between sexes and used the park-specific MARK estimates of survival (Table 2) in our simulations.
Population viability
λ varied among parks in a consistent manner: at a given level of post-fledging survival (SD = 0.645, 0.726, or 0.808) and survival from parental independence to first breeding (SI = 0.7*SA, 0.8*SA, 0.9*SA, or 1.0*SA), λ was always highest at WPDX and lowest at SPBK (Fig. 2). Every combination of SD and SI yielded positive growth (λ > 1.0) at WPDX, but at SPBK, half of the combinations of SD and SI resulted in population declines (λ < 1.0; Fig. 2). On average, λs were second highest at LSSR (> 1.0 in 11 of 12 combinations of SD and SI), followed by MARC (> 1.0 in 6 of 12 combinations of SD and SI). To summarize, with 4 parks and 4 levels of SI, 16 estimates of λ were generated for all 3 values of 30-day post-fledging survival (SD). At SD of 0.645 (mortality due to domestic cats assumed to exist at all parks), 6 of 16 λs were significantly below replacement (1.0). This improved to only 3 of 16 when SD increased to 0.726 to reflect removal of cat-induced mortality and partial compensation. Only 1 of 16 simulations generated a λ < 1.0 when cat-induced mortality was eliminated and post-fledging mortality was fully additive (SD = 0.808).
Simulated population growth rate (i.e., lambda; means ±95 % confidence intervals) of Spotted Towhees in four parks in Portland, OR, USA, under three conditions of mortality in the 30-day post-fledging period (SD) and four levels of mortality in the subsequent ~10-month period of independence just prior to the first breeding season (SI). Baseline conditions (a) reflect loss of fledglings to all sources of mortality, including domestic cats, reported by Shipley et al. (2013), whereas mortality due to domestic cats was removed and either partial compensatory mortality (b) or additive mortality (c) of fledglings was assumed. For all three scenarios of SD we also modeled four levels of SI such that offspring in the 10-month period between obtaining independence and recruiting into the breeding population survived at either 70 %, 80 %, 90 % or 100 % of survival rate exhibited by adults. First year survival (fledging to recruitment) is thus equal to SD*SI. Horizontal dashed line at lambda equal 1.0 represents a stable population that just replaces itself, while lambdas above and below 1.0 represent growing and declining populations, respectively
Vital rates did not change between simulations with and without immigration, and therefore as expected, the 6 populations that exhibited population declines in our set of 16 baseline models (SD = 0.645) also exhibited λs < 1.0 when limited immigration was allowed (on average, 1 female every other year). Within park comparisons of λ for simulations with and without immigration also never differed (Fig. 3a). By contrast, 3 of 6 populations exhibited significant positive growth when immigration was allowed while overlap of the 95 % CI of the MARC population at SI = 0.7*SA with relative final population size of 0.25 indicated that it could not be distinguished from a stable population (Fig. 3b)
Comparison of population growth rate (lambda; a) and final population size after a decade of growth (b) for Spotted Towhees in four parks in Portland, OR, USA, when modeled as either closed (i.e., no immigration) or open populations with, on average, one immigrant female arriving every other year. Comparisons limited to populations in decline when mortality during the 30-day postfledging period (SD) reflected losses of fledglings to all sources of mortality (SD = 0.645; Shipley et al. 2013). Values are means ±95 % confidence intervals (CI), with pairwise comparisons made between closed and open populations. Labels on the abscissa designate the combinations of park (LSSR = Lesser, MARC = Maricara, and SPBK = Springbrook) and mortality during the 10-month period of juvenile independence (SI) that yielded declining populations when they were assumed to be closed to immigration. Dashed horizontal line in (b) represents starting population size (0.25*carrying capacity) such that populations whose 95 % CI does not include the dashed line and are below or above the line represent populations that declined or grew over the decade long simulation, respectively
Discussion
Population simulations using our empirically determined and park-specific vital rates showed that 2 of the 4 parks under study were self-sustaining. Moreover, by incorporating a wider range of realistic combinations of post-fledging survival during the period of parental dependence (SD) and then independence over the first year (SI) we also showed that a majority of simulated towhee populations were self-sustaining. The excess recruits produced under most combinations of SD and SI (Fig. 2) were available to disperse to supplement numbers at other parks. When combined with our other finding that low-level, but steady, immigration reversed declines in most populations with vital rates that, based on birth and death rates yielded λs < 1.0, this suggests strongly that immigration from peri-urban/rural sites was not necessary to maintain towhee populations within the Southwest Hills of Portland. Rather, towhee populations in the Southwest Hills likely function as a viable system of sources and sinks (sensu Pulliam 1988). The strength of our conclusions rests heavily on the quality of our data and the reliability of the assumptions upon which our models were based. We have high confidence in our estimates of annual towhee productivity and survival, and below we address our assumptions.
First year survival
As with all population studies of passerine birds (e.g., Shutler et al. 2006), measurement of survival to age at first reproduction presented a challenge. To help generate the best estimate possible we separated the first year into the period of parental dependence that follows fledging, and the longer period of independent living that precedes first breeding. Our radio telemetry studies from SPBK indicated that nearly two-thirds of towhee young survived the ~30-day period of parental dependence (Shipley et al. 2013). We assumed this estimate was temporally stable (i.e., applied to earlier years) and was applicable to other parks. Important predators of fledglings at SPBK included Cooper’s hawks (Accipiter cooperii), western screech owls (Megascops kennicottii) and domestic cats (Shipley et al. 2013). Cooper’s hawks were present in all four parks. Screech owls are abundant in Portland and we suspect they too were found in all four parks. We are less certain, however, of the extent of cat predation on fledgling towhees at other parks. This uncertainty was, in large part, the impetus behind our modeling effort. By assuming cat predation was equally strong at all parks we may have created a worst case scenario. Modeling scenarios of relaxed domestic cat predation thus allowed us to assess the extent to which domestic cats may effect towhee populations.
An even greater uncertainty existed for survival over the ~10-month period of independence (SI). We assumed that the disappearance of most juveniles from their natal park did not represent complete mortality. Rather, most towhee juveniles, like other juvenile passerines, presumably left their natal site and dispersed to nearby but unsurveyed sites (Fig. 1), or like most passerines, well beyond the limits of the study site (Weatherhead and Forbes 1994). To account for this uncertainty we incorporated a series of SI values based on empirical data (Siriwardena et al. 1998) to allow for a realistic range of estimates for SYr1. When we modeled the worst case, but conceivable, combination of SD (= 0.645) and SI (= 0.7*SA), WPDX was the only viable population. Low seasonal productivity of young in part explains the inability of SPBK and possibly MARC to maintain numbers, but low first year survival (= SD*SI) is a major concern because it reflects the degree to which of our assumptions matched reality.
An SI of 0.7 is at the low end of estimates reported by Siriwardena et al. (1998), while the baseline SD recorded at SPBK (0.645) reflects contribution from domestic cats to fledgling mortality (37 % of all fledgling deaths). Whittaker and Marzluff (2009) reported substantially higher post-fledging survival rates of towhees in Seattle, Washington (0.835) where losses of towhees to domestic cats were not recorded (K. Whittaker, pers. Comm.). Removing losses to domestic cats raised our estimate of SD to a level (0.808) very similar to that reported for Seattle. When we modeled an SD of 0.808 and the next highest SI (0.8*SA), that was nonetheless still below the average SI reported by Siriwardena et al. (1998), we found that populations in 3 of 4 parks grew, while SPBK maintained its numbers (Fig. 2). The latter scenario assumed complete additivity of post-fledging mortality. Relaxing this assumption to allow for partial compensation (SD = 0.726) left SPBK as the only park with declining numbers (Fig. 2). We thus view our baseline results (Fig. 2a) as worst case scenario as losses to domestic cats might well be lower at other parks, or lower in the future at SPBK if domestic cat activity could be eliminated.
If domestic cats were more abundant at SPBK than elsewhere, this may explain both lower seasonal productivity and low female SA at SPBK. Predator activity has potential effects beyond the direct killing of individuals (Lima 2009). The mere threat of predators may cause parent birds to alter incubation patterns (Zanette et al. 2011), reduce parental feeding rate of young (Dunn et al. 2010, Zanette et al. 2011), and reduce seasonal productivity (Zanette et al. 2011). The commotion caused by parental alarm calling and active defense in response to predator approach also has the potential to draw the attention of other predators. Simulation studies predict that indirect sublethal effect of predators on breeding birds may be greater than their direct effects (Beckerman et al. 2007), and Bonnington et al. (2013) verified that parent European blackbirds (Turdus merula) reduced food delivery after exposure to taxidermic models of a domestic cat, and that nest loss to corvid predators was higher following exposure to cat models than to controls. Similar adjustments to predator exposure may occur in towhees as our comparisons of towhee parental behavior at nests 2 to 3 days before potential fledging showed that feeding rate to young was significantly lower at nests that eventually failed than at nests that eventually fledged young (McKay 2008). Not only was complete nest failure more common at SPBK, but productivity of successful nests was also lowest. Nest failure and low productivity are associated with increased dispersal in passerine birds, especially among females (Murphy 1996, Hoover 2003). Permanent dispersal beyond study sites cannot be distinguished from death (Lebreton et al. 1992), and given the more frequent nest failure and lower productivity at SPBK than at all other parks, we suspect that permanent emigration rather than mortality explains the lower apparent survival of female towhees at SPBK.
Additive or compensatory mortality?
We are unaware of any studies that have examined whether mortality of young birds in the post-fledging period is additive or compensatory, but theory predicts that mortality is likely to be additive when a new source of mortality overlaps a relatively brief period of already heavy natural mortality (Clark 1987, Sandercock et al. 2011). The first 3 weeks of post-fledging life, when towhee losses to domestic cats occurred (Shipley et al. 2013), is an especially heavy period of mortality for young birds (reviewed in Cox et al. 2014). We thus suspect that elimination of domestic cat mortality at SPBK would likely enhance towhee fledgling survival because it is probably additive to mortality caused by native predators (Shipley et al. 2013).
Whether mortality is additive or compensatory is also an important consideration for possible between-season interactions of SD and SI. Compensatory mortality is predicted to be most likely among species characterized by high fecundity but low survival when new sources of mortality occur in the season preceding the normal period of high mortality (for young birds, presumably winter; Conroy and Krementz 1990, Sandercock et al. 2011). A large pulse of newly independent towhee young resulting from low post-fledging mortality due to low predation (i.e, high SD) may in effect produce only a “doomed surplus” (Errington 1946) destined to die in the winter prior to breeding because of lack of space or food, or losses to parasites or predators (i.e., low SI). Hatch-year birds often suffer greater mortality than adult birds during periods of stress (Arcese et al. 1992, Robinson et al. 2007), and work on European tit species has shown that stability of population size through nonbreeding season mortality often occurs through disproportionately high mortality of hatch-year birds (Perrins 1979, Ekman 1984). Interseasonal compensatory mortality in towhees may thus occur. We acknowledge this uncertainty and the need for more work to address the possibility that between-season compensatory mortality may affect population persistence, especially as N0 approaches K.
Density-dependence and dispersal
Although we recognized that a carrying capacity existed for each park, and that reproduction and/or survival were likely subject to density-dependence, our model did not incorporate density-dependent effects on population growth. However, for the following reasons we do not believe this compromised our results. First, population size varied little from year-to-year at each park, and appeared to be very high in relation to park area and average territory size of a sister species (see Methods). Populations in all parks thus appeared to be operating at essentially carrying capacity, and as a consequence, the vital rates that we measured and used in our models likely reflected effects of life at high density. Incorporating negative density-dependence would have resulted in higher reproductive and/or survival rates at the simulation’s starting population sizes of 0.25*K. The effect would have been more rapid initial population growth, but as population size grew we would have ultimately returned to the same vital rates upon which the model was based. Consequently, the conclusions regarding the sustainability of each population would have been unchanged.
Opening populations to the possibility of low-level immigration (in reality, immigration slightly greater than emigration) had important implications for population persistence as 3 of the 6 declining populations modeled with baseline conditions of SD (0.645) grew despite having λs < 1.0, while a fourth was able to maintain its numbers. We emphasize that the immigration rates that we modeled were low (1 female every other year). For LSSR, the park with the smallest population, this amounted to essentially 2.5 % of the population over a two year period (1 immigrant over 2 years for a population with a K = 20); for the largest population (SPBK) it amounted to 1.4 % of the population over a two year period. Towhees can breed successfully in greenspaces as small as 1 ha that are surrounded by pavement (S. Bartos Smith, unpubl. Data), and also nest in yards of private residences. We do not know if the latter are sufficient for full life cycle needs of towhees, but regardless, the abundant tree cover in the matrix surrounding all four parks (Fig. 1) exceeded that which Tremblay and St. Clair (2011) identified as minimally necessary (20–40 %) for the movement of urban-sensitive birds across developed landscapes. Thus, at the growth rates detected at LSSR, and especially WPDX, dispersal to other sites should be possible for individuals produced in the latter parks when populations were at carrying capacity. Immigration is therefore no doubt a critical factor in maintaining towhees as an abundant breeder throughout the Portland landscape.
Summary and recommendations
The generally poor representation of ground nesting birds in urban bird communities (Hedblom and Söderström 2010) suggests that developed landscapes present unique challenges to this nesting guild. That said, the ground nesting spotted towhee appears capable of maintaining its presence in the Portland metropolitan area without input from surrounding peri-urban/rural populations. The explanations for their success are likely attributable to their omnivorous diet, use of edge habitats (Bartos Smith et al. 2012), and potential for high productivity (i.e., ability to raise up to 3 broods/year), but also to features of the biotic communities, habitats and landscape of Portland. Snakes, which commonly cause nest failure for ground nesters (Patten and Bolger 2003, Cox et al. 2013), are virtually absent from Portland’s parks (MT Murphy, unpubl. Data). At the same time ground cover and shrubs, which provide cover from avian predators (Patten and Bolger 2003), are abundant. The absence of snakes, while beneficial to birds, is an unfortunate outcome of habitat fragmentation in a highly urbanized landscape (Patten and Bolger 2003). By contrast, maintenance of a dense native species understory is an active management goal of Portland’s park system not common in most urban parks. For the continued success of towhees, and other ground and low shrub nesters, we recommend a continuation and expansion of programs to promote natural habitat features in city parks. Our simulations also suggested that dispersal enhances persistence of towhee populations in some parks, and the abundant tree cover in Portland’s Southwest Hills provides safe passage through what might otherwise be inhospitable development. Benefits of increased densification of housing due to infill should thus be weighed against the threats to wildlife posed by reduced habitat connectivity because infill removes potentially valuable greenspaces where parks may not exist. As a final recommendation we urge that steps be taken to eliminate wildlife mortality caused by domestic and feral animals. Annual losses of birds and other wildlife in the United States to domestic and feral cats are staggering (Loss et al. 2013). Steps should be taken to educate the public as to the threats posed by their pets and the ineffectiveness of trap-neuter-release programs because the latter fail to halt growth of feral populations (Anderson et al. 2004, Longcore et al. 2009), and do not recognize the indirect sublethal effects on wildlife from exposure to potential predators (Beckerman et al. 2007, Lima 2009, Zanette et al. 2011, Bonnington et al. 2013).
References
Anderson KE, Fujiwara M, Rothstein SI (2012) Demography and dispersal of juvenile and adult brown-headed cowbirds (Molothrus ater) in the eastern sierra Nevada, California, estimated using multistate models. Auk 129:307–318
Anderson MC, Martin BJ, Roemer GW (2004) Use of matrix models to estimate the efficacy of euthanasia versus trap-neuter-return for management of free-roaming cats. J Am Vet Med Assoc 225:1871–1876
Arcese P, Smith JNM, Hochachka WH, Rogers CM, Ludwig D (1992) Stability, regulation, and the determination of abundance in an insular song sparrow population. Ecol 73:805–822
Arlt D, Forslund P, Jeppsson T, Pärt T (2008) Habitat-specific population growth of a farmland bird. PLoS One 8:e3006
Aronson MFJ, La Sorte FA, Nilon CH, Katti M, Goddard MA, Lepczyk CA, Warren PS, Williams NSG, Cilliers S, Clarkson B, Dobbs C, Dolan R, Hedblom M, Klotz S, Kooijmans JL, Kühn I, MacGregor-Fors I, McDonnell M, Mörtberg U, Pyšek P, Siebert S, Sushinsky J, Werner P, Winter M (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc Roy Soc B 281:1–8
Baker PJ, Molony SE, Stone E, Cuthill IC, Harris S (2008) Cats about town: is predation by free-ranging pet cats Felis catus likely to affect urban bird populations? Ibis 150(Suppl. 1):86–99
Balogh AL, Ryder TB, Marra PP (2011) Population demography of gray catbirds in the suburban matrix: sources, sinks and domestic cats. J Ornith 152:717–726
Bartos Smith S, Greenlaw JS (2015) Spotted Towhee (Pipilo maculatus), The Birds of North America Online, (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu.bnaproxy.birds.cornell.edu/bna/species/263
Bartos Smith S, McKay JE, Richardson JK, Murphy MT (2012) Edges, trails, and reproductive performance of Spotted Towhees in urban greenspaces. In: Lepczyk CA, Warren PS (eds) Urban Bird Ecology and Conservation. Studies in Avian Biology (45). University of California Press, Berkeley, CA, pp. 162–182
Beckerman AP, Boots M, Gaston KJ (2007) Urban bird declines and the fear of cats. Anim Conserv 10:320–325
Blair RB, Launer AE (1997) Butterfly diversity and human land use: species assemblages along an urban gradient. Biol Cons 80:113–125
Bonnington C, Gaston KJ, Evans KL (2013) Fearing the feline: domestic cats reduce avian fecundity through trait-mediated indirect effects that increase nest predation by other species. J Appl Ecol 50:15–24
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landscape Urban Plan 74:46–69
Chamberlain DE, Cannon AR, Toms MP, Leech DI, Hatchwell BJ, Gaston KJ (2009) Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151:1–18
Clark WM (1987) Effects of harvest on annual survival of muskrats. J Wildl Manag 51:265–272
Conroy MJ, Krementz DG (1990) A review of the evidence for the effects of hunting on American black duck populations. Trans North Am Wildl Nat Res Conf 55:501–517
Cox WA, Thompson III FR, Reidy JL (2013) The effects of temperature on nest predation by mammals, birds, and snakes. Auk 130:784–790
Cox WA, Thompson III FR, Cox AS, Faaborg J (2014) Post-fledging survival in passerine birds and the value of post-fledging studies to conservation. J Wildl Manag 78:183–193
Crook KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566
Crooks KR, Suarez AV, Bolger DT (2004) Avian assemblages along a gradient of urbanization in a highly fragmented landscape. Biol Cons 115:451–462
Dhondt AA, Kast TL, Allen PE (2002) Geographical differences in seasonal clutch size variation in multi-brooded bird species. Ibis 144:646–651
Dinsmore SJ, Wunder MB, Dreitz VJ, Knopf FL (2010) An assessment of factors affecting population growth of the Mountain Plover. Avian Cons Ecol 5(1): 5. [online] URL: http://www.ace-eco.org/vol5/iss1/art5/
Dunn JC, Hamer KC, Benton TG (2010) Fear for the family has negative consequences: indirect effects of nest predators on chick growth in a farmland bird. J Appl Ecol 47:994–1002
Ekman J (1984) Density-dependent seasonal mortality and population fluctuations of the temperate-zone willow tit (Parus montanus). J Anim Ecol 53:119–134
Errington PL (1946) Predation and vertebrate populations. Quart R Biol 21:145–177
Evans KL, Gaston KJ, Sharp SP, McGowan A, Simeoni M, Hatchwell BJ (2009) Effects of urbanisation on disease prevalence and age structure in blackbird Turdus merula populations. Oikos 118:774–782
Fischer JD, Cleeton SH, Lyons TP, Miller JR (2012) Urbanization and the predation paradox: the role of trophic dynamics in structuring vertebrate communities. Bioscience 62:809–818
Fleischer Jr AL, Bowman R, Woolfenden GE (2003) Variation in foraging behavior, diet, and time of breeding of Florida scrub-jays in suburban and wildland habitats. Condor 105:515–527
Fuller RA, Warren PH, Gaston KJ (2007) Daytime noise predicts nocturnal singing in urban robins. Biol Letters 3:368–370
Germaine SS, Wakeling BF (2001) Lizard species distributions and habitat occupation along an urban gradient in Tucson, Arizona, USA. Biol Cons 97:229–237
Greenlaw JS. (2015) Eastern Towhee (Pipilo erythrophthalmus), The Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu.bnaproxy.birds.cornell.edu/bna/species/262. doi:10.2173/bna.262
Grzybowski JA (2005) An estimate of juvenile survival in Black-capped Vireos and its implications to source-sink analyses of songbirds. USDA Forest Serv Gen Tech Rep PSW-GTR-191
Guénard B, Cardinal-De Casas A, Dunn RR (2015) High diversity in an urban habitat: are some animal assemblages resilient to long-term anthropogenic change? Urban Ecosyst 18:449–463
Hedblom M, Söderström B (2010) Landscape effects on birds in urban woodlands: an analysis of 34 Swedish cities. J Biogeogr 37:1302–1316
Hoover JP (2003) Decision rules for site fidelity in a migratory bird, the prothonotary warbler. Ecol 84:416–430
Horak P, Lebreton J-D (1998) Survival of adult great tits Parus major in relation to sex and habitat: a comparison of rural and urban populations. Ibis 40:205–209
Kempenaers B, Borgström P, Löes P, Schlicht E, Valcu M (2010) Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr Biol 20:1735–1739
Keyser AJ, Keyser MT, Promislow DEL (2004) Life-history variation and demography in western bluebirds (Sialia mexicana). Auk 121:118–133
Klem Jr D, Farmer CJ, Delacretaz N, Gelb Y, Saenger PG (2009) Architectural and landscape risk factors associated with bird-glass collisions in an urban environment. Wilson J Ornith 121:126–134
Klomp JE, Murphy MT, Bartos Smith S, McKay JE, Ferrera I, Reysenbach A-L (2008) Cloacal microbial communities of female spotted towhees: microgeographic variation and individual sources of variability. J Avian Biol 39:530–538
Kostecke RM, Cimprich DA (2008) Adult and juvenile survival of black-capped vireos within a large breeding population in Texas. Condor 110:251–259
Lebreton J-D, Burnham KP, Clobert J, Anderson DR (1992) Modelling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Lepczyk CA, Mertig AG, Liu J (2003) Landowners and cat predation across rural-to-urban landscapes. Biol Cons 115:191–201
Leston LFV, Rodewald AD (2006) Are urban forests ecological traps for understory birds? An examination using northern cardinals. Biol Cons 131:566–574
Lima SL (2009) Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol Rev 84:485–513
Longcore T, Rich C, Sullivan LM (2009) Critical assessment of claims regarding management of feral cats by trap-neuter-return. Con Biol 23:887–894
Loss SR, Will T, Marra PP (2013) The impact of free-ranging domestic cats on wildlife of the United States. Nat Commun 4:1396
Loss SR, Will T, Marra PP (2014a) Estimation of bird-vehicle collision mortality on U.S. roads. J Wildl Manag 78:763–771
Loss SR, Will T, Loss SS, Marra PP (2014b) Bird-building collisions in the United States: estimates of annual mortality and species vulnerability. Condor: Ornith Appl 116:8–23
Mannan RW, Steidl RJ, Boal CW (2008) Identifying habitat sinks: a case study of cooper’s hawks in an urban environment. Urban Ecosyst 11:141–148
McDonald RI, Kareiva P, Forman RTT (2008) The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol Conserv 141:1695–1703
McKay J (2008) An analysis of parental care in Spotted Towhee populations located in urban greenspaces. In: Unpublished Masters Thesis. Portland State University, Portland, OR USA
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
Murphy MT (1996) Survivorship, breeding dispersal and mate fidelity in eastern kingbirds. Condor 98:82–92
Neil K, Wu J (2006) Effects of urbanization on plant flowering phenology: a review. Urban Ecosyst 9:243–257
Partecke J, Van’t Hoff T, Gwinner E (2004) Differences in the timing of reproduction between urban and forest European blackbirds (Turdus merula): result of phenotypic flexibility or genetic differences? Proc Roy Soc B 271:1995–2001
Partecke J, Schwabl I, Gwinner E (2006) Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87:1945–1952
Patten MA, Bolger DT (2003) Variation in top-down control of avian reproductive success across a fragmentation gradient. Oikos 101:479–488
Payton ME, Greenstone MH, Schenker N (2003) Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci 3:34
Perrins CM (1979) British tits. Collins, London
Pulliam HR (1988) Sources, sinks, and population regulation. Am Nat 132:652–661
Ramalho CE, Hobbs RJ (2012) Time for a change: dynamic urban ecology. Trends Ecol Evol 27:179–188
Redmond LJ, Murphy MT (2012) Using complementary approaches to estimate survival of juvenile and adult eastern kingbirds. J Field Ornith 83:247–259
Ricklefs RE (1969) An analysis of nesting mortality in birds. Smithsonian Contr Zool 9
Robb GN, McDonald RA, Chamberlain DE, Bearhop S (2008) Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front Ecol Environ 6:476–484
Robinson RA, Baillie SR, Crick HQP (2007) Weather-dependent survival: implications of climate change for passerine population processes. Ibis 149:357–364
Rodewald AD, Kearns LJ, Shustack DP (2011) Anthropogenic resource subsidies decouple predator-prey relationships. Ecol Appl 21:936–943
Roux KE, Marra PP (2007) The presence and impact of environmental lead in passerine birds along an urban to rural land use gradient. Arch Environ Contam Tox 53:261–268
Sandercock BK, Nilsen EB, Brøseth H, Pedersen HC (2011) Is hunting mortality additive or compensatory to natural mortality? Effects of experimental harvest on the survival and cause-specific mortality of willow ptarmigan. J Anim Ecol 80:244–258
Schaub M, Aebischer A, Gimenez O, Berger S, Arlettaz R (2010) Massive immigration balances high anthropogenic mortality in stable eagle owl populations: lessons for conservation. Biol Cons 143:1911–1918
Schaub M, Jakober H, Stauber W (2013) Strong contribution of immigration to local population regulation: evidence from a migratory passerine. Ecology 94:1828–1838
Shochat E (2004) Credit or debit? Resource input changes population dynamics of city-slicker birds. Oikos 106:622–626
Seto KC, Fragkias M, Guneralp B, Reilly MK (2011) A meta-analysis of global urban land expansion. PLoS One 6:e23777
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Nat Acad Sc USA 109:16083–16088
Shipley AA, Murphy MT, Elzinga AH (2013) Residential edges as ecological traps: postfledging survival of spotted towhees in an urban park. Auk 130:501–511
Shutler D, Clark RG, Fehr C, Diamond AW (2006) Time and recruitment costs as currencies in manipulation studies on the costs of reproduction. Ecology 87:2938–2946
Sibly RM, Hone J (2002) Population growth rate and its determinants: an overview. Phil Trans Roy Soc B 357:1153–1170
Sims V, Evans KL, Newson SE, Tratalos JA, Gaston KJ (2008) Avian assemblage structure and domestic cat densities in urban environments. Div Distrib 14:387–399
Siriwardena GM, Baillie SR, Wilson JD (1998) Variation in the survival rates of some British passerines with respect to their population trends on farmland. Bird Study 45:276–292
Snep RPH, Opdam PFM, Baveco JM, WallisDeVries MF, Timmermans W, Kwak RGM, Kuypers V (2006) How peri-urban areas can strengthen animal populations within cities: a modeling approach. Biol Cons 127:345–355
Stracey CM, Robinson SK (2012) Are urban habitats ecological traps for a native songbird? Season-long productivity, apparent survival, and site fidelity in urban and rural habitats. J Avian Biol 43:50–60
Tarof SA, Kramer PM, Hill III JR, Tautin J, Stutchbury BJM (2011) Brood size and late breeding are negatively related to juvenile survival in a Neotropical migratory songbird. Auk 128:716–725
Tarwater CE, Ricklefs RE, Maddox JD, Brawn JD (2011) Pre-reproductive survival in a tropical bird and its implications for avian life histories. Ecology 92:1271–1281
Tremblay MA, St. Clair CC (2011) Permeability of heterogenous urban landscape to the movements of forest songbirds. J Appl Ecol 48:679–688
Van Heezik Y, Smyth A, Adams A, Gordon J (2010) Do domestic cats impose an unsustainable harvest on urban bird populations? Biol Cons 143:121–130
Weatherhead PJ, Forbes MRL (1994) Natal philopatry in passerine birds: genetic or ecological influences? Behav Ecol 5:426–433
Weatherhead PJ, Blouin-Demers G (2004) Understanding avian nest predation: why ornithologists should study snakes. J Av Biol 35:185–190
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46 Supplement 120–138
Whittaker KA, Marzluff JM (2009) Species-specific survival and relative habitat use in an urban landscape during the postfledging period. Auk 126:288–299
Zanette LY, White AF, Allen MC, Clinchy M (2011) Perceived predation risk reduces the number of offspring songbirds produce per year. Sci 334:1398–1401
Zimmerman GS, Gutiérrez RJ, Lahaye WS (2007) Finite study areas and finite rates: sampling effects on estimates of spotted owl survival and population trends. J Appl Ecol 44:963–971
Acknowledgments
We thank the many field assistants who helped us with nest searching, and Portland Parks and Recreation and Lake Oswego Parks and Recreation for access to our study areas. Financial support for our research was provided by a USFW grant to MTM, an EPA GRO Fellowship to SBS, and grants from the American Ornithologists’ Union and the American Museum of Natural History’s Chapman Fund to SBS. All research was conducted in compliance with Portland State University Animal Care and Use protocols, and bird banding and handling was done under permission of the USGS Bird Banding Laboratory and the Oregon Department of Fish and Wildlife. Comments by Joseph A. Grzybowski and two anonymous reviewers substantially clarified our thinking and improved the manuscript. The research described in this paper has been funded in part by the United States Environmental Protection Agency (EPA) under the Greater Research Opportunities (GRO) Graduate Program. EPA has not officially endorsed this publication and the views expressed herein may not reflect the views of the EPA.
Author information
Authors and Affiliations
Corresponding author
Appendix 1. Structure of the population model
Appendix 1. Structure of the population model
Finite rate of increase (lambda; λ) is the metric that best describes population performance (Sibly and Hone 2002). Its value lies in its use of vital rates to calculate a rate of population change that can be used to assess current population status (e.g., Arlt et al. 2008) and project future population trends (a population with λ = 1.0 just replaces itself, while values < and >1.0 represent declining and growing populations, respectively). Among others, Pulliam (1988) described a simple population model in which λ was calculated using estimates of annual adult survival (SA), seasonal production of young (F: reduced by 50 % to represent only females [F/2]), and survival of juveniles to their first year of breeding (SYr1):
Few good estimates of SYr1 exist (but see Keyser et al. 2004, Zimmerman et al. 2007, Arlt et al. 2008, Tarof et al. 2011, Tarwater et al. 2011, Anderson et al. 2012, Redmond and Murphy 2012) because of low natal philopatry in most birds (Weatherhead and Forbes 1994) and the fact that permanent emigration cannot be separated from true mortality. Depending upon the species, improvements in the estimate of SYr1 might be possible if SYr1 could be subdivided into periods during which survival is either conveniently measured and/or which reflect distinct periods when survival is likely to differ (e.g., migration vs. winter residency for a migratory bird). Tarof et al. (2011), for instance, separated first-year survival of Purple Martins (Progne subis) into the post-fledging, pre-migratory roost period, and then migration-overwinter survival and derived estimates of survival for each.
Good estimates of offspring survival for the few weeks immediately following fledging exist for an increasing number of species (see Cox et al. 2014 for review), and the usual pattern is for mortality to be relatively heavy in the week after young leave the nest, then stabilize and remain relatively high for the remainder of the period of dependence (typically 2–4 weeks). It is the period between gaining independence and the next breeding season for which the greatest uncertainty in offspring survival exists. We therefore chose to identify two periods between fledging and first opportunity to breed to estimate SYr1: the immediate post-fledging period when young are dependent on parents for care, and the period of independence that follows and ends with the beginning of the next breeding season. For spotted towhees (Pipilo maculatus; hereafter towhees), these encompass ~30 days and ~10 months, respectively (about one month is spent in the nest as eggs/young). Towhees fledge young from early May to early August, and therefore fledglings must survive between 11 months (May to April) and 9 (August to April) before their first opportunity to breed. Given that we used total seasonal productivity as our estimate of F, we chose the midpoint of 10 months to estimate SI.
We modified the basic growth equation to incorporate separate estimates of survival during the period of dependence (SD) and independence (SI) for young in their first year,
and then used STELLA (v. Stella 10.0.3) to simulate variation in all vital rates to calculate λ and assess the viability of towhee populations in all parks. Adult site fidelity was very high as we did not record a single instance of adult breeding dispersal among parks. By contrast, low natal site fidelity resulted in few detections of juvenile recruits to their natal parks. This is common in passerines (see Weatherhead and Forbes 1994); we assume that low recruitment of locally hatched juveniles represented normal dispersal patterns and that an equal number of first-year birds hatched elsewhere entered the parks The assumption that immigration and emigration were equal is equivalent to assuming that populations are closed, which is important because it enabled us to use the simulations to ask the question of whether park-specific estimates of SA and F/2 were sufficient to maintain populations.
Our estimate of SD came from Shipley et al.’s (2013) study of towhees breeding in SPBK. We assumed this was applicable to other parks, and then explored, through simulation, the effect on λ of a range of values for SI that varied as a proportion of SA. Siriwardena et al. (1998) reported survival rates for hatch-year birds captured as independent individuals from their summer of hatch to the next breeding season for 28 passerine species. Survival was reported for adults captured at the same time. After we omitted four species with small sample sizes (adult + hatch-year birds <200 individuals), the average ratio of SI to SA was 0.86 (SE = 0.025, N = 24), with a range from 0.70 to over 1.00. Two studies of Black-capped Vireos (Vireo atricapilla) exhibited a very similar ratio of independent hatch year survival to adult survival (0.83: Kostecke and Cimprich 2008; 0.70 to 0.89: Grzybowski 2005). We therefore chose to explore the effect of SI on λ when SI was 70 %, 80 %, 90 %, or 100 % of SA.
Our model begins with the seeding of each a park with a number of individuals, which we standardized at 1/4 of “carrying capacity” (K). K was based on the highest estimated count of breeding pairs in each park over the period of study. We built K into the model to prevent unrestrained growth in a habitat that would clearly have a ceiling on population size. This added realism, but the actual value used would not affect our ability to address the question of whether the observed vital rates in a park were sufficient to maintain population size. We simulated a decade of population change, and at the start of each annual iteration of the model, population size (NTotal) was compared to K at NB (number of breeders) in Fig. 1. When we later opened the populations to permit immigration (see below), the immigrants were added to NTotal before testing for whether K had been exceeded so that regardless of the sources, NB could never exceed K. The excess were removed as emigrants (E) before determination of each year’s NB.
Vital rates were not modeled to be density-dependent. Although we could have done so, the strength and form of the relationship would have been pure speculation and therefore we chose to assume that birth and death rates did not vary with population size. We suggest that this makes our models conservative as regards their ability to predict a population’s ability to sustain itself because the vital rates used were taken from populations in the parks that were likely at or near capacity, and thus already experiencing any negative effects of high density. Adding density-dependence would have led to higher F and/or SA at lower N. The effect of this would have been to accelerate growth, but we would have ultimately arrived at the same vital rates as N approached K. The model randomly chose a value of F/2, SA, and SD from a range of values falling within ±2 standard errors of mean F/2 and SA and ±20 % of mean SD. To determine SI for each park, the model calculated monthly survival rate (MSR) for the randomly chosen SA from each park individually at each iteration. That monthly survival rate was raised to the power of 10 to determine the equivalent survival rate for an adult for the 10 month period preceding a recruit’s first opportunity to breed (1 July to 1 April). The latter was then multiplied by the designated proportion (0.7, 0.8, 0.9, or 1.0) to obtain SI. Survival from the point of fledging to the following breeding season (SYr1) was the product of SD and SI. The product of F/2 and SYr1 equaled the number of new recruits, which when added to SA yielded λ. Dinsmore et al. (2010) described a 2-stage (juvenile and adult) Leslie matrix for a birth pulse population censused in the postbreeding period. The towhee data conformed to these conditions, and we found that our estimates of λ were identical to that generated by Dinsmore et al.’s (2010) model when we ran our model with constant parameter values (i.e., as a deterministic model). Our STELLA model thus generated accurate estimates of λ but with the incorporation of stochasticity in all vital rates.
The 10 estimates of λ from the decade-long simulation were averaged and the population size in the final year (Nfinal) also averaged to produce a single data point for both SA and NFinal. Five hundred such simulations were run for each park at all levels of SI (0.7*SA, 0.8*SA, etc.), but only every fifth estimate was used so as to reduce possible serial correlation among randomly chosen values. Consequently, the number of simulations used to calculate grand mean λ and NFinal for each park at each category of SI was 100 in our initial modelling efforts. However, we reduced the sample size to 50 in all subsequent simulations (see below) because comparison of the mean of the running average of λ at iterations 46 through 50 to that at iterations 96 through 100 showed that the differences in λ to be well below 1 % (mean difference = 0.22 [SE = 0.047 %], n = 16 comparisons). In short, iterations beyond 50 did not improve the accuracy of our estimates of λ.
As noted above, we also changed the population from being closed (i.e., immigration = emigration) to open to immigration (i.e., immigration slightly > than emigration) to incorporate the possibility of immigration in some models to evaluate whether low levels of immigration might rescue populations from local extinction. Sink populations (i.e., λ < 1.0) can persist with sufficient immigration (Schaub et al. 2010, 2013) and therefore to test for this contingency we allowed immigration to occur at a random rate. A number between 0 and 1 was generated randomly, and no immigration occurred if the number was ≤0.5, but one immigrant was allowed to enter the population if the randomly generated value fell between 0.51 and 1.0. This in in effect resulted in the addition, on average, of one female every other year. All other aspects of the model were unchanged (Fig. 4).
Visual representation of STELLA model used to project population size of spotted towhees nesting in urban parks in Portland, OR USA. Circles are functions (“converters” in STELLA) that contain expressions describing seasonal fecundity (F/2), survival of adults (SA), survival of young during the 30 day period of post-fledging parental dependence (SD), survival of young over the ensuing 10 month period between independence and start of the next breeding season (SI), survival over the period between fledging and start of the next breeding season (SYr1 = SD*SI), monthly survival rate (MSR) of adults, and population growth rate (λ). MSR equals 10(logS A )/12 and SI = P*MSR10, where P represents the proportional difference between adult and first-year survival (i.e., 0.7, 0.8, 0.9, or 1.0). NB is number of breeders, while the flow G represents growth of the population (= λ*NB). The rectangle is a stock that contains total population size (NTotal), while flow O removes each year’s growth to prevent the summation of successive annual increments of growth. Possible immigrants are generated by the converter I by randomly generating a value between 0 and 1 at each iteration. No immigration occurs if I ≤ 0.5, but one immigrant enters the population if the randomly generated value falls between 0.51 and 1.0. At NB, NTotal and I are summed and compared to K. If the sum is < K then NB equals the sum. If K is exceeded the excess are removed as emigrants (“dispersers”)
Rights and permissions
About this article
Cite this article
Smith, S.B., McKay, J.E., Richardson, J.K. et al. Demography of a ground nesting bird in an urban system: are populations self-sustaining?. Urban Ecosyst 19, 577–598 (2016). https://doi.org/10.1007/s11252-016-0532-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-016-0532-6