Abstract
The exploration of the effects of urbanization on bird demography has attracted much attention, and several studies found lower reproductive success in towns, which suggested strong environmental constraints. Here, we conducted a 3-year study to explore the consequences of urbanization on the breeding success of two species that originated in forests, the Blue Tit Cyanistes caeruleus and the Great Tit Parus major. In two replicates of urban and forest habitats, we studied the components of reproductive success. In one replicate of each habitat, we quantified nestling growth over the three breeding seasons, and we collected data on egg quality during one breeding season. The general picture that emerges from our finding is that in urban sites breeding success was lower with smaller clutch sizes, higher clutch, higher brood failure rates and lower survival rates. Our results also showed reduced growth in urban habitats, at the embryonic and nestling stages, with potential adverse consequences on fitness. Crucial ecological factors could explain the observed contrasts between the habitats, and food limitation is among the most likely. Overall, we demonstrated the negative effects of urbanization on the reproductive success of forest birds, and our results were consistent between species and geographic areas for these negative effects. Our results suggest a mismatch between urban environments and the habitat exploitation abilities that birds have evolved in their native forest ecosystems.
Zusammenfassung
Vom Ei zum flüggen Jungvogel: negativer Einfluss von urbanem Lebensraum auf die Reproduktion bei zwei Meisenarten
Die Erforschung der Effekte von Urbanisierung auf Vogeldemografie hat viel Aufmerksamkeit auf sich gezogen und einige Studien haben einen niedrigeren Reproduktionserfolg innerhalb von Städten gefunden. Dies weist auf starke Umwelteinflüsse hin. In unserer dreijährigen Studie haben wir die Folgen der Urbanisierung auf den Bruterfolg von Blaumeisen C. caeruleus und Kohlmeisen P. major, welche beide ursprünglich in Wäldern vorkamen, erforscht. Auf je zwei Untersuchungsflächen je Lebensraum haben wir Komponenten des Reproduktionserfolgs untersucht. In einem Gebiet pro Brutsaison haben wir über je drei Jahre das Kükenwachstum aufgenommen, zudem in einem Jahr die Eiqualität untersucht. Das generelle Bild ist, dass in urbanem Gelände Bruterfolg und Gelegegröße geringer, Gelegeverluste und Brutverluste höher und die Überlebensraten geringer waren. Zudem war die Wachstumsrate in urbanen Lebensräumen während der embryonalen Phase und der Nestlingsphase geringer, was potentiell negative Auswirkungen auf die Fitness hat. Die beobachteten Unterschiede zwischen den Lebensräumen könnten von wichtigen ökologischen Faktoren erklärt werden, wobei Nahrungsbegrenzung vermutlich einer der wahrscheinlichsten ist. Wir haben den negativen Einfluss von Urbanisierung auf den Reproduktionserfolg von Waldvögeln gezeigt. Diese negativen Effekte stimmten zwischen den Arten und den geografischen Gebieten überein. Unsere Ergebnisse zeigen eine Diskrepanz zwischen urbaner Umwelt und den Fähigkeiten der Vögel, diesen Lebensraum zu nutzen, welche die welche Vögel in ihren ursprünglichen Lebensräumen evolviert haben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization is among the most extreme forms of anthropogenic modification of habitats, but towns are yet colonized by many wild species (McKinney and Lockwood 1999). Abiotic and biotic factors differ strongly between urbanized areas and natural or subnatural habitats: for example, cities have higher temperatures, louder noise and often lower parasite prevalence than rural habitats (Grimm et al. 2008; Geue and Partecke 2008; Slabbekoorn 2013). The ecological and evolutionary consequences of urbanization have stimulated a growing interest, particularly for birds (Marzluff 2001; Chace and Walsh 2006), because organisms should experience environmental pressures that contrast strongly between towns and their native habitats.
The effects of urbanization on bird demography has led to the exploration of the components of breeding success in urban environments. Interestingly, lower breeding success has frequently been reported in urban birds compared with their rural conspecifics (Hõrak 1993; Hõrak et al. 2000; Mennechez and Clergeau 2006; Kaliński et al. 2009; Seress et al. 2012). For example, several studies revealed that urban birds lay fewer eggs (Solonen 2001; Grégoire 2003; Mennechez and Clergeau 2006), perhaps in response to characteristics of the urban habitat, such as reduced prey availability or increased competition or predation risks due to high local densities (Chace and Walsh 2006; Chamberlain et al. 2009). Additionally, most studies report lower reproductive success in towns with a lower proportion of fledglings, which should indicate strong environmental constraints (Chamberlain et al. 2009).
Because egg and offspring numbers are directly associated with reproductive success, they are critical components that mirror habitat suitability and constraints. Other breeding parameters, such as egg quality or nestling growth, may reliably reflect environmental pressures experienced by birds at different stages of the reproduction process. Egg quality is a key component that is strongly influenced by female condition and the environmental characteristics experienced by females which transfer into egg nutrients for embryo and nestling development (Blount et al. 2000; Groothuis et al. 2005; Krist 2011). Several works have emphasized the importance of yolk carotenoids for the development and maturation of embryonic tissues, with positive consequences for egg hatchability and nestling growth and maturation (Surai et al. 2001; Blount et al. 2002). Additionally, carotenoid yolk concentration may affect survival prospects of offspring (McGraw et al. 2005; Marri and Richner 2014). Because birds cannot synthesise carotenoids, yolk concentration depends to a large extent on the dietary intake of the female and thus on the availability of these compounds in the local environment (Biard et al. 2005; Hargitai et al. 2006). Egg mass and eggshell thickness are other important characteristics with direct consequences on breeding success. Because some females provide larger eggs with greater reserves, several studies have documented significant advantages of larger eggs for offspring survival (Williams 1994; Styrsky et al. 1999; Krist 2011). Moreover, eggs with too thin shells can be more easily accidentally broken by the parents (Ratcliffe 1970) or exposed to dehydration (Drent and Woldendorp 1989) during incubation. The availability and the quality of resources have been observed in many studies to affect nestling growth and survival (Mennechez and Clergeau 2006; Blondel 2007; Peach et al. 2008). Therefore, an accurate description of the growth curve during the nestling stage may provide reliable information on constraints experienced by birds breeding in urban areas.
In this study, we explored the consequences of urbanization during three consecutive years on the breeding success of two species that originated in forests, the Blue Tit Cyanistes caeruleus and the Great Tit Parus major. Both species have been extensively studied to determine the demographic consequences of contrasted forest habitats (van Balen 1973; Kilgas et al. 2007; Blondel 2007; Sanz et al. 2010; Porlier et al. 2012), and, to a lesser extent, to compare populations between urban areas and forests (Hõrak 1993; Hõrak et al. 2000; Solonen 2001; Hõrak et al. 2002; Marciniak et al. 2007; Isaksson and Andersson 2007; Kaliński et al. 2009; Bańbura et al. 2010). Here, we compared a suite of components of reproductive success between Great Tits and Blue Tits in either urban or forest habitat at two study sites in France, but also collected novel data on egg quality and nestling growth to try to identify the sources of any variation in fitness we observed.
Methods
Study sites
Two cities (Dijon, 47°32N, 5°02E; Besançon, 47°25N, 6°03E) and two forests (Forêt d’Auxonne, 47°10N, 5°26E; Forêt de Chaux, 47°09N, 5°68E) were studied in two regions of eastern France, Burgundy and Franche-Comté, respectively. Dijon and Besançon are two middle-sized cities (about 200,000 inhabitants) whose economy is characterized by tertiary activities (http://www.insee.fr). The Forêt d’Auxonne and the Forêt de Chaux are deciduous forests of 7800 and 20,493 ha, respectively. The most abundant tree species are oaks (Quercus petrae and Q. robur), beech (Fagus sylvatica) and hornbeam (Carpinus sp.). Nest boxes were installed in medium (50-year-old) to old (100-year-old) growth stands and at least 1 km from the edge of the forests. The two cities and the two forest sites were approximately 100 and 40 km apart, respectively. Each site received around 150 artificial nestboxes (Nestbox 1B with protective front panel, 12 cm diameter and 20 cm high; Schwegler, Germany). The entrance hole diameter was 28 and 32 mm, and was accepted by both species. Nestboxes in cities were dispersed in parks, squares and along streets, with each patch containing 1–25 nestboxes separated by at least 50 m. The vegetation of the parks has for the most part been formed artificially. The tree cover is patchy with tree-free areas mainly covered by grass and paved surfaces. Tree patches are composed of deciduous and coniferous species of different stands including both native (oaks, maples Acer spp., limes Tilia spp., beech, and birches Betula spp. as most common species) and exotic species (poplars Populus spp., cedars Cedrus spp., cherry laurel Prunus laurocerasus, and thujas Thuja spp. as the most common species). Each study site covers an average area of 5.6 km long and 2.8 km wide. In the forests, nestboxes were placed along pathways to facilitate access, with the same minimal distance between two nestboxes as in cities.
Reproduction and growth
Reproduction of Great and Blue Tits was followed during three consecutive years (2012–2014) and only first breeding attempts were analyzed. In 2012, reproduction was studied in all sites, while the Burgundy sites were only followed in 2013 and 2014 for logistical constraints. For similar logistical reasons, we monitored a subsample of Blue Tits to assess nestling growth, and we did not consider all reproductive variables for this species in 2013 and 2014. Meteorological conditions varied between years, with 2013 spring being cold and wet, and 2014 being warm and dry (see Supplementary information).
Females of both species started to lay in late March/early April (see Tables 1, 2) as described for continental Europe (Cramp et al. 1993). Nests were inspected once a week from 20 March to record the laying date of the first egg. When more than one egg was found in the nest, the laying date of the first egg was back-calculated assuming that one egg was laid per day. Incubation was assumed to begin 1 day before clutch completion, and indeed we did not observe asynchronous hatching (except sometimes for one egg, see below). The nests were not visited again until the estimated hatching date (13 days after initiation of incubation), and broods that had not hatched were then checked every 2 days until they did. Hatching took place within 1 day for the whole brood except for one egg (probably the last laid) in some occasions. The incubation period was the time between initiation of incubation and hatching.
At the Burgundy sites in 2012, one of the first three eggs to be laid was removed for quality analysis (see below) and replaced with a fake egg (Isaksson et al. 2008). Two or three days after the eggs had hatched, the dummy egg was removed and replaced with one nestling of the same age (taken from broods not included in the present study) so that the parents would have the same number of nestlings to care for as they would have done had the original egg hatched. The number of nestlings present was noted on day 1 (D1), 7 (D7) and 13 (D13) post-hatching. In Burgundy, nestling body mass was measured on D1, D7 and D13 with a Pesola spring balance (±0.05 g), and their tarsus length was measured on D7 and D13 with an electronic callipers (±0.1 mm). Morphological data were not collected from nestlings in Franche-Comté due to logistical constraints. To obtain individual identification 1 day post-hatching, the nestlings within each brood were identified by cutting a specific tuft of fluff on their head, shoulder and back in a unique pattern. These tufts were present in hatchlings and disappeared gradually with feather growth. This method was a reliable alternative to the use of hypoallergenic ink which needs to be refreshed every 2 or 3 days. The nests were not visited until nestlings were 7 days old when they were fitted with individually numbered metal leg rings (Muséum National d’Histoire Naturelle, CRBPO, Paris, France).
We defined clutch size as the number of eggs laid (including any removed eggs in 2012). Hatching success was the proportion of all eggs laid that hatched, after excluding the removed egg from the calculations for Burgundy in 2012 (Seress et al. 2012), and hatching rate was the proportion of eggs that hatched in the nests that did not fail at the egg stage. We defined the clutch failure rate as the proportion of clutches that had no hatchlings. We defined the survival until D13 as the proportion of hatched nestlings that were still alive on D13 including the compensatory nestling added to the nests in Burgundy in 2012, the brood failure rate as the proportion of broods in which all nestlings died before D13, and the survival rate as the proportion of chicks alive at D13 in nests where at least one nestling survived until D13.
Egg quality
The eggs collected in Burgundy in 2012 were weighed on a precision balance (±0.01 mg) and then the yolks were separated and stored at −80 °C until analysis. The shells were stored in individual tubes.
The carotenoids were extracted from the yolk of 60 Great Tit eggs and 58 Blue Tit eggs following Surai and Speake (1998). First, the proteins were precipitated from the homogenised yolks by vortexing them in 300 µl of ethanol/water (2:1, vol:vol) for 5 min. Then, 500 µl of hexane was added to extract the carotenoids. The solution was vortexed for 5 min then centrifuged at 4 °C for 4 min at 12,000 rpm. The supernatant was removed and transferred to a new tube. The extraction procedure was repeated on the remaining residue to extract as many carotenoids as possible. The supernatants from the two extractions were pooled, and the liquid was evaporated under nitrogen gas in darkness so that only the residue of the carotenoids remained. The carotenoids were then resuspended in 120 µl of absolute ethanol and the optical density of this solution was measured with a spectrophotometer at 450 nm to correspond to the peak absorbance of carotenoids. The carotenoid concentration was assessed from a standard curve of lutein, which was obtained by serial dilution of an initial solution of lutein at 20 μg ml−1 diluted seven times to 0 μg ml−1. This technique provided reliable assessments comparable with an HPLC analysis (Alonso-Alvarez et al. 2004).
The shell thicknesses of 58 Great Tit eggs and 36 eggs Blue Tit were measured using a scanning electron microscope (Hitachi TM-1000). For each egg, three or four small freshly broken fragments of shell (roughly 0.2 × 0.5 cm) were fixed in gum, and two fragments in which the edge was clearly visible were chosen for the measurements. Five measurements of shell thickness were recorded for each fragment. Thirty-six randomly-selected eggs were remeasured using this technique, and the intra-fragment and inter-fragment variation were 3.2 and 4.2 %, respectively.
Statistical analyses
The analyses were performed separately for the two species of tits and for the 3 years of study in Burgundy.
The variation in first egg date, clutch size, and incubation period were analysed with generalised linear models (GLMs) with Poisson error distributions (Zuur et al. 2009). The first egg date was standardised as the number of days between the first egg laying date and 1 March. GLMs with binomial distributions and logit link functions were used for hatching success and hatching rate, as well as survival until D13 and survival rate (Warton and Hui 2011). Models included habitat (urban vs. forest), regions for 2012 data (Burgundy vs. Franche-Comté) and the habitat:region interaction for 2012 data. For clutch failure rate and brood failure rate, the interaction between habitat and region was not considered because probabilities reached 0 % in one of the forest sites (Tables 1, 2). For incubation time, hatching success, hatching rate, and clutch failure rate, as well as survival until D13, survival rate and brood failure rate, the first egg date was added as a covariate (Cresswell and McCleery 2003; Blondel 2007). Because overdispersion was detected, the standard errors were corrected using a quasi-GLM model (Zuur et al. 2009).
The differences in egg mass, yolk carotenoids and eggshell thickness between habitats in Burgundy (2012 data only) were analysed with linear models (LMs). First egg date, clutch size, and egg mass were included as covariates for yolk carotenoids and eggshell thickness analyses to avoid collinearity among variables (Isaksson et al. 2008). First egg date and clutch size were included as covariates for egg mass analyses (Isaksson et al. 2008). To achieve normality, yolk carotenoid concentrations in Great Tit eggs, and yolk carotenoid concentrations, egg mass and eggshell thicknesses for Blue Tit eggs were log-transformed.
In Burgundy, the habitat effect on nestling growth, nestling mass at D1, and body condition at D13 was analysed with linear mixed models (LMMs) that controlled for a brood effect as a random factor. First egg date and brood size were included in the analyses as covariates. Because only three measurements were available to monitor nestling growth (D1, D7 and D13), growth was assumed to be linear. According to the historical data of van Balen (1973), growth is actually weakly sigmoidal. However, our measurements took place during the rapid phase of nestling growth that shows quite a linear profile (from 2 days post-hatching to 15 days post-hatching). The nestling age was defined as a fixed effect and nestling identity was nested in brood as a random factor to take account for the non-independence of the measurements within a brood (one nestling was measured three times during growth). Only the chicks that were measured at each age were included in the analyses of growth. The residuals of the linear regression between log-transformed body mass and tarsus length were used for a global estimation of body condition at D13 in Burgundy. Sample sizes for BC condition analysis were generally higher than for growth because additional broods and nestlings for which we have only D13 data were added in both species (Table 6). For LMMs as for LMs models, we have plotted residuals against fitted value to identify violation of homogeneity following the recommendation from Zuur et al. (2009). We did not observe any violation of homogeneity for all of our models.
Each analysis was performed using the full model with software R v.3.15.1 (R Development Core Team, Vienna, 2014). Statistical significance was set at p < 0.05 for all results. The models containing a random factor were analysed with the package lme4 (Bates 2010) and the pbkrtest package for the estimation of degrees of freedom for GLMMs (Kenward and Roger 1997). If not significant, interactions were removed from the models to gain in statistical power. Variables (habitat and region) and covariates (first egg date, clutch size, egg mass and brood size) of interest for our investigations were kept in the models, even if they were not significant (Tables 3, 4, 5, 6).
Results
Reproductive parameters
In Great as well as in Blue Tits, first egg date did not differ between the two habitats in 2013 and in 2014, but there was a significant interaction between habitat and region in 2012 with females laying later in the forest of Franche-Comté in both species (Tables 1, 2, 3).
In 2012, urban Great Tits in Burgundy laid approximately 3 eggs less than their counterparts in the forest, while no difference was observed in Franche-Comté as shown by a significant interaction between habitat and region (Tables 1, 3). The difference in clutch size was still observed in Burgundy in 2013 and 2014 for this species (Tables 1, 3). In 2012, there was a global effect of habitat on Blue Tit clutch size with urban females laying on average 0.8 less eggs than forest ones when grouping data from the two regions (Tables 2, 4). In addition, Blue Tit clutch sizes were larger in Franche-Comté than in Burgundy (Tables 2, 4). In Burgundy, urban Blue Tits also laid less eggs than forest ones in 2013 and 2014, but did not appear significant in 2014 (Tables 2, 4). Finally, incubation period in 2012 was significantly longer in urban areas for Blue Tits (mean ± SE: urban: 14.7 days ± 3.6; forest: 13.1 days ± 2.0; GLM analysis: β ± SE = 0.11 ± 0.04, F 1,183 = 6.7, p < 0.01), and Great Tits showed the same pattern with a difference close to significance (urban: 14.1 days ± 2.1; forest: 13.0 days ± 1.9; GLM analysis: β ± SE = 0.08 ± 0.05, F 1,153 = 3.2, p = 0.07).
In 2012, Great Tits hatching success was 10 % lower and clutch failure rate was 6 % higher in cities than in forests, but hatching rates did not differ between habitats despite a strong tendency (Tables 1, 3). Additionally, clutch failure rate was positively associated with first egg date, and hatching success was negatively associated with first egg date: late clutches were less prone to hatch (Table 3). In 2013 and 2014, hatching success was also lower (18 and 11 %, respectively) and clutch failure rate was higher (12 and 11 %, respectively) in the urban area than in forest in Burgundy (Tables 1, 3). Hatching rate of Great Tits was significantly lower in Dijon in 2013 (8 %), but did not differ between habitats in 2014 (Tables 1, 3). In 2012, Blue Tits also had a lower hatching success (7 %) and a higher clutch failure rate (7 %) in urban sites, with higher values in Franche-Comté than in Burgundy (Tables 2, 4). For this species, hatching rate did not differ between urban and forest habitats during the 3 years (Tables 2, 4).
In 2012, Great Tit nestlings had 34 % lower survival until D13 and 16 % lower survival rate in cities, where, in addition, brood failure rate was 21 % higher than in forests, when grouping data from the two regions (Tables 1, 3). In addition, survival until D13 and survival rate were higher in Franche-Comté than in Burgundy (Tables 1, 3). In 2013 and 2014, survival until D13 was also lower (37 and 18 %, respectively) and brood failure rate was higher (25 and 32 %, respectively) in the urban area than in the forest in Burgundy. Survival rate of Great Tit nestlings was also 18 % lower in city in 2013 despite the difference was not significant, but did not differ in 2014 (Tables 1, 3). In Blue Tits, survival until D13 and survival rate were lower for urban nestlings than for forest ones in 2012 (31 and 16 %, respectively) when grouping data from the two regions (Tables 2, 4). In 2013, the same difference between habitats was observed for these two variables in Burgundy (25 and 21 %, respectively), while 2014 data showed a strong but not significant tendency (Tables 2, 4). In 2012, Blue Tit brood failure rate was 18 % higher in cities. In addition, brood failure rate was positively and fledging success negatively associated with first egg date: nestlings from late-initiated clutches were less prone to survive (Table 4). In Burgundy, data from 2013 to 2014 showed that Blue Tit brood failure rate was not significantly higher in urban habitat, despite a very strong tendency in 2014.
Egg quality
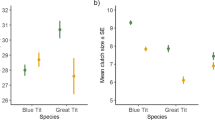
Egg weight and yolk carotenoid concentrations did not differ between habitats in Great Tits, whereas eggs of forest Blue Tits were on average lighter and contained fewer carotenoids (Fig. 1a, b). In Blue Tits, yolk carotenoid concentrations increased with egg mass (Table 5). Shell thickness did not differ between habitats in Great Tits (mean ± SD; forest: 43.62 µm ± 3.80 and urban: 43.90 µm ± 3.81) and Blue Tits (mean ± SD; forest: 39.47 µm ± 3.82 and urban: 40.30 µm ± 4.75), but was positively associated with egg mass (Table 5).
Egg and chick characteristics (mean ± SE) of Great and Blue Tits in urban and forest habitats in Burgundy: a egg mass (g), b egg yolk carotenoid concentrations (µg/mg), and c nestling mass on D1 (g). Sample sizes are indicated in the figures, and for the nestling data, brood numbers are given in parentheses. An asterisk indicates a significant difference
Growth
In 2012, Great Tit nestlings from the forest were heavier at D1 than those from Dijon (LMM analysis: β ± SE = −0.45 ± 0.13, F 1,21.41 = 12.29, p < 0.01), whereas egg mass did not differ between the two sites (see above) (Fig. 1c). For Blue Tits, the nestling mass at D1 was not significantly different between the two habitats (LMM analysis: β ± SE = 0.08 ± 0.08, F 1,37.15 = 0.85, p = 0.36) (Fig. 1c), while eggs were heavier in Dijon (Fig. 1a). At D1, Great Tit nestlings from early clutches were the heaviest (LMM analysis: β ± SE = −0.04 ± 0.01, F 1,23.25 = 6.69, p = 0.02). Additionally, 13-day-old nestlings of both species showed a higher body condition in the forest than in the city during the 3 years of the study (Table 6; Fig. 2). In Blue Tits, the nestling body condition was negatively affected by brood size, and the nestlings from early clutches were in better condition (Table 6). Interestingly, the highly significant interaction between age and habitat showed that growth of Tit nestlings was significantly lower in the urban site of Burgundy than in the forest site (Table 6; Fig. 3).
Discussion
Our results show that several components of reproductive success in Great and Blue Tits are reduced in urban habitats compared to forests. In urban sites, breeding success was lower, with, in most cases, smaller clutch sizes, higher clutch and higher brood failure rates and lower nestling survival rates. Additionally, growth rate and nestling body condition in the period leading up to fledging were all reduced in urban birds, with potential adverse consequences for fitness. The general picture that emerges from our findings is a negative effect of urban habitat on reproduction.
A reduced clutch size is perhaps the most commonly reported negative effect of urbanization upon bird reproductive success (Chamberlain et al. 2009). Among species that evolved in forests, females laid fewer eggs in towns than in control wooded areas (Grégoire 2003; Mennechez and Clergeau 2006; Chamberlain et al. 2009). This pattern has been reported from several previous studies of tits with urban birds laying clutches that were 10–20 % smaller than forest birds, as reported in our study (Hõrak 1993; Hõrak et al. 2000, 2002; Solonen 2001; Marciniak et al. 2007). Females are known to reduce clutch size according to their own body condition and/or in response to local constraints (Both and Visser 2003; Marzal et al. 2005; Fontaine and Martin 2006). Because cities are described as providing fewer adequate resources than forests and are characterised by several anthropogenic alterations, such as noise, artificial light and contaminant circulation, the urban environment could exert strong constraints on bird reproduction (Peach et al. 2008; Dominoni et al. 2013). Under these circumstances, females might optimise their fitness by laying fewer eggs but then increasing the amount of resources they devote to those eggs and the subsequent nestlings in order to increase their survival prospects (Krist 2011). In our case study, Blue Tits might have adopted this strategy because egg mass and carotenoid content were higher in urban habitats while body mass of females Blue Tits did not differ between urban and forest habitats [mean ± SD; forest: 11.2 ± 0.4 g (n = 38); urban: 11.0 ± 0.7 g (n = 36), detailed data not shown here]. However, Great Tit eggs did not differ between habitats. An alternative interpretation was that the females laid fewer eggs to conserve energy and resources for a second nesting attempt (Farnsworth et al. 2001; Parejo and Danchin 2006; Verhulst and Nilsson 2008). This strategy might increase breeding success, particularly if the risk of nest failure was high (Faivre et al. 2001; Weggler 2006; Lambrechts et al. 2008). Kaliński et al. (2009) observed that pairs of Great Tits in urban areas were more likely to initiate a second brood (50 % against 15 % in forest), as observed in other species, such as the Blackbird Turdus merula (Grégoire 2003; Luniak 2004; Chamberlain et al. 2009). In our study, Tits in urban habitats had most of their investment in first clutches exposed to a higher complete failure risk and a higher nestling mortality rate than in forests. This is also consistent with studies that found that Great Tits nesting in stressful conditions (e.g. inclement weather with low temperatures and heavy rainfall, or low abundance of food) were more likely to desert their first attempt in order to renest (Hõrak et al. 1999; Ouyang et al. 2012). This strategy could improve breeding success if adults find alternative resources later in the season (for instance, in the herb layer) as it is well known that prey availability is seasonally highly variable (Marciniak et al. 2007; Arnold et al. 2010). In the framework of our 3-year project, second broods were also monitored in Burgundy in 2014. Lower breeding success was still observed in the urban habitat (unpublished data). Therefore, second broods did not compensate for the overall difference of breeding success between habitats.
Maternal allocation to eggs is also a key element that determines fitness. Indeed, in several bird species (reviewed by Krist 2011), including the Great Tit (Marri and Richner 2014), nestling growth and survival were strongly related to egg quality, with delayed effects, even after rearing. Eggshell thickness, yolk carotenoids and egg mass are three characteristics that indicate female qualitative and quantitative investment in the early steps of reproduction, and several works have shown that egg mass and carotenoids positively affected the development of nestlings (Styrsky et al. 1999; Koutsos et al. 2003; Marri and Richner 2014). Because birds cannot synthesise carotenoids or store calcium in the long term, the quality of their eggs depends on their ability to acquire them from the local environment (Graveland and Berends 1997; Biard et al. 2005; Hargitai et al. 2006). Additionally, females may modulate yolk carotenoid deposition into their eggs according to laying sequence (Hõrak et al. 2002; Saino et al. 2002), habitat (Hõrak et al. 2002; Eising et al. 2008; Safran et al. 2010), and their own condition (Blount et al. 2000; Christians 2002). In our case study, our results did not indicate any negative effects of urban habitat on egg quality, in accordance with recent findings on Great and Blue Tits (Isaksson et al. 2008; Bańbura et al. 2010). Furthermore, the urban Blue Tits laid larger and more carotenoid-rich eggs, which suggested that they were not constrained to invest crucial resources in eggs. Urban tits may not benefit from laying eggs of better (Blue Tit) or similar (Great Tit) quality than in the forest because they produced similar (Blue Tit) or lighter (Great Tit) nestlings at D1, which suggested a slower embryonic development in both species. Obviously, other aspects of egg quality, such as hormone concentrations (Groothuis and Schwabl 2008), temperature or incubation period (Nilsson et al. 2008), are known to affect embryo development with consequences for nestling phenotypes. Here, we observed a longer incubation period in urban habitats for Blue Tits and a strong similar tendency for Great Tits. Temporary disruption of incubation temperature and slowed incubation with delayed hatching might have compromised embryo development in both species and hatching success in Great Tits (Monrós et al. 1998; Naef-Daenzer et al. 2004). Factors that interrupted incubation (e.g. human disturbance and longer foraging activities of females) remain to be determined for further interpretation. Delayed initiation of incubation after clutch completion might have also altered embryo development without an increase of incubation period per se. Finally, nestling mass at D1 might better reflect the early stage of post-hatching growth rather than embryonic development, with faster early growth in forests than in towns. The assessment of mass at hatching would provide a definitive answer but is difficult to obtain because all eggs do not hatch exactly at the same time. However, because yolk residuals serve as an important energy and nutrient source for hatchlings during the early stage of their life (Starck and Ricklefs 1998), the contribution of food delivered by parents to early growth was probably less crucial than later in the nestling life.
A higher nestling mortality also contributed to the poorer reproductive performance of urban Tits we observed here. The complete brood failure and brood reduction that we observed are in agreement with previous findings on other urban birds (Chamberlain et al. 2009), including Tits (Hõrak 1993; Solonen 2001; Kaliński et al. 2009). In our case study, this may be a consequence of the reduced D1 weights in urban Great Tits, as nestling weight in early life could affect the probability of survival until fledging (Cleasby et al. 2010). Other crucial ecological factors might also explain the observed contrast, and parasitism and/or food availability deserve attention (Martin 1987). Ectoparasites such as the hen flea (Siphonaptera: Ceratophyllidae) and blowfly larvae (Diptera: Calliphoridae) are known to strongly affect survival in young Tits (Richner et al. 1993; Eeva et al. 1994; Blondel 2007). Our observations did not support a role for ectoparasites because we did not detect any during our handling of the chicks. Food availability is more likely because the density and size of deciduous trees, which support caterpillars, were low in towns. Caterpillars are an essential component of breeding Tit diet. Caterpillar-poor habitats are known to constrain nestling condition and ultimately breeding success (Visser et al. 2006; Blondel 2007), and a long-term study in Poland found a lower density of caterpillars in urban parks than in the nearby forest (Marciniak et al. 2007; Kaliński et al. 2009). Moreover, studies on essential nutrients, such as carotenoids, also found lower carotenoid concentration in urban caterpillars during the breeding season (Isaksson and Andersson 2007; Isaksson 2009).
The reduced nestling growth that we observed in both species and in both years is another illustration of the constraints exerted by the urban habitat. To our knowledge, our study and that of Richner (1989) in nestling Carrion Crows (Corvus corone) are the only ones that compared growth between urban and rural habitats during the nestling stage. Our results were also consistent with those of Kaliński et al. (2009) who described a lower pre-fledging body condition in urban Great Tits compared with birds from woodlands. Food limitation was again a relevant interpretation for the reduced growth that we observed here (Schew and Ricklefs 1998).
These results may lead to the addressing of interesting issues on population status. Indeed, the reduced breeding success in towns suggests that urban populations may represent sinks that could be sustained by immigration from forests surrounding the cities. Molecular analyses over several pairs of urban and forest sites distributed across species ranges will be required to elucidate this question. Because urban habitats may be attractive especially during winter (by offering additional resources and warm temperatures), we may also hypothesise that towns are ecological traps. In this case, individuals chose inferior habitat to breed (i.e. cities) preferentially over what is actually superior habitat (i.e. forests), and performed badly (Battin 2007). Again, molecular approaches in combination with site fidelity within and between years could be helpful to investigate this issue (Stracey and Robinson 2011).
Overall, we demonstrated the negative effects of urbanization on the reproductive success components of forest birds, and our results were globally consistent between years, species and geographic areas for these effects. Further work, such as experimental feeding, would be required to determine the mechanisms of the negative effects of urbanisation on the reproduction of free-ranging bird populations.
References
Alonso-Alvarez C, Bertrand S, Devevey G et al (2004) An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am Nat 164:651–659. doi:10.1086/424971
Arnold KE, Ramsay SL, Henderson L et al (2010) Seasonal variation in diet quality: antioxidants, invertebrates and Blue Titis Cyanistes caeruleus. Biol J Linn Soc 99:708–717
Bańbura M, Sulikowska-Drozd A, Kaliński A et al (2010) Egg size variation in Blue Tits Cyanistes caeruleus and Great Tits Parus major in relation to habitat differences in snail abundance. Acta Ornithol 45:121–129. doi:10.3161/000164510X551264
Bates D (2010) lme4: Mixed-effects modeling with R. http//lme4.r-forge.r-project.Org/b. Accessed 27 Sept 2013
Battin J (2007) When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv Biol 18:1482–1491
Biard C, Surai PF, Møller AP (2005) Effects of carotenoid availability during laying on reproduction in the Blue Tit. Oecologia 144:32–44. doi:10.1007/s00442-005-0048-x
Blondel J (2007) Coping with habitat heterogeneity: the story of Mediterranean Blue Tits. J Ornithol 148:3–15. doi:10.1007/s10336-007-0161-1
Blount JD, Houston DC, Moller AP (2000) Why egg yolk is yellow. Trends Ecol Evol 15:47–49
Blount JD, Surai PF, Nager RG et al (2002) Carotenoids and egg quality in the Lesser blackbacked gull Larus fuscus: a supplemental feeding study of maternal effects. Proc R Soc Lond B 269:29–36. doi:10.1098/rspb.2001.1840
Both C, Visser ME (2003) Density dependence, territoriality, and divisibility of resources: from optimality models to population processes. Am Nat 161:326–336. doi:10.1086/346098
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plan 74:46–69. doi:10.1016/j.landurbplan.2004.08.007
Chamberlain DE, Cannon AR, Toms MP et al (2009) Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151:1–18. doi:10.1111/j.1474-919X.2008.00899.x
Christians JK (2002) Avian egg size: variation within species and inflexibility within individuals. Biol Rev Camb Philos Soc 77:1–26
Cleasby IR, Nakagawa S, Gillespie DOS, Burke T (2010) The influence of sex and body size on nestling survival and recruitment in the House sparrow. Biol J Linn Soc 101:680–688. doi:10.1111/j.1095-8312.2010.01515.x
Cramp S, Perrins CM, Brooks DJ (1993) The birds of western Palearctic—volume VII. Handbook of the birds of Europe, the Middle East and North Africa. Oxford University Press, New York, pp 225–281
Cresswell W, McCleery RH (2003) How Great Tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J Anim Ecol 72:356–366. doi:10.1046/j.1365-2656.2003.00701.x
Dominoni D, Quetting M, Partecke J (2013) Artificial light at night advances avian reproductive physiology. Proc R Soc Lond B 280:20123017
Drent PJ, Woldendorp JW (1989) Acid rain and eggshells. Nature 339:431
Eeva T, Lehikoinen E, Nurmi J (1994) Effects of ectoparasites on breeding success of Great Tits (Parus major) and Pied flycatchers (Ficedula hypoleuca) in an air pollution gradient. Can J Zool 72:624–635. doi:10.1139/z94-085
Eising C, Pavlova D, Groothuis T (2008) Maternal yolk androgens in European Starlings: affected by social environment or individual traits of the mother? Behaviour 145:51–72
Faivre B, Préault M, Théry M et al (2001) Breeding strategy and morphological characters in an urban population of Blackbirds, Turdus merula. Anim Behav 61:969–974. doi:10.1006/anbe.2000.1669
Farnsworth G, Simons T, Brawn J (2001) How many baskets? Clutch sizes that maximize annual fecundity of multiple-brooded birds. Auk 118:973–982
Fontaine JJ, Martin TE (2006) Parent birds assess nest predation risk and adjust their reproductive strategies. Ecol Lett 9:428–434. doi:10.1111/j.1461-0248.2006.00892.x
Geue D, Partecke J (2008) Reduced parasite infestation in urban Eurasian Blackbirds (Turdus merula): a factor favoring urbanization? Can J Zool 86:1419–1425. doi:10.1139/Z08-129
Graveland J, Berends AE (1997) Timing of the calcium intake and effect of calcium deficiency on behaviour and egg laying in captive Great Tits, Parus major. Physiol Zool 70:74–84
Grégoire A (2003) Démographie et différenciation chez le Merle Noir Turdus merula: lien avec l’habitat et les relations hôtes-parasites. Doctoral thesis, University of Bourgogne, Dijon
Grimm NB, Faeth SH, Golubiewski NE et al (2008) Global change and the ecology of cities. Science 319:756–760. doi:10.1126/science.1150195
Groothuis TGG, Schwabl H (2008) Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos Trans R Soc Lond B 363:1647–1661. doi:10.1098/rstb.2007.0007
Groothuis TGG, Müller W, von Engelhardt N et al (2005) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 29:329–352. doi:10.1016/j.neubiorev.2004.12.002
Hargitai R, Matus Z, Hegyi G et al (2006) Antioxidants in the egg yolk of a wild passerine: differences between breeding seasons. Comp Biochem Physiol B 143:145–152. doi:10.1016/j.cbpb.2005.11.001
Hõrak P (1993) Low fledging success of urban Great Tits. Ornis Fenn 70:168–172
Hõrak P, Jenni-Eiermann S, Ots I (1999) Do Great Tits (Parus major) starve to reproduce? Oecologia 119:293–299
Hõrak P, Vellau H, Ots I, Møller a P (2000) Growth conditions affect carotenoid-based plumage coloration of Great Tit nestlings. Naturwissenschaften 87:460–464
Hõrak P, Surai P, Moller A (2002) Fat-soluble antioxidants in the eggs of Great Tits Parus major in relation to breeding habitat and laying sequence. Avian Sci 2:123–130
Isaksson C (2009) The chemical pathway of arotenoids: from plants to birds. Ardea 97:125–128
Isaksson C, Andersson S (2007) Carotenoid diet and nestling provisioning in urban and rural Great Tits Parus major. J Avian Biol 38:564–572. doi:10.1111/j.2007.0908-8857.04030.x
Isaksson C, Johansson A, Andersson S (2008) Egg yolk carotenoids in relation to habitat and reproductive investment in the Great Tit Parus major. Physiol Biochem Zool 81:112–118. doi:10.1086/522650
Kaliński A, Wawrzyniak J, Bańbura M et al (2009) Haemoglobin concentration and body condition of nestling Great Tits Parus major: a comparison of first and second broods in two contrasting seasons. Ibis 151:667–676. doi:10.1111/j.1474-919X.2009.00959.x
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. doi:10.2307/2533558
Kilgas P, Tilgar V, Mänd R (2007) Physiological condition of incubating and brood rearing female Great Tits Parus major in two contrasting habitats. Acta Ornithol 42:129–136
Koutsos EA, Clifford AJ, Calvert CC, Klasing KC (2003) Maternal carotenoid status modifies the incorporation of dietary carotenoids into immune tissues of growing chickens (Gallus gallus domesticus). J Nutr 133:1132–1138
Krist M (2011) Egg size and offspring quality: a meta-analysis in birds. Biol Rev Camb Philos Soc 86:692–716. doi:10.1111/j.1469-185X.2010.00166.x
Lambrechts MM, Rieux A, Galan M-J et al (2008) Double-brooded Great Tits (Parus major) in Mediterranean oak habitats: do first broods always perform better than second broods? Russ J Ecol 39:516–522. doi:10.1134/S1067413608070084
Luniak M (2004) Synurbization–adaptation of animal wildlife to urban development. In: Shaw WW, Harris LK, Vandruff L (eds) Proceedings of 4th international symposium urban wildlife conservation, Tucson, pp 50–55
Marciniak B, Nadolski J, Nowakowska M et al (2007) Habitat and annual variation in arthropod abundance affects Blue Tit Cyanistes caeruleus reproduction. Acta Ornithol 42:53–62
Marri V, Richner H (2014) Yolk carotenoids increase fledging success in Great Tit nestlings. Oecologia 176:371–377. doi:10.1007/s00442-014-3051-2
Martin T (1987) Food as a limit on breeding birds: a life-history perspective. Annu Rev Ecol Syst 18:453–487
Marzal A, de Lope F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545. doi:10.1007/s00442-004-1757-2
Marzluff J (2001) Worldwide urbanization and its effects on birds. In: Marzluff JM, Bowman R, Donnelly R (eds) Avian ecology and conservation in an urbanizing world. Springer, New York, pp 19–47
McGraw KJ, Adkins-Regan E, Parker RS (2005) Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften 92:375–380. doi:10.1007/s00114-005-0003-z
McKinney M, Lockwood J (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453
Mennechez G, Clergeau P (2006) Effect of urbanisation on habitat generalists: starlings not so flexible? Acta Oecol 30:182–191. doi:10.1016/j.actao.2006.03.002
Monrós J, Belda E, Barba E (1998) Delays of the hatching dates in Great Tits Parus major: effects on breeding performance. Ardea 86:213–220
Naef-Daenzer L, Nager R, Keller LF, Naef-Daenzer B (2004) Are hatching delays a cost or a benefit for Great Tit Parus major parents? Ardea 92:229–238
Nilsson J, Stjernman M, Nilsson J (2008) Experimental reduction of incubation temperature affects both nestling and adult Blue Tits Cyanistes caeruleus. J Avian Biol 39:553–559. doi:10.1111/j.2008.0908-8857.04199.x
Ouyang JQ, Quetting M, Hau M (2012) Corticosterone and brood abandonment in a passerine bird. Anim Behav 84:261–268. doi:10.1016/j.anbehav.2012.05.006
Parejo D, Danchin E (2006) Brood size manipulation affects frequency of second clutches in the Blue Tit. Behav Ecol Sociobiol 60:184–194. doi:10.1007/s00265-005-0155-z
Peach WJ, Vincent KE, Fowler JA, Grice PV (2008) Reproductive success of House sparrows along an urban gradient. Anim Conserv 11:493–503. doi:10.1111/j.1469-1795.2008.00209.x
Porlier M, Charmantier A, Bourgault P et al (2012) Variation in phenotypic plasticity and selection patterns in Blue Tit breeding time: between-and within-population comparisons. J Anim Ecol 81:1041–1051. doi:10.1111/j.1365-2656.2012.01996.x
Ratcliffe DA (1970) Changes attributable to pesticides in egg breakage frequency and eggshell thickness in some British birds. J Appl Ecol 7:67–115
Richner H (1989) Habitat-specific growth and fitness in Carrion crows (Corvus corone corone). J Anim Ecol 58:427–440
Richner H, Oppliger A, Christe P (1993) Effect of an ectoparasite on reproduction in Great Tits. J Anim Ecol 62:703–710
Safran RJ, McGraw KJ, Pilz KM, Correa SM (2010) Egg-yolk androgen and carotenoid deposition as a function of maternal social environment in Barn swallows Hirundo rustica. J Avian Biol 41:470–478. doi:10.1111/j.1600-048X.2010.04962.x
Saino N, Bertacche V, Ferrari RP et al (2002) Carotenoid concentration in Barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc R Soc Lond B 269:1729–1733. doi:10.1098/rspb.2002.2088
Sanz J, García-Navas V, Ruiz-Peinado J (2010) Habitat type and nest-site characteristics on the breeding performance of Great and Blue Tits (Parus major and P. caeruleus) in a Mediterranean landscape. Ornis Fenn 87:1–11
Schew W, Ricklefs R (1998) Developmental plasticity. In: Starck JM, Ricklefs RE (eds) Avian growth and development. Oxford University Press, New York, pp 288–304
Seress G, Bókony V, Pipoly I et al (2012) Urbanization, nestling growth and reproductive success in a moderately declining House sparrow population. J Avian Biol 43:403–414. doi:10.1111/j.1600-048X.2012.05527.x
Slabbekoorn H (2013) Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim Behav 85:1089–1099. doi:10.1016/j.anbehav.2013.01.021
Solonen T (2001) Breeding of the Great Tit and Blue Tit in urban and rural habitats in southern Finland. Ornis Fenn 78:49–60
Starck JM, Ricklefs RE (1998) Avian growth and development: evolution within the altricial-precocial spectrum. Oxford University Press, New York
Stracey CM, Robinson SK (2011) Are urban habitats ecological traps for a native songbird? Seson-long productivity, apparent survival, and site fidelity in urban and rural habitats. J Avian Biol 43:50–60
Styrsky JD, Eckerle KP, Thompson CF (1999) Fitness-related consequences of egg mass in nestling House wrens. Proc R Soc Lond B 266:1253. doi:10.1098/rspb.1999.0771
Surai PF, Speake BK (1998) Distribution of carotenoids from the yolk to the tissues of the chick embryo. J Nutr Biochem 9:645–651. doi:10.1016/S0955-2863(98)00068-0
Surai P, Speake B, Sparks N (2001) Carotenoids in avian nutrition and embryonic development. 2. Antioxidant properties and discrimination in embryonic tissues. J Poult Sci 38:117–145
Van Balen JH (1973) A comparative study of the breeding ecology of the Great tit, Parus major, in different habitats. Ardea 61:1–93
Verhulst S, Nilsson J-A (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos Trans R Soc Lond B 363:399–410. doi:10.1098/rstb.2007.2146
Visser ME, Holleman LJM, Gienapp P (2006) Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147:164–172. doi:10.1007/s00442-005-0299-6
Warton D, Hui F (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Weggler M (2006) Constraints on, and determinants of, the annual number of breeding attempts in the multi-brooded Black redstart Phoenicurus ochruros. Ibis 148:273–284. doi:10.1111/j.1474-919X.2006.00527.x
Williams TD (1994) Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol Rev Camb Philos Soc 69:35–59. doi:10.1111/j.1469-185X.1994.tb01485.x
Zuur AF, Ieno EN, Walker NJ et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
The Regional Council of Burgundy and the Center for Research and Higher Education provided financial support for this work. The study site in Franche-Comté belongs to the Long Term Ecological Research (LTER) Network “Zone Atelier Arc Jurassien” (http://zaaj.univ-fcomte.fr/). We thank S. Garnier who helped with the fieldwork. We thank G. Sorci, P. Christ, A. Grégoire, S. Cornet and M. Galipaud for statistical analyses and their comments on the results. Many thanks to I. Stewart for his comments on an earlier version of this manuscript. The authors also greatly thank D. Dominoni and the anonymous reviewer for their helpful comments on this paper. The study conforms to the legal requirements of France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Electronic supplementary material
Meteorological conditions during the tit reproduction season (March–June) in Burgundy and Franche-Comté for the 3 years of study (2012–2014): minimum and maximum monthly temperatures (°C); cumulative rainfall per month (mm); cumulative sunshine per month (h). Meteorological stations used were Dijon-Longvic for Burgundy sites (47°27N, 5°09E) and Besançon-Thise for Franche-Comté sites (47°25N, 5°99E). Data obtained with permission from http://www.infoclimat.fr (all rights reserved).
Rights and permissions
About this article
Cite this article
Bailly, J., Scheifler, R., Berthe, S. et al. From eggs to fledging: negative impact of urban habitat on reproduction in two tit species. J Ornithol 157, 377–392 (2016). https://doi.org/10.1007/s10336-015-1293-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1293-3