Abstract
Aspects influencing the distribution patterns of mammals are particularly important for species living in human altered landscapes. The current study investigated the relationship between occurrence patterns of large spotted genets (Genetta tigrina) with various environmental variables believed to influence their detection and site occupancy in an urban environment. Presence/absence data was collected from 28 camera trap stations between June 2012 and October 2013 in Kloof/Hillcrest suburbs, Durban, South Africa. Average estimated occupancy of G. tigrina was 0.62 ± 0.14 with a detection probability 0.19 ± 0.03. The naïve occupancy was 0.607. Model selection indicated that bush cover and placement of camera traps on wide paths negatively influenced G. tigrina occupancy. Both winter and fringe habitats influenced the site occupancy of G. tigrina positively. Furthermore, bush cover was negatively associated with detection probability of G. tigrina. The current camera surveys provided baseline data for long-term species observations within suburban Kloof/Hillcrest. The present study indicated the importance of a variety of factors on the distribution of G. tigrina, particularly in landscapes where natural habitats are threatened with changing land use and increasing human populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, there has been an increase in the human population living in urban areas and subsequently an increase in the amount of land urban areas occupy (Riem et al. 2012). Urbanization results in the anthropogenic conversion and altering of natural habitats into commercial, industrial and residential uses and has been linked to a worldwide decline in native biodiversity (Adams 2005; Randa and Yunger 2006; Riem et al. 2012). Native vegetation is replaced with anthropogenic structures (buildings, pavement) and often non-native vegetation (Riem et al. 2012). Furthermore, the reduction in available habitats for wildlife subsequently reduces species richness, abundance, distribution and genetic diversity (Bettigole et al. 2013). As urbanization continues to alter and degrade natural habitats, animals are forced into close proximity to human populations (Bateman and Fleming 2012; Lowry et al. 2013). The urban landscape is characterized by reduced landscape heterogeneity which influences both ecosystem functioning and structuring and the introduction of environmental stressors such as pollutants and elevated human activity (Randa and Yunger 2006; Jung and Kalko 2010). This often results in many species withdrawing into reduced ranges in response to spread of urban environments (Bateman and Fleming 2012). Urban landscapes often reflect a gradient of land use varying from the highly urbanized city centers through to the suburban areas and less altered rural landscapes (Riem et al. 2012). The ability of these patches to sustain wildlife populations varies spatially between open residential areas and highly developed central business districts or industrial areas (Baker and Harris 2007). The degree of habitat modification and land use generally declines with increasing distance from the highly urbanized city center (Randa and Yunger 2006). This creates an urban–rural gradient that can be assessed to determine the effect that increasing urbanization is having on biotic communities. Urban-gradient studies generally reflect a reduction of native species and an increase of non-native species towards the centre of urban areas (McKinney 2002). Native habitats and species are often eliminated from the city centers and these areas are associated with high rates of extinction (McKinney 2002; Randa and Yunger 2006). Furthermore, species richness decreases across the urban–rural gradient, with the lowest species richness recorded in urban centers (McKinney 2002). Increasing distance from the urban center often results in a decline in habitat modification. This gives rise to a mosaic of natural and semi-natural habitats such as residential gardens, golf courses, parks, green belts and conservancies (patches of semi-natural habitats) with a lower land use (Baker and Harris 2007; Maruani and Amit-Cohen 2007). The presence of these natural green areas provide numerous habitat possibilities for wildlife, are essential for sustaining wildlife populations and can increase urban biodiversity (Savard et al. 2000; Shultz et al. 2012; Bateman and Fleming 2012). Connections of relatively undisturbed habitat patches within urban areas act as refugia for a variety of species and can also alleviate the impact of urbanization on wildlife populations (Baker and Harris 2007; Ordeñana et al. 2010; Bateman and Fleming 2012). Urban green spaces can provide long-term habitat stability in a rapidly changing urban environment and often support high species diversity (McKinney 2002; Shultz et al. 2012). However in most instances patches of remaining natural habitats are highly isolated with no connection to other patches thus creating abrupt boundaries that generally hinder species diversity (McKinney 2002; Randa and Yunger 2006). A good knowledge of species abundance, distribution and occurrence is imperative for effective planning and implementation of conservation strategies (Tobler et al. 2008; Mugerwa et al. 2012). Furthermore, aspects that influence species distribution patterns such as competition, predation, topography and land-use changes, provide important knowledge for future conservation and ecological studies (Macdonald and Rushton 2003; Mugerwa et al. 2012; Msuha et al. 2012). Recent monitoring techniques have implemented camera trapping to determine animal presence, abundance and habitat use (Stein et al. 2008; Mugerwa et al. 2012). Camera trapping is particularly useful in habitats that present difficult field conditions for other assessment methods, and provide important information that would otherwise be difficult to obtain using traditional field techniques (Cutler and Swann 1999; Abi-Said and Amr 2012; Anile et al. 2012; McCallum 2012). Camera trapping data have recently been used to estimate the landscape capacity to support wildlife through occupancy modeling (MacKenzie et al. 2006; Long et al. 2010; Ramesh et al. 2012; Bettigole et al. 2013; Ramesh et al. 2013). Occupancy modeling is a monitoring technique based on the detection/non detection of a target species at a particular site and allows individuals to estimate the probability of occurrence (ψ) at any number of locations, based on the habitat characteristics of each site.(MacKenzie et al. 2006; Ramesh et al. 2012; Bettigole et al. 2013). These detection/non detection surveys allow for the assessment and distribution of secretive species by detecting changes in their occupancy (Ramesh et al. 2012). The large spotted genet (Genetta tigrina) is a nocturnal generalist carnivore adapted to a semi arboreal lifestyle (Roberts et al. 2007). They are distributed throughout most of southern Africa in a variety of habitats including savannah, woodlands, forest and is adaptable to human settlements (Rowe-Rowe 1992; Skinner and Chimimba 2005; Roberts et al. 2007; Ramesh and Downs 2014; Widdows and Downs 2015). However, the distribution of most members of African Viverridae are speculated due to the lack of recent scientific studies (Skinner and Chimimba 2005). Despite their extensive range they are poorly studied in comparison to other small African carnivores. This is mainly due to their nocturnal and elusive habits coupled with their small size. The limited studies conducted on G. tigrina in Africa have focused on diet (Roberts et al. 2007; Widdows and Downs 2015), habitat use (Ramesh and Downs 2014), movements and activity patterns in Orno National Park, Ethiopia (Ikeda et al. 1982; Fuller et al. 1990), as vectors for disease and genetics (Gaubert et al. 2005; Gaubert et al. 2009) with no studies focusing on their distribution in an urban environment.
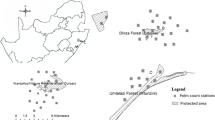
Research focusing on mammals in urban landscapes in South Africa is very limited particularly in the province of KwaZulu-Natal. Wildlife communities and humans in Kloof/Hillcrest suburbs, Durban, South Africa (Fig. 1) live in close proximity due to the large conservancy situated within this urban landscape. Habitat requirements and landscape features influencing G. tigrina have not been studied in an occupancy framework. In the current study, we used camera trap data to estimate the site occupancy and detection probabilities of G. tigrina within an urban landscape of Kloof/Hillcrest. This analysis has enhanced the understanding of the habitat requirements of urban genets and provided basic ecological data for population management and conservation.
Methods
Study area
The study was conducted in the urban suburbs of Kloof/ Hillcrest (S 29.785982 E 30.829841) in Durban, KwaZulu-Natal, South Africa. The mean annual minimum and maximum temperature is 13.9 and 24 °C respectively and the mean annual rainfall is 974 mm (http://en.climate-data.org/location/27097/). Most rainfall occurs in the summer. Frost is infrequent whereas mist is common and provides additional moisture (Mucina and Rutherford 2006). The altitude of the study site is ≥ 560 m ASL. The vegetation in the study area is dominated by KwaZulu-Natal sandstone sourveld scattered with low shrubs and patches of riverine forests (Mucina and Rutherford 2006). 68 % of this grassland biome has been transformed due to plantations, cultivations and urban development (Mucina and Rutherford 2006). This landscape has numerous urban nature reserves such as the Kranzkloof Nature Reserve, Glenholme, Ipithi and Springside Nature Reserves.
Camera traps
We used five digital camera traps (LtI Acorn ® 6210MC, China) triggered by passive infrared sensors, set up at 28 sites during June 2012 – October 2013. Each camera was mounted to a fixed point such as a tree, cross beam, fence or a pole placed in the ground. We set camera height between 30 and 40 cm above the ground in order to increase the probability of detecting all mammalian species that are encountered. Cameras were placed in relatively concealed areas to avoid theft or vandalism. Instances where camera sensors were triggered by unwarranted motion by vegetation or foliage, the cameras were subsequently elevated up to one meter and angled down (Stein et al. 2008). Furthermore, vegetation was cleared around the camera sites to minimize false triggers and avoid blank images and to facilitate the capturing of unobstructed images. The cameras were operational continuously over the 24 h cycle recording the date and time of each photograph. The cameras were programmed to delay one minute between successive photographs and the motion sensor was programmed to “medium sensitivity. This time interval prevented numerous images and videos during one triggering episode. Videos allow for accurate identification of smaller mammals. All camera trap sites were marked and georeferenced using a Global Positioning System (GPS, Garmin Nuvi) (Fig. 1). Each site was visited once a week to check the suitability of the selected site and to verify that the cameras were working normally. In areas with a high site activity, some cameras were required to be checked more frequently. Over the study period each camera remained at a site for 21 nights (SD = 1.37). Photos from the camera traps were downloaded onto a laptop (Lenovo G530, China) in the field on a weekly basis. These weekly visits also ensured the continuous operation of the cameras, routine maintenance and to replace the batteries in the cameras if required.
Habitat measurements
We determined habitat use of G. tigrina by measuring habitat features around each camera location. The variables were selected to test various landscape characteristics concerned with the species habitat use. Percent canopy cover, leaf litter and bush cover were evaluated in a 30-m radius plot from the center of each camera location. Canopy cover was defined as percent of ground covered by the horizontal projection of tree crowns within the plot (Ramesh et al. 2013). Leaf litter was visually assessed in 1 × 1 m subplots placed in four cardinal directions and mean of these four values represented the entire plot. The three different habitat types were scaled according to the increasing human disturbance, namely residential (situated within the urban landscape with no connection to green areas), fringe (situated on the border of residential properties and green belts) and greenbelt habitats (situated within green belts). Surface water bodies both natural (rivers and streams) and artificial (pools and water features) and tarred roads were plotted and the Euclidean distance function in ArcGIS 9.3 was used to calculate the distance (meters) from each camera location to the nearest water source and tarred roads. The presence (1) and absence (0) of domestic dogs (Canis domesticus) and cats (Felis catus) at a camera location and the placement of camera-trap on a narrow trail or large trail were also recorded. Certain locations comprised on degraded grasslands and this may influence the occurrence of large spotted genets. Therefore, we recorded degraded grassland habitats at each camera site as presence (1) and absence (0) scale. As the study covered all seasons, we added all seasons under presence (1) and absence scale (0) in our analysis. A relative abundance index (RAI) of domestic pets (dogs and cats) was estimated from each camera site.
Data analyses
Genetta tigrina occupancy was estimated using a likelihood based method (MacKenzie et al. 2002). A detection history for large spotted genets was created for each camera location, consisting of binary values with ‘1’ indicating species detection during the sampling occasion and ‘0’ indicating non detection (Otis et al. 1978). The event of photographing an individual from the camera traps operating at a single camera station was considered to be an independent record/detection. On some occasions, individuals were captured repeatedly at a camera station during a time period (≤1 min). To avoid pseudo-replications, the first capture of the individual within one minute time frame was considered as an independent record/detection (O’ Brien et al. 2003). Photographs detecting multiple individuals were counted as a single detection. It was assumed that each site was independent and no individual would move between sites during the survey period. We considered each camera location as an independent site and each sampling occasion was treated as a temporal repeat of the survey. The program PRESENCE 5.5 (Hines 2006) was used to model site occupancy and detection probability of G. tigrina with its covariates. We also included covariates measured at camera points: log transformed RAI for domestic dogs and cats. We hypothesized that site occupancy and detection were related to the given covariates. We used continuous site covariates: canopy cover, leaf litter, bush cover, land-use type, distance to the nearest water source (both artificial and natural), and roads as well as four categorical variables presence/absence of domestic dogs, cats, narrow trail, and large trail. Categorical variables were recorded as ‘1’, where the particular variable was present and ‘0’ when absent. Correlations among independent variables were tested to avoid problems with multicollinearity (Graham 2003). All continuous covariates were log transformed prior to the analysis. A global model was generated that contained all potential covariates for occupancy and allowed detection probability (p) to vary by all covariates. P was initially modeled and then followed by ψ. The potential covariates for occupancy were allowed to vary, individually or in combination, whereas detection was either maintained in the global model or remained constant, i.e.,ψ(covariate)p(covariate), or ψ(covariate)p(.). The simplest model was also considered, where both occupancy and detection probability remained constant, ψ(.)p(.). This information was then used to rank and compare candidate models (MacKenzie et al. 2002). Models with ΔAICc ≤ 2 were considered to have strong support (Burnham and Anderson 2002). Model selection, calculation of model weights, and averaging of parameters were conducted according to Burnham and Anderson (2002). We examined covariates that best explained overall G. tigrina occupancy (ψ). We ran 10,000 bootstrap samples (ĉ > 1.0 indicated that there was more variation in the observed data than expected) and Pearson goodness-of fit tests (White and Burnham 1999) to assess the fit of the models to the data. Due to the ratio of sample sizes (n) to the maximum number of estimated parameters (k) bring less than 40, models were ranked according to AICc (AIC adjusted for small sample size). The model-averaged parameters were calculated using Akaike weights (ωi) for both proportion of sites used and detection probabilities. In order to determine the relative influence of each covariate on occurrence, model weights were summed over all models containing the particular covariate
Results
A total of 118 independent photographs captured G. tigrina across all camera sites from 582 camera trap nights. Overall G. tigrina was detected at 17 camera trap locations producing a naïve occupancy of 0.607. Based on a model with all parameters held constant, the site occupancy and detection probability of G. tigrina was 0.61 ± 0.09 and 0.20 ± 0.02 respectively. There was no major difference between the naïve occupancy and the estimated occupancy of G. tigrina (Table 1). In addition a range of other mammals were identified using the camera traps (Appendix 1).
Four of the variables measured were significantly associated with G. tigrina occurrence (ΔAICc ≤ 2, Table 2). The goodness-of-fit test for the global model showed no lack of fit (ĉ = 0.6). The model that produced the greatest support for G. tigrina occurrence (ΔAICc = 0) was ψ(BC + WP + WN + F),p(BC)(Table 1, highest wi = 0.115). This suggests that the variables, fringe habitats (β = 1.71 ± 1.26) and the winter season (β = 1.98 ± 1.41) positively influenced occupancy and variation in bush cover (β = −2.32 ± 1.40, R2 = 0.47; Fig. 2) and wide paths (β = −2.24 ± 1.51) negatively influenced occupancy. In this model, the detection probability of G. tigrina was 0.19 ± 0.029 (Table 1). Of the 10 top ranked models (ΔAICc ≤ 2) occupancy of three models were positively influenced by camera locations at fringe sites and one model was influenced by distance to roads positively. In all top 10 models (ΔAICc ≤ 2) the detection probability of G. tigrina was negatively influenced by increasing bush cover (β = −0.41 ± 0.25, Table 2; Fig. 3).
The summed model weights for each factor with respect to occupancy of G. tigrina were bush cover (wi = 0.87), wide path (wi = 0.45), winter (wi = 0.47), fringe habitats (wi = 0.42) distance to road (wi = 0.18) and distance to natural water source (wi = 0.07). Across all models, the variables that best predicted detection probability of G. tigrina was bush cover (wi = 0.82), whereas there was little support for the distance to road (wi = 0.056) (Table 1). Therefore, the average estimated site occupancy (0.62 ± 0.14) and detection probability (0.19 ± 0.03) of G. tigrina were selected as the final estimates. This corresponded to a difference of 1.3 % from the naïve site occupancy. The detection probability (0.19 ± 0.03) of G. tigrina was low.
Discussion
This study indicated the importance of a variety of landscape factors that influence the distribution and detection probability of G. tigrina, despite having a broad distribution in southern Africa. The importance of these factors is of particular relevance for the implementation of conservation strategies within altered human landscapes. Areas of high bush cover are thought to provide adequate refuge and cover and subsequently reduce predation risk. However, our modeling indicated that the occurrence of G. tigrina was negatively associated with increasing bush cover. This result is contrary to previous studies that have indicated the preference of G. tigrina to well-covered, woodland habitats (Rowe-Rowe 1992; Skinner and Chimimba 2005). Previous research focusing on G. tigrina has occurred in protected areas, which are occupied by other large carnivores. Large carnivores can exert interspecific and predation pressure on the occurrence of small carnivores such as G. tigrina (Caro and Stoner 2003; Virgos and Casanovas 1997). However, human persecution and habitat loss associated with urban areas has resulted in the exclusion of many large carnivores from the suburbs of Kloof/Hillcrest. The reduced predation pressure could allow G. tigrina to move through a variety of habitats and not be confined to areas with high bush cover. Furthermore, G. tigrina is a visual predator and rely on their keen eye sight (Skinner and Chimimba 2005). Areas with high bush cover could inhibit the ability of G. tigrina to locate and capture prey. With the reduction of bush cover in urban landscapes due to habitat loss and modification, the reduced reliance in this habitat type could be a contributing factor to the success of G. tigrina within urban Kloof/ Hillcrest. Our results suggest that areas with high bush cover hinder the site occupancy and distribution of G. tigrina within an urban landscape. Furthermore, there has been increasing reports of G. tigrina using anthropogenic structures such as buildings as daytime resting places further reducing the reliance on areas with high bush cover. Wide paths had a negative effect on the site occupancy of G. tigrina. The avoidance of wide paths could be a strategy to avoid humans and domestic dogs that are more likely to use wider paths as opposed to narrow paths. In a study conducted in a California urban nature reserve by George and Crooks (2006), the detection and frequency probabilities of bobcats (Lynx rufus) declined on wider paths that had high human and domestic dog activity. This indicates spatial displacement due to human and domestic animal activity. In combination with bush cover and wide paths, G. tigrina occupancy was positively associated with fringe habitats. This suggests that the spatial design of this habitat may be structuring the distribution patterns of G. tigrina in urban landscapes. These habitats had no experience the high modification associated with the urban centers. The positive association is not surprising as fringe habitats provide numerous habitat possibilities, refugia, reduced human and domestic activity and provide alleviation from the effects of urbanization (Savard et al. 2000; Bateman and Fleming 2012; Shultz et al. 2012). Furthermore, fringe habitats allow G. tigrina to access the urban environment where they are able to make use of a variety of anthropogenic resources such as food, shelter and supplementary feeding stations provided by residents. It’s the ability to colonize and adapt to both natural and urban landscapes that has facilitated the urban genet population and expansion into the suburbs of Kloof/Hillcrest. Genetta tigrina occupancy experienced seasonal influences and was positively associated with winter. During winter the main prey items of G. tigrina mainly invertebrates, become less abundant (Scholtz and Holm 1986; Anu et al. 2009). This reduction in food availability may result in G. tigrina increasing their foraging bouts and distances in search of alternative food resources in the urban environment. This trend was observed when analyzing the scats of G. tigrina in the suburbs of Kloof/Hillcrest. During winter there was a reduction in invertebrates and a significant increase in anthropogenic waste in the scats. The reduction in the main prey items of G. tigrina and subsequent increased reliance on less desirable food items could account for the increased site occupancy of G. tigrina during winter. The detection probability of G. tigrina was low (0.19 ± 0.03) and this could be due to several factors. Firstly, G. tigrina are considered to be semi arboreal in nature (Skinner and Chimimba 2005) and as a result may avoid moving on the ground where camera traps were positioned. Secondly, camera trap height and position may influence detection probability and site occupancy of G. tigrina due to their diminutive body size. Furthermore, interspecies interactions and spatial avoidance with humans and domestic pets may also have influenced the detection probability and site occupancy. Bush cover had a negative influence on the detection probability of G. tigrina. In a study conducted by Ramesh and Downs (2014) bush land had a positive association with Genetta genetta detection probability as this habitat type provides shelter and reduced predation risk. Throughout the suburbs of Kloof/Hillcrest, G. tigrina have been observed utilizing anthropogenic structures such as roofs for day time resting sites. The availability of this novel resting sites and subsequent benefits (reduced predation risk and presence of prey items associated with human dwellings) may reduce the reliance on areas with high bush cover. As human populations continue to rise and urban landscapes continue to decline natural habitats, it is imperative to conduct surveys of carnivores living in close proximity to human settlements, in order to gain a greater understanding of the factors that influence their distribution. Occupancy modeling provides this information by calculating the relationship between species and various habitat characteristics (Bailey et al. 2004). Our occupancy modeling provided an insight into the landscape features that influence G. tigrina within an urban landscape. Future research should include other mammals that were documented during the urban survey such as caracal, blue duiker and porcupine in order to obtain information on their occurrence within a human dominated landscape.
References
Abi-Said M, Amr ZS (2012) Camera trapping in assessing diversity of mammals in Jabal Moussa Biosphere Reserve, Lebanon. Vert Zool 62:145–152
Adams LW (2005) Urban wildlife ecology and conservation: a brief history of the discipline. Urban Ecosyst 8:139–156
Anile S, Amica C, Ragni B (2012) Population density estimation of the European wildcat (Felis silvestris silvestris) in Sicily using camera trapping. Wildl Biol 8:1–12
Anu A, Sabu TK, Vineesh PJ (2009) Seasonality of litter insects and relationship with rainfall in a wet evergreen forest in south Western Ghats. J Insect Sci 9:1–10
Bailey LL, Simons TR, Pollock KH (2004) Estimating site occupancy and species detection probability parameters for terrestrial salamanders. J Appl Ecol 14:692–702
Baker PJ, Harris S (2007) Urban mammals. What does the future hold? An analysis of factors affecting patters of use of residential gardens in Great Britain. Mammal Rev 37:297–315
Bateman PW, Fleming PA (2012) Big city life: carnivores in urban environments. J Zool 287:1–23
Bettigole CA, Donovan TM, Manning R, Austin J, Long R (2013) Acceptability of residential development in a regional landscape: potential effects on wildlife occupancy patterns. Biol Conserv 169:401–409
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Caro TM, Stoner J (2003) The potential for interspecific competition among African carnivores. Biol Conserv 110:67–75
Cutler TL, Swann DE (1999) Using remote photography in wildlife ecology: a review. Wildl Soc Bull 27:571–581
Friedmann Y, Daly B (2004) Red data book of the mammals of South Africa: a conservation assessment. CBSG-EWT, Johannesburg
Fuller TK, Biknevicius AR, Kat PW (1990) Movements and behaviour of large spotted genets (Genetta maculata Gray 1830) near Elmenteita, Kenya (Mammalia, Vivveridae). Trop Zool 3:13–19
Gaubert P, Taylor PJ, Veron G (2005) Integrative taxonomy and phylogenetic systematics of the genets (Carnivora, Viverridae, Genetta): a new classification of the most speciose carnivoran genus in Africa. In: Huber BA, Sinclair BJ, Lampe K-H (eds) African biodiversity: molecules, organisms, ecosystems. Springer, New York, pp 371–383
Gaubert P, Godoy JA, Del Cerro I, Palomares F (2009) Early phases of a successful invasion: mitochondrial phylogeography of the common genet (Genetta genetta) within the Mediterranean Basin. Biol Invasions 11:523–546
George SL, Crooks KR (2006) Recreation and large mammal activity in an urban nature reserve. Biol Conserv 133:107–117
Graham MH (2003) Confronting multicollinearity in ecological multiple regression. Ecology 84:2809–2815
Hines JE (2006) PRESENCE – Software to estimate path occupancy and related parameters. USGS-PWRC.http://www.mbr-pwrc.usgs.gov/software/presence.html
Ikeda H, Ono Y, Baba M, Doi T, Iwamoto T (1982) Ranging and activity pattern of three nocturnal viverrids in Omo National Park, Ethiopia. Afr J Ecol 20:179–186
Jung K, Kalko EKV (2010) Where forest meets urbanization: foraging plasticity of aerial insectivorous bats in an anthropogenically altered environment. J Mammal 91:144–153
Long RA, Donovan TM, MacKay P, Zielinski WJ, Buzas JS (2010) Predicting carnivore occurrence with noninvasive surveys and occupancy modeling. Landsc Ecol 26:327–340
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549
Macdonald DW, Rushton S (2003) Modelling space use and dispersal of mammals in real landscapes: a tool for conservation. J Biogeogr 30:607–620
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling – inferring patterns and dynamics of species occurrence. Elsevier, Burlington
Maruani T, Amit-Cohen I (2007) Open space planning models: a review of approaches and methods. Landsc Urban Plan 81:1–13
McCallum J (2012) Changing use of camera traps in mammalian field research: habitats, taxa and study types. Mammal Rev 43:196–206
McKinney ML (2002) Urbanization, biodiversity and conservation. Bioscience 52:883–890
Msuha MJ, Carbone C, Pettorelli N, Durant SM (2012) Conserving biodiversity in a changing world: land use change and species richness in northern Tanzania. Biodivers Conserv 21:2747–2759
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 10. South African National Biodiversity Institute, Pretoria
Mugerwa B, Sheil D, Ssekiranda P, Van Heist M, Ezuma P (2012) A camera trap assessment of terrestrial vertebrates in Bwindi Impenetrable Nation Park, Uganda. Afr J Ecol 51:21–31
O’ Brien TG, Kinnard MF, Wibisono HT (2003) Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv 6:131–139
Ordeñana MA, Crooks KR, Boydston EE, Fisher RN, Lyren LM, Siudyla S, Haas C, Harris S, Hathaway SA, Turschak GM, Miles KA, Van Vuren DH (2010) Effects of urbanization on carnivore species distribution and richness. J Mammal 91:1322–1331
Otis DL, Burnham KP, White GC, Anderson DR (1978) Statistical inference from capture data on closed animal populations. Wildl Monogr 62:1–135
Ramesh T, Downs CT (2014) Modelling large spotted genet (Genetta tigrina) and slender mongoose (Galerella sanguinea) occupancy in a heterogeneous landscape of South Africa. Mamm Biol 79:331–337
Ramesh T, Kalle R, Sankar K, Qureshi Q (2012) Factors affecting habitat patch use by sloth bears in Mudumalai Tiger Reserve, Western Ghats, India. Ursus 23:78–85
Ramesh T, Kalle R, Sankar K, Qureshi Q (2013) Dry season factors determining habitat use and distribution of mouse deer (Moschiola indica) in the Western Ghats. Eur J Wildl Res 59:271–280
Randa LA, Yunger JA (2006) Carnivore occurrence along an urban–rural gradient: a landscape-level analysis. J Mammal 87:1154–1164
Riem JG, Blair RB, Pennington DN, Solomon NG (2012) Estimating mammalian species diversity across an urban gradient. Am Midl Nat 168:315–332
Roberts PD, Somers MJ, White RM, Nel JAJ (2007) Diet of South African large spotted genet Genetta tigrina (Carnivore, Viverridae) in a coastal dune forest. Acta Theriol 52:45–53
Rowe-Rowe DT (1992) The carnivores of Natal. Natal Parks Board, Pietermaritzburg
Savard JPL, Clergeau P, Mennechez G (2000) Biodiversity concepts in urban ecosystems. Landsc Urban Plan 48:131–142
Scholtz CH, Holm E (1986) Insects of Southern Africa. Butterworths, Johannesburg
Shultz AJ, Tingley MW, Bowie RCK (2012) A century of avian community turnover in an urban green space in northern California. Condor 114:258–267
Skinner JD, Chimimba CT (2005) The mammals of Southern Africa subregion, 3rd edn. Cambridge University Press, New York, pp 412–426
Stein AB, Fuller TK, Marker LL (2008) Opportunistic use of camera traps to assess habitat-specific mammal and bird diversity in north central Namibia. Biodivers Conserv 17:3579–3587
Tobler MW, Carrillo-Percastegui SE, Pitman RL, Mares R, Powell G (2008) An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Anim Conserv 11:169–178
Virgos E, Casanovas JG (1997) Habitat selection of genet (Genetta genetta) in the mountains of Central Spain. Acta Theriol 42:169–177
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–139
Widdows C, Downs CT (2015) A genet drive-through: Are large spotted genets using urban areas for “fast food”? A dietary analysis. Urban Ecosyst. doi:10.1007/s11252-015-0438-8
Acknowledgments
P. Candotti of the Kloof Conservancy, C. Ive and T. McClurg are thanked for helping with the setting up of the camera traps. We would also like to thank all the residents that gave us permission to carry out this research on their properties. The University of KwaZulu-Natal and National Research Foundation (NRF) are also thanked for the financial funding for the study.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Widdows, C.D., Ramesh, T. & Downs, C.T. Factors affecting the distribution of large spotted genets (Genetta tigrina) in an urban environment in South Africa. Urban Ecosyst 18, 1401–1413 (2015). https://doi.org/10.1007/s11252-015-0449-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-015-0449-5