Abstract
Ecology of Asia's smallest ungulate, the Indian chevrotain or mouse deer (Moschiola indica), has been poorly assessed. We used camera-trapping data to investigate habitat use of mouse deer in Mudumalai Tiger Reserve. Presence/absence data, collected under a systematic sampling framework, were used to test a priori hypotheses incorporating covariates believed to influence mouse deer occurrence and detection. The average occupancy rate of mouse deer in the study area was 0.56 (SE = 0.22) with a low detection probability (0.29, SE = 0.14). Model selection indicated that presence of moist bamboo brakes positively influenced while percent leaf litter negatively influenced mouse deer occupancy. Placement of camera-traps along narrow trails positively influenced detection probability of mouse deer. Future conservation efforts in India should focus on preservation of bamboo vegetation and dense forest cover which provide refuge for the mouse deer. Our results illustrate that occupancy can be suitable for monitoring elusive, forest dwelling, small ungulates; however, caution is needed when applying these models on small ranging species, as our study identifies the limitations in our survey design and its improvement for future monitoring which are applicable for similar-sized species across a range of habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecology of Asia's smallest ungulate, the Indian chevrotain or mouse deer (Moschiola indica), is a poorly known ungulate belonging to the family Tragulidae, an ancient paraphyletic assemblage of primitive ruminants with a history dating back to the Miocene (Webb and Taylor 1980; Janis 1987; Gentry and Hooker 1988; Groves and Meijaard 2005). They are small-bodied, primarily nocturnal, crepuscular, and elusive in nature (Barrette 1987; Cranbrook 1987; Nowak 1991; Geist 1998; Matsubayashi et al. 2003). Presently, three genera are left, of which two, Moschiola and Tragulus, occur in the South and Southeast Asian region (Meijaard and Groves 2004). The mouse deer fulfills important ecological roles as seed dispersers (Feer 1995; Devy 2006; Prasad and Sukumar 2010) and prey for small and large carnivores (Mudappa 1999; Madhukumar 2002; Ramesh 2010; Sanei and Zakaria 2011). It predominantly feeds on fallen fruits (Bodmer 1990; Prasad et al. 2010) and leaves. High energy food resources such as fruits play an important role in how small-bodied ungulates selectively use habitats at smaller scales. They usually browse the understory vegetation and consume a variety of ancillary food items including fungi, tubers, shoots, and animal matter from the forest floor (Hofmann 1985). They often utilize dense vegetation cover while resting during the day and in the night; individuals are observed spending most of their time on the open forest floors without undergrowth (Matsubayashi et al. 2003).
Habitat requirements of mouse deer are rarely measured in an occupancy framework (McShea et al. 2009; Sridhara 2010), yet literature on its ecology and behavior from anecdotes and sight records (Prater 1971; Eisenberg and Lockhart 1972; Krishnan 1972; Ramachandran et al. 1986; Paulraj 1995; Gokula 1997) provide important clues to identify ecological factors that might predict distribution of the species. Although mouse deer is listed as “Least Concern” by IUCN (Duckworth et al. 2008), they are under severe threat from hunting pressure by indigenous tribal communities, rapid habitat loss, and forest fragmentation along the Western and Eastern Ghats (Madhusudan and Karanth 2000, 2002; Raman 2004). In addition to human-induced changes in habitat configuration and composition, poaching for bush meat is another serious concern for wildlife species in Asian tropical forests (Bennett 2001).
Among the current monitoring methods, camera-trapping has proven to be successful in determining abundance, occupancy, and habitat use of elusive ungulates (Carbone et al. 2001; Bowkett et al. 2007; Krishna et al. 2009; Nag 2008; Rovero and Marshall 2009; Tobler et al. 2009). Data gathered using cameras for population estimation of target species allow researchers to concomitantly quantify ecological aspects of non-target species as well. Studies on the mouse deer are critical for understanding tropical forest ecosystems and ungulate evolution and conservation in India. Such results highlight the apparent need for further studies to enhance our understanding of the habitat needs of this species in regions across its distribution range. Occupancy surveys are usually unbiased, cost-effective monitoring methods applied based on the detection of a species at a particular site providing reasonable estimates of population status and trends (MacKenzie et al. 2002; Ramesh et al. 2012). Unaccounted detection probability of a species could influence habitat-use models resulting in biased estimates of habitat effects or ambiguous inferences about the conservation importance of different habitats (Tyre et al. 2003; Gu and Swihart 2004). Detection/non-detection surveys therefore present a new technique for assessing the status of elusive wild animals by detecting changes in their occupancy estimates. Conducting systematic surveys over time is important for species monitoring; management and conservation efforts should be made to continually evaluate and refine methodologies.
The deciduous and evergreen forests in Protected Areas of South India are large and contiguous, having immense potential to support high densities of mouse deer (Raman 2004). With this goal, we apply a rigorous statistical approach to estimate site occupancy and detection probabilities based on mark–recapture theory (MacKenzie et al. 2002). We aimed to use camera trap data to determine mouse deer occupancy as a function of various site variables hypothesized to influence its probability of occupancy and detection in Mudumalai Tiger Reserve (Mudumalai). The main objectives of this research were to (1) establish a reliable estimate of mouse deer occupancy in Mudumalai and (2) to identify associations between mouse deer occupancy and habitat characteristics. This information is likely to provide basic ecological data to facilitate appropriate conservation management for mouse deer in India.
Methods
Study area
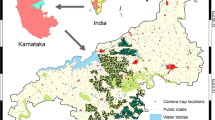
Our study was conducted in Mudumalai Tiger Reserve (11° 32′–11° 43′ N; 76° 22′–76°45′ E), which lies in the Nilgiri Biosphere Reserve of South India. Mudumalai is situated at the tri-junction of Tamil Nadu, Karnataka, and Kerala states in India with Wayanad Wildlife Sanctuary on the west, Bandipur Tiger Reserve in the north, and the Nilgiri North Forest Division in the south (Fig. 1). The topography is undulating, interspersed with hills, valleys, ravines, water courses, and swamps. Elevation varied from 960 to 1,266 m. There is a spatio-temporal rainfall gradient from east to west bringing corresponding changes in the vegetation. The eastern part of this reserve falls in the rain shadow region of Western Ghats. The reserve spans 321 km2 including dry thorn (ca. 60 km2), dry deciduous (ca. 87 km2), moist deciduous (ca. 60.82 km2), semi-evergreen (ca. 58.3 km2), moist bamboo brakes, and riparian fringe forests (Champion and Seth 1968). The forest is drained by the Moyar River, which flows along the eastern boundary of the reserve. The reserve receives rainfall from the southwest monsoon which starts in May and ends in August while the north-east monsoon starts in September and ends in December. The climate is monsoonal with dry season (January to April), first wet (May to August), and second wet (September to December) seasons. Eastern areas receive the least amount of heaviest rains (1,000–2,000 mm). The mean temperature ranges from 15 °C to 32 °C in the dry season, 17 °C to 30 °C in the first wet season, and 16 °C to 26 °C in the second wet season (Centre for Ecological Sciences, Indian Institute of Science). The primary threat to this area comes from immense biotic pressure (cattle grazing, cultivations, settlements, collection of fuel wood, non-timber forest products, etc.) exerted by the ever-expanding human population. The variability in forest structure and degree of deciduousness in the dry forests is a function of moisture availability and anthropogenic disturbance (Pascal 1986). Forest fires (natural and anthropogenic) occur annually, wiping off acres of forests in Mudumalai. These forests are also subject to major proliferation of invasive plants such as Lantana camara, Eupatorium odoratum, Parthenium hysterophorus, and Opuntia dillenii. In addition to mouse deer, other ungulates found in Mudumalai are chital (Axis axis), sambar (Rusa unicolor), muntjac (Muntiacus muntjak), wild pig (Sus scrofa), gaur (Bos gaurus), four-horned antelope (Tertracerus quadricornis), and blackbuck (Antelope cervicapra).
Camera trapping
We deployed “heat-in-motion” sensor 35-mm film camera traps, DEERCAM DC300 (Non Typical, Inc., Park Falls, Wisconsin, USA.) in three intensive study areas of Mudumalai Tiger Reserve. Camera trap data presented here were “by-catch” from our project with a primary objective to study sympatric large carnivores (tiger, leopard, and dhole) in Mudumalai. According to the proportion of major forest types, 20 trap stations were deployed in deciduous forest (107 km2) from 1 January to 9 February 2010; 13 in the dry thorn forest (39.2 km2) from 12 February to 23 March 2010, and 17 in the semi-evergreen forest (39.6 km2) from 27 March to 5 May 2010 during the dry season. The average inter-trap distance between stations was 1.8 km. Cameras were placed in way such that the outermost ones formed an approximate oval-shaped boundary around the trapping area. Trap stations were operated 24 h per day. Each station comprised a pair of cameras to maximize capture probability of mouse deer. Cameras were placed approximately 35 cm above the ground and 5–8 m away from the center of the trail. We programmed cameras to take photographs every 30 s, and the sensitivity of the motion sensor was set to high. Cameras were placed along roads, trails, stream-beds, and near water holes to maximize photo-captures. No bait, lure, or attractant was used at any camera location. Photographs provided information on the date and time of the picture taken. Trap stations were visited every 5 days to replace film and check their maintenance. Although some cameras were damaged by elephants (Elephas maximus), we replaced them with new ones to avoid loss of data.
Habitat measurements
We determined habitat use by measuring habitat features around each camera location. The variables were chosen to test different hypotheses concerned with the species habitat specialization. Percent canopy cover, leaf litter, fallen fruits, and understory cover were evaluated in a 10-m radius plot from the center of each camera location. Percent values were classified as ≤25 %, 26–50 %, 51–75 %, or > 75 %. Canopy cover was defined as percent of ground covered by the horizontal projection of tree crowns within the plot. Leaf litter and fallen fruits were visually assessed in 1 × 1 m subplots placed in four cardinal directions; mean of these four values represented the plot. We visually judged how well combined understory cover ≤1 m height from the ground would hide a mouse deer 10 m away from the center of the plot in four cardinal directions; we then used the mean of these four values to represent the plot. The three different forest types were scaled according to the increasing moisture gradient as follows—0 for dry thorn, 0.5 for deciduous, and 1 for semi-evergreen forest. Surface water bodies (rivers, water holes, streams) and disturbance sites (villages, settlements, and plantations) were plotted over a topographical (Survey of India) map using Global Positioning System location data collected during field surveys. The Euclidean distance function in ArcGIS 9.3 Spatial Analyst, a Geographic Information System software program developed by ESRI, was used to calculate the distance (meters) from each camera location to the nearest water source and disturbance site. The presence/absence of moist bamboo (Bambusa arundinaceae) brakes within a radius of 100 m from a camera location, placement of camera-trap on the road, and narrow trail or large trail were also recorded.
Data analysis
Mouse deer occupancy was estimated using a likelihood-based method (MacKenzie et al. 2002). We created mouse deer detection history (1100100) for each camera location, consisting of binary values with ‘1’ indicating species detection during the sampling occasion and ‘0’ indicating non-detection (Otis et al. 1978). The event of capturing an individual photographed from either of the two cameras operating or by both camera traps at a single camera station was considered to be an independent record/detection. On some occasions, individuals were captured repeatedly at a camera station during a time period (≤30 s); thus, to avoid pseudo-replications, we considered the first capture of the animal within that time frame as an independent record/detection (O’Brien et al. 2003). Photographs with more than a single individual were counted as a single detection. MacKenzie et al. (2002) model assumes that mouse deer distribution within a season is “closed” (i.e., there are neither colonizations nor extinctions). It is assumed that each site is independent and no animal will move between sites during the survey period. In this case, the movement of mouse deer is very small compared with the selected grid size. Hence, setting up a grid system and using these models for a small-ranging species like the mouse deer may not violate occupancy closure at each site.
Trapping duration was limited to 40 days to minimize the likelihood of occupancy changes. The 40-day datasets were then divided into eight sampling periods of 5 days each. This was necessary in order to increase the detection probability for each sampling period. As we considered each camera location an independent site, each sampling occasion was a temporal repeat of the survey. Detection histories were pooled from the three intensive study areas and entered together into PRESENCE v.2.4 software (Proteus Wildlife Research Consultants, New Zealand; http://www.proteus.co.nz, accessed 6 September 2012) with the single-season option (MacKenzie et al. 2006). Sites with camera malfunctions, insufficient survey time, or incomplete data were discarded in the analyses.
We used continuous site covariates: canopy cover, leaf litter, fallen fruits, understory cover, habitat type, distance to the nearest water source, and disturbance site as well as four categorical variables presence/absence of bamboo, road, narrow trail, and large trail. Categorical variables were recorded as ‘1’, where the particular variable was present and ‘0’ when absent. Previous studies showed that mouse deer occurrence varied with forest types (Duckworth et al. 2008) and microhabitat resources (Heydon and Bulloh 1997). Hence, we defined a small set of candidate models incorporating these variables (covariates) based on a priori hypothesis from our experience and available literature. Correlations among independent variables were tested to avoid problems with multicollinearity (Graham 2003). In this case, percent canopy cover and understory cover were highly correlated with each other (R 2 = 0.55), hence, we used only understory cover in our analysis. Mouse deer are likely to use resources near dense forest floor in crown-gap areas such as fallen fruits and short vegetation (Matsubayashi et al. 2003; Jayasekara et al. 2007). High leaf litter and understory cover provide refuge and necessary forage supplies for small-bodied ungulates (Hanley 1984). Water sources like rivers and streams are critical resources for wild animals especially during the dry season.
All continuous covariates were standardized to z-scores prior to analysis, allowing model coefficients to be interpreted as the change in the log-odds ratio of occupancy relative to a 1 standard deviation change in the covariate from its mean (Cooch and White 2005). We produced a global model that contained all potential covariates for occupancy and allowed detection probability (p) to vary by all covariates. We followed a two-step procedure where we initially modeled p and then modeled ψ. The potential covariates for occupancy were then allowed to vary, individually or in combination, while detection was either maintained in the global model or remained constant, i.e., ψ(covariate)p(covariate), or ψ(covariate)p(.). We also considered the simplest model, where both occupancy and detection probability remained constant, ψ(.)p(.).
Using this information, we followed a single-season occupancy modeling framework (MacKenzie et al. 2002) to rank and compare candidate models. We considered models with ΔAICc ≤ 2 to have strong support (Burnham and Anderson 2002). Model selection, computation of model weights, and averaging of parameters followed the framework of Burnham and Anderson (2002). We examined covariates that best explained overall mouse deer occupancy (ψ). Because the data set was small, we kept the candidate models simple. We ran 10,000 bootstrap samples (ĉ > 1.0 indicated overdispersion of data) and Pearson goodness-of-fit tests (White and Burnham 1999) to assess the fit of the models to our data. Because the ratio of sample sizes (n) to the maximum number of estimated parameters (k) was < 40, models were ranked according to AICc (AIC adjusted for small sample size). We calculated model-averaged parameters using Akaike weights for proportion of sites used and detection probabilities. To infer the relative influence of each covariate on occurrence, model weights were summed over all models containing the particular covariate.
Results
In study areas dry thorn, deciduous, and semi-evergreen forests, 12, 27, and 11 independent mouse deer photographs were recorded from 800, 520, and 680 camera-trap nights, respectively (Table 1, Fig. 2). Overall, mouse deer was detected in 23 sites producing a naive occupancy of 0.46. Based on a model with all parameters held constant, our site occupancy and probability of mouse deer was 0.79 (SE = 0.39) and 0.22 (SE = 0.13), respectively. The difference between the naive proportion of sites occupied and the estimated proportion of sites occupied varied after accounting for imperfect detection (Table 2).
Of the variables measured, only five were substantially associated with mouse deer occurrence (ΔAICc ≤ 2). The goodness-of-fit test for the global model indicated no lack of fit (ĉ = 0.6). The occurrence model with the greatest support (ΔAICc = 0) was ψ(leaf litter + bamboo),p(.) (Table 2, highest w i = 0.16) suggesting that the variables, bamboo presence influenced occupancy positively (β = 10.55 ± 9.25, Table 3) and percent leaf litter influenced occupancy negatively (β = −5.45 ± 5.08, R 2 = 0.41, Fig. 3, Table 3). In this model, the probability of detecting mouse deer was 0.30 (SE = 0.12, 95 % CI = 0.12–0.56). The second most parsimonious model was ψ(.),p(.) (Table 2), which suggested that mouse deer occupancy could be independent of covariates. Since five models had ΔAICc ≤ 2, none of the models could be judged as the best. The third-ranked model ψ(leaf litter + bamboo),p(distance to disturbance) included probability of detection as a function of distance to disturbance site giving a detection probability 0.31 (SE = 0.15, Table 2). In this model, detection probability was negatively affected by distance to disturbance site (β = −0.59 ± 0.44, R 2 = 0.74, Fig. 4). In the fourth-ranked model ψ(leaf litter + bamboo),p(narrow trail) detection probability of mouse deer was positively affected by presence of camera along narrow trails (β = 1.13 ± 0.82) and in the fifth-ranked model ψ(leaf litter + bamboo),p(large trail) detection probability of mouse deer was negatively influenced by presence of camera along large trails (β = −1.32 ± 0.31).
The summed model weights for each factor with respect to occupancy are percent leaf litter 52 %, presence of moist bamboo brakes 45 %, percent fallen fruits 9 %, understory cover 7 %, and habitat 3 %. Across all models, the variables that best predicted detection probability of mouse deer was distance to disturbance site (w i = 0.36), whereas there was little support for the presence of narrow trails (w i = 0.13) and large trails (w i = 0.14) (Table 2). Therefore, averaged model occupancy (0.56, SE = 0.22) and detection probability (0.29, SE = 0.14) values were chosen as the final estimates which corresponds to a difference of 10 % from the naive site occupancy.
Discussion
Habitat use by mouse deer
This study emphasizes the feasibility of rigorously assessing mouse deer populations in one of India’s finest conservation landscapes by estimating their detection probability and site occupancy. Our modeling showed that mouse deer occurrence was positively associated with presence of moist bamboo brakes. In Borneo, the lesser mouse-deer (Tragulus javanicus), preferred crown-gap areas with dense undergrowth of creeping bamboo (Dinochloa spp.) as resting and foraging sites during the day (Matsubayashi et al. 2003). Even during foot-based field surveys in the day, the first and second authors often encountered pairs of mouse deer flushing through bamboo vegetation. These observations support our modeling results on the positive effect of presence of moist bamboo brakes, suggesting that the spatial layout of this habitat may be structuring distribution pattern of mouse deer. Fallen leaves of crown-gap areas are thought to be good food resources for many terrestrial mammals because many pioneer plants in gap areas are reported to save energy in production of secondary metabolites and/or mechanical protection to achieve high growth rate (Coley et al. 1985; Swaine and Whitmore 1988), yet our modeling showed a negative association between leaf litter and mouse deer occupancy. This supposedly suggests that the moist bamboo habitats provide sufficient refuge, cover, and forage substrates for mouse deer. Day-resting habits by mouse deer from radio-telemetry studies in Sabah have reported the species resting often in dense undergrowths (Matsubayashi et al. 2003). Mouse deer usually prefer staying in dense undergrowth (Barrette 1987; Nowak 1991), yet understory cover did not influence its occupancy in our modeling probably because there was only a marginal difference in understory cover between sites occupied (mean ± SE, 50.35 ± 6.01) and unoccupied (35.26 ± 4.33) by the species. Most parts of the reserve have comparatively less understory cover in the dry season except for areas close to water sources or those in evergreen habitats. If cameras were deployed in resting sites or day-bed sites, then we could have expected the effect of understory cover on species occupancy. Future hypothesis should be framed incorporating fine scale microhabitat variables like temperature, moisture, and humidity across multi-season occupancy surveys. High energy resources such as fruits play an important role in the diet of small-bodied ungulates (Bodmer 1990; Miura et al. 1997) and have been reported to even broaden their dietary spectrum; yet our study did not show any influence of fallen fruits on species occupancy.
A recent multi-species occupancy modeling by McShea et al. (2009) in Sarawak, Malaysia, reported that the mean distance for detection of mouse deer was closer to secondary forest blocks indicating the importance of secondary forests for the species. The mouse deer also occurs in some fragmented and disturbed areas, such as plantations, rural gardens, and degraded forests in the country. Tropical vertebrate species respond in various ways to forest disturbance, some by declining, others by increasing in abundance (Johns 1992) and or just by being tolerant. The negative influence of distance to disturbance sites on detection probability of mouse deer was unexpected. Disturbance like wood cutting, lopping, cattle-grazing, and fuel-wood extraction occurs from 10 am to 4 pm inside the Tiger Reserve; hence, we presume that this is very unlikely to cause much disturbance to a strictly nocturnal small mammal like the mouse deer, especially if the animal occupies secluded narrow trails in dense vegetation that are usually not traversed by people. The positive affect of narrow trails and negative effect of large trails on detection probability of mouse deer are supportive of this fact and could be a strategy to minimize its chances of exposure to human presence, large predators, or megaherbivores that usually frequent larger trails. Yang et al. (1990) reported that the absence of large predators such as tigers and leopards in Pulau Ubin, Singapore, had reduced predation pressure on the greater mouse deer (Tragulus napu) and later accelerated population recovery of the mouse deer. Baral et al. (2009) reported that chevrotain numbers may have dropped due to extensive habitat conversion, and the species at present may be very rare in Nepal. Mouse deer may be among the most frequently hunted animals in the forests of south India (Kumara and Singh 2004), but the real effects of hunting are still unknown, and most forest ungulate populations cannot recover easily from heavy offtakes (Robinson and Bennett 2000; Baral et al. 2009). As a forest-associated species, forest conversion has a major role in altering the behavior and habitat use of secretive small ungulates. Hence, it is imperative to conduct systematic surveys even in fragmented forests across the landscape to get a better insight on its ecology and conservation needs.
Occupancy modelling had the ability to indicate a clear understanding of elusive ungulates by examining relationships between species and habitat characteristics (Bailey et al. 2004). However, we state several reasons for the low detection probability of mouse deer achieved in our study. Camera-trap height and placement to mainly capture large carnivores might affect site occupancy and detection probability of mouse deer due to its small body size, its affinity towards densely vegetated forest floor, and possibly interspecies interactions, temporal, and or spatial avoidance with carnivores. Future research should incorporate environmental variables at a landscape level for improvement in model precision. Mark–recapture population estimates of the species and radio-telemetry for future studies will greatly expand our knowledge on their ecology. It should be noted that the detection probabilities of species may vary among years and seasons (MacKenzie et al. 2003). For example, in the future, we need to take seasonal variation in water availability into consideration since most of the streambeds dry up in the dry season which may affect movement patterns in mouse deer. This gives an indication for conducting multi-season occupancy surveys to better explain the variable influence of covariates. Despite these caveats in our study, the results confirm the value of camera trapping for studying elusive forest mammals and obtaining useful information on their occurrence and conservation status.
Conservation recommendations
The present study emphasized the feasibility of rigorously assessing mouse deer population in an important conservation landscape in the Western Ghats, India, by estimating their site occupancy and detection probability. Yet, in the Indian scenario, it is extremely challenging for forest managers to manage the spread of anthropogenic activities and simultaneously ensure protection of wildlife. Forest managers must stop further encroachment by people into the eastern zone of the Tiger Reserve which is mainly an open forest because in the long-term, overgrazing might lead to several unproductive barren patches in the landscape that could wipe out dense forest dwelling small mammals like the mouse deer. The best possible solution is to have plantation drives during the monsoon, habitat-restoration, maintain a seed bank or a nursery with key native plant species that regenerate faster in low nutrient soil. Controlled livestock grazing can be used to enhance foraging conditions for wild ungulates by modifying plant communities that are preferred by wild ungulates and increase structural diversity in the landscape. Planting fruiting trees, maintenance of swamps, riparian vegetation, planting bamboo seeds, and maintenance of salt-licks and water holes are likely to increase protection for mouse deer populations. Therefore, we recommend forest managers that the integrity of the network of moist bamboo vegetation and maintenance of narrow trails with dense vegetation cover may be considered explicitly in management decisions for conservation of mouse deer. Development in camera trapping protocols and analytical frameworks is very important as these methods are relevant to a majority of similar-sized forest mammals. Results obtained from the present survey could be used to improve survey methodologies for small ungulates, by reducing the grid size in proportion to its home-range. Furthermore, a standardized survey procedure will aid in comparing occupancy rates and habitat associations when conducted at the landscape level. The next step would be to gather more information about the abundance and distribution of the mouse deer and to establish a species conservation plan along with forest managers. Mudumalai Tiger Reserve is one of the few important Protected Areas in the world surrounded by 3,300 km2 of contiguous intact forest (Silori and Mishra 2001). The species is still thought to be locally extinct in certain parts of the country, and such misconceptions prevail because there has not been any recent survey specifically for the mouse deer. It may suffice for field managers and conservation biologists to monitor trends in populations or trends in occupancy as a response to specific conservation/management interventions amongst a wide array of habitat conditions, forest management practices, and disturbance gradients across the species distribution in India.
References
Bailey LL, Simons TR, Pollock KH (2004) Estimating site occupancy and species detection probability parameters for terrestrial salamanders. Ecol Appl 14:692–702. doi:10.1890/03-5012
Baral HS, Shah KB, Duckwotha JW (2009) Clarification of the status of Indian Chevrotain Moschiola indica in Nepal. Vert Zool 59:197–200
Barrette C (1987) The comparative behaviour and ecology of chevrotains, musk deer, and morphologically conservative deer. In: Wemmer CW (ed) Biology and management of the Cervidae. Smithsonian Institution Press, Washington DC, pp 200–213
Bennett EL (2001) Timber certification: where is the voice of the biologist? Conserv Biol 15:308–310. doi:10.1046/j.1523-1739.2001.015002308.x
Bodmer RE (1990) Ungulate frugivores and the browser-grazer continuum. Oikos 57:319–325
Bowkett AE, Rover F, Marshall AR (2007) The use of camera-trap data to model habitat use by antelope species in the Udzungwa Mountain forests, Tanzania. Afr J Ecol 46:479–487. doi:10.1111/j.1365-2028.2007.00881.x
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Second edition. Springer–Verlag, New York
Carbone C, Christie S, Conforti K, Coulson T, Franklin N, Ginsberg JR, Griffiths M, Holden J, Kawanishi K, Kinnaird M, Laidlaw R, Lynam A, Macdonald DW, Martyr D, McDougal C, Nath L, O'Brien T, Seidensticker J, Smith DJL, Sunquist M, Tilson R, Wan Shahruddin WN (2001) The use of photographic rates to estimate densities of tigers and other cryptic mammals. Anim Conserv 4:75–79. doi:10.1017/S1367943001001081
Champion HG, Seth SK (1968) A revised survey of the forest types of India. Government of India Press, New Delhi
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899. doi:10.1126/science.230.4728.895
Cooch E, White G (2005) Program Mark: a gentle introduction. http://www.phidot.org/software/mark/docs/book. Accessed 5 September 2012
Cranbrook E (1987) Riches of the wild: land mammals of South-East Asia. Oxford University Press, Singapore
Devy, MS (2006) Effects of fragmentation on a keystone tree species in the rainforest of Kalakad-Mundanthurai Tiger Reserve, India. Project Report, Ashoka Trust for Research in Ecology and the Environment, Bangalore. http://www.ruffordsmallgrants.org/files/Devy%20Report.pdf Accessed 3 October 2012
Duckworth JW, Baral HS, Timmins RJ (2008) Moschiola indica. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.1. <www.iucnredlist.org>. Downloaded on 28 August 2011
Eisenberg JF, Lockhart M (1972) An ecological reconnaissance of Wilpattu National Park, Ceylon. Smithson Contr Zool 101:1–118. doi:10.5479/si.00810282.101
Feer F (1995) Seed dispersal in African forest ruminants. J Trop Ecol 11:683–689 doi:10.1017/S0266467400009238
Geist V (1998) Deer of the world: their evolution, behaviour, and ecology. Stackpole Books, Mechanicsburg
Gentry AW, Hooker JJ (1988) Chapter 9: the phylogeny of the Artiodacyla. In: Benton J (ed) The phylogeny and classification of the tetrapods, volume 2. Systematics Association special volume 35B. Clarendon Press, Broadbridge, pp 235–272
Gokula V (1997) Notes on foetuses of mouse deer Tragulus meminna in Mudumalai Wildlife Sanctuary, Tamil Nadu, South India. J Bombay Nat Hist Soc 94:148
Graham, MH (2003) Confronting multicollinearity in ecological multiple regression. Ecology 84:2809–2815. http://dx.doi.org/10.1890/02-3114
Groves CP, Meijaard E (2005) Interspecific variation in Moschiola, the Indian chevrotain. Raffles Bull Zool 12:413–421
Gu W, Swihart RK (2004) Absent or undetected? Effects of non-detection of species occurrence on wildlife-habitat models. Biol Conserv 116:195–203. doi:10.1016/S0006-3207(03)00190-3
Hanley TA (1984) Habitat patches and their selection by wapiti and black-tailed deer in a coastal montane coniferous forest. J Appl Ecol 21:423–436. http://www.jstor.org/stable/2403419
Heydon MJ, Bulloh P (1997) Mousedeer densities in a tropical rainforest: the impact of selective logging. J Appl Ecol 34:484–496. http://www.jstor.org/stable/2404892
Hofmann RR (1985) Digestive physiology of the deer—their morpho-physiological specialization and adaptation. Roy Soc New Zealand Bull 22:393–407
Janis CM (1987) Grades and clades in hornless ruminant evolution: the reality of the Gelocidae and the systematic position of Lophiomeryx and Bachitherium. J Vertebr Paleontol 7:200–216. doi:10.1080/02724634.1987.10011653
Jayasekara P, Weerasinghe UR, Wijesundara S, Takatsuki S (2007) Identifying diurnal and nocturnal frugivores in the terrestrial and arboreal layers of a tropical rain forest in Sri Lanka. Ecotropica 13:7–15
Johns AD (1992) Vertebrate response to selective logging: implications for the design of logging systems. Phil Trans R Soc Lond B 335:437–442
Krishna YC, Clyne PJ, Krishnaswamy J, Kumar NS (2009) Distributional and ecological review of the four horned antelope, Tetracerus quadricornis. Mammalia 73:1–6. doi:10.1515/MAMM.2009.003
Krishnan M (1972) An ecological survey of the larger mammals of Peninsular India. J Bombay Nat Hist Soc 69:469–501
Kumara HN, Singh M (2004) The influence of differing hunting practices on the relative abundance of mammals in two rainforest areas of the Western Ghats, India. Oryx 38:321–327. doi:10.1017/S0030605304000560
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. doi:10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2
MacKenzie DI, Nichols JD, Hines JE, Knutson MG, Franklin AB (2003) Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 84:2200–2207. doi:10.1890/02-3090
MacKenzie DI, Nichols JD, Royle JA, Pollock KP, Bailey LL, Hines JE (2006) Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Academic Press, San Diego, California, USA
Madhukumar JJ (2002) Hunting attempt by Nilgiri marten Martes gwatkinsi Horsfield, family Mustelidae, in Periyar Tiger Reserve, Kerala. J Bombay Nat Hist Soc 99:286
Madhusudan MD, Karanth KU (2000) Hunting for an answer: is local hunting compatible with large mammal conservation in India. In: Robinson JG, Bennett EL (eds) Hunting for sustainability in the tropics. Columbia University Press, New York, pp 339–355
Madhusudan MD, Karanth KU (2002) Local hunting and the conservation of large mammals in India. Ambio 31:49–54. doi:10.1579/0044-7447-31.1.49
Matsubayashi H, Bosi E, Kohshima S (2003) Activity and habitat use of lesser mouse-deer (Tragulus javanicus). J Mammal 84:234–242. doi:10.1644/1545-1542(2003)084<0234:AAHUOL>2.0.CO;2
McShea, WJ , Stewart C, Peterson L, Erb P, Stuebing R, Giman B (2009) The importance of secondary forest blocks for terrestrial mammals within an Acacia/secondary forest matrix in Sarawak, Malaysia. Biol Conserv 142:3108–3119. http://dx.doi.org/10.1016/j.biocon.2009.08.009
Meijaard E, Groves CP (2004) A taxonomic revision of the Tragulus mouse-deer (Artiodactyla). Zool J Linn Soc 140:63–102. doi:10.1111/j.1096-3642.2004.00091.x
Miura S, Yasuda M, Ratnam LC (1997) Who steals the fruits? Monitoring frugivory of mammals in a tropical rain forest. Malay Nat J 50:183–193
Mudappa D (1999) Lesser-known carnivores of the Western Ghats. In: Hussain SA (ed) ENVIS Bulletin: Wildlife and protected areas, mustelids, viverrids and herpestids of India, pp 65–70
Nag K (2008) Assessing animal abundance from photographic capture data using an occupancy approach. M.Sc thesis, The Manipal Academy of Higher Education, Manipal University, India
Nowak RM (1991) Walker's mammals of the world, 5th ed. vol. II. The John Hopkins University Press, Baltimore
O’brien TG, Kinniard MF, Wibisono HT (2003) Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv 6:131–139. doi:10.1017/S1367943003003172
Otis DL, Burnham KP, White GC, Anderson DR (1978) Statistical inference from capture data on closed animal populations. Wildl Monogr 62:1–135
Pascal JP (1986) Explanatory booklet on the forest map of south India. French Institute, Pondicherry
Paulraj S (1995) Prey–predator relationships with special reference to the tiger, panther, and dhole competitions in Kalakad-Mundanthurai Tiger Reserve (Tirunelveli District–Tamil Nadu). Indian Forester 121:922–930
Prasad S, Sukumar R (2010) Context-dependency of a complex fruit-frugivore mutualism: temporal variation in crop size and neighborhood effects. Oikos 119:514–523. doi:10.1111/j.1600-0706.2009.17971.x
Prasad S, Pittet A, Sukumar R (2010) Who really ate the fruit? A novel approach to camera trapping for quantifying frugivory by ruminants. Ecol Res 25:225–231. doi:10.1007/s11284-009-0650-1
Prater SH (1971) The book of Indian animals. Bombay Natural History Society, Oxford University Press, Bombay
Ramachandran KK, Nair PV, Easa PS (1986) Ecology of larger mammals of Periyar Wildlife Sanctuary. J Bombay Nat Hist Soc 83:505–524
Raman TRS (2004) Mouse deer (Moschiola meminna Erxleben, 1777). ENVIS Bulletin: Wildlife Institute of India, Dehra Dun 7:131–140
Ramesh T (2010) Prey selection and food habits of large carnivores: tiger Panthera tigris, leopard Panthera pardus and dhole Cuon alpinus in Mudumalai Tiger Reserve, Tamil Nadu. Ph.D. Thesis, Saurashtra University, Gujarat, Rajkot
Ramesh T, Kalle R, Sankar K, Qureshi Q (2012) Factors affecting habitat patch use by sloth bears in Mudumalai Tiger Reserve, Western Ghats, India. Ursus 23:78–85.doi: http://dx.doi.org/10.2192/URSUS-D-11-00006.1
Robinson JG, Bennett EL (2000) Hunting for sustainability in tropical forests. Colombia University Press, New York
Rovero F, Marshall AR (2009) Camera trapping photographic rate as an index of density in forest ungulates. J Appl Ecol 46:1011–1017. doi:10.1111/j.1365-2664.2009.01705.x
Sanei A, Zakaria M (2011) Occupancy status of Malayan leopard prey species in a fragmented forest in Selangor, Malaysia. Asia Life Sciences Suppl 7:41–55
Silori CS, Mishra BK (2001) Assessment of livestock grazing pressure in and around the elephant corridors in Mudumalai Wildlife Sanctuary, south India. Biodivers Conserv 10:2181–2195. doi:10.1023/A:1013285910650
Sridhara S (2010) Habitat use of the Indian chevrotain (Moschiola indica) in Someshwara Wildlife Sanctuary. M.Sc. dissertation, National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, India
Swaine MD, Whitmore TC (1988) On the definition of ecological species groups in tropical rain forests. Vegetatio 75:81–86. doi:10.1007/BF00044629
Tobler MW, Carrillo-Percastegui SE, Powell G (2009) Habitat use, activity patterns and use of mineral licks by five species of ungulate in south-eastern Peru. J Trop Ecol 25:261–270. doi:10.1017/S0266467409005896
Tyre AJ, Tenhumberg B, Field SA, Niejalke D, Paris K, Possingham HP (2003) Improving precision and reducing bias in biological surveys: estimating false negative error rates. Ecol Appl 13:1790–1801. doi:10.1890/02-5078
Webb SD, Taylor BE (1980) The phylogeny of hornless ruminants and a description of the cranium of Archaeomeryx. Bull Am Mus Nat Hist 167:121–154
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–139. doi:10.1080/00063659909477239
Yang CM, Yong K, Lim KKP (1990) Wild mammals of Singapore. In: Chou LM, Ng PKL (eds) Essays in zoology: papers commemorating the 40th anniversary of the Department of Zoology. National University of Singapore, Singapore University Press, Singapore, pp 1–23
Acknowledgments
We thank the Director and Dean, Wildlife Institute of India, for continuous support during our study. We thank the Chief Wildlife Warden, Tamil Nadu, for granting us permission to work in Mudumalai. We sincerely thank the two anonymous reviewers for constructive comments on earlier versions of the manuscript. We are indebted to assistance in the field from C. James, M. Kethan, M. Mathan, and Forest Department staff for their hard work and support during field data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Aguirre
Rights and permissions
About this article
Cite this article
Ramesh, T., Kalle, R., Sankar, K. et al. Dry season factors determining habitat use and distribution of mouse deer (Moschiola indica) in the Western Ghats. Eur J Wildl Res 59, 271–280 (2013). https://doi.org/10.1007/s10344-012-0676-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-012-0676-5