Abstract
Exploring factors related to species distribution is important to better understand their natural histories and provide their effective conservation. However, the ecology of some threatened species remains poorly understood. Here, we conducted the first quantitative investigation of species-habitat relationships for the Brazilian three-banded armadillo, Tolypeutes tricinctus, a threatened species endemic to Brazil. We combined camera traps and active searches to explore the influence of ecological and methodological factors, including human-related habitat features, on T. tricinctus occupancy and detection probabilities in a human-modified landscape in northeastern Brazil. The T. tricinctus occupancy probability was high throughout the study area, whereas its detection probability was eight times higher by active searches than camera trapping, which should be considered when designing studies on T. tricinctus ecology in the future. Our results suggest that T. tricinctus can be widely distributed in human-modified landscapes under moderate levels of hunting and habitat loss and highlight the importance of the engagement of local people into research and conservation projects for better outcomes, as we found by counting on their local ecological knowledge for the conduction of active searches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exploring factors affecting species distributions is important to better understand their biology and for decision-making processes in population monitoring, management, and conservation (Jonzén 2008). These factors include habitat features, such as resource availability and interactions with other species (Ferreguetti et al. 2016; Dias et al. 2019). Given the current human-induced biodiversity crisis worldwide, many studies have been focusing on understanding human-related factors that affect species distributions and may lead to their extinction, such as habitat loss and overexploitation (Rodrigues and Chiarello 2018; Dobbins et al. 2020).
The study of habitat relationships of some species is hampered by their low densities and/or elusive behavior, which is the case of armadillos (Xenarthra: Cingulata) (McDonough and Loughry 2008; Superina et al. 2014). They usually present low population densities and solitary habits and spend most of the time underground inside their burrows (McDonough and Loughry 2008). Although armadillos are subjected to many conservation challenges, few studies have focused on exploring their habitat requirements, and many species are still understudied (Superina et al. 2014; Superina and Abba 2020). For taxa such as armadillos, the evaluation of factors influencing species occurrence (or occupancy) can be suitable to assess habitat relationships, as it requires only presence-absence data while accounting for false absences caused by imperfect detection (MacKenzie et al. 2002). However, this methodological approach is not yet common in existing studies focused on this group (but see Ferreguetti et al. 2016; Rodrigues and Chiarello 2018).
The Brazilian three-banded armadillo, Tolypeutes tricinctus (Linnaeus, 1758) (Cingulata: Chlamyphoridae) (Gibb et al. 2016), is a threatened species endemic to northeastern Brazil (Miranda et al. 2014; Feijó et al. 2015; Reis et al. 2015; Santos et al. 2019b; Hannibal et al. 2021; Schetino et al. 2021). Tolypeutes tricinctus is remarkable for the ability to roll up its body into a ball as a defense mechanism (Online Resource 1 — Fig. S1); however, it also makes this species easy to hunt (Santos et al. 1994). Hunting and habitat loss are considered the main threats to T. tricinctus and may have led to its extinction from different localities (Miranda et al. 2014; Feijó et al. 2015; Reis et al. 2015). Consequently, it is thought to be currently restricted to less disturbed areas (Miranda et al. 2014). Tolypeutes tricinctus occurs in the Caatinga dry forests of northeastern Brazil and adjacent areas in the Cerrado savannas (Feijó et al. 2015). The areas in the Cerrado where T. tricinctus occurs have been increasingly converted into sugarcane and soybean plantations (Miranda et al. 2014). Meanwhile, the Caatinga is a historically neglected region for research and conservation initiatives (Santos et al. 2011) and has been experiencing an expansion of wind farms (Neri et al. 2019), which can impose negative impacts on wildlife, such as barrier effects to animal dispersal and increased levels of hunting and road-kills (Helldin et al. 2012; Dias et al. 2019, 2020).
Despite this dramatic scenario, only recently T. tricinctus has received conservation attention with the creation of a national action plan for its conservation (ICMBio 2014). In addition, little is still known about its biology. For instance, only recently the burrowing activity of Tolypeutes spp. and the T. tricinctus predation by native predators have been described in the literature (Attias et al. 2016; Magalhães et al. 2021). Most of the available information about T. tricinctus comes from unpublished works and field observations (e.g., Marinho-Filho et al. 1997), and no published study has systematically assessed its habitat relationships so far. The combination of restricted distribution, high threat level, low conservation attention, and lack of knowledge led T. tricinctus to be considered a priority species for research and conservation efforts (Superina et al. 2014).
Here, we provide the first systematic assessment of species-habitat relationships for T. tricinctus. We combined camera traps, active searches, and occupancy models to investigate how different environmental and human-related factors may influence its local distribution (Table 1). We worked with a recently discovered population of T. tricinctus in an unprotected area, where human activities, such as cattle raising, hunting, and wind power production are present.
Materials and methods
Study area
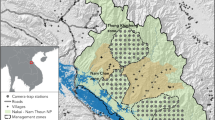
This study was conducted in a private area of 53 km2 in the municipality of Brotas de Macaúbas, state of Bahia, in the Caatinga ecoregion of northeastern Brazil (Fig. 1). The climate is altitude tropical, with a warm rainy season (November–March) and cold dry season (April–October), and altitudes between 1000 and 1200 m. a. s. l. (SEI 2014). The soil types vary from Litholic Neosols (or Leptosols) at higher elevations and Red-yellow Latosols (or Ferralsols) at lower elevations (Online Resource 2). The native vegetation consists mainly of Caatinga dry forests and some small patches of Cerrado savannas and Rupestrian Grasslands (Fig. 1; Online Resource 3 — Figs. S4 and S5). Anthropized areas comprise wind farm infrastructure, roads, and former croplands in regeneration. The latter is characterized by zones of exposed soil partially covered by ruderal herbaceous and/or shrubby vegetation. In addition to habitat loss, hunting is performed by local people; however, T. tricinctus is not a frequent hunting target (R. A. Magalhães, unpublished data). The western portion of the area is partially within a biodiversity priority area (MMA 2017).
Location of sampling units or sites (black dots) used to sample for Tolypeutes tricinctus in the studied area (dashed polygon) in northeastern Brazil. Green is native bushy-arboreal vegetation. Pale brown is anthropized open areas, such as former croplands, and pink is other open areas, such as rocky outcrops. The shaded area is a biodiversity priority area. The insert shows the location of the study area (orange triangle) according to the Caatinga distribution (in gray) and Brazilian states. Biodiversity priority areas follow MMA (2017), and land cover and Caatinga distribution follow Projeto Mapbiomas (2021). Geographic Coordinate System and Datum: WGS84
Data collection
We randomly distributed 24 sampling units (or sites) 1.5 km apart in the area. We installed one Bushnell® digital camera trap at each site approximately 30 cm above the ground and angled it slightly downwards to avoid missing closer records. All cameras were set to operate 24 h a day with a 1-min interval between 15-s-long videos. Sampling occurred between April and July 2019, totaling 90 days. In the middle of the sampling period, we visited all sites to check the cameras.
We conducted 30-min active searches for individuals of T. tricinctus and their signs (tracks and excavations; Online Resource 1 — Fig. S2) in a 10-m radius (63 m2) buffer surrounding each site during camera trap checking and removal, thus resulting in two active searches at each site. We defined this area due to logistic constraints, as it could be sampled in 30 min, thereby not compromising our field logistics. During each active search, we registered whether any evidence of T. tricinctus was found (1) or not (0). Active searches were conducted with the help of three previously identified specialists from local communities. They presented substantial knowledge of the local fauna, were able to track T. tricinctus individuals, and precisely described their signs. At each site, one of the three local specialists was responsible for performing the active searches. To avoid issues with false-positive detections and sign decay during active searches (Rhodes et al. 2011), we only considered fresh tracks and excavations that could be unambiguously attributed to T. tricinctus and erased all after camera trap installation and the first active search. This procedure prevented us from registering signs that could have been left before our sampling and from recounting the same signs during the second active search.
Occupancy modeling covariates
We selected covariates that could influence T. tricinctus occupancy probability based on the literature (Table 1). To assess the effect of habitat loss on T. tricinctus occupancy, we calculated the amount of native and anthropogenic habitat at each site (Regolin et al., 2021).
Using 30-m resolution land cover maps generated by Projeto Mapbiomas (2021) for the year 2019, we calculated the proportion of pixels of native and anthropogenic vegetation inside buffers of 1 ha, 10 ha, 20 ha, 50 ha, and 100 ha around each site (number of pixels/buffer area). We tested for correlation between pairs of buffers and because all were highly correlated (|r|> 0.70; Online Resource 4 — Table S2), we used only the scale that presented greater variation throughout the sites for occupancy analysis, which was 1 ha. We also measured the minimum distance from each site to biodiversity priority areas, wind turbines, permanent water sources, roads, and residences. All geoprocessing analyses were conducted in program QGIS v.3.16.7 (QGIS Development Team 2021). We also computed the detection rate of cattle (independent records/day) for each site. Independent records comprised those obtained at the same site with at least a 1-h interval. To evaluate the influence of soil sand content on T. tricinctus occupancy probability (Table 1), we collected soil samples (200 g) in depths of 0–20 cm and 20–40 cm at each site. We performed granulometric analysis using sieving and sedimentation techniques to assess the proportion of each soil particle size-class (in mass fraction): boulders and stones (> 2 mm), sand (2–0.05 mm), silt (0.05–0.002 mm), and clay (< 0.002 mm) (Donagema et al. 2011).
To evaluate the influence of methodological covariates on T. tricinctus detection probability, we used the type of method employed in each sampling occasion at each site as a categorical covariate (Table 1). We also expected detection to vary according to the sampling effort. For each active search, we fixed the sampling effort in 5 days for each occasion, i.e., we assumed that tracks and forage burrows recorded were left up to 5 days before each active search. During this period, they could remain identifiable before being naturally erased. The tracks and forage burrows of T. tricinctus would hardly remain identifiable for more than a few days because the area is very windy, and its soils are sandy and friable (Online Resource 2). For camera trapping, we computed the number of days the camera operated at each site and each 5-day occasion as an effort covariate. Only uncorrelated covariates (|r|≤ 0.70) were used in the analysis (Online Resource 4 — Table S3). As the distance to wind turbines was highly correlated with cattle detection rate and distance to residences, we removed wind turbines from the analysis. We also removed the cattle detection rate from the analysis because it was highly correlated with the proportion of native shrubby-arboreal vegetation, which indicates habitat loss, one of the main threats to T. tricinctus. Consequently, we used eight covariates to model T. tricinctus occupancy and detection probabilities (Table 1).
Data analysis
We combined detections into 20 5-day occasions (18 for camera-trapping and two for active searches) to compose detection histories for each site. Specifically, we recorded whether T. tricinctus was detected (1) or not (0) during each occasion. We used a single-season occupancy model for analysis (MacKenzie et al. 2002).
We built models representing our a priori hypotheses for occupancy (Ψ) and detection probabilities (p) (Table 1) and fit them in Program MARK (White and Burnham 1999). We adopted a “stepdown” strategy to build the models (Lebreton et al. 1992). Using an additive structure of the most parametrized model for Ψ, we first modeled p using only one covariate for each model. Then, using the most parsimonious model for p, we modeled Ψ using only one covariate for this parameter as well. We opted to run models with only one covariate due to our relatively small sample size, thus avoiding over-parameterization. The models were ranked according to the value of the Akaike information criterion adjusted for small sample sizes (AICc), for which the best models are those with the lowest values (Burnham and Anderson 2002). When the difference in AICc value between a given model and the best-supported model was less than two (ΔAICc < 2.00), we considered it as well supported by our data (Burnham and Anderson 2002). Consequently, all models with ΔAICc < 2.00 were identified as best supported. As more than one model presented ΔAICc < 2.00 for Ψ (model uncertainty), we calculated model-averaged estimates for this parameter (Burnham and Anderson 2002). Finally, we also checked for overdispersion using the most parametrized model for both p and Ψ (the full or global model) by applying the goodness-of-fit test developed for occupancy analyses (MacKenzie and Bailey 2004) in Program PRESENCE (Hines 2006).
Results
We obtained 10 records of T. tricinctus by camera trapping at 10 sites and detected the species by active searches at 11 of them, at six exclusively by this method. Tolypeutes tricinctus thus presented a naïve occupancy of 0.67. It was detected once at 11 sites, twice at four sites, and three times at one site.
The goodness-of-fit test revealed no evidence of overdispersion (χ2 = 910.87; ĉ = 1.00; P = 0.61). Our analysis showed uncertainty among Ψ model structures, four of which were better supported (AICc < 2.00; Table 2). Although the covariates distance to roads (β = 0.015), distance to the nearest residence (β = − 0.004), and soil sand content (β = − 20.000) were included in the most parsimonious models, the null model structure was supported by our data (ΔAICc = 0.00), predicting that none of these covariates had a strong influence on T. tricinctus occupancy probability, which was high and constant according to our model-averaged estimates (Ψ = 1.00; 95% CI = 0.95–1.00).
The detection probability of T. tricinctus varied according to the sampling method (Table 2), being active searches the most efficient. The detection probability by active searches (p = 0.25; 95% CI = 0.15–0.39) was eight times higher than by camera trapping (p = 0.03; 95% CI = 0.01–0.05).
Discussion
We found the T. tricinctus occupancy probability close to one, indicating that this species potentially occupies all sites in the study area. This result highlights that, although T. tricinctus may have disappeared or become rare over much of its range (Miranda et al. 2014; Reis et al. 2015), it can still be widely distributed at localities where it is not subjected to intense hunting pressure and habitat loss, as discussed by Miranda et al. (2014). However, the occupancy probability we found might be overestimated due to the very low detection probability obtained by camera trapping (MacKenzie et al. 2002). The T. tricinctus detection rate by camera trapping in our study area (0.005 records/camera-day) is similar to that found elsewhere in the Caatinga (Campos et al. 2019) and higher than for T. matacus in the Pantanal wetlands (about 0.001 records/camera-day; Porfirio et al. 2014). Considering this apparent generally low detectability of Tolypeutes by camera traps, increasing sampling effort in future research with T. tricinctus should help to improve the precision and accuracy of occupancy estimates (MacKenzie et al. 2002). Nevertheless, even the T. tricinctus naïve occupancy in our study area was high when compared to those already found for other armadillos (Ferreguetti et al. 2016; Rodrigues and Chiarello 2018). Moreover, the high occupancy probability of T. tricinctus we found is supported by interview data from a rural community in our study area, whose residents consider this species abundant and widely distributed (R. A. Magalhães, unpublished data).
We recorded T. tricinctus in contrasting habitats, from forested sites either farther from roads and residences to sites closer to them. Tolypeutes tricinctus has been observed in different habitats, including croplands and managed forests in human-modified landscapes under moderate habitat loss and hunting intensity (Marinho-Filho et al. 1997; Bocchiglieri et al. 2010). However, previous studies have not systematically evaluated its distribution. Our results indicate that T. tricinctus can occupy less or more disturbed sites in such landscapes, thus supporting previous observations.
Although T. tricinctus is regarded as one of the most sensible armadillos to habitat alterations (Marinho-Filho and Reis 2008), we did not find effects of hunting and habitat loss on its occupancy probability. Hunting and habitat loss are considered the most important threats to T. tricinctus (Miranda et al. 2014; Reis et al. 2015). They have been shown to negatively affect the distribution of other armadillos (Ferreguetti et al. 2016; Rodrigues and Chiarello 2018). However, studies with T. matacus have indicated that this species can be common in areas where it is intensively hunted (Noss et al. 2008; Cuéllar 2008). In our study area, T. tricinctus is not a frequent hunting target (R. A. Magalhães, unpublished data), which likely explains why its distribution was not affected by proximity to roads and residences. Similarly, despite the existence of anthropogenic habitats, our studied landscape still holds a substantial amount of native vegetation (Fig. 1). Therefore, the current levels of hunting and habitat loss appear not to be severe enough to affect T. tricinctus distribution. Broader spatial and temporal scales may be required to detect such effects (Santos et al. 2019a).
The detection probability of T. tricinctus was eight times higher by active searches than by camera trapping. Although camera trapping is a non-intrusive method, whose efficiency does not vary significantly according to field conditions, it presents higher initial costs (Silveira et al. 2003). Active searches for signs are efficient to record species occurrence but depend heavily on environmental conditions and experienced personnel (Silveira et al. 2003). During 24 h of active searches, we detected T. tricinctus signs at 46% of the sites, whereas we spent ~ 46,100 camera-hours to detect the species at 42% of them. The costs with field personnel for both methods were equal, as they were applied simultaneously. However, camera trapping involved additional costs with equipment of more than 9000 USD and more time to process data. Active searches thus presented greater cost–benefit when compared to camera trapping to register T. tricinctus occurrence and can even improve occupancy estimates by increasing detection probabilities.
Our sampling design may have favored the detection of T. tricinctus by active searches when compared to camera trapping. First, camera traps were installed ~ 30 cm above the ground. Although angling them downwards may have favored detection (Rowcliffe et al. 2011), lower heights than we used (30 cm) might increase the detection of closer records. Second, active searches covered a 63-m2 buffer surrounding each site, expanding the sampling to outside the camera trap detection zone. This method also allowed us to record different signs of T. tricinctus. Finally, we counted on skilled people from local communities to conduct active searches, which may have also increased detection (Silveira et al. 2003). The inclusion of local specialists reinforces the importance of local ecological knowledge and engagement of local communities in research and conservation initiatives to increase their effectiveness.
In summary, we found T. tricinctus widely distributed in a human-modified landscape subjected to moderate levels of hunting and habitat loss, supporting previous field observations. We also found active searches more effective than camera trapping to detect T. tricinctus occurrence and that both methods can be successfully applied together. Thus, we recommend considering these differences in effectiveness for designing studies on T. tricinctus ecology. Based on our results, we support the engagement of local people in research and conservation projects for better outcomes.
Data availability
All data used for analysis is cited in the text, available as Online Resource, and/or available from the authors on request.
Code availability
Not applicable.
References
Attias N, Miranda FR, de Sena LMM et al (2016) Yes, they can! Three-banded armadillos Tolypeutes sp. (Cingulata: Dasypodidae) dig their own burrows. Zoologia (Curitiba) 33:e20160035. https://doi.org/10.1590/S1984-4689zool-20160035
Bocchiglieri A, Mendoncça AF, Henriques RPB (2010) Composição e diversidade de mamíferos de médio e grande porte no Cerrado do Brasil central. Biota Neotrop 10:169–176. https://doi.org/10.1590/s1676-06032010000300019
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Campos CB, Esteves CF, Dias DM, Rodrigues FHG (2019) Medium and large sized mammals of the Boqueirão da Onça, North of Bahia State Brazil. Pap Avulsos Zool 59:e20195912. https://doi.org/10.11606/1807-0205/2019.59.12
Cuéllar E (2008) Biology and ecology of armadillos in the Bolivian Chaco. In: Vizcaíno SF, Loughry WJ (eds) The biology of the Xenarthra. University Press of Florida, Gainesville, pp 306–312
Dias DM, Massara RL, Campos CB, Rodrigues FHG (2019) Human activities influence the occupancy probability of mammalian carnivores in the Brazilian Caatinga. Biotropica 51:253–265. https://doi.org/10.1111/btp.12628
Dias DM, Ferreguetti AC, Rodrigues FHG (2020) Using an occupancy approach to identify poaching hotspots in protected areas in a seasonally dry tropical forest. Biol Conserv 251:108796. https://doi.org/10.1016/j.biocon.2020.108796
Dobbins M, Sollmann R, Menke S et al (2020) An integrated approach to measure hunting intensity and assess its impacts on mammal populations. J Appl Ecol 57:2100–2111. https://doi.org/10.1111/1365-2664.13750
Donagema GK, de Campos DVB, Calderano SB et al (2011) Manual de métodos de análises de solos, 2nd edn. Embrapa Solos, Rio de Janeiro
Feijó A, Garbino GST, Campos BATP et al (2015) Distribution of Tolypeutes Illiger, 1811 (Xenarthra: Cingulata) with comments on its biogeography and conservation. Zool Sci 32:77–87. https://doi.org/10.2108/zs140186
Ferreguetti AC, Tomas WM, Bergallo HG (2016) Density and niche segregation of two armadillo species (Xenarthra: Dasypodidae) in the Vale Natural Reserve, Brazil. Mamm Biol 81:138–145. https://doi.org/10.1016/j.mambio.2015.10.007
Fleischner TL (1994) Ecological costs of livestock grazing in western North America. Conserv Biol 8:629–644. https://doi.org/10.1046/j.1523-1739.1994.08030629.x
Gibb GC, Condamine FL, Kuch M et al (2016) Shotgun mitogenomics provides a reference phylogenetic framework and timescale for living xenarthrans. Mol Biol Evol 33:621–642. https://doi.org/10.1093/molbev/msv250
Hannibal W, Zortéa M, Calaça AM et al (2021) Checklist of mammals from Goiás, central Brazil. Biota Neotrop 21:e20201173. https://doi.org/10.1590/1676-0611-BN-2020-1173
Helldin JO, Jung J, Neumann W et al (2012) Effects of wind power on terrestrial mammals: a synthesis. Swedish Environmental Protection Agency, Bromma
Hines, JE (2006) PRESENCE - Software to estimate patch occupancy and related parameters. USGS-PWRC. http://www.mbr-pwrc.usgs.gov/software/presence.html. Accessed 17 Oct 2021
ICMBio – Instituto Chico Mendes de Conservação da Biodiversidade (2014) Sumário Executivo do Plano de Ação Nacional para a Conservação do Tatu-bola. ICMBio/MMA, Brasília.
Jonzén N (2008) Habitat selection: implications for monitoring, management, and conservation. Isr J Ecol Evol 54:459–471. https://doi.org/10.1560/IJEE.54.3-4.459
Lebreton J, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118. https://doi.org/10.2307/2937171
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. J Agric Bio Environ Stat 9:300–318. https://doi.org/10.1198/108571104X3361
MacKenzie DI, Nichols JD, Lachman GB et al (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. https://doi.org/10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2
Magalhães RA, de Sena LMM, Rodrigues FHG (2021) First records of Brazilian three-banded armadillo (Tolypeutes tricinctus, Mammalia, Cingulata, Chlamyphoridae) predation by jaguar (Panthera onca, Mammalia, Carnivora, Felidae). Pap Avulsos Zool 61:e20216158. https://doi.org/10.11606/1807-0205/2021.61.58
Marinho-Filho J, Reis ML (2008) Tolypeutes tricinctus Linnaeus, 1758. In: Chiarello AG, Aguiar LMS, Cerqueira R (eds) Livro vermelho da fauna brasileira ameaçada de extinção. MMA/Biodiversitas, Brasília/Belo Horizonte, pp 709–710
Marinho-Filho J, Guimarães M, Reis ML et al (1997) The discovery of the Brazilian three-banded armadillo in the Cerrado of Central Brazil. Edentata 3:11–13
McDonough CM, Loughry W (2008) Behavioral ecology of armadillos. In: Vizcaíno SF, Loughry WJ (eds) The biology of the Xenarthra. University Press of Florida, Gainesville, pp 281–293
Miranda F, Moraes-barros N, Superina M, Abba AM (2014) The IUCN red list of threatened species. Tolypeutes tricinctus, Brazilian three-banded armadillo. https://www.iucnredlist.org/species/21975/47443455. Accessed 17 Oct 2021
MMA – Ministério do Meio Ambiente (2017) 2a atualização das Áreas Prioritárias para Conservação da Biodiversidade 2018. http://areasprioritarias.mma.gov.br/2-atualizacao-das-areas-prioritarias. Accessed 9 Sep 2021
Neri M, Jameli D, Bernard E, Melo FPL (2019) Green versus green? Adverting potential conflicts between wind power generation and biodiversity conservation in Brazil. Perspect Ecol Conserv 17:131–135. https://doi.org/10.1016/j.pecon.2019.08.004
Noss AJ, Cuéllar RL, Cuéllar E (2008) Exploitation of xenarthrans by the Guaraní-Isoseño indigenous people of the Bolivian Chaco: comparisons with hunting by other indigenous groups in Latin America, and implications for conservation. In: Vizcaíno SF, Loughry WJ (eds) The biology of the Xenarthra. University Press of Florida, Gainesville, pp 244–254
Porfirio G, Sarmento P, Filho NLX et al (2014) Medium to large size mammals of southern Serra do Amolar Mato Grosso do Sul Brazilian Pantanal. Check List 10:473–482. https://doi.org/10.15560/10.3.473
Projeto Mapbiomas (2021). Coleção 5 da série anual de mapas de cobertura e uso de solo do Brasil. https://mapbiomas.org/en/colecoes-mapbiomas-1?cama_set_language=en. Accessed 9 Sep 2021
QGIS Development Team (2021) QGIS Geographic Information System. OpenSource Geospatial Foundation Project. http://qgis.osgeo.org. Accessed 5 Oct 2022
Regolin AL, Oliveira-Santos LG, Ribeiro MC, Bailey LL (2021) Habitat quality, not habitat amount, drives mammalian habitat use in the Brazilian Pantanal. Landscape Ecol 36:2519–2533. https://doi.org/10.1007/s10980-021-01280-0
Reis ML, Chiarello AG, Campos CB et al (2015) Avaliação do risco de extinção de Tolypeutes tricinctus (Linnaeus, 1758) no Brasil. In: ICMBio – Instituto Chico Mendes de Conservação da Biodiversidade (ed) Avaliação do risco de extinção de xenartros brasileiros. ICMBio, Brasília, pp 237–248
Rhodes JR, Lunney D, Moon C et al (2011) The consequences of using indirect signs that decay to determine species’ occupancy. Ecography 34:141–150. https://doi.org/10.1111/j.1600-0587.2010.05908.x
Rodrigues TF, Chiarello AG (2018) Native forests within and outside protected areas are key for nine-banded armadillo (Dasypus novemcinctus) occupancy in agricultural landscapes. Agric Ecosyst Environ 266:133–141. https://doi.org/10.1016/j.agee.2018.08.001
Rowcliffe JM, Carbone C, Jansen PA et al (2011) Quantifying the sensitivity of camera traps: an adapted distance sampling approach. Methods Ecol Evol 2:464–476. https://doi.org/10.1111/j.2041-210X.2011.00094.x
Santos IB, da Fonseca GAB, Rigueira SE, Machado RB (1994) The rediscovery of the Brazilian three banded armadillo and notes on its conservation status. Edentata 1:11–15
Santos JC, Leal IR, Almeida-Cortez JS et al (2011) Caatinga: the scientific negligence experienced by a dry tropical forest. Trop Conserv Sci 4:276–286. https://doi.org/10.1177/194008291100400306
Santos PM, Bailey LL, Ribeiro MC et al (2019a) Living on the edge: forest cover threshold effect on endangered maned sloth occurrence in Atlantic Forest. Biol Conserv 240:108264. https://doi.org/10.1016/j.biocon.2019.108264
Santos PM, Bocchiglieri A, Chiarello AG et al (2019b) Neotropical xenarthrans: a data set of occurrence of xenarthran species in the Neotropics. Ecology 100:e02663. https://doi.org/10.1002/ecy.2663
Schetino MAA, Campos DP, Anacleto TCS, Santos FR (2021) New records of Tolypeutes tricinctus Linnaeus, 1758 (Cingulata: Tolypeutinae) in northern Minas Gerais state, Brazil. Lundiana 14:01–04
SEI – Superintendência de Estudos Econômicos e Sociais da Bahia (2014). Tipologia climática Köppen. SEI, Salvador
Silveira L, Jácomo ATA, Diniz-Filho JAF (2003) Camera trap, line transect census and track surveys: a comparative evaluation. Biol Conserv 114:351–355. https://doi.org/10.1016/S0006-3207(03)00063-6
Superina M, Abba AM (2020) Conservation perspectives for a highly disparate lineage of mammals: the Xenarthra. Mastozool Neotrop 27:48–67
Superina M, Pagnutti N, Abba AM (2014) What do we know about armadillos? An analysis of four centuries of knowledge about a group of South American mammals, with emphasis on their conservation. Mammal Rev 44:69–80. https://doi.org/10.1111/mam.12010
Taber FW (1945) Contribution on the life history and ecology of the nine-banded armadillo. J Mammal 26:211–226
Trombulak SC, Frissell CA (2000) Review of ecological effects of roads on terrestrial and aquatic communities. Conserv Biol 14:18–30. https://doi.org/10.1046/j.1523-1739.2000.99084.x
Tudge SJ, Brittain S, Kentatchime F, et al (2022) The impacts of human activity on mammals in a community forest near the Dja Biosphere Reserve in Cameroon. Oryx 1–9. https://doi.org/10.1017/S0030605321000806
White GC, Burnham KP (1999) Program mark: survival estimation from populations of marked animals. Bird Study 46:120–139
Acknowledgements
The companies Azurit Engenharia and Statkraft provided logistical support in the study area. We thank the field assistants Cosme, Lourisvaldo, and Olavo for their indispensable help with field activities.
Funding
This work was supported by the Arizona Center for Nature Conservation (ACNC)/Phoenix Zoo Conservation & Science Grant Program, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [grant numbers: 88887.199565/2018–00; 88882.316024/2019–01], and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number: 301289/2019–0].
Author information
Authors and Affiliations
Contributions
Conceptualization: Rodolfo A. Magalhães, Rodrigo L. Massara, Fábio S. de Oliveira, and Flávio H. G. Rodrigues. Methodology: Rodolfo A. Magalhães, Rodrigo L. Massara, Fábio S. de Oliveira, and Flávio H. G. Rodrigues. Formal analysis and investigation: Rodolfo A. Magalhães, Rodrigo L. Massara, and Fábio S. de Oliveira. Writing — original draft preparation: Rodolfo A. Magalhães. Writing — review and editing: Rodrigo L. Massara, Fábio S. de Oliveira, and Flávio H. G. Rodrigues. Funding acquisition: Rodolfo A. Magalhães, Rodrigo L. Massara, and Flávio H. G. Rodrigues.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Thales Renato Ochotorena de Freitas.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Magalhães, R.A., Massara, R.L., de Oliveira, F.S. et al. The Brazilian three-banded armadillo is widely distributed in a human-modified landscape in northeastern Brazil. Mamm Res 68, 53–62 (2023). https://doi.org/10.1007/s13364-022-00651-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-022-00651-5