Abstract
Coxiella burnetii, or Q fever agent, has notable implications for human and livestock health. Infections in cattle primarily manifest through reproductive issues where infected animals shed the bacterium in birth fluids, placental tissues, and milk, serving as potential sources of transmission. Bovine herds become reservoirs, contributing to the environmental contamination of farming areas. Comprehensive studies on the prevalence, transmission routes, and associated risk factors among cattle contribute to the development of effective control strategies, ultimately safeguarding both livestock and public health.Here we determine the prevalence of Coxiella burnetii antibodies against in dairy cattle farms from Kabylia (northern Algeria) and identify the associated risk factors. Bulk tank milk samples from 184 farms were analyzed by indirect ELISA technique, 49 of them were tested positive which corresponds to a prevalence rate of 26.63% (95% CI 20.25–33.01%). Multivariate analysis by logistic regression showed that the risk factors associated with detection of anti-Coxiella burnetii antibodies are: cohabitation of cattle with small ruminants(OR = 3.74 95% CI [1.41–8.92]), exposure to prevailing winds (OR = 5.12 95% CI [2.11–13.45]), and the veterinarian visits frequency(OR = 5.67 95% CI [2.55–13.60]). These findings underscore the susceptibility of dairy cattle to Q fever in the Kabylia region, highlighting practices that pose risks. We recommend the implementation of hygienic measures and adherence to proper farming conditions to mitigate the transmission of Q fever and reduce the associated zoonotic risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Q fever, a bacterial zoonosis caused by Coxiella burnetii, an obligate intracellular bacterium, is found worldwide except in New Zealand (Pexara et al., 2018). This bacterium has been identified in a wide range of vertebrates including wild mammals, marine mammals, birds, and reptiles (Eldin et al., 2017), and has also been found in over 40 species of ticks which can serve as both reservoir and vectors (España et al., 2020).
Animals can become infected with the agent through contact with other infected animals or their environment (Rousset et al., 2003). Infection is mainly transmitted by inhalation of contaminated aerosols, but can also occur through the digestive and transcutaneous routes, including through the bite of infected ticks (Alsaleh et al., 2011). The presence of C. burnetii has been confirmed by PCR in bull (Kruszewska and Tylewska-Wierzbanowska, 1997) and sheep semen (Ruiz-Fons et al., 2014), although sexual transmission in ruminants has been strongly suspected but not yet proven (Pellerin et al., 2018). In cattle, C. burnetii infection is most often asymptomatic (Maurin and Raoult, 1999) and can remain latent for several years (Rousset et al., 2003). While clinical manifestations are more commonly observed in sheep and goats, they may be less apparent in cattle (Anastácio et al., 2016), and can include abortions, stillbirths, and stunted newborns (Agerholm, 2013). Infected females may excrete large numbers of the bacteria into the environment during parturition or abortion, with shedding in parturition products, urine, feces, and milk (Mori et al., 2017). C. burnetii can persist to be excreted for several months in vaginal mucus, feces, urine, and milk (Rodolakis, 2009). Bacteria can also be aerosolized and carried long distances by the wind (Mori et al., 2017).
In ruminants, the complement fixation test (CFT) is still used in some laboratories for the diagnosis of Q fever, but the most commonly used tests are the ELISA and the rt-PCR technique. For serological diagnosis, ELISA is the method of choice because it is automated and more sensitive than CFT (Boarbi et al., 2016). For herd-wide diagnosis, ELISA and rt-PCR are used on bulk tank milk (Rodolakis, 2009). Bulk milk testing for C. burnetii antibodies by ELISA is an appropriate method to determine herd exposure and status (Agger et al., 2010; Muskens et al., 2011). The results are comparable to those obtained from serum samples, as antibodies are transferred from blood to milk in lactating cows (Nielsen et al., 2011).
In Algeria, the demand for milk is constantly growing, and dairy farming struggles to meet this exponential demand. Among the factors hindering the development of this sector are infectious pathologies. Although Q fever has been reported in both humans and animals. The seroprevalence of Q fever in the human population of Setif is 15.5%, living in a rural area or having occupational exposure are factors significantly associated with seropositivity in the tested population (Lacheheb and Raoult, 2009). In cattle, individual seroprevalence ranged from 10.6% (Agag et al., 2016) to 11.6% (Menadi et al., 2020), at herd level, seroprevalence varied from 22% (Agag et al., 2016) to 45.56% (Menadi et al., 2020). The only one study using bulk tank milk and targeting a large number of cattle farms for the detection of antibodies against C. burnetii is available, a prevalence of 37% have been reported (Menadi et al., 2022). Studies have also demonstrated the circulation of C. burnetii among sheep flocks, with variable seroprevalences reported at individual level ( 12,4% to 24,9%) and at herd level ( 35,9% to 66,7%) ( Hireche et al., 2020; Khaled et al., 2016; Belhouari et al., 2022). These studies revealed that reproductive disorders were significantly associated with seropositivity, and made it possible to identify certain risk factors, but further studies are needed to fully understand the epidemiology of the disease.
The current study aimed to estimate the prevalence of antibodies against C. burnetii in bulk tank milk of dairy cattle herds using indirect ELISA test, and identify associated risk factors in the Kabylia region (northern Algeria).
Materials and methods
Study location
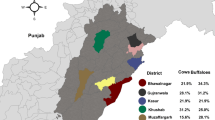
The current study was conducted from June 2022 to October 2022 in the departments of Bejaia (36°12’ and 36°53’ N, 4°21’ and 5°29’ E, 3223,48 Km2) and Tizi-Ouzou (36° 28’ and 36° 55’ N, 03° 45’ and 04° 31’ E, 2957,93 Km2) in northern Algeria. The study area is situated between the Mediterranean Sea to the north, the departments of Setif, Bordj Bouareridj, and Bouira to the south, Jijel to the East, and Boumerdes to the West (Fig. 1). The area is characterized by a Mediterranean climate and mountainous terrain, which covers 90% of its surface. The two departments have a cattle population of 42,693 and 70,998 head for Bejaia and Tizi-Ouzou respectively, and a milk production of around 415,013,000 L and 147,993,000 L for Bejaia and Tizi-Ouzou respectively in 2019 (MADR., 2021).
Milk sampling strategy
A total of 184 bulk tank milk samples were collected from 92 farms in the department of Bejaia and 92 farms in the department of Tizi-Ouzou. These farms had a total of 2779 heads, with an average of 15 heads per farm. The sample size was determined based on an expected herd prevalence of 22% (Abdelhadi et al., 2015) and a desired level of precision of 6% at a 95% confidence level, resulting in a requirement of 183 herds. During each farm visit, a 50 mL sample of bulk tank milk was collected in sterile tubes, identified, processed under cold conditions, and then frozen at -20 °C for later use.
ELISA-analysis
The frozen samples were thawed to return to room temperature. Then, each sample was centrifuged for 15 min at 7500 rpm to separate the cream from skimmed milk. A commercial ID Screen® Q Fever Indirect Multi-species kit (IDVet, Grabels, France) was used as recommended by the manufacturer.
Samples are used undiluted, and controls are diluted 1:50 in dilution buffer 2, then 100 µl of positive and negative controls, 100 µl of each sample were distributed in the microplates, the microplates were covered and incubated for 45 min at 21 °C. The wells were emptied and then washed, filled a first time with 300 µl of washing solution for 2 min, followed by 2 conventional washes with no waiting time. Concentrated conjugate 10X the was diluted 1:10 in buffer dilution 3, 100 µl of diluted conjugate were then distributed to each well, and the plates were covered and incubated for 30 min at 21 °C. The wells were emptied and then washed with 300 µl of washing solution, 100 µl of substrate solution were distributed to each well, and the microplates were covered and incubated for 15 min at 21 °C. To stop the reaction, 100 µl of stop solution were distributed to each well.
The optical densities (OD) were measured at 450 nm, the test was validated, and for each sample, the percentage SP (%) was calculated according to the following formula:
SP% = Sample OD - CN OD / CP OD - CN OD X 100.
According to the manufacturer’s manual, milk samples with S/P% ≤ 30% are considered negative, those with S/P% > 40% positive, and are considered doubtful those with SP% 30% < S/P% ≤ 40%.
Data analysis
The prevalence of anti-C. burnetii antibodies and its 95% confidence interval were calculated as the ratio of the number of positive herds to the total number of herds tested. Information about breeding practices, such as the number of heads, lactating females, address, screening and vaccination, were collected for each farm included in the study. A questionnaire was also completed on site for each farm and it was divided into three parts: (1) The first part focused on the pathological antecedents of the farms during the 12 last months, including any history of abortion (farms are considered suspect for Q fever if they recorded at least 2 or more cases of abortion per month or 3 cases of abortion during the last 12 months (Sidi-Boumedine et al.,2010)), this part of the questionnaire also includes questions about antecedents of retained placenta, repeat breeding (No gestation after 3 artificial inseminations), metritis, mastitis and respiratory pathologies. For these last pathological antecedents, only responses from farms with a high incidence of these disorders (> 40%) are taken into account; responses from farms with sporadic cases are not taken into account because these disorders are not specific and can have various origins. (2) The second part related to the breeding conditions, such as borrowing of breeding stock, cohabitation with small ruminants, tick infestation, sharing of equipment with other farms, presence of domestic carnivores and rodents in the barn, exposure to prevailing winds, presence of calving pens, hygienic precautions at the entrance to the farm, protective measures during calving or abortion, and presence of livestock in the vicinity. These first two parts contain questions with binomial answers (Presence or Absence) and (Yes or No). (3) The last part includes questions relating to certain risk practices, such as the frequency of visits by the attending veterinarian, the fate of parturition or abortion products (fetal membranes, runts, among others.), the frequency of manure evacuation, the method of reproduction and the origin of water on the farm.
The chi-square test (or Fisher’s exact test/ Yate’s corrected Chi-Squared test for small samples sizes) was performed to evaluate the correlation between the positive detection of anti-C. burnetii antibodies in bulk tank milk and the risk factors studied. The results were considered significant if P < 0.05. Finally, the different parameters with a significant influence (P < 0.05) were subjected to multiple logistic regression analyses, using R software version 4.0.4 to determine their possible role as risk factors for the detection of antibodies against C. burnetii in bulk tank milk of studied farms. P ≤ 0.05 was considered significant, with CI at 95%.
Results
Prevalence of antibodies against C. burnetii in bulk tank milk
A total of 49 /184 herds were positive with a prevalence of 26.63% (95% CI: 20.25–33.01%), 10 /184 herds provided a doubtful result, and 125 herds were negative. The prevalence of antibodies anti-C. burnetii in the department of Bejaia was 31.52% (26.68 − 36.36%, 95% CI), based on 29 positive cases out of 92 tested (29/92), and that recorded in the department of Tizi-Ouzou was 21.73% (17.44-26.02%, 95% CI), with 20 positive cases out of 92 tested (20/92). There was no significant difference between the results of these two departments (P = 0.133).
Univariate analysis
The relation between the detection of antibodies anti-C. burnetii in bulk tank milk and variables were analyzed using chi-square test.
The results obtained revealed a significant association between the detection of anti C. burnetii antibodies and abortion antecedents (P = 0.001), retained placenta antecedents (P = 0.009) and repeat breeding antecedents (P = 0.008). Regarding the other variables related to the pathological history of the herds, no significant association was detected (Table 1).
Regarding the breeding conditions, several significant associations with the positive detection of antibodies against C. burnetii were revealed:, cohabitation of cattle with small ruminants (P = 0.01), tick infestation (P = 0.033), exposure to prevailing winds (P = 0.005) and the livestock presence in the vicinity (P < 0.001). The other husbandry parameters studied did not show a significant association with positive result in ELISA analysis (Table 2).
The analysis of risky practices using chi-square test revealed that the frequent manure evacuation and the frequent visits of veterinarian to the farms are significantly associated with the detection of anitbodies against C. burnetii in bulk tank milk (P = 0.044 and P < 0.001 respectively) ( Table 3).
Multivariate logistic regression analysis
Multivariate analysis of husbandry-related parameters showed that: cohabitation of cattle with small ruminants, exposure to prevailing winds, and veterinarian visits frequency were potential risk factors for detection of antibodies against C.burnetii in bulk tank milk of dairy cattle herds (P < 0.05) (Table 4).
Discussion
To the best of our knowledge, this study is the first to use bulk tank milk to detect C. burnetii antibodies in the Kabylia region of northern Algeria. The ELISA test used in this study is based on the use of C. burnetii phase I and II antigens, which were isolated from the bovine abortion placenta. The test using C. burnetii antigens isolated from ruminants are more sensitive than tests using antigens from the Nine Mile reference strain isolated from ticks (EFSA 2010). The use of bulk tank milk for antibody testing appears to be an accurate tool for determining the status of dairy herds against C. burnetii infection (Ruiz-Fons et al., 2011).
In this survey, 26.63% of the studied farms were positive for the presence of anti-C. burnetii antibodies, based on the analysis of 184 samples of bulk tank milk in the Kabylia region using a commercial ELISA test kit. This result is higher than the one recorded in 2017 in the Bejaïa region (22%) (Agag et al., 2016) using ELISA technique on blood serums. This difference may be explained by the fact that in this study, bulk tank milk was used to test all lactating cows on the farm, unlike in the previous study where only a few cows were selected for sampling. The recorded prevalence is higher than that reported in Sweden (8.2%) (Ohlson et al., 2014), and England and Wales (21.2%) (Paiba et al., 1999), but lower than that reported in the Setif region of northeastern Algeria (37%) (Menadi et al., 2022), Iran (45.4%) (Khalili et al., 2011), Spain (66.9%) (Astobiza et al., 2012), and the Netherlands (78.6%) (Muskens et al., 2011).
A significant association was found between the cohabitation of cattle with small ruminants and a positive result of bulk tank milk analysis by ELISA test (P < 0.05; OR = 3.74 [1.41–8.92]). Menadi et al., (2020) also observed this association in their study. This can be attributed to the duration of excretion of C. burnetii particularly during parturition or abortion, which can extend over several weeks in ewes (Berri et al., 2001) or goats (Rousset et al., 2009), increasing the risk of contamination. Additionally, this practice is common in farms (72/184) and is often done for ritual purposes.
Exposure to prevailing winds was identified as a risk factor for the presence of specific antibodies to C. burnetii in bulk tank milk by logistic regression (P < 0.05; OR = 5.12 [2.11–13.45]). The involvement of wind in the spread of C. burnetii has been suggested since the 1950s (Tissot-Dupont et al., 2004), with studies showing that exposure to strong winds is a major risk factor for C. burnetii infection for both humans (Schimmer et al., 2010) and animals (Nusinovici et al., 2017). People can become infected through exposure to aerosols carrying Coxiella during the movement of infected animals, contaminated manure, or other products, even if they have no contact with animals or animal products (Woldehiwet, 2004). The mountainous nature of the study area increases its exposure to strong winds. Proper management of parturition/abortion products and building livestock structures in the opposite direction to the prevailing wind could limit the spread and contamination of humans and animals by C. burnetii.
The logistic regression analysis revealed that frequent visits by veterinarians were a risk factor for positive findings of antibodies against C.burnetii in bulk tank milk (P < 0.05,5.67 [2.55–13.60]). These results are consistent with those of the study carried out in Denmark, who proposed that veterinarians may act as mechanical vectors of C. burnetii from infected to healthy farms(Agger et al., 2010). Implementing hygiene measures by veterinarians, such as disinfecting equipment, changing boots and installing foot baths at the entrance to the farm, as well as restricting access to outsiders, could reduce the passive transport and introduction risks of C. burnetii.
This is the first investigation in the Kabylia region C. burnetii in dairy cattle herds using bulk tank milk. It provided the prevalence of antibodies but had also identified the associated risk factors. Further studies, employing molecular biology techniques to determine the proportion of herds shedding C. burnetii, are recommended for a more comprehension. In the absence of robust epidemiological data, other researches on the disease in small ruminants and humans are needed. This holistic approach aims to fully comprehend the Q fever epidemiology and formulate comprehensive control plans integrating hygienic and biosecurity measures for both human and animal health within a “one health” framework.
Data availability
The datasets generated and/or analyzed in this study are not publicly available for administrative reasons( pending thesis defense), but can be obtained from the corresponding author upon reasonable request.
Change history
17 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11250-024-04075-y
References
Abdelhadi, F.Z., Abdelhadi, S.A., Niar, A., Benallou, B., Meliani, S., Smail, N.L., Mahmoud, D., 2015. Abortions in Cattle on the Level of Tiaret Area (Algeria). Global Veterinaria, 14, 638–645.
Agag, S., Kaidi, R., Khelef, D., 2016. Séroprévalence de la fièvre Q chez les bovins de la région de Bejaïa (Algérie). Revue d’élevage et de médecine vétérinaire des pays tropicaux, 69, 155–159.
Agerholm, J.S., 2013. Coxiella burnetii associated reproductive disorders in domestic animals-a critical review. Acta Veterinaria Scandinavica, 55, 13.
Agger, J.F., Christoffersen, A.-B., Rattenborg, E., Nielsen, J., Agerholm, J.S., 2010. Prevalence of Coxiella burnetii antibodies in Danish dairy herds. Acta Veterinaria Scandinavica, 52, 5.
Alsaleh, A., Pellerin, J.-L., Rodolakis, A., Larrat, M., Cochonneau, D., Bruyas, J.-F., Fieni, F., 2011. Detection of Coxiella burnetii, the agent of Q fever, in oviducts and uterine flushing media and in genital tract tissues of the non pregnant goat. Comparative Immunology, Microbiology and Infectious Diseases, 34, 355–360.
Anastácio, S., Carolino, N., Sidi-Boumedine, K., Da Silva, G.J., 2016. Q fever dairy herd status determination based on serological and molecular analysis of bulk tank milk. Transboundary and Emerging Diseases, 63, e293–e300.
Astobiza, I., Ruiz-Fons, F., Pinero, A., Barandika, J.F., Hurtado, A., Garcia-Perez, A.L., 2012. Estimation of Coxiella burnetii prevalence in dairy cattle in intensive systems by serological and molecular analyses of bulk-tank milk samples. Journal of Dairy Science, 95, 1632–1638.
Belhouari, A., Souames, S., Berrama, Z., Ouchene, N., 2022. Seroprevalence of Q fever among ewes and associated risk factors in Ain Defla region, North-central Algeria. Comparative Immunology, Microbiology and Infectious Diseases, 87, 101853.
Berri, M., Souriau, A., Crosby, M., Crochet, D., Lechopier, P., Rodolakis, A., 2001. Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep. The Veterinary Record, 148, 502–505.
Boarbi, S., Fretin, D., Mori, M., 2016. Coxiella burnetii, agent de la fièvre Q. Canadian Journal of Microbiology, 62, 102–122.
EFSA, Panel on Animal Health and Welfare (AHAW), 2010. Scientific Opinion on Q fever. EFSA Journal, 8, 1–114. http://www.efsa.europa.eu/en/efsajournal/pub/1595. Accessed 23 Dec 2022.
Eldin, C., Mélenotte, C., Mediannikov, O., Ghigo, E., Million, M., Edouard, S., Mege, J.-L., Maurin, M., Raoult, D., 2017. From Q fever to Coxiella burnetii infection: a paradigm change. Clinical Microbiology Reviews, 30, 115–190.
España, P.P., Uranga, A., Cillóniz, C., Torres, A., 2020. Q fever (Coxiella burnetii). Seminars in respiratory and critical care medicine, 41, 509–521.
Hireche, S., Agabou, A., Bouaziz, O., 2020. Seroprevalence of Coxiella burnetii among ewes and associated risk factors in Constantine (Northeastern Algeria). Journal of the Hellenic Veterinary Medical Society, 71, 2383–2390.
Khaled, H., Sidi-Boumedine, K., Merdja, S., Dufour, P., Dahmani, A., Thiéry, R., Rousset, E., Bouyoucef, A., 2016. Serological and molecular evidence of Q fever among small ruminant flocks in Algeria. Comparative Immunology, Microbiology and Infectious Diseases, 47, 19–25.
Khalili, M., Sakhaee, E., Aflatoonian, M.R., Shahabi-Nejad, N., 2011. Herd–prevalence of Coxiella burnetii (Q fever) antibodies in dairy cattle farms based on bulk tank milk analysis. Asian Pacific Journal of Tropical Medicine, 4, 58–60.
Kruszewska, D., Tylewska-Wierzbanowska, S., 1997. Isolation of Coxiella burnetii from bull semen. Research in Veterinary Science, 62, 299–300.
Lacheheb, A., Raoult, D., 2009. Seroprevalence of Q-fever in Algeria. Clinical Microbiology and Infection, 15, 167–168.
MADR (Ministère de l’Agriculture et du Développement Rural). Statistique agricole superficies et productions SERIE « B » 2019. 2021. https://madr.gov.dz/wp-content/uploads/2022/04/SERIE-B-2019.pdf. Accessed 23 Dec 2022.
Maurin, M., Raoult, D. 1999. Q fever. Clinical Microbiology Reviews, 12, 518–553.
Menadi, S.E., Mura, A., Santucciu, C., Ghalmi, F., Hafsi, F., Masala, G., 2020. Seroprevalence and risk factors of Coxiella burnetii infection in cattle in northeast Algeria. Tropical Animal Health and Production, 52, 935–942.
Menadi, S.E., Chisu, V., Santucciu, C., Di Domenico, M., Curini, V., Masala, G., 2022. Serological, Molecular Prevalence and Genotyping of Coxiella burnetii in Dairy Cattle Herds in Northeastern Algeria. Veterinary Sciences, 9, 40.
Mori, M., Mertens, K., Cutler, S.J., Santos, A.S., 2017. Critical aspects for detection of Coxiella burnetii. Vector-Borne and Zoonotic Diseases, 17, 33–41.
Muskens, J., Van Engelen, E., Van Maanen, C., Bartels, C., Lam, T., 2011. Prevalence of Coxiella burnetii infection in Dutch dairy herds based on testing bulk tank milk and individual samples by PCR and ELISA. The Veterinary Record, 168, 79.
Nielsen, K.T., Nielsen, S.S., Agger, J.F., Christoffersen, A.-B., Agerholm, J.S., 2011. Association between antibodies to Coxiella burnetii in bulk tank milk and perinatal mortality of Danish dairy calves. Acta Veterinaria Scandinavica, 53, 64.
Nusinovici, S., Hoch, T., Brahim, M.L., Joly, A., Beaudeau, F., 2017. The Effect of Wind on Coxiella burnetii Transmission Between Cattle Herds: A Mechanistic Approach. Transboundary and Emerging Diseases, 64, 585–592.
Ohlson, A., Malmsten, J., Frössling, J., Bölske, G., Aspán, A., Dalin, A.-M., Lindberg, A., 2014. Surveys on Coxiella burnetii infections in Swedish cattle, sheep, goats and moose. Acta Veterinaria Scandinavica, 56, 39.
Paiba, G.A., Green, L.E., Lloyd, G., Patel, D., Morgan, K.L., 1999. Prevalence of antibodies to Coxiella burneti (Q fever) in bulk tank milk in England and Wales. The Veterinary Record, 144, 519–522.
Pellerin, J.L., Alsaleh, A., Mermillod, P., Souza-Fabjan, J.M.G., Rodolakis, A., Rousset, E., Dubreil, L., Bruyas, J.F., Roux, C., Fieni, F., 2018. Attachment of Coxiella burnetii to the zona pellucida of in vitro produced goat embryos. Theriogenology, 106, 259–264.
Pexara, A., Solomakos, N., Govaris, A.Q., 2018. Q fever and seroprevalence of Coxiella burnetii in domestic ruminants. Veterinaria Italiana, 54, 265–279.
Rodolakis, A., 2009. Q fever in dairy animals. Annals of the New York Academy of Sciences, 1166, 90–93.
Rousset, E., Arricau-Bouvery, N., Souriau, A., Huard, J., Rodolakis, A., Pepin, M., Aubert, M. Les modalités de transmission de la fièvre Q à l’Homme. In: Bulletin épidemiologique. Agence Française de sécurité sanitaire des aliments (AFSSA). 2003. https://be.anses.fr/sites/default/files/BEP-mg-BE7_0.pdf, accessed 23 Dec 2022.
Rousset, E., Berri, M., Durand, B., Dufour, P., Prigent, M., Delcroix, T., Touratier, A., Rodolakis, A., 2009. Coxiella burnetii shedding routes and antibody response after outbreaks of Q fever-induced abortion in dairy goat herds. Applied and Environmental Microbiology, 75, 428–433.
Ruiz-Fons, F., Astobiza, I., Barandika, J.F., Juste, R.A., Hurtado, A., Garcia-Perez, A.L., 2011. Measuring antibody levels in bulk-tank milk as an epidemiological tool to search for the status of Coxiella burnetii in dairy sheep. Epidemiology and infection, 139, 1631–1636.
Ruiz-Fons, F., González-Barrio, D., Aguilar-Ríos, F., Soler, A.J., Garde, J.J., Gortázar, C., del Rocío Fernández-Santos, M., 2014. Infectious pathogens potentially transmitted by semen of the black variety of the Manchega sheep breed: Health constraints for conservation purposes. Animal Reproduction Science, 149, 152–157.
Schimmer, B., Ter Schegget, R., Wegdam, M., Züchner, L., de Bruin, A., Schneeberger, P.M., Veenstra, T., Vellema, P., Van Der Hoek, W., 2010. The use of a geographic information system to identify a dairy goat farm as the most likely source of an urban Q-fever outbreak. BMC Infectious Diseases, 10, 69.
Sidi-Boumedine, K., Rousset, E., Henning, K., Ziller, M., Niemczuck, K., Roest, H., Thiéry, R., 2010. Development of harmonised schemes for the monitoring and reporting of Q-fever in animals in the European Union. EFSA Supporting Publications, 7, 48.
Tissot-Dupont, H., Amadei, M.-A., Nezri, M., Raoult, D., 2004. Wind in November, Q fever in December. Emerging Infectious Diseases, 10, 1264–1269.
Woldehiwet, Z., 2004. Q fever (coxiellosis): epidemiology and pathogenesis. Research in Veterinary Science, 77, 93–100.
Acknowledgements
We wish to thank Dr Bensafia Mounir, Dr Oudafal Abdelhakim, Dr Foughali Asma Amina for their valuable help in the field; the staff of the biochemistry laboratory of the Biotechnology Research Centre for their technical assistance.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Salah Agag, Bachir Medrouh and Hamza Khaled. The first draft of the manuscript was written by Salah Agag and revised by Hamza Leulmi, Hanene Djeghim and Hacène Medkour Conceptualisation of study was performed by Rachid Kaidi, Djamel Khelef and Salah Agag. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical statement
All animal owners declared their oral consent before the collection of the milk samples as well to the related survey questions.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original version of this article, the 4th author’s name was duplicated and appeared again as the 9th author in the contributor section.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agag, S., Medrouh, B., Khaled, H. et al. Prevalence and associated risk factors of anti-Coxiella burnetii antibodies in dairy cattle herds using bulk tank milk analysis in Kabylia area, north Algeria. Trop Anim Health Prod 56, 106 (2024). https://doi.org/10.1007/s11250-024-03950-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-024-03950-y