Abstract
Resistin is one of the most important adipocytokines in mammalian cells due to its involvement in insulin resistance, obesity, and autoimmune diseases. Resistin is encoded by RETN gene that is primarily expressed in adipose tissues. Mutations in this gene have been associated with several productive traits in animals. This study was conducted to assess the possible biomarker capacity of RETN by evaluating its association with growth traits in two economically important sheep in the Middle East. Genotyping was conducted using PCR-single strand conformation polymorphism (SSCP), and the polymorphism of RETN was associated with several growth traits for three months intervals starting from birth until one year of age. In a total of 190 Karakul sheep and 245 Awassi sheep, only one SNP (233A > C) was detected in the RETN gene. The identified novel SNP showed significant associations with all growth traits at the ages of six, nine, and twelve months. At the age of six months onward, lambs with AC and CC genotypes showed respectively lower body weight and length, chest and abdominal circumferences, and wither and rump heights than those with AA genotype. Due to the remarkable association between RETN;233A > C and lower growth traits, this genotype is suggested as a promising marker to assess growth traits in Karakul and Awassi sheep. This is the first study that demonstrated the importance of RETN as a possible tool for evaluating growth traits in two breeds of sheep with a possibility to be applied to other breeds via large-scale association analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth traits indices are key economic indicators due to their vital roles in assessing the productive potential of sheep. While various factors, such as dietary components (Cao et al., 2021), animal management (Alcock and Hegarty, 2011), and external conditions (Ramírez-Ramírez et al., 2021), play a role in shaping animals' growth traits, genetic variations hold the most decisive factors in controlling sheep production (Gootwine, 2020). Due to the multifactorial factors that contribute to growth traits, various genetic loci have been employed in these measurements. Due to their direct or indirect participation in growth and development, an increasing number of loci have been consistently linked to the various growth traits in sheep (Kalds et al., 2022). Whereas other variable genetic candidates have not yet been suggested to be linked to growth traits despite they play a variety of critical activities in the body (Cheng et al., 2020; Martinez-Gonzalez et al., 2020). One of these critical loci that has not been explored yet in terms of its possible association with growth traits is the RETN gene, the gene encodes for resistin.
Resistin is a cytokine made by adipose tissue that regulates weight, insulin sensitivity, inflammation, and reproductive traits (Biernat et al., 2018). It is a polypeptide hormone with a high cysteine content and made of only 109 amino acid residues in sheep. Resistin is encoded by the RETN gene, which is positioned on chromosome 5 in sheep and spans a total length of 1315 bp (GenBank accession no. NC_065058.1). Resistin can impact insulin target areas including fat, liver, and skeletal muscle, while also reducing glucose absorption by tissues (Shi et al., 2019). It may also affect the transcription of enzymes involved in insulin signaling and metabolism via influencing the regulation of glucose and lipid metabolism (Yu et al., 2021). Resistin exhibits noticeable alterations in metabolic and gonadal tissues in various metabolic disorders. Its functions are always linked to the specific tissue or organ where it is situated (Dall’Aglio et al., 2019). Resistin has been found to affect reproductive activity in males and females, in addition to its direct association with various metabolic disorders like diabetes and obesity (Tovar et al., 2005). Due to the presence of resistin in the ovarian granulosa cells of cows, it can potentially modify steroidogenesis and cellular proliferation making it a possible metabolic signal for regulating reproductive activity (Maillard et al., 2011). Owing to its involvement in oocytes' steroidogenesis, proliferation, maturation, and apoptosis, resistin (RETN) gene has been demonstrated to play a significant role in animal production (Rak-Mardyła et al., 2013). Based on its effect on steroidogenesis, it is suggested that resistin is a novel regulator of ovarian function in prepubertal animals (Rak-Mardyła et al., 2013). Irrespective of the versatile metabolic roles of resistin, the polymorphisms of RETN gene have not been associated with any economic traits in sheep. Due to this non-highlighted association between RETN and the productive traits in sheep, it is worth exploring this potential link in economically significant breeds such as Awassi and Karakul, which exhibit distinct differences in growth measurements.
The fat-tailed Awassi is one of the most well-known sheep breeds in the Middle East (Al-Thuwaini et al., 2020a, b; Khazaal et al., 2022; Mohammed et al., 2022). This breed is known for its distinctive fat tail, which can weigh up to 30% of the animal's total weight. Due to its traditional use in the production of meat, milk, and wool, it serves as a triple-purpose breed (Gootwine, 2011). Awassi sheep are well-suited to the arid climates of the Middle East. They are also known for their hardiness and adaptability to a range of environments. In addition to Iraq, Awassi breed is found in many other countries in the region, including Jordan, Syria, and Saudi Arabia. However, their sexual and profitability traits differ depending on the habitat in which they usually survive (Al-Thuwaini et al., 2020a, b). The Karakul breed, on the other hand, is a fat-tailed breed found throughout Asia and Africa. They are known for their distinctive curly, glossy black or brown fur, which is highly prized for its softness and durability. Karakul sheep are also well adapted to harsh desert environments and are able to survive on sparse vegetation and limited water. The capacity to resist extreme environments distinguishes this breed over many other breeds in the region. Karakul sheep have been exploited for pelt, meat, and wool production (Pourlis, 2011; Mirhoseini et al., 2015). It has recently been demonstrated that Karakul lambs have greater growth trait values than Awassi lambs (Aljubouri et al., 2020). Taking this information in mind, this study was conducted to evaluate the possible association between the genetic variations of RETN gene with the growth traits of both investigated Middle-East fat-tailed breeds.
Materials and methods
Animals
This study was conducted on two important breeds that had noticeable differences in their growth trait measurements. The two populations of sheep that were examined belonged to 190 Karakul and 245 Awassi breeds. All the traits were recorded in the Barakat Abu al Fadhl Al-Abbas (BAFAS) Station for raising sheep, in which both breeds were reared (Al-Khafeel co., Holy Karbala, Iraq). This station is located at a longitude of 32.6027° N, a latitude of 44.0197° E, and 32 m above mean sea level. The local climate in this region is characterized by hot summers and mild to cold winters. The annual rainfall may be less than 100 mm per year, making it a very arid region. The newborn lambs were kept with their mothers in individual boxes for two weeks and received special care. Within this period, it was confirmed that the newborn lambs were provided with colostrum. At the age of 3 weeks, they started to be fed a diet consisting of Alfalfa leaves and oatmeal starter. After 3 months, lambs were weaned off breast milk completely. The ethical standards for the care and use of farm animals were followed during the performance of animal experiments (Vaughn, 2012). All animal-related procedures were authorized by the animal welfare committee in the college of agriculture, Al-Qasim Green University. Both populations under examination were housed at the designated breeding station in the same conditions. Both breeds were managed by the same staff during the period of the field experimental procedures (Oct. 2020 to Nov. 2021).
Data collection
Each lamb's ear was marked with a unique identifying number. Several growth characteristics in the tested lambs of the Karakul and Awassi breeds were documented from birth to one year of age. To avoid any possible confusion in the recorded phenotype, the measurements of the growth traits were made by the same individuals using the same standards (Zhao et al., 2017). Body weight (BW), body length (BL), wither height (WH), rump height (RH), chest circumference (CHC), and abdominal circumference (ABC) were used to describe the features that were examined. All included traits were measured under similar conditions and when the sheep is standing and relaxed. A tape measure was used to determine the CHC and ABC, while BL, WH, and RH measurements were taken with a measuring stick. A weighing scale was used to measure the BW in kg units, while the other measurements were based on cm units (Suppl. material). Five different age intervals were considered in the present study for collecting phenotype records, starting from the day of birth until one year of age (birth, 3, 6, 9, and 12 months) following the procedure of (Malik et al., 2016).
Genomic DNA extraction
Genomic DNA was collected from the whole blood samples of all the sheep that were being studied, and any possible discomfort experienced by the animals during blood collection was minimized. Genomic DNA was manually isolated using a salting-out technique (Al-Shuhaib, 2017). Gel electrophoresis was used to assess the extracted genomic DNA's integrity (0.8% agarose in 1 × TAE buffer). Using a Nanodrop spectrophotometer (BioDrop LITE; Biodrop, UK), the quality and quantity of genomic DNA were verified.
PCR primers
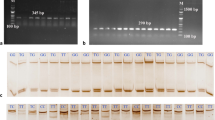
Three pairs of specific PCR primers were designed online using the default parameters of NCBI primer BLAST web server (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Ye et al., 2012). The designed PCR fragments covered the majority of the RETN gene (GenBank NC_065058.1). The first PCR fragment was made up of 222 bp, which was designed to cover the downstream portion of 5ʹ-UTR, exon-1, and the upstream portion of intron-1. The second PCR fragment was made up of 285 bp and was designed to cover the downstream portion of intron-1, exon-2, and the upstream portion of intron-2. The third PCR fragment was made up of 227 bp and was designed to cover the downstream portion of intron-2, exon-3, and the upstream portion of 3ʹ-UTR (Fig. 1a). More details about the sequences of the designed PCR primers, positions within the genomic sequences, and their empirically determined annealing temperatures are shown in Table 1.
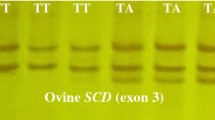
A schematic diagram for the RETN gene-based genotyping using PCR-SSCP method in Awassi and Karakul sheep. a PCR design of three fragments for the amplification of 222 bp, 285 bp, and 227 bp respectively. b Genotyping by PCR-SSCP method, only the 285 bp PCR products showed three different genotypes. c Sequencing electropherograms confirmations of the identified three variants for the polymorphic locus
PCR conditions
PCR amplifications were performed in a gradient thermal cycler, Nexus, Eppendorf, Germany. PCR-ready PreMix with a 20µL capacity was used for PCR experiments according to the manufacturer’s instructions (Cat# K-2012, Bioneer, South Korea). The PCR was performed under the following conditions: an initial denaturation step at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, primer annealing at 45 s (specific to each PCR fragment as indicated in Table 1), extension at 72 °C for 30 s, and a final extension at 72 °C for 5 min. The expected lengths of PCR fragments (222 bp, 285 bp, and 227 bp) were confirmed by 1.5% (w/v) agarose gel electrophoresis before being subjected to genotyping.
SSCP
In this protocol, PCR products of 222 bp, 285 bp, and 227 bp were denatured with equal volumes of SSCP denaturing-loading buffer (95% formamide, 0.05% xylene cyanol dye, 0.05% bromophenol blue dye, 20 mM EDTA pH 8) at 95 °C for 8 min. The denatured PCR amplicons were rapidly chilled on ice for at least 10 min. Subsequently, two µL of each PCR product was loaded into mini–wide gel electrophoresis of 10 cm (length) × 20 cm (width) × 0.1 mm thickness (Cleaver Scientific, Rugby, UK). SSCP electrophoresis was conducted on neutral polyacrylamide gels (acrylamide/bisacrylamide 37.5/1 with 7% glycerol, in 1X TBE) using 200 mA and 100 V until the tracking dye reached the bottom of the gel (Al-Shuhaib et al., 2018, 2019). The polyacrylamide gels were stained using a quick and sensitive silver staining procedure (Byun et al., 2009).
Sequencing
Five representatives of each identified PCR-SSCP pattern were sequenced from both termini by Macrogen (Geumchen, Seoul, South Korea). Only the clear regions of the representative electropherograms were aligned with the RETN genomic sequences (GenBank accession no. NC_065058.1) using BioEdit Software, ver. 7.1 (DNASTAR; Madison, USA). The identified single nucleotide polymorphism (SNP) was manually highlighted in its electropherogram using SnapGene Viewer, ver. 4.0.4 (http://www.snapgene.com).
Genetic diversity analysis
The observed heterozygosity (Ho) and expected heterozygosity (He) indices were calculated to assess genetic diversity in both examined breeds. The Chi-square test was used to identify the possible deviation from Hardy–Weinberg equilibrium (HWE) assumptions for genotype distribution. PopGene32 software, ver. 1.31, was used to compute the genetic population criteria of Ho, He, and Chi-square test. The frequencies of the identified genotypes were determined through direct counting. A population is assumed to have a low or high capacity for genetic diversity when the Ho values are lower or higher than the He values, respectively (Al-Shuhaib et al., 2017). The Gene-Calc web-based server (https://gene-calc.pl/pic) was used to determine the polymorphism information content (PIC). PIC values of 0.25, > 0.25—0.5, and > 0.5 designate loci in the studied population with low, moderate, and high genetic polymorphism capacities, respectively (Ren et al., 2010).
Statistical analysis
Karakul and Awassi breeds' growth traits, including BW, BL, WH, RH, CHC, and ABC, were examined beginning with the birth. At three months intervals, these traits were measured frequently until the lambs turned one year of age. The following general linear model (GLM) was employed to evaluate any possible connection between genotypes and growth traits in both Karakul and Awassi sheep using IBM SPSS Statistics software, ver. 24.0 (IBM, NY, USA):
where; Yijkl represents the phenotypic value of the traits, µ represents the overall mean, Gi is the fixed effect of the ith genotype (i = 1, 2, and 3 for AA, AC, and CC genotypes), Bj signifies the fixed effect of the jth breed of sheep (j = 1 and 2 for Awassi and Karakul), GiBj reflects the interaction between genotype and breed effects, Sk signifies the fixed impact of the kth sex of lambs (l = 1 and 2 for male and female), and eijkl is the random residual effect of each observation. The Tukey–Kramer test was utilized to compare means, and a P-value of 0.05 or less was considered statistically significant for all analyses. The effects of the age of dams, the season of birth, and the parity were not considered in the final analysis since preliminary statistical analysis revealed that these variables had no significant impact on the traits under consideration.
Results
SNP detection techniques
Out of three PCR amplicons (RETN-1, RETN-2, and RETN-3) in the study, only one of them (RETN2) showed genetic polymorphism. Based on the PCR-SSCP approach, three electrophoretic patterns were identified (Fig. 1b). The identified electrophoretic patterns were subjected to Sanger sequencing to verify the detected genotypes in polyacrylamide gels. To confirm the three identified genotypes found in polyacrylamide gels, Sanger sequencing was applied to each of the detected electrophoretic patterns. These genotypes were validated by sequencing reactions, which revealed the presence of one SNP as the source of the three observed variants. The identified SNP was given the designation 233A > C based on its location in RETN-2 amplicons (Fig. 1c). As a result, three genotypes were observed in this polymorphic locus, namely AA (the major homozygous genotype), AC (the heterozygous genotype), and CC (the minor homozygous genotype). After checking the positioning of the identified 233A > C SNP in the NCBI database (NC_065058.1:g.14244921A > C), it was revealed that this SNP was located in the intron-2 sequences of the RETN gene. To see whether it had already been deposited online, the position of this intron SNP was checked by Ensembl genome browser 108 (https://asia.ensembl.org/index.html) (Howe et al., 2021). By checking the positioning of this SNP, it was found that this SNP had not previously been deposited in the ovine RETN gene. Based on the ensemble data of the RETN gene (ENSOARG00020009710), it was revealed that this SNP is a novel intronal variation within the ovine RETN gene. The identified variations were submitted to the NCBI, and three GenBank accession codes (OQ320793, OQ320794, and OQ320795) were obtained to denote the RETN:AA, RETN:AC, and RETN:CC genotypes, respectively.
Genetic diversity of the identified SNP
Allele A showed higher frequencies (0.6342 and 0.5898) than allele C (0.3658 and 0.4102) in Karakul and Awassi breeds, respectively. The homozygous AA genotype had the highest frequency (0.44) in the Karakul breed, followed by the heterozygous AC (0.39) and minor homozygous CC (0.17) genotypes. In Awassi sheep, the heterozygous AC genotype had a higher frequency (0.44) than the major homozygous AA (0.37) and minor homozygous CC (0.19) genotypes, respectively. Genetic diversity analysis revealed that both populations were in HWE. The observed Ho values for Karakul (0. 3947) and Awassi (0. 4449) were both lower than their corresponding He values in Karakul (0. 4652) and Awassi (0.4849), indicating that both populations have a significant potential for genetic variation. However, because the measured PIC values (0.3563 in Karakul and 0.3668 in Awassi) were greater than 0.25 and less than 0.5, the discovered polymorphic locus included only moderate quantities of polymorphism (Table 2).
Effect of the detected SNP on growth traits
Prior to exploring how the 233A > C SNP may relate to growth traits, the impacts of sex and breed interference with the measured traits were assessed in both populations under study. After conducting an association analysis to examine the association between breed/sex and growth traits, it was found that both factors had different effects on the growth characteristics that were investigated. At the age of birth, significant associations of both factors were constrained on WH and RH (P < 0.05). Whereas no association was observed with the other growth traits measured at this age interval. Starting at 3 months of age, the influence of breed on most of the assessed growth characteristics began to show significantly stronger correlations. In both age intervals of 3 and 6 months, the breed of sheep showed significant (P < 0.05) to highly significant (P < 0.001) association with BW, BL, WH, RH, and ABC. The significance of these associations was extended to include all growth traits measured at the age of 9 months onward. According to these significant associations, Karakul lambs showed higher indices for all growth traits than Awassi lambs. Whereas the effect of the sex of the animal was restricted to BW at the ages of 6 months (Table 3). In this age interval, males showed significantly (P < 0.05) higher indices of BW traits than females. Whereas no significant interference was observed from the sex factor on the other investigated growth traits, whether in 6 months intervals or other age intervals.
The genotype–phenotype information revealed various degrees of associations between the identified 233A > C SNP and the growth traits of the studied population. In the age of birth, no significant association was identified between this SNP and any of the measured traits, and the examined indices did not reach the threshold level among AA, AC, and CC genotypes. At the age of 3 months, 233A > C SNP showed significant (P < 0.05) association with CHC and ABC, to highly significant (P < 0.001) levels of association with BW. From the age of 6 months onward, the associations between the identified SNP with growth traits were largely extended to include all the indices with higher levels of significance. The lambs with the AA genotype had the highest means of growth traits in these three age intervals, compared to the lambs with the CC and CC genotypes, respectively (Table 4).
Association analyses showed little effect of the breed interaction factor in intervening with the genotype associations with the growth traits measured. The breed interaction factor showed a significant effect on only two growth traits measured at the age of 6 months, in which Karakul lambs showed higher BL and CHC indices than Awassi lambs (P < 0.05). Furthermore, this effect was reduced at the ages of 9 months and 12 months to include only one trait, in which Karakul lambs displayed higher CHC indices than Awassi lambs (P < 0.05) (Table 5).
Discussion
In this work, the polymorphism of the RETN gene was screened for potential associations with growth traits in two economically important breeds of sheep with different growth characteristics. Owing to the lack of any previous study reporting possible associations between RETN gene and any reproductive or productive trait in sheep, this study genotyped RETN gene in 435 sheep to identify any possible SNP that might be associated with the remarkable phenotypic differences between both investigated breeds. To do so, PCR-SSCP was used due to its lower cost, rapid screening, and higher capacity to identify numerous variants that cannot be identified by the other commonly used post-PCR methods (Kakavas, 2021). Three pairs of PCR primers were designed to be within the optimal resolution lengths of PCR-SSCP method (Badi et al., 2021). However, in both populations under investigation, there was only one SNP identified. Three genotypes (AA, AC, and CC) were detected at the 233A > C SNP location in the RETN gene through SSCP-based electrophoretic patterns and subsequently confirmed through Sanger sequencing. The SNP was found to be located in intron-2 of the RETN gene and was designated as a novel variation within the investigated ovine gene. The identification of the intronal SNP is not unusual due to the well-established fact that non-coding SNPs occur at higher frequencies than coding regions (Vignal et al., 2002; Capriotti et al., 2013).
Both sex and breed had a variety of effects on the traits measured in the investigated population. Given that male lambs normally develop faster and weigh more than female lambs (Mirderikvandi et al., 2016), male lambs had greater values for several examined growth traits at different age ranges. These data indicate that the sex of the lambs had some influence on growth traits. Karakul lambs have much higher values for most growth traits than Awassi lambs (Aljubouri et al., 2021a, b), so the breed impact was mostly found to be significant in this study. However, this is not the first instance where there were apparent differences in growth traits between the two breeds. According to several recent studies comparing the two populations, it has been reported that Karakul lambs have higher growth trait indices than Awassi lambs. However, the already identified differences in growth characteristics between Karakul and Awassi sheep is the key point behind conducting such association study between RETN and growth traits in both breeds. Accordingly, it is interesting to find out the causative SNP(s) that might be behind these already documented phenotypic differences.
Although the majority of the recently assessed growth traits have regularly shown obvious Karakul superiority over Awassi sheep (Aljubouri et al., 2021a, b), this study found that the impact of the identified 233A > C SNP could not be excluded from the studied variables. This is due to the significant connections established between growth traits and the identified 233A > C-based AA, AC, and CC genotypes, regardless of the breed under study. As a result, the currently reported effect of 233A > C SNP can perform its function without being influenced by the type of breed investigated. However, the effect of this SNP increases gradually with age. Though no potential link was detected between the AA, AC, and CC genotypes and all growth traits at birth, a significant association emerged at the first three months of age with specific growth traits. In contrast, the significant effect of the identified 233A > C SNP was extended to include all growth traits in the later recorded months. The non-significant role of the detected 233A > C SNP is most likely due to the difficulties of determining the characteristics of growth attributes at birth compared to other age intervals (Baneh and Hafezian, 2009; Hickford et al., 2010; Koncagül et al., 2013). The identified SNP's causal impact becomes apparent after the lambs reach three months of age and beyond. However, this could be due to an increase in RETN gene expression at these ages. However, resistin expression has not yet been extensively studied in sheep. Nonetheless, our findings are consistent with previous research on nonhuman primates that found that the expression of the RETN gene varies with age (Sarmento-Cabral et al., 2017). Furthermore, age-dependent resistin expression has also been supported by several studies conducted on rats (Nogueiras et al., 2003; Morash et al., 2004).
Owing to its critical role in growth traits, our results provide evidence for the importance of 233A > C SNP in marker-assisted selection. It has been observed that RETN genetic variations have been associated with several quantitative traits in domestic animals, such as fatness in pigs (Cieslak et al., 2009), and meat quality in Chinese cattle (Gao et al., 2011).
According to the results of our association analyses, lambs with the CC genotype grew at a slower rate than those with the CA and AA genotypes. It was concluded from this study that sheep with the CC genotype had significantly lower indices of BW, BL, WH, RH, CHC, and ABC at 6 months of age and onward than sheep with the CA and AA genotypes, respectively. This was caused by the replacement of A with C at the intron-2 of the RETN gene (NC 056,058.1;g.14244921A > C) Due to this replacement, lambs with the major homozygous AA genotype considerably outperformed lambs with the heterozygous AC and minor homozygous CC genotypes in all evaluated growth traits. Given the advantage of the wild-type over the other two genotypes, the negative effect of the identified SNP may be anticipated.
Though our experiments have demonstrated the negative effect of the RETN:233A > C SNP in two breeds of sheep, several in vitro experiments are necessary to understand the mechanisms through which this SNP causes such negative effects on the mRNA sequences of the RETN gene.
There has been no prior work that has described the association of the identified 233A > C SNP with any productive or reproductive traits in domestic animals. However, this identified SNP has been demonstrated to be connected with growth traits in sheep; neither the Middle East nor any other region of the world has documented any correlation between RETN and ovine productive features. Accordingly, our study is the first to show a correlation between sheep growth traits and RETN polymorphism. In light of this, our findings on RETN variants may directly be used in marker-assisted selection to enhance growth trait measures in both analyzed breeds, with potential extension to other sheep breeds.
Conclusions
All growth trait assessments in the Karakul and Awassi breeds were strongly impacted by the identified RETN:233A > C SNP starting at the sixth month of age. Our association studies have shown that this intron SNP has a negative effect on sheep growth traits and development. The significant growth trait differences observed in lambs with CC and AC genotypes compared to those with AA genotype can be used in future breeding efforts to improve animal production. More experiments are required to validate this finding, though.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Shuhaib, M.B.S., 2017. A universal, rapid, and inexpensive method for genomic DNA isolation from the whole blood of mammals and birds. Journal of Genetics, 96, 171–176
Al-Shuhaib, M.B.S., Al-Fihan, R.A., Al-Qutbi, A.A. and Al-Thuwaini, T.M., 2017. Potential Consequences of DGAT2 and BTN Genes Polymorphism in Iraqi Holstein Cattle. Scientia Agriculturae Bohemica, 48, 127–141
Al-Shuhaib, M.B.S., Al-Kafajy, F.R. and Al-Jashami, G.S., 2019. A computational approach for explaining the effect of the prl gene polymorphism on prolactin structure and biological activity in Japanese quails. Animal Biotechnology, 32, 273–281
Al-Shuhaib, M.B.S., Al-Lamy, S.M.A., Al-Tayy, H.M.A., Al-Thuwaini, T.M. and Radhi, A.H., 2018. Single Nucleotide Polymorphism (SNP) of leptin gene in holstein cattle. Thai Journal of Veterinary Medicine, 48, 187–201
Al-Thuwaini, T.M., Al-Shuhaib, M.B.S. and Hussein, Z.M., 2020. A novel T177P missense variant in the HSPA8 gene associated with the low tolerance of Awassi sheep to heat stress. Tropical Animal Health and Production, 52, 2405–2416
Al‐Thuwaini, T.M., Al‐Shuhaib, M.B.S., Lepretre, F. and Dawud, H.H., 2020. Two co‐inherited novel SNPs in the MC4R gene related to live body weight and hormonal assays in Awassi and Arabi sheep breeds of Iraq. Veterinary Medicine and Science, 7, 897–907
Alcock, D.J. and Hegarty, R.S., 2011. Potential effects of animal management and genetic improvement on enteric methane emissions, emissions intensity and productivity of sheep enterprises at Cowra, Australia. Animal Feed Science and Technology, 166, 749–760
Aljubouri, T.R.S., Al-Khafaji, F.M. and Al-Shuhaib, M.B.S., 2021. Relationship of some metabolic Hormones with Increased Live Body Weight of Male and Female of Karakul and Awassi Lambs. Basrah Journal of Agricultural Sciences, 34, 107–117
Aljubouri, T.R.S., Hassan, A.F. and Al-Shuhaib, M.B.S., 2021. Remarkable correlation of non-genetic factors with growth traits in two breeds of sheep. Iraqi Journal of Agricultural Sciences, 52, 309–314
Aljubouri, T.R.S., Hassan, A.F., Al-Shuhaib, M.B.S. and Mahyari, S.A., 2020. Association of GnRH1 Gene with Growth Traits in Two Breeds of Sheep. Agricultural Research, 10, 285–293
Badi, M.A., Al-Shuhaib, M.B.S., Aljubouri, T.R.S., Al-Thuwaini, T.M., Dawud, H.H., Hussein, T.H., Al-Nafii, A.T., Abdulmalek, D., Altamemi, M.K.A. and Fadhil, I.A., 2021. Rapid and optimized protocol for efficient PCR-SSCP genotyping for wide ranges of species. Biologia, 76, 2413–2420
Baneh, H. and Hafezian, S.H., 2009. Effects of environmental factors on growth traits in Ghezel sheep. African Journal of Biotechnology, 8, 2903–2907
Biernat, W., Kirsz, K., Szczesna, M. and Zieba, D.A., 2018. Resistin regulates reproductive hormone secretion from the ovine adenohypophysis depending on season. Domestic animal endocrinology, 65, 95–100
Byun, S.O., Fang, Q., Zhou, H. and Hickford, J.G.H., 2009. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Analytical biochemistry, 385, 174–175
Cao, Y., Yao, J., Sun, X., Liu, S. and Martin, G.B., 2021. Amino acids in the nutrition and production of sheep and goats. Amino Acids in Nutrition and Health, 1285, 63–79
Capriotti, E., Calabrese, R., Fariselli, P., Martelli, P.L., Altman, R.B. and Casadio, R., 2013. WS-SNPs&GO: a web server for predicting the deleterious effect of human protein variants using functional annotation. BMC genomics, 14, S6
Cheng, J., Cao, X., Hanif, Q., Pi, L., Hu, L., Huang, Y., Lan, X., Lei, C. and Chen, H., 2020. Integrating genome-wide CNVs into QTLs and high confidence GWAScore regions identified positional candidates for sheep economic traits. Frontiers in Genetics, 11, 569
Cieslak, J., Nowacka-Woszuk, J., Bartz, M., Fijak-Nowak, H., Grzes, M., Szydlowski, M. and Switonski, M., 2009. Association studies on the porcine RETN, UCP1, UCP3 and ADRB3 genes polymorphism with fatness traits. Meat science, 83, 551–554
Dall’Aglio, C., Scocco, P., Maranesi, M., Petrucci, L., Acuti, G., De Felice, E. and Mercati, F., 2019. Immunohistochemical identification of resistin in the uterus of ewes subjected to different diets: Preliminary results. European Journal of Histochemistry: EJH, 63, 3020
Gao, L., Ujan, J.A., Zan, L., Xue, M. and Camus, A., 2011. A novel polymorphism of resistin gene and its association with meat quality traits in Chinese Bos taurus. African Journal of Biotechnology, 10, 1252–12256
Gootwine, E., 2020. Genetics and breeding of sheep and goats In:, Animal Agriculture, (Elsevier Inc.), Chapter 10, 183–198. https://doi.org/10.1016/b978-0-12-817052-6.00010-0
Gootwine, E., 2011. Mini review: breeding Awassi and Assaf sheep for diverse management conditions. Tropical animal health and production, 43, 1289–1296
Hickford, J.G.H., Forrest, R.H., Zhou, H., Fang, Q., Han, J., Frampton, C.M. and Horrell, A.L., 2010. Polymorphisms in the ovine myostatin gene (MSTN) and their association with growth and carcass traits in New Zealand Romney sheep. Animal genetics, 41, 64–72
Howe, K.L., Achuthan, P., Allen, James, Allen, Jamie, Alvarez-Jarreta, J., Amode, M.R., Armean, I.M., Azov, A.G., Bennett, R. and Bhai, J., 2021. Ensembl 2021. Nucleic acids research, 49, D884–D891
Kakavas, K. V, 2021. Sensitivity and applications of the PCR Single-Strand Conformation Polymorphism method. Molecular Biology Reports, 48, 3629–3635
Kalds, P., Zhou, S., Gao, Y., Cai, B., Huang, S., Chen, Y. and Wang, X., 2022. Genetics of the phenotypic evolution in sheep: a molecular look at diversity-driving genes. Genetics Selection Evolution, 54, 1–27
Khazaal, N.M., Alghetaa, H.F., Al-Shuhaib, M.B.S., Al-Thuwaini, T.M. and Alkhammas, A.H., 2022. A novel deleterious oxytocin variant is associated with the lower twinning ratio in Awassi ewes. Animal Biotechnology, In Press.https://doi.org/10.1080/10495398.2022.2152038
Koncagül, S., Vural, M.E., Karataş, A., Akca, N. and Bingöl, M., 2013. Reproductive performance of ewes and growth characteristics of lambs in Zom sheep reared in Karacadağ District. Kafkas Üniv Vet Fak Derg, 19, 63–68
Maillard, V., Froment, P., Ramé, C., Uzbekova, S., Elis, S. and Dupont, J., 2011. Expression and effect of resistin on bovine and rat granulosa cell steroidogenesis and proliferation. Reproduction, 141, 467–479
Malik, Z.S., Dalal, D.S., Dahiya, S.P., Patil, C.S. and Dahiya, R., 2016. Genetic analysis of growth traits in Harnali sheep. Veterinary World, 9, 128–132
Martinez-Gonzalez, L.J., Antúnez-Rodríguez, A., Vazquez-Alonso, F., Hernandez, A.F. and Alvarez-Cubero, M.J., 2020. Genetic variants in xenobiotic detoxification enzymes, antioxidant defenses and hormonal pathways as biomarkers of susceptibility to prostate cancer. Science of The Total Environment, 730, 138314
Mirderikvandi, M., Masoudi, A., Khaldari, M., Bojarpour, M. and Nazari, P., 2016. The effect of some factors on growth performance of Lori Bakhtiari lambs. Livestock Research for Rural Development, 28, 212
Mirhoseini, S.Z., Zare, J., Hossein-Zadeh, N.G., Khanzadeh, H., Seidavi, A., Laudadio, V., Dario, C., Tufarelli, V. and Selvaggi, M., 2015. Estimation of genetic parameters for body weight traits and pelt quality score in Iranian Karakul sheep. Small Ruminant Research, 132, 67–71
Mohammed, M.M., Al-Thuwaini, T.M. and Al-Shuhaib, M.B.S., 2022. A novel p. K116Q SNP in the OLR1 gene and its relation to fecundity in Awassi ewes. Theriogenology, 184, 185–190
Morash, B.A., Ur, E., Wiesner, G., Roy, J. and Wilkinson, M., 2004. Pituitary resistin gene expression: effects of age, gender and obesity. Neuroendocrinology, 79, 149–156
Nogueiras, R., Gualillo, O., Caminos, J.E., Casanueva, F.F. and Diéguez, C., 2003. Regulation of resistin by gonadal, thyroid hormone, and nutritional status. Obesity research, 11, 408–414
Pourlis, A.F., 2011. A review of morphological characteristics relating to the production and reproduction of fat-tailed sheep breeds. Tropical Animal Health and Production, 43, 1267–1287
Rak-Mardyła, A., Durak, M. and Łucja Gregoraszczuk, E., 2013. Effects of resistin on porcine ovarian follicle steroidogenesis in prepubertal animals: an in vitro study. Reproductive Biology and Endocrinology, 11, 45
Ramírez-Ramírez, A.I., Delgado-Tiburcio, G., Cruz-Espinoza, F., Corredor, A.C.H. and Gallegos-Sánchez, J., 2021. Photoperiod and its relationship to sheep reproduction. AGROProductividad, 14, 3–11
Ren, G., Chen, H., Zhang, L.Z., Lan, X.Y., Wei, T.B., Li, M.J., Jing, Y.J., Lei, C.Z. and Wang, J.Q., 2010. A coding SNP of LHX4 gene is associated with body weight and body length in bovine. Molecular biology reports, 37, 417–422
Sarmento-Cabral, A., Peinado, J.R., Halliday, L.C., Malagon, M.M., Castaño, J.P., Kineman, R.D. and Luque, R.M., 2017. Adipokines (leptin, adiponectin, resistin) differentially regulate all hormonal cell types in primary anterior pituitary cell cultures from two primate species. Scientific reports, 7, 43537
Shi, J., Fan, J., Su, Q. and Yang, Z., 2019. Cytokines and abnormal glucose and lipid metabolism. Frontiers in endocrinology, 10, 703
Tovar, S., Nogueiras, R., Tung, L.Y.C., Castaneda, T.R., Vázquez, M.J., Morris, A., Williams, L.M., Dickson, S.L. and Diéguez, C., 2005. Central administration of resistin promotes short-term satiety in rats. European Journal of Endocrinology, 153, R1–R5
Vaughn, S.E., 2012. Review of the third edition of the guide for the care and use of agricultural animals in research and teaching. Journal of the American Association for Laboratory Animal Science, 51, 298–300
Vignal, A., Milan, D., SanCristobal, M. and Eggen, A., 2002. A review on SNP and other types of molecular markers and their use in animal genetics. Genetics selection evolution, 34, 275–305
Ye, J., Coulouris, G., Zaretskaya, I., Cutcutache, I., Rozen, S. and Madden, T.L., 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC bioinformatics, 13, 134
Yu, J., Liu, L., Li, Z., Wang, Y., Zhang, W., Jin, Y., He, L., Chen, Y. and Yao, Y., 2021. Association of single nucleotide polymorphisms in ADIPOQ gene with risk of hypertension: a systematic review and meta-analysis International. Journal of Molecular Epidemiology and Genetics, 12, 90
Zhao, H., He, S., Zhu, Y., Cao, X., Luo, R., Cai, Y., Xu, H. and Sun, X., 2017. A novel 29 bp insertion/deletion (indel) variant of the LHX3 gene and its influence on growth traits in four sheep breeds of various fecundity. Archives Animal Breeding, 60, 79–85
Acknowledgements
The authors would like to thank the breeders of BAFAS Station, Al-Khafeel co., Karbala, Iraq for their support and help throughout all the experimental procedures of this study.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TRA; recorded growth traits, collected blood samples, extracted DNA, conducted PCR, performed PCR-SSCP genotyping, and performed statistical calculations. MBSA; designed PCR primers, analyzed data, and wrote the manuscript. Both authors approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aljubouri, T.R.S., Al-Shuhaib, M.B.S. The identification of a novel SNP in the resistin (RETN) gene associated with growth traits in Karakul and Awassi sheep. Trop Anim Health Prod 55, 165 (2023). https://doi.org/10.1007/s11250-023-03595-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-023-03595-3