Abstract
Limited information exists on the application of human chorionic gonadotropin (hCG) post insemination in the Indian crossbred dairy cows. This study aimed to evaluate the effect of four hCG administration protocols on luteal characteristics and pregnancy outcomes following artificial insemination (AI). Using block randomization, 50 healthy lactating crossbred cows were allocated in equal numbers (n = 10 cows per group) to receive either 5 mL normal saline (control) or 1500 IU hCG on the day of AI (hGG-0), day 7 post AI (hCG-7), day 14 post AI (hCG-14), or days 0, 7, and 14 post AI (hCG-0,7,14). All cows were scanned using sequential transrectal ultrasound examinations to evaluate primary luteal parameters, development of accessory corpora lutea, and pregnancy. Serial blood samples were collected to measure plasma progesterone concentrations. Data were analyzed using repeated measures ANOVA and Fisher’s exact tests. The mean primary luteal area, total luteal area, and total luteal diameter values were significantly greater in the hCG-treated cows. Compared to the control, the hCG-14 group had a significantly higher percentage of cows with an accessory corpora luteum. However, there were no significant differences in the mean progesterone concentrations or the first service conception rates between any of the groups. Overall, the results of this study indicate that while hCG administration post AI in healthy Indian crossbred cows may enhance primary luteal dimensions or induce the formation of accessory corpora lutea, it does not appear to have any beneficial effect on luteal function or pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gradual reduction in fertility of dairy cows observed over the last several decades has been attributed to a variety of reasons, including low progesterone concentrations in the first 3 weeks post insemination (Walsh et al. 2011). Elevated progesterone concentrations are associated with better conceptus elongation (Garrett et al. 1988; Carter et al. 2008), greater interferon-tau secretion (Mann and Lamming 1999, 2001), and increased pregnancy rates (Stronge et al. 2005; McNeill et al. 2006). Due to its luteotropic effect (Price and Webb 1989), human chorionic gonadotropin (hCG) has been used in multiple studies on purebred dairy cows to evaluate its effect on luteal function and pregnancy (Nascimento et al. 2013). Although the responses vary greatly between studies, hCG has been shown to promote primary luteal function, induce formation of accessory corpora lutea, and improve conception rates ( Rajamahendran and Sianangama 1992; Sianangama and Rajamahendran 1992; Beltran and Vasconcelos 2008; Nascimento et al. 2013). The variability may be partly due to different hCG doses or days of administration used in different studies and partly due to breed differences and other factors including parity, days in milk, and total milk production.
Crossbred cattle constitute about 26.6% of the total cattle population in India, as mentioned in the latest census report published by the Department of Animal Husbandry and Dairying in 2019. Information on the application of hCG post insemination in Indian crossbred dairy cows is limited to a couple of studies in repeat breeding cows (Pandey et al. 2016; Kumar et al. 2020). However, the effects of hCG administration have not been investigated in healthy Indian crossbred cows. The objective of this study was to evaluate luteal characteristics, plasma progesterone, and pregnancy in normal cyclic crossbred cows administered 1500 IU hCG on single or multiple specific days post insemination.
Materials and methods
All animal handling and experimental procedures used in this study were approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the Institutional Ethics Committee (IAEC/VGO/CVASc/181).
Animals and management
This study was conducted at the Instructional Dairy Farm (IDF), Nagla, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, India. Fifty healthy cyclic crossbred dairy cows [age 4 to 7 years, body condition score (BCS) between 3 and 4 on a scale of 1 to 5 in which 1 represents emaciated and 5 represents obese/extremely fat (Edmonson et al. 1989)] were enrolled in the study. The enrolled cows were at least 60 days in milk (DIM) and free of any apparent reproductive abnormalities detected through transrectal ultrasonography and the white side test (Bhat et al. 2014). All animals were kept under uniform housing and feeding management throughout the study.
Experimental design
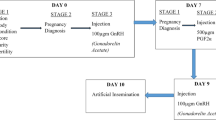
Cows were observed twice a day (morning and evening) for behavioral signs of standing estrus. Cows suspected to be in estrus were examined using transrectal ultrasonography and those with a preovulatory follicle (≥ 10-mm diameter) were bred twice through AI at 12 and 24 h after standing estrus. Using block randomization, the inseminated cows were allocated in equal numbers (n = 10 cows per group) to receive either 5 mL normal saline IM on days 0, 7, and 14 (control) or 1500 IU hCG (Chorulon®, MSD Animal Health, Pune, India) IM on the day of AI (hGG-0), day 7 post AI (hCG-7), day 14 post AI (hCG-14), or days 0, 7, and 14 post AI (hCG-0,7,14).
A portable ultrasound scanner (DIGI-600 M, PROVET; S.S. Medical Systems (I) Private Limited, India) equipped with a 5.0-MHz linear-array transducer was used for transrectal ultrasonography. All cows were examined on days 7, 14, 20, 25, 30, 35, 40, and 60 to evaluate primary luteal parameters (primary luteal diameter and primary luteal area) and development of accessory corpora lutea. Diameter and area of the primary and accessory corpora lutea were measured using the inbuilt measurement functions in the ultrasound machine. Total luteal diameter and total luteal area were calculated by adding the dimensions of primary corpus luteum and any accessory corpora lutea. Pregnancy diagnosis was performed on day 60. All ultrasound examinations were performed by the same operator.
Blood samples were collected through jugular venipuncture in heparinized test tubes on days 0, 1, 2, 7, 14, 20, 25, 30, 35, 40, and 60. Plasma was harvested by centrifugation at 3000 rpm for 15 min and stored at − 20 °C. Progesterone concentration was estimated using C.T. RIA kits (M/s Beckman Coulter IM 1188) with a highly specific progesterone antibody and analytical sensitivity of 0.03 ng/mL.
Statistical analysis
Statistical analysis of the data was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Data on continuous outcome variables (primary luteal diameter, primary luteal area, total luteal diameter, total luteal area, and plasma progesterone concentrations) were tested for normality through the Shapiro-Wilk test and plotted to identify any outliers before analyses. Differences in means of these variables between groups were evaluated for statistical significance through repeated measures ANOVA with least significant difference (LSD) post hoc tests. Differences in proportions of pregnant cows and cows with accessory corpus luteum between groups were tested for statistical significance using Fisher’s exact test. A P value of less than 0.05 was considered statistically significant. Results are presented as means ± SEM unless otherwise stated.
Results
Primary luteal diameter and area
Analysis of the data on primary luteal diameter revealed neither a group effect nor a group-by-day interaction (Fig. 1). In contrast, there was a group-by-day interaction (P < 0.05) for the primary luteal area. Compared to the control, a significantly higher mean primary luteal area was observed on day 20 post AI in the hCG-0 group; days 20, 40, and 60 post AI in the hCG-7 group; days 20 and 30 post AI in the hCG-14 group; and days 40 and 60 post AI in the hCG-0,7,14 group (Fig. 2).
Mean (± SEM) primary luteal area of control cows and cows treated with 1500 IU hCG on the day of AI (hGG-0), day 7 post AI (hCG-7), day 14 post AI (hCG-14), or days 0, 7, and 14 post AI (hCG-0,7,14). Asterisks indicate the days on which a significant difference was observed in the mean values between groups
Total luteal diameter and area
A group-by-day interaction (P < 0.05) was observed for both total luteal diameter (Fig. 3) and total luteal area (Fig. 4). Compared to the control, a significantly higher mean total luteal diameter was observed on days 14 and 20 post AI in the hCG-7 group, days 20 and 25 post AI in the hCG-14 group, and days 20, 25, 30, 35, 40, and 60 post AI in the hCG-0,7,14 group (Fig. 3). Similarly, compared to the control, a significantly higher mean total luteal diameter was observed on days 14, 20, 40, and 60 post AI in the hCG-7 group; days 20, 25, 30, and 60 post AI in the hCG-14 group; and days 20, 25, 30, 35, 40, and 60 post AI in the hCG-0,7,14 group (Fig. 4).
Mean (± SEM) total luteal diameter of control cows and cows treated with 1500 IU hCG on the day of AI (hGG-0), day 7 post AI (hCG-7), day 14 post AI (hCG-14), or days 0, 7, and 14 post AI (hCG-0,7,14). Asterisks indicate the days on which a significant difference was observed in the mean values between groups
Mean (± SEM) total luteal area of control cows and cows treated with 1500 IU hCG on the day of AI (hGG-0), day 7 post AI (hCG-7), day 14 post AI (hCG-14), or days 0, 7, and 14 post AI (hCG-0,7,14). Asterisks indicate the days on which a significant difference was observed in the mean values between groups
Plasma progesterone concentration
There was neither a group effect nor a group-by-day interaction for plasma progesterone concentration (Fig. 5).
Accessory corpus luteum
The proportions of cows with an accessory corpus luteum in each of the five groups are presented in Fig. 6. Compared to the control group, the proportion of cows with an accessory corpus luteum was significantly higher in the hCG-14 group.
Pregnancy
As shown in Fig. 7, there were numerical differences in the proportions of pregnant cows between the groups but these differences did not attain statistical significance.
Discussion
The results of this study suggest that hCG administration post insemination is effective in enhancing the development of primary corpus luteum and inducing accessory corpora lutea in healthy Indian crossbred cows. These findings are consistent with the reported luteinizing effects of hCG treatment in dairy cows, as reviewed previously (De Rensis et al. 2010). An increase in the primary and total luteal dimensions of cows has been reported after hCG administration on various days post AI (Rajamahendran and Sianangama 1992; Santos et al. 2001; Maillo et al. 2014). The stimulatory effect of hCG administration on the primary luteal structural characteristics could be attributed to its effects on the growth and transformation of small luteal cells into large luteal cells (Farin et al. 1988).
The higher mean total luteal diameter and area in the hCG-treated groups were partly due to the luteotropic action of hCG on the primary CL and partly due to the formation of accessory corpora lutea. Our findings are in agreement with the previous studies in dairy cows that reported accessory CL formation after hCG treatment (Price and Webb 1989; Rajamahendran and Sianangama 1992; Santos et al. 2001; Stevenson et al. 2007). The rate of accessory CL formation has been shown to be greater with hCG administration during the early luteal phase compared to the follicular and midluteal phases (Price and Webb 1989; Rajamahendran and Sianangama 1992). In the present study, the best response for accessory CL formation was observed in cows treated with hCG on day 14 post AI. This variation may be due to subtle differences in the follicular dynamics of Indian crossbred cows. Future studies in hCG-treated normal cyclic Indian crossbred cows are warranted to investigate the underlying follicular dynamics that might explain the variation in response.
In contrast to the stimulatory effect of hCG on primary CL and formation of accessory corpora lutea, none of the hCG treatments had any effect on the luteal function as assessed by measuring plasma concentrations. While a lack of hCG treatment effect on plasma progesterone concentration has been reported previously in dairy cows (Sianangama and Rajamahendran 1996), a vast majority of studies showed an increase in concentration after hCG treatment (Rajamahendran and Sianangama 1992; Sianangama and Rajamahendran 1992; Santos et al. 2001; Beltran and Vasconcelos 2008; Nascimento et al. 2013). Higher progesterone concentrations have also been reported in hCG-treated repeat breeding Indian crossbred cows (Pandey et al. 2016). Although the lack of higher progesterone concentrations in hCG-treated cows despite the increase in luteal dimensions is surprising, the finding may be explained by previously reported inconsistent relationships between luteal structural dimensions and plasma progesterone concentrations (Assey et al. 1993; Yung et al. 1996; Sartori et al. 2002).
Similar to the findings of two previous studies (Helmer and Britt 1986; Rajamahendran and Sianangama 1992), hCG treatment did not have a significant effect on pregnancy. In contrast, other studies have shown an increase in conception rate after hCG treatment (Sianangama and Rajamahendran 1992; Santos et al. 2001; Stevenson et al. 2007; Nascimento et al. 2013; Pandey et al. 2016). The absence of a statistically significant difference in first service conception rates between groups in the present study could be attributed to smaller sample sizes or a lack of requirement for additional luteotropic support in healthy animals.
In conclusion, the results of this study suggest that while hCG administration post AI in healthy Indian crossbred cows may enhance primary luteal dimensions or induce the formation of accessory corpora lutea, it does not appear to have any beneficial effect on luteal function or pregnancy. Further studies involving larger sample sizes are required to determine conclusively if hCG administration post AI has any beneficial effect on the reproductive performance of healthy Indian crossbred cows.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Assey, R.J., Purwantara, B., Greve, T., Hyttel, P. and Schmidt, M.H., 1993. Corpus luteum size and plasma progesterone levels in cattle after cloprostenol-induced luteolysis. Theriogenology, 39, 1321–1330
Beltran, M.P. and Vasconcelos, J.L.M., 2008. Conception rate in Holstein cows treated with GnRH or hCG on the fifth day post artificial insemination during summer. Arquivo Brasileiro de Medicina Veterinaria e Zootecnia, 60, 580–586
Bhat, F.A., Bhattacharyya, H.K. and Hussain, S.A., 2014. White side test: a simple and rapid test for evaluation of nonspecific bacterial genital infections of repeat breeding cattle. Veterinary research forum : an international quarterly journal, 5, 177–180
Carter, F., Forde, N., Duffy, P., Wade, M., Fair, T., Crowe, M.A., Evans, A.C.O., Kenny, D.A., Roche, J.F. and Lonergan, P., 2008. Effect of increasing progesterone concentration from day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reproduction, Fertility and Development, 20, 368–375
De Rensis, F., López-Gatius, F., García-Ispierto, I. and Techakumpu, M., 2010. Clinical use of human chorionic gonadotropin in dairy cows: an update. Theriogenology, 73, 1001–1008
Edmonson, A.J., Lean, I.J., Weaver, L.D., Farver, T. and Webster, G., 1989. A body condition scoring chart for Holstein dairy cows. Journal of Dairy Science, 72, 68–78
Farin, C.E., Moeller, C.L., Mayan, H., Gamboni, F., Sawyer, H.R. and Niswender, G.D., 1988. Effect of luteinizing hormone and human chorionic gonadotropin on cell populations in the ovine corpus luteum. Biology of Reproduction, 38, 413–421
Garrett, J.E., Geisert, R.D., Zavy, M.T. and Morgan, G.L., 1988. Evidence for maternal regulation of early conceptus growth and development in beef cattle. Journal of Reproduction and Fertility, 84, 437–446
Helmer, S.D. and Britt, J.H., 1986. Fertility of dairy cattle treated with human chorionic gonadotropin (hCG) to stimulate progesterone secretion. Theriogenology, 26, 683–695
Kumar, A., Kumar, S., Shivhare, M., Aich, R. and Nema, S.P., 2020. Comparative therapeutic efficacy of gonadotropin releasing hormone (GnRH), human chorionic gonadotropin (HCG) analogues and progesterone in non-infectious repeat breeding crossbred cows. International Journal of Current Microbiology and Applied Sciences, 9, 24–30.
Maillo, V., Duffy, P., O’Hara, L., De Frutos, C., Kelly, A.K., Lonergan, P. and Rizos, D., 2014. Effect of hCG administration during corpus luteum establishment on subsequent corpus luteum development and circulating progesterone concentrations in beef heifers. Reproduction, Fertility and Development, 26, 367–374
Mann, G.E. and Lamming, G.E., 1999. The influence of progesterone during early pregnancy in cattle. Reproduction in Domestic Animals, 34, 269–74
Mann, G.E. and Lamming, G.E., 2001. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction, 121, 175–180
McNeill, R.E., Diskin, M.G., Sreenan, J.M. and Morris, D.G., 2006. Associations between milk progesterone concentration on different days and with embryo survival during the early luteal phase in dairy cows. Theriogenology, 65, 1435–1441
Nascimento, A.B., Bender, R.W., Souza, A.H., Ayres, H., Araujo, R.R., Guenther, J.N., Sartori, R. and Wiltbank, M.C., 2013. Effect of treatment with human chorionic gonadotropin on day 5 after timed artificial insemination on fertility of lactating dairy cows. Journal of Dairy Science, 96, 2873–2882.
Pandey, N.K.J., Gupta, H.P., Prasad, S. and Sheetal, S.K., 2016. Plasma progesterone profile and conception rate following exogenous supplementation of gonadotropin-releasing hormone, human chorionic gonadotropin, and progesterone releasing intra-vaginal device in repeat-breeder crossbred cows. Veterinary World, 9, 559–562
Price, C.A. and Webb, R., 1989. Ovarian response to hCG treatment during the oestrus cycle in heifers. Journal of Reproduction and Fertility, 86, 303–308
Rajamahendran, R. and Sianangama, P.C., 1992. Effect of human chorionic gonadotrophin on dominant follicles in cows: formation of accessory corpora lutea, progesterone production and pregnancy rates. Journal of Reproduction and Fertility, 95, 577–584
Santos, J.E.P., Thatcher, W.W., Pool, L. and Overton, M.W., 2001. Effect of human chorionic gonadotropin on luteal function and reproductive performance of high-producing lactating Holstein dairy cows. Journal of Animal Science, 79, 2881–2894
Sartori, R., Rosa, G.J.M. and Wiltbank, M.C., 2002. Ovarian structures and circulating steroids in heifers and lactating cows in summer and lactating and dry cows in winter. Journal of Dairy Science, 85, 2813–2822
Sianangama, P.C. and Rajamahendran, R., 1992. Effect of human chorionic gonadotropin administered at specific times following breeding on milk progesterone and pregnancy in cows. Theriogenology, 38, 85–96
Sianangama, P.C. and Rajamahendran, R., 1996. Effect of hCG administration on day 7 of the estrous cycle on follicular dynamics and cycle length in cows. Theriogenology, 45, 583–92
Stevenson, J.S., Portaluppi, M.A., Tenhouse, D.E., Lloyd, A., Eborn, D.R., Kacuba, S. and DeJarnette, J.M., 2007. Interventions after artificial insemination: conception rates, pregnancy survival, and ovarian responses to gonadotropin-releasing hormone, human chorionic gonadotropin, and progesterone. Journal of Dairy Science, 90, 331–340
Stronge, A.J.H., Sreenan, J.M., Diskin, M.G., Mee, J.F., Kenny, D.A. and Morris, D.G., 2005. Post-insemination milk progesterone concentration and embryo survival in dairy cows. Theriogenology, 64, 1212–1224
Walsh, S.W., Williams, E.J. and Evans, A.C.O., 2011. A review of the causes of poor fertility in high milk producing dairy cows. Animal Reproduction Science, 123, 127–138
Yung, M.C., VandeHaar, M.J., Fogwell, R.L. and Sharma, B.K., 1996. Effect of energy balance and somatotropin on insulin-like growth factor I in serum and on weight and progesterone of corpus luteum in heifers. Journal of Animal Science, 74, 2239–2244
Acknowledgements
The authors are thankful to the staff of the Instructional Dairy Farm (IDF), Nagla, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, India for their assistance with the handling and restraint of the cows during this study.
Author information
Authors and Affiliations
Contributions
Sanjay Agarwal: conceptualization, methodology, investigation, writing—original draft preparation. Harihar Prasad Gupta: funding acquisition, project administration, supervision. Shiv Prasad: project administration, resources, supervision. Pawan Kumar Verma: data curation, formal analysis. Afroza Khanam: data curation, formal analysis, visualization. Firdous Ahmad Khan: conceptualization, formal analysis, software, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection Dairy Science and Health in the Tropics
Rights and permissions
About this article
Cite this article
Agarwal, S., Gupta, H.P., Prasad, S. et al. Effect of various hCG treatment protocols on luteal characteristics, plasma progesterone concentration, and pregnancy in normal cyclic Indian crossbred dairy cows. Trop Anim Health Prod 53, 220 (2021). https://doi.org/10.1007/s11250-021-02665-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-021-02665-8