Abstract
The objective of this article was to investigate the efficiency of GnRH administrations at different time points after induced luteolysis on pregnancy rates in low-yielding subfertile cows. One thousand six hundred and ten healthy and subfertile dairy cows of different ages and races were used in this study. Cows were randomly divided into 4 groups. Estrus cycles were synchronized by two, with 11-day intervals, injections of the prostaglandin F2α-analogue (PG). The artificial inseminations (AIs) of all animals were achieved at the 72nd and 96th hours following the last PG injection. The animals in groups I (n 257), II (n 337), and III (n 675) were used for the administration of a single dose of GnRH at different time points. Accordingly, GnRH was applied at 48th, 64th, and 72nd hours following the last PG injection in groups I, II, and III, respectively. Group IV was accepted as a control without GnRH injection (n 341). The pregnancy rates in groups I, II, III, and IV after transrectal pregnancy examinations were found to be 89.88%, 91.09%, 83.25%, and 77.12%, respectively. In our study, maximal pregnancy rates could be obtained with GnRH injections performed at 48th and 64th hours following luteolysis induction (P < 0.001). There was a 6–8% decrease in pregnancy rates due to the injection of GnRH in the 72nd hour (P < 0.001). These dramatic losses and gains in pregnancy rates in our study emphasized the necessity of taking the time of injection into account when using GnRH to stimulate ovulation. It can be said that the success of GnRH stimulation of ovulation is directly related to the follicle wave dynamics at the time of injection point and the character of a dominant follicle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
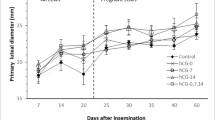
An important indication of gonadotropin-releasing hormone (GnRH) in dairy cows, within the scope of stimulating ovulation with artificial insemination (AI), is to increase the chances of pregnancy (Thatcher et al. 1993). However, it can be seen that pretty different results can be obtained in studies of this type of use of GnRH (Wiltbank and Pursley 2014). This issue is being debated in many ways today and remains a focus of interest, particularly where fertility losses may occur, depending on whether GnRH injections are made at the appropriate time. The success of GnRH in stimulating ovulation is directly related to the diameter of the dominant follicle (DF). Studies in cattle have shown that ovulation of small-diameter DFs may result in lower pregnancy rates than large DFs. This is assumed to be due to the lack of oocyte maturation due to GnRH injection, which was not performed at the appropriate time. Follicle diameter plays a key role in the success of GnRH stimulation. Failure to control this follicle diameter may adversely affect the pregnancy rates (Kastelic and Mapletoft 1998). Since the DF diameter has been proven to be different in both cows and heifers, also in two or three wave cycles (Sirois and Fortune 1988; Ireland et al. 2000), it is not always possible to control the size of the DF according to GnRH stimulation. Moreover, the fact that luteolysis is either natural (Ireland et al. 2000; Savio et al. 1993; Knopf et al. 1989; Ginther et al. Ginther et al. 1989a, 1989b) or induced (Kastelic et al. 1990; Kastelic and Ginther 1991) affects the diameter of the DF. In this case, GnRH injection time will be of critical importance by controlling the ovarian functions and positively contributing to pregnancy rates. For example, significant reductions in pregnancy rates have been observed (Schmitt et al. 1996) due to the shortening of the estrus cycle (Stevens et al. 1993) as a result of GnRH stimulation following 24 h of prostaglandin (PG) administration. This phenomenon has been observed after PG injections during the dominant period of the first follicular wave (Taponen 2003). Therefore, the objective of this study was to investigate the efficiency of GnRH administrations at different time points after induced luteolysis on pregnancy rates in low-yielding subfertile local cows.
Materials and methods
Animals and management
The study was carried out under field conditions, during the artificial insemination (AI) examinations (between February and May), in the central villages of Van Province (Turkey) (38.5972516, 43.3894878). One thousand six hundred and ten healthy and subfertile (this concept will be described in detail below) lactating local cows of different ages (3–6 years) and races (710 native black cattle, 376 south-eastern Anatolian red cattle, 188 Swiss brown, and 336 cattle from different crossbreeds) were used, cows which had given birth at least one time before. Average daily milk yield was 7.2 ± 3.5 kg and the animals were 109 ± 26 days (mean ±S.D.) in lactation. Animals used in this study belonged to various small and poor family farms. The number of animals ranged from 2 to 11. In these groups, 2–5 of the animals were dairy cows. There was no animal registry system. These animals were housed in stalls during the year from October to May. During this period, there was no ration that was arranged according to the needs of the feeding of animals. The animals were fed with inadequate and low-quality straw, barley, and clover. From June to September, the animals were grazed on very low-quality grasslands in the region, so all the animals had various degrees of protein, energy, and mineral deficiency. The stalls where the animals housed were generally too narrow and too dark. Also, milking was done by hand.
Reproductive state of the animals
According to the general examinations of all animals, there were no health problems in all animals. The most recent births of these animals were normal and carried no problems. No problems were encountered during the postpartum periods. However, there were two reproductive problems according to both the report of farmers and our examination of the genital organs, which are listed below:
- 1.
The animals whose external heat symptoms cannot be observed by farmers: in the rectal ultrasonographic examinations of these animals, two different groups were determined according to the ovarian findings: (1) small ovaries and ovaries without functional structures (like corpus luteum (CL) and Graaf follicle) (animals with inactive ovarian) and (2) ovaries with an active CL (animals with subestrus or silent heat).
- 2.
The animals whose external heat symptoms can be observed by farmers: however, despite the correct timing of single dose AI (without ovulation control), pregnancy could not be achieved in these animals. In the rectal ultrasonographic examinations of these animals, no abnormalities were found in the genital system (animals with probable ovulation problems).
Experimental design
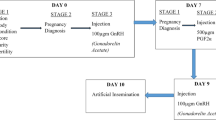
Cows were divided into 4 groups. Groups were formed according to the number of animals examined within the regions visited. In this way, homogenous injection groups were formed in small family farms close to each other. Estrus cycles (regardless of the presence of Cl in the ovary) were synchronized by two, with 11-day intervals, injections of the Prostaglandin F2α-analogue (PG, 25 mg dinoprost tromethamine, Dinolytic®, Zoetis). The AI (Specifications Semen; motility is at least 70%, density 20 × 106/dose, dead live rate 5%, abnormal sperm rate 3%.) of all animals, without estrus observations, was performed at the 72nd and 96th hours following the last PG injection. The animals in groups I (n 257), II (n 337), and III (n 675) were used for the administration of single-dose GnRH (100 mcg buserelin acetate, Ovarelin®, Ceva). Accordingly, GnRH was applied at 48th, 64th, and 72nd hours following the last PG injection. Group IV was the control without GnRH injection (n 341). Pregnancy examination by the transrectal ultrasonography was performed once between the days 30 and 40 after AI.
Statistical analysis
Descriptive statistics for the continuous variables were presented as mean, standard deviation, and minimum and maximum values while count and percentages were representing for categorical variables. Either Z test or Chi-square test was used to compare pregnancy rates of the groups. In addition, logistic regression analysis was also performed to determine the relationships between pregnancy rate and the explanatory variables of age, parity, and milk yield. The statistical significance level considered was 5% and SPSS statistical program was used for all statistical computations.
Results
The transrectal ultrasonographic results between 30 and 40 days after AI and the statistical comparisons of these values are summarized in Tables 1 and 2. The pregnancy rates for groups I, II, III, and VI were 89.88%, 91.09%, 83.25%, and 77.12% respectively. The results in the groups showed that pregnancy rates in subfertile dairy cows could be increased by 6–14% with GnRH treatments. And also, the different pregnancy rates obtained proved that the injection time points of GnRH were also very effective in stimulating ovulation following luteolysis (P < 0.001). Our study showed that there may be a 2–8% (P < 0.05) reduction in pregnancy rates due to injections of GnRH at different time points to stimulation of the ovulation after PG-induced luteolysis. In this study, maximal pregnancy rates could be obtained with GnRH injections at the 48th and 64th hours following the luteolysis (P < 0.001). GnRH injections at the 72nd hour (during AI) resulted in a 6–8% reduction in pregnancy rates (P < 0.001). These dramatic losses and gains in pregnancy rates emphasized the necessity of taking the timing of injection point into account when using GnRH to stimulate ovulation. According to the logistic regression analysis, age, parity, and milk yield of the animal variables did not have an extra effect on pregnancy rates (Table 2).
Discussion
Estrus and ovulation following the PG-induced luteolysis in healthy cattle have been widely reported in the literature (Silvia et al. 1991; Skarzynski et al. 2008; Ginther et al. 2009). In the present study, luteolysis was successfully achieved in subfertile cows by the use of PG injections with 11-day intervals. With the removal of progesterone influence, animals could be clinically observed to enter the follicular phase (Xu et al. 1997). The current study showed that luteal structures formed by spontaneous ovulations are highly sensitive to PG, despite the lack of coordination between the hypothalamus and pituitary in subfertile cows. However, our results also revealed the need for GnRH support after luteolysis for optimal ovulation and pregnancy rates (P < 0.001). In double-dose PG-induced cows, low progesterone levels prior to the second PG injection after luteolysis are responsible for fertility losses due to inadequate (Savio et al. 1993; Sirois and Fortune 1990; Stock and Fortune 1993) spontaneous preovulatory luteinising hormone (LH) release (Xu et al. 1997). In the present study, the proposed GnRH injection following PG-induced luteolysis may be a prophylactic intervention against this loss. In addition, double-dose PG + GnRH synchronization may be a significant alternative to the extremely high pregnancy rates (80–90%) obtained in the experimental groups like a much quite popular Ovsynch protocol which has been used twice as GnRH (Pursley et al. 1995).
Ovulation is probably the most important and critical stage of reproductive physiology in mammals. LH plays a key role in initiating this process. LH leads to the ovulation and causes ovulatory follicle lutealization (Algire et al. 1992). GnRH is a decapeptide that regulates both LH and FSH release in most mammals (Thatcher et al. 1993; Schally et al. 1971). Induction of ovulation of the DF is possible (Silcox et al. 1993) by the use of either exogenous GnRH or its agonists and it was reported that ovulation occurred between 24 and 32 h after GnRH injection in a synchronization protocol (Pursley et al. 1995). The concentration of FSH and LH in the blood remains elevated for 3–5 h upon injection of exogenous GnRH or its agonists (Juengel et al. 2000). In this way, ovulation in cattle can be controlled by stimulating them with GnRH. We also compared the pregnancy rates of GnRH administrations at different time points after induced luteolysis in this study. Our goal here was to determine the more appropriate injection time point, for maximal pregnancy rate, of the GnRH. We especially preferred subfertile animals as materials because the role of GnRH in indirectly altering the rate or efficiency of oocyte maturation in low-fertility cows warrants study (Thatcher et al. 1993).
In this study, it was observed that GnRH contributed significantly to pregnancy rates by stimulating ovulation in subfertile cows (P < 0.001). This result indirectly draws attention to the presence of some defects in the mechanism of ovulation in subfertile cows, the inability to release spontaneous GnRH. To our knowledge, inadequate secretion of GnRH from the hypothalamus in dairy cows is mainly due to major diseases, stress, and inadequate nutrition. In addition, significant imbalances in the times that animals are exposed to light and darkness also caused the inadequate release of GnRH. Indeed, this animal population used in our research was housed in dark stalls for about 6 months depending on the climatic conditions of the Van Province–Turkey. These animals also had important nutritional problems.
This study showed that the pregnancy rates can be increased by 6–14% with the support of GnRH in the animals hosted in such climate and maintenance conditions. The current study, however, also proved that the injection time of GnRH in inducing ovulation following luteolysis was also very effective, except the embryo losses, in pregnancy rates (P < 0.001). Our study showed that there may be a 2–8% (P < 0.05) reduction in pregnancy rates due to injections of GnRH at different times to stimulation of the ovulation following PG-induced luteolysis. In this study, maximal pregnancy rates could be obtained with GnRH injections performed at 48th and 64th hours, especially after luteolysis induction (P < 0.001). GnRH injections at the 72 h (during AI) resulted in a 6–8% reduction in pregnancy rates (P < 0.001). These dramatic losses and gains in pregnancy rates in our study emphasized the necessity of taking the time of injection point into account when using GnRH to stimulate ovulation. It can be said that the success of GnRH stimulation of ovulation is directly related to the follicle wave dynamics at the time of injection point and the character of the dominant follicle. Administration of GnRH during the bovine estrous cycle causes regression or ovulation of the dominant follicle and initiates the emergence of a new wave of follicular growth within two to 3 days following treatment (Silcox et al. 1993; Kohram et al. 1998). Atresia or ovulation of the dominant follicle depends on the status of the dominant follicle (growing, static, or regressing) at the time of GnRH injection (Twagiramungu et al. 1994; Dirandeh et al. 2009). The reaction during the injection of follicle against GnRH injection can be explained by the LH receptor capacity. There are no LH receptors expressed by granulosa cells in the first 2 days of a follicular wave. Follicles acquire LH receptors during the deviation process (Ginther et al. 1996) that are required for ovulation (Xu et al. 1997).
Oocytes that are ovulated with GnRH injection but are not mature for fertilization are still in our minds as the factors that can cause lower pregnancy rates in all experimental groups. Our study presented in this case would argue that the success of exogenous GnRH should be based on pregnancy outcomes rather than ovulation. This can be supported by Pursley et al. (1995)‘s study. These investigators detected the presence of ovulation by GnRH injections performed after 48 h in each of the 20 animals (100%), but the pregnancy rates were limited to only 50%. In this study (Pursley et al. 1995), it cannot be said that a 50% reduction in the pregnancy rate is due to definitely immature oocytes. However, no doubt, “the oocytes were all mature and the cause of pregnancy rate losses was 100% of other poly-factors” would also not reflect the truth.
It should be noted that geologic localization, the physiological status of the animal, race, nutrition, herding management, and general herd fertility can also be influential in the significant variations in success rates achieved with GnRH-supported studies under the AI protocol (Morgan and Lean 1993).
As a result
Studies have shown that there are strong relationships among the fertility in milk cows and physiological life span and dominance of the follicle. In our study results, with different and important pregnancy rates, we also estimate that it is effective in determining the ovulation and pregnancy rates of the dominant follicle developmental stage at the time of GnRH injection, following the PG-induced luteolysis. Our study suggests that breeders may be advanced in order to obtain pregnancies of over 90% by the single injection of GnRH (100 mcg buserelin) at the 64th hour following a double dose of PG-induced luteolysis in cows. This rate is particularly satisfactory for subfertile animals. However, it should not be forgotten that the different results (higher or lower pregnancy rates) obtained in similar applications in the field may be due to the specific character of the follicular wave number and the preovulatory dominant follicle.
Since our present study was carried out on a large animal population spread over different regions in the field, the functional structures of both hormones and ovaries before and after GnRH injection could not be investigated with ultrasound examination. In the future, similar GnRH studies with endocrinological and ultrasonography-assisted studies may provide quantitative benefits as well as clinical findings in order to understand the highly complex mechanism of ovulation. Considering the harsh climate conditions of our study, the long-lasting winter might have an effect on the different results of subfertile animals while the temperature stress will be of another interest for future studies. However, it should not be forgotten that in vitro production and transfer of immature oocytes obtained by follicular aspiration may be an alternative solution in cases where the desired fertility values are not achieved with ovulation induced by GnRH injection.
References
Algire, J.E., Srikandakumar, A., Guilbault, L.A., Downey, B.R., 1992. Preovulatory changes in follicular prostaglandins and their role in ovulation in cattle. Canadian Journal of Veterinary Research; 56(1): 67. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1263505/. Accessed 04/08/2019
Dirandeh, E., Kohram, H., Shahneh, A. Z., 2009. GnRH injection before artificial insemination (AI) alters follicle dynamics in Iranian Holstein cows. African Journal of Biotechnology; 8 (15), pp. 3672–3676. https://academicjournals.org/journal/AJB/article-abstract/50718947153. Accessed 04/08/2019
Ginther, O.J., Kastelic, J.P., Knopf, L., 1989a. Composition and characteristics of follicular waves during the bovine estrous cycle. Animal Reproduction Science; 20: 187–200. https://doi.org/10.1016/0378-4320(89)90084-5
Ginther, O.J., Knopf, L., Kastelic, J.P., 1989b. Temporal associations among ovarian events in cattle during estrous cycles with two and three follicular waves. Journal of Reproduction and Fertility; 87: 223–30. https://www.ncbi.nlm.nih.gov/pubmed/2621698. Accessed 04/08/2019
Ginther, O.J., Wiltbank, M.C., Fricke, P.M., Gibbons, J.R., Kot, K., 1996. Selection of the dominant follicle in cattle. Biology of Reproduction; 55:1187–94. https://doi.org/10.1095/biolreprod55.6.1187
Ginther, O., Araujo, R.M., Palhao, M., Rodrigues, B., Beg, M., 2009. Necessity of sequential pulses of prostaglandin F2alpha for complete physiologic luteolysis in cattle, Biology of Reproduction; 80:641–48. https://doi.org/10.1095/biolreprod.108.072769
Ireland, J.J., Mihm, M., Austin, E., Diskin, M.G., Roche, J.F., 2000. Historical perspective of turnover of dominant follicles during the bovine estrous cycle: key concepts, studies, advancements, and terms. Journal of Dairy Science; 83(7):1648–58. https://doi.org/10.3168/jds.S0022-0302(00)75033-8
Juengel, J.L., Haworth, J.D., Rollyson, M.K., Silva, P.J., Sawyer, H.R. and Niswender, G.D., 2000. Effect of dose of prostaglandin F2α on steroidogenic components and oligonucleosomes in ovine luteal tissue. Biology of Reproduction; 62(4): 1047–51. https://doi.org/10.1095/biolreprod62.4.1047
Kastelic, J.P. and Ginther, O.J., 1991. Factors affecting the origin of the ovulatory follicle in heifers with induced luteolysis. Animal Reproduction Science; 26: 13–24. https://doi.org/10.1016/0378-4320(91)90062-5
Kastelic, J.P., Mapletoft, R.J., 1998. Ovarian follicular responses in dairy cows treated with GnRH and cloprostenol. The Canadian Veterinary Journal; 39:107–109. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1539901/pdf/canvetj00148-0049.pdf. Accessed 04/08/2019
Kastelic, J.P., Knopf, L., Ginther, O.J., 1990. Effect of day of prostaglandin F2alpha treatment on selection and development of the ovulatory follicle in heifers. Animal Reproduction Science; 23: 169–180. https://doi.org/10.1016/0378-4320(90)90001-V
Knopf, L., Kastelic, J.P., Schallenberger, E., Ginther, O.J., 1989. Ovarian follicular dynamics in heifers: a test of the two-wave hypothesis by ultrasonically monitoring individual follicles. Domestic Animal Endocrinology; 6: 111–9. https://doi.org/10.1016/0739-7240(89)90040-4
Kohram, H., Bousquet, D., Durocher, J., Guilbault, L.A., 1998. Alteration of follicular dynamics and superovulatory responses by gonadotropin releasing hormone and follicular puncture in cattle: a field trial. Theriogenology; 49(6):1165–74. https://doi.org/10.1016/S0093-691X(98)00064-8
Morgan, W.F., Lean, I.J., 1993. Gonadotrophin-releasing hormone treatment in cattle: a metaanalysis of the effects on conception at the time of insemination. Australian Veterinary Journal; 70: 205–9. https://doi.org/10.1111/j.1751-0813.1993.tb03304.x
Pursley, J.R., Mee, M.O., Wiltbank, M.C., 1995. Synchronization of ovulation in dairy cows using PGF2x and GnRH. Theriogenology; 44:915–23. https://doi.org/10.1016/0093-691X(95)00279-H
Savio, J.D., Thatcher, W.W., Morris, G.R., Entwistle, K., Drost, M., Mattiacci, M.R., 1993. Effects of induction of low plasma progesterone concentrations with a progesterone-releasing intravaginal device on follicular turnover and fertility in cattle. Journal of Reproduction and Fertility; 98:77–84. https://www.ncbi.nlm.nih.gov/pubmed/8345482. Accessed 04/08/2019
Schally, A.V., Arimura, A., Kastin, A.J., Matsuo, H., Baba, Y., Redding, T.W., Nair, R.M.G. and Debeljuk, L., 1971. Gonadotropin releasing hormone: One polypetide regulates secretion of luteinizing and follicle-stimulating hormones. Science; 173: 1036–8. https://doi.org/10.1126/science.173.4001.1036
Schmitt, E.J.P., Diaz, T., Drost, M., Thatcher, W.W., 1996. Use of a gonadotropin-releasing hormone agonist or human chorionic gonadotropin for timed insemination in cattle. Journal of Animal Science;74: 1084–91. https://doi.org/10.2527/1996.7451084x
Silcox, R.W., Powell, K.L., Kiser, T.E., 1993. Ability of dominant follicles (DF) to respond to exogenous GnRH administration is dependent on their stage of development. Journal of Animal Science;71(Suppl 1), 219.
Silvia, W., Lewis, G., McCracken, J., Thatcher, W., Wilson, L. Jr., 1991. Review: Hormonal regulation of uterine secretion of prostaglandin F2 alpha during luteolysis in ruminants. Biology of Reproduction; 45: 655–63. https://doi.org/10.1095/biolreprod45.5.655
Sirois, J, Fortune, J.E., 1988. Ovarian follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography. Biology of Reproduction; 39: 308–17. https://doi.org/10.1095/biolreprod39.2.308
Sirois, J. and Fortune, J.E., 1990. Lengthening the bovine estrous cycle with low levels of exogenous progesterone: a model for studying ovarian follicular dominance. Endocrinology; 127: 916–925. https://doi.org/10.1210/endo-127-2-916
Skarzynski, D., Ferreira-Dias, G., Okuda, K., 2008. Regulation of luteal function and corpus luteum regression in cows: hormonal control, immune mechanisms and intercellular communication. Reproduction in Domestic Animals; 43:57–65. https://doi.org/10.1111/j.1439-0531.2008.01143.x
Stevens, R.D., Seguin, B.E., Momont, H.W., 1993. Simultaneous injection of PGF2alpha and GnRH into diestrous dairy cows delays return to estrus. Theriogenology;39: 73–380. https://doi.org/10.1016/0093-691X(93)90380-N
Stock, A.E., Fortune, J.E., 1993. Ovarian follicular dominance in cattle: relationship between prolonged growth of the ovulatory follicle and endocrine parameters. Endocrinology; 132:1108–14. https://doi.org/10.1210/endo.132.3.8440173
Taponen, J., 2003. Ovarian function in dairy cattle after gonadotropin-releasing hormone treatments during perioestrus. Academic Dissertation, University of Helsinki, Finland. http://ethesis.helsinki.fi/julkaisut/ela/kliin/vk/taponen/ovarianf.pdf. Accessed 04/08/2019
Thatcher, W.W., Drost, M., Savio, J.D., Macmillan, K.L., Entwistle, K.W., Schmitt, E.J., de la Sota, R.L., Morris, G.R., 1993. New clinical uses of GnRH and its analogs in cattle. Animal Reproduction Science, 33:27–49. https://doi.org/10.1016/0378-4320(93)90105-Z
Twagiramungu, H., Guilbault, L.A., Proulx, J.G., Dufour, J.J., 1994. Influence of corpus luteum and induced ovulation on ovarian follicular dynamics in postpartum cyclic cows treated with buserelin and cloprostenol. J Anim Sci; 72(7): 1796–805. https://doi.org/10.2527/1994.7271796x
Wiltbank, M.C., Pursley, J.R., 2014. The cow as an induced ovulator: Timed AI after synchronization of ovulation. Theriogenology, 81(1): 170–185. https://doi.org/10.1016/j.theriogenology.2013.09.017
Xu, Z.Z., Burton, L.J., Macmillan, K.L., 1997. Reproductive performance of lactating dairy cows following estrus synchronization regimens with PGF2α and progesterone. Theriogenology; 47: 687–701. https://doi.org/10.1016/S0093-691X(97)00027-7
Acknowledgements
The authors would like to thank Prof. Dr. Opsomer (University of Gent) and Prof. Dr. Sıddık Keskin (University of Van YYÜ) for their contribution to the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uslu, B.A., Kocyigit, A., Sendag, S. et al. The effect of GnRH on the pregnancy ratio in low-yielding local race cows: comparison of different injection times. Trop Anim Health Prod 52, 497–502 (2020). https://doi.org/10.1007/s11250-019-02034-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-02034-6