Abstract
Epidemiological data on trypanosomosis and piroplasmosis of horses are lacking in southeastern Nigeria. The prevalence of trypanosome and piroplasm infections in horses and resistance profile of isolated trypanosomes to diminazene and isometamidium salts were investigated. For the cross-sectional study of horses billed for slaughter, 304 horses were randomly sampled. Approximately 2 ml of blood was collected into anticoagulant-treated bottles for haematocrit (HCT) determination, direct microscopic examinations, and rat inoculation. Gender, body condition scores (BCS), age groups, and body weights of sampled horses were noted. Two isolates of Trypanosoma brucei recovered from the cross-sectional study were profiled for resistance to isometamidium hydrochloride and diminazene diaceturate in 36 BALB/c mice. Standardized protocols were used (Eisler et al., Veterinary Parasitology 97:171–182, 2001). 19.1% of horses (95% confidence interval 14.7–23.5%) were positive for haemoparasite infections including Theileria equi (16.1%) and Babesia caballi (3.9%). Only two (0.66%) Trypanosoma brucei infections were seen, being from active cases. Associations between age or gender, and presence of haemoparasites were only random. Haemoparasite-infected horses had significantly (p < 0.05) lower mean HCT and body weights and poorer BCS. From resistance profiling, for each isolate, all mice in control groups were parasitaemic by day 6 post-inoculation, while mice in test groups remained aparasitaemic over 60-day observation period. The study showed the endemicity and weights of Trypanososma spp. and piroplasm infections and among horses within the area. Furthermore, circulating strains of Trypanosoma brucei in the area are still susceptible to isometamidium and diminazene salts in mice. The pharmacoepidemiological significances of these findings were discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The country-wide population of horses in Nigeria, as of 2014, comprising of several breeds, was estimated to be about 300,000 (Turaki et al. 2014); currently, the figure may have more than doubled. This horse population supports the nation’s equestrian life where they are aptly used for recreational sporting, traditional ceremonies, and biomedical research, as well as in military troops and police squads. As a secondary use, spent horses are consumed by humans as a source of animal protein mainly in the southern part of Nigeria (Ardo and Abubakar 2016). The geographical belt hosting the vectors of nagana (a form of equine trypanosomosis) and equine piroplasmosis traverses Nigeria; hence, both diseases pose an unforgiving health challenge to the horse population in the region and that is besides the economic loss to owners accruing from shortfall in productivity and expensive veterinary care.
The African animal trypanosomoses of horses, nagana, are mainly haemolymphatic and neuropathologic diseases caused by Trypanosoma vivax, Trypanosoma congolense, or Trypanosoma brucei. ‘Surra’ refers strictly to equine trypanosomosis caused by Trypanosoma evansi, which is majorly reported in Southern and Central America, while ‘dourine’ refers to sexually transmitted equine trypanosomosis caused by Trypanosoma equiperdum (Gizaw et al. 2017; Buscher et al. 2019). Nagana has similar clinical manifestations with trypanosomosis of other domestic species, including fluctuating pyrexia, anaemia, ventral oedema, and lymphadenopathy of superficial lymph nodes (Giordani et al. 2016; Nweze et al. 2017; Aregawi et al. 2019). Drug resistance is one of the major causes of treatment failures in trypanosomosis in domestic animal species, and the increasing number of cases of therapeutic failures following use of isometamidium or diminazene salts has been reported (Gall et al. 2004; Mungube et al. 2012; Moti et al. 2015; Okolo et al. 2019). Available trypanocides labelled for use in domestic animal species are over four decades old, and drug-resistant strains of Trypanosoma spp. continue to spread (Geerts et al. 2001; Giordani et al. 2016). Okolo et al. (2020) remarked that high cost of research and development, clinical trials, drug licencing, and unpredictability of returns on investment are major disincentives to the development of novel trypanocides by big pharmaceutical firms.
Equine piroplasmosis (equine malaria) is a tick-borne intracellular haemoparasitic infection of equids caused by Babesia caballi or Theileria equi or both, characterized by icterus, anaemia, fever, oedema, splenomegaly, and hepatomegaly (Wise et al. 2013; Sumbria et al. 2014). The disease is designated endemic in countries within several continents including Africa, Asia, Europe, and America where the tick vector thrives (Cantú-Martínez et al. 2012; Sumbria et al. 2014; Del Pino et al. 2016).
Though equine trypanosomosis and equine piroplasmosis are known to be endemic in Nigeria (Mshelia et al. 2016; Giordani et al. 2016; Onyiche et al. 2019) and could in fact be fatal when left untreated, reports on their presence, weights, and associated risk factors especially in regions within southeastern Nigeria are wanting. Buscher et al. (2019) emphasized that epidemiological data on equine trypanosomosis in certain regions are needed but are lacking. Such epidemiological variables, when evaluated at regular intervals, are essential for design, implementation, monitoring, and review of successful intervention strategies against equine piroplasmosis and equine trypanosomosis. In addition, monitoring the resistance of trypanosomes to commonly used trypanocides provides very useful pharmacoepidemiological information towards control of the disease. Our main aim, therefore, was to evaluate whether equine haemoparasites (causing piroplasmosis and nagana) are prevalent within the region or not, and to evaluate whether circulating strains of Trypanosoma spp. within the region have developed resistance to standard dosages of diminazene diaceturate and isometamidium hydrochloride in mice models.
Materials and methods
Study location
The sampling location for the cross-sectional study was the Obollo-Afor horse market. The Obollo-Afor horse market represents the largest concentration of equids within Enugu State, and probably southeastern Nigeria. It is located within Udenu Local Government Area of Enugu State, South Eastern Nigeria, with geographical coordinates 6.9153° N and 7.5139° E (Okoh et al. 2012). Laboratory analysis of samples, as well as the resistance profiling study, was carried out at the research laboratory of Department of Veterinary Medicine, University of Nigeria Nsukka.
Study design
Cross-sectional study design was used for the evaluation of prevalence rates of piroplasms and Trypanosoma spp. infections in the horses, while a randomized controlled experimental design was used to evaluate trypanocide resistance profile of isolated trypanosomes. For profiling of each isolate, the mice were randomly divided into three groups (n = 6). Groups A and B received 20 mg/kg diminazene diaceturate (Dimivet®, India) and 1 mg/kg isometamidium hydrochloride (Intromidium®, Netherlands), respectively, while group C was the infected control. Further details are described subsequently.
Determination of sample size and sampling procedure
The sample size was determined using the model described by Thrusfield (2007).
where n = required sample size, Pexp = expected prevalence, and d = desired absolute precision.
The following values were set for the parameters: confidence interval = 95%; absolute precision = 5%, and expected prevalence = 22% (Oladipo et al. 2015). Prevalence rates of 22% and 0.8% were previously reported for piroplasms (Oladipo et al. (2015) and Trypanosoma spp. infections (Ehizibolo et al. 2012) of horses in Nigeria, respectively. The larger prevalence rate (22%) was used as the expected prevalence rate for sample size calculation in this study so as to have a larger sample size and improved accuracy.
The sample size calculation gave a total of three hundred and four (304) horses. Horses billed for slaughter were sampled between February and September 2019. Simple random sampling technique was used to select a total of three hundred and four (304) horses for sampling. On each day of sampling, numbers were assigned to all the horses billed for slaughter. Non-repetitive random numbers generated using the ‘RANDBETWEEN’ function of computer software, Microsoft Excel (Microsoft Corporation, USA), were used for making random selection of 15 horses to be sampled on each particular day. About 2 ml of blood was collected from each subject into labelled EDTA-treated bottles via jugular venipuncture. The samples were transported within 1 h to the research laboratory of the Department of Veterinary Medicine for further processing. The heart girth and body length measured by means of a measuring tape were used for estimation of body weights of subjects by normogram method (Caroll and Huntington 1988). The body condition scores of the subjects were observed by the same clinician throughout the study. Standard scoring system was used (Caroll and Huntington 1988) where 0 represents very poor, 1 poor, 2 moderate, 3 good, 4 fat, and 5 very fat. The selected horses were grouped into age groups based on physical examination of the dentition as described in Jeffrey (1996). In addition, the gender of the horses was observed and noted.
Determination of haematocrit, inoculation into immunosuppressed rats, and screening for Trypanosoma spp. and equine piroplasms
Packed cell volumes of the horses were determined using micro-haematocrit method (Thrall and Weiser 2002). Also, 1.5 ml of blood samples from horses with lower haematocrits (< 26%) was inoculated intra-peritoneally into Sprague-Dawley rats within 1 h of collection and determination of haematocrit. This was warranted by the need to isolate live trypanosomes for resistance profiling and the need to increase the overall chances of detection of parasitaemia from the subjects with very low levels of Trypanosoma spp. infections. The rats were immunosuppressed with intraperitoneal cyclophosphamide injection at 30 mg/kg body weight 24 h before inoculation. The rats were monitored for parasitaemia from 24 h post-inoculation using wet mount technique (Woo 1969) for 20 days. The buffy coat technique was used to screen for the presence of Trypanosoma spp. in all the sampled horses. Briefly, the buffy coat layers of centrifuged micro-haematocrit tubes containing blood samples from subjects were carefully cut onto a clean microscopic slide and covered with a cover slip to make a wet mount preparation for examination under the light microscope. Examination for equine piroplasms was done by preparing a thin and thick blood smear of each sample which were air dried and thereafter stained using Giemsa stain. The stained slides were examined with oil immersion objective of the light microscope. Assessments for Trypanosoma spp. or piroplasm infections were carried out by separate observers blinded to clinical data, and positive cases were determined by examining the microscopic morphologies of the parasites as described in Taylor et al. (2016), Uilenberg (1998), and Soulsby (1982).
Resistance profiling of Trypanosoma brucei isolates
The two Trypanosoma spp. isolated from the cross-sectional study above were identified to be Trypanosoma brucei based on their microscopic morphology as described in Uilenberg (1998) and Soulsby (1982). Thirty-six BALB/c mice weighing between 25 and 30 g were used for trypanocide resistance profiling of two field isolates of Tryponosoma brucei from the cross-sectional study above. The mice were housed in fly proof cages and were offered proprietary animal feed and drinking water ad libitum.
The resistance profiles of the two isolates to diminazene diaceturate and isometamidium hydrochloride were evaluated using the single-dose test protocol described by Eisler et al. (2001). Briefly, following 2 weeks of acclimatization, mice in each group for each isolate were infected with approximately 1 × 105 Trypanosoma brucei harvested from two donor Sprague-Dawley rats infected with each of the isolates separately. The Trypanosoma brucei were suspended in phosphate-buffered saline and inoculated intra-peritoneally in the test mice. Twenty-four hours post-inoculation, groups A and B for each of the isolates were treated intra-peritoneally with diminazene diaceturate at 20 mg/kg and isometamidium hydrochloride at 1.0 mg/kg body weight, respectively, while group C for each isolate was left untreated. The parasitological monitoring of the test mice was done thrice a week for the first 2 weeks and subsequently once a week for 60 days. The number of days to first detection of parasitaemia in group C (infected control) for both isolates was noted; thereafter, the mice in the group were humanely sacrificed.
Data analysis and interpretation
Data generated from the cross-sectional study were analysed using descriptive statistics, and results were presented as percentages with 95% confidence interval of point estimates. Chi-square and Fisher’s exact tests were used to check for association between risk factors and the presence or absence of haemoparasites in horses. Student’s t test was used to analyse variation in mean haematocrit and body weight between infected and non-infected horses. Significant associations or differences were accepted at p < 0.05. Data generated from the resistance profile study was analysed using descriptive statistics and interpreted as described in Eisler et al. (2001).
Result
Prevalence and associated risk factors of haemoparasites of horses at Obollo-Afor
Following parasitological screening of the trade horses (N = 304), a total of 58 horses (19.1%; 95% confidence interval [CI] 14.7–23.5%) were positive for Trypanosoma spp. or piroplasm infection or both. The prevalence rates of the haemoparasites were as follows: Trypanosoma brucei 0.66% (95% CI − 0.3–1.6%), Theileria equi 16.1% (CI 11.9–20.3%), and Babesia caballi 3.9% (CI 1.8–6.1%). Mixed haemoparasite infections were present in only 5 horses (1.6%, 95% confidence interval 0.2–3.1%) (Fig. 1).
Of the male horses sampled (total number of male horses, n = 149), 18% harboured one form of haemoparasite or the other, while 20% of female horses examined (total number of female horses, n = 155) were positive for haemoparasite(s). Horses belonging to age groups 5–7 (n = 8), 8–11 (n = 219), and ≥ 12 (n = 77) years old had 25%, 17.8%, and 22% positive cases for haemoparasite infections, respectively (Table 1). Horse with body condition scores graded poor (n = 22), moderate (n = 72), and good (n = 210) had 13.6%, 9.7%, and 22.9% positive cases for haemoparasite infections, respectively (Fig. 2).
Furthermore, for horses infected with haemoparasites, there was no statistically significant association between the presence of the infection and gender (X2 = 0.073; p = 0.786) or age group (Fisher’s exact = 1.185; p = 0.587) (Table 1). Statistically significant associations were seen between body condition and presence of haemoparasite infection in the horses (Fisher’s exact = 6.476; p = 0.042) (Fig. 2). Horses without haemoparasite infections had significantly higher mean haematocrit values [t (df 302) = 4.641; p < 0.01] and mean body weight [t (df 302) = 2.39; p = 0.01] when compared to those of horses with haemoparasite infections (Table 2).
The in vivo resistance profiles of trypanosomes isolated from horses at Obollo-Afor to diminazene diaceturate and isometamidium hydrochloride
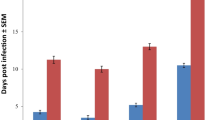
Two isolates of Trypanosoma brucei (isolates I and II) were recovered from the cross-sectional survey for haemoparasites in trade horses in Nsukka. Infections with Trypanosoma brucei, isolate I, established in 2 of 6 mice by day 4 post-inoculation, and by day 6 post-inoculation, all mice in the infected control (group C) were parasitaemic. Mice in groups A (infected + 20 mg/kg diminazene diaceturate) and B (infected + 1 mg/kg isometamidium chloride) were aparasitaemic over a 60-day observation period (Table 3). Infections with Trypanosoma brucei, isolate II, established in 4 of 6 mice by day 4 post-inoculation, and by day 6 post-inoculation, all mice in the infected control (group C) were parasitaemic. Likewise, mice in groups A (infected + 20 mg/kg diminazene diaceturate) and B (infected + 1 mg/kg isometamidium chloride) were aparasitaemic over a 60-day observation period (Table 4). Hence, the circulating strains of Trypanosoma brucei recovered from the horses were susceptible to treatments with either diminazene diaceturate or isometamidium hydrochloride at recommended dosages in mice models, considering that infection could not establish in all treated mice groups.
Discussion
Nearly one in every five horses sampled (N = 304) was infected by at least one of the haemoparasites examined for. The prevalence of Theileria equi infections (16.1%) was approximately four times higher than that of Babesia caballi (3.9%); a similar pattern has been reported by several workers in different countries where the disease is found (Sumbria et al. 2016; Mahmoud et al. 2016; Nugraha et al. 2018). Four of the horses examined had mixed infections involving B. caballi and T. equi; it has been established that concurrent infections by the two agents are not rare (Wise et al. 2013; Onyiche et al. 2019). Ixodid ticks and tsetse flies, which are vectors to equine piroplasms, are commonly found in the tropical rain forest of Nigeria, and their presence is strongly linked to the prevalence of equine piroplasmosis (Onyiche et al. 2019). Turaki et al. (2014) reported a combined prevalence of 41.7% for Babesia caballi and Theileria equi infection in horses within Borno, Gombe, and Taraba states of North Eastern Nigeria; this value is higher than the combined prevalence rate obtained in our study (20% for B. caballi and T. equi). Using direct microscopic technique, Mshelia et al. (2016) reported a prevalence rate of 10.3% and 2.7% for Theileria equi and Babesia caballi, respectively, in horses (n = 156) within Northern Nigeria from 2007 to 2010, whereas by using c-ELISA techniques, prevalence rates of 77.8% and 4.8% were reported for Theileria equi and Babesia caballi, respectively, in horses (n = 252) within the same region by 2011 (Mshelia et al. 2016). The prevalence rates found from our study are similar to those reported by Mshelia et al. (2016) using direct microscopy technique, but the prevalence of Theileria equi from their study, as evaluated using c-ELISA, was remarkably higher than the value reported in our study. Enzyme-linked immunosorbent assay has been adjudged to be an important tool for epidemiological monitoring of equine piroplasmosis. However, unlike direct microscopy technique, cross-reactivity, horses with ‘carrier’ status, and false positive results pose a problem to the accuracy of c-ELISA technique, with risk of elevating the number of positive cases (Onyiche et al. 2019).
Of the 304 horses examined, only two horses (0.66%) were infected by Trypanosoma spp., specifically Trypanosoma bruceis. The only two positive cases were from horses that appeared apparently ill, showing clinical signs of acute trypanosomosis including depression, prescapular lymphadenopathy, pale ocular mucous membrane, and mucopurulent ocular discharge.
The very low prevalence rate of 0.66% for nagana seen in this study may be due to low disease burden in the area or because parasitaemia in subjects were below the detection threshold for the parasitological techniques used. Simplicity and very high specificity are desirable features of the buffy coat examination technique; however, its lower sensitivity when compared to molecular techniques could have posed a diagnostic limitation in the current study. Compared to molecular techniques, rat inoculation remains a more useful diagnostic and parasite recovery technique especially when in vivo trypanocide resistance profiling is intended. In separate experiments, inoculation in rodents was reported to have sensitivities of 95% and 88% in detecting T. brucei and T. evansi infections (Luckins 1992); however, in the current study, this technique did not elicit any meaningful increase in the number of positive cases. Extended period of time between blood sample collection and inoculation in rodents can lead to negative results; this possible source of error was minimized in the current study by keeping the blood collection and inoculation time within 1 h. Furthermore, if the horses had low-grade infections with Trypanosoma vivax not detectable by direct microscopy, rat inoculation would have still failed to detect the infection bearing in mind that T. vivax does not survive in rodents (Uilenberg 1998). This is another limitation that may have contributed to the low prevalence rate observed for Trypanosoma spp. infection in the area.
The remarkably low prevalence rate (0.66%) of Trypanosoma spp. reported in this study is similar to values reported by Ehizibolo et al. (2012) who showed that out of 243 horses that were screened for the presence of haemoparasites in northern Nigeria, only two horses (one single and one mixed infection) were positive for Trypanosoma vivax, giving a prevalence rate of 0.82%. Nagana caused by Trypanosoma congolense or T. brucei are geographically restricted to tsetse flies zone within sub-Saharan Africa while nagana caused by T. vivax infections are found in Africa as well as Latin America (Buscher et al. 2019). Nagana is characterized by intermittent fever, anaemia, ventral oedema, emaciation, and sometimes, neurological disorders. The exact burden and distribution of acute nagana in horses would still remain vague because the disease is under-reported by African countries to the World Organization for Animal Health (OIE) (Giordani et al. 2016; Buscher et al. 2019).
There were no meaningful associations between sex or age groups and prevalence of the haemoparasites in the horses examined. This means that either male or female horses, or horses belonging to the described age groups (5–7, 8–11, and ≥ 12 years) could equally be infected with any of the haemoparasite(s). Young horses less than 9 months of age are usually immune to infectious challenge with Theileria equi or Babesia caballi owing to passive transfer of immunity from dam to foal via colostrum (Sumbria et al. 2014); hence, in nature, a meaningful association exists between age and presence of piroplasmosis, but in this study, such association must have been muffled by the preponderance of adult horses (over 95%) in the sample population. Data from the study also indicated that haemoparasitic infections were associated with poor body condition score; furthermore, horses infected with haemoparasites had significantly lower mean body weights when compared to the uninfected. Poor body condition, poor performance, and loss of body weight are classical signs of chronic equine piroplasmosis (Laus et al. 2015), and this may not be unconnected to the inappetence or anorexia that dominates the clinical picture of most animals suffering from the disease. Horses that were infected with haemoparasite(s) had significantly lower mean packed cell volumes when compared to uninfected horses. Piroplasmosis is characterized by haemolytic anaemia (Wise et al. 2013), while anaemia remains a cardinal feature of African animal trypanosomosis (Nweze et al. 2017; Okolo et al. 2019). This finding highlights the untoward effects of trypanosomosis or piroplasmosis on the haematopoietic system of equine patients.
The resistance profiling of two isolates of Trypanosoma brucei recovered from the cross-sectional study was carried out using the single-dose multi-drug test as described by Eisler et al. (2001). The two trypanocides used for the profiling remains the two most commonly used trypanocides for prophylaxis or treatment in Nigeria (Anene et al. 2001). Infection established successfully in all mice within the control groups of the isolates of Trypanosoma brucei profiled for resistance. This validated the test protocol (Eisler et al. 2001). Although by days 55 and 34 post-inoculation for isolates I and II, respectively, one mouse was lost to mechanical injury in different test groups, in each case bringing the number of test mice in the group to five, the resistance profiling was still valid provided the surviving five mice remained aparasitaemic for over 60 days (Eisler et al. 2001). The test result indicates that the circulating strains of Trypanosoma brucei implicated in nagana of horses within the area are still susceptible to the commonly used trypanocides (diminazene diaceturate and isometamidium hydrochloride) at recommended dosages in mice.
Multi-dose resistance profiling test was not necessary inasmuch as the two isolates were found susceptible using the single-dose multi-drug test. Resistance to trypanocides is an established problem in the chemotherapy of human and animal trypanosomosis (Delespaux and de Koning 2007; Giordani et al. 2016). Although the results from drug resistance test in mice models must be interpreted cautiously for domestic species, the test was developed to reflect the problem of drug resistance in the field (Eisler et al. 2001). The pharmacoepidemiological significance of the resistance profile observed in this study is the submission that chances are high that standard dosage of diminazene diaceturate (3.5–7.0 mg/kg) or isometamidium (0.5 mg/kg) used for prophylaxis or treatment of equine nagana are still effective in the area. The pathogenesis of nagana in horses may be such that favours little or no drug resistance selection pressure on offending trypanosomes, hence their susceptibility to standard dosages of trypanocides.
In conclusion, piroplasmosis and nagana are somewhat prevalent haemoparasitic disease of horses at Obollo-Afor southeastern Nigeria posing serious challenge to the health, welfare, and productivity of horses. Examinations for Trypanosoma spp. infections in horses using buffy coat examination and rat inoculation techniques showed a very low prevalence rate of 0.66%. Resistance profiling of two isolates of Trypanosoma brucei recovered showed susceptibility to diminazene diaceturate and isometamidium hydrochloride in mice. Measures to slow down or prevent possible development of drug resistance to trypanocides in future are therefore strongly recommended: these include prevention of unauthorized access to trypanocides, product quality control and regulations to avoid adulterations, avoiding the administration of sub-standard dosages of the drugs, ensuring only labelled use of trypanocides, pharmacoepidemiological monitoring, and vector control.
References
Anene, B.M., Onah D.N. and Nawa, Y., 2001. Drug resistance in pathogenic African trypanosomes: what hopes for the future? Veterinary Parasitology, 96, 83–100
Ardo, B. and Abubakar, D.M. 2016. Seroprevalence of horse Equus caballus brucellosis on the Mambilla plateau of Taraba State, Nigeria. Journal of Equine Science, 27, 1-6.
Aregawi, W.G., Agga, G.E., Abdi, R.D. and Büscher, P., 2019. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi, Parasite and Vectors, 12, 67-79
Buscher, P., Gonzatti, M.I., Hébert, L., Inoue N., Pascucci, I., Schnaufer, A., Suganuma, K., Touratier, L. and Van Reet, N., 2019. Equine trypanosomosis: enigmas and diagnostic challenges, Parasites and Vectors, 12, 234.
Cantú-Martínez, M.A., Segura-Correa, J.C., Silva-Páez, M.L., Avalos-Ramírez, R. and Wagner, G.G. 2012. Prevalence of antibodies to Theileria equi and Babesia caballi in horses from northeastern Mexico. Journal of Parasitology, 98, 869–870.
Caroll, C.L., and Huntington, P.J. (1988). Body condition scoring and weight estimation of horses. Equine Veterinary Journal 20(1): 41-45
Del Pino, L.E.B., Roberto, N., Vincenzo, V., Francesca, I., Antonella, C., Luca, A.G. and Teresa, S.M. , 2016. Babesia caballi and Theileria equi infections in horses in Central-Southern Italy: Sero-molecular survey and associated risk factors, Ticks and Tick-borne Diseases, 7, 462–469.
Delespaux, V. and de Koning, H.P., 2007. Drugs and drug resistance in African trypanosomiasis, Drug Resistance Updates, 10, 30–50.
Ehizibolo, D.O., Kamani, J., Ehizibolo, P.O., Egwu, K.O., Dogo, G.I. and Salami-shinaba, J.O., 2012. Prevalence and significance of parasites of horses in some states of northern Nigeria, Journal of Equine Science, 23, 1 – 4.
Eisler, M.C., Brandt, J., Bauer, B., Clausen, P.H., Delespaux, V., Holmes, P.H., Ilemobade, A., Machila, N., Mbwambo, H., McDermott, J., Mehlitz, D., Murilla, G., Ndungu, J.M., Peregrine, A.S., Sidibe, I., Sinyangwe, L. and Geerts, S., 2001. Standardised tests in mice and cattle for the detection of drug resistance in tsetse-transmitted trypanosomes of African domestic cattle, Veterinary Parasitology, 97, 171–182.
Gall, Y., Woitag, T., Bauer, B., Sidibe, I., McDermott, J., Mehlitz, D. and Clausen, P.H., 2004. Trypanocidal failure suggested by PCR results in cattle field samples, Acta Tropica 92, 7–16.
Geerts, S., Holmes, P. H., Eisler, M. C. and Diall, O., 2001. African bovine trypanosomiasis: the problem of drug resistance, Trends in Parasitology, 17, 25–28.
Giordani, F., Morrison, L.J., Rowan, T.G., De Koning, H.P. and Barrett, M.P., 2016. The animal trypanosomiasis and their chemotherapy: a review, Parasitology, 143, 1862–1889.
Gizaw, Y., Megersa, M. and Fayera, T., 2017. Dourine: a neglected disease of equids, Tropical Animal Health and Production, 49, 887-897.
Jeffrey, D., 1996. Horse dentistry, the theory and practice of equine dental maintenance, 2nd ed. (Norfolk printing company, Nebraska).
Laus, F., Spaterna, A., Faillace, V., Veronesi, F., Ravagnan, S., Beribé, F. and Tesei, B., 2015. Clinical investigation on Theileria equi and Babesia caballi infections in Italian donkeys, BMC Veterinary Research, 11, 100-107.
Luckins, A.G., 1992. Methods for diagnosis of trypanosomosis in livestock, World Animal Review, 71, 15-20.
Mahmoud, M.S.; El-Ezz, N.T.A.; Abdel-Shafy, S.; Nassar, S.A.; El Namaky, A.H., Khalil,W.K. and Suarez, C.E. 2016. Assessment of Theileria equi and Babesia caballi infections in equine populations in Egypt by molecular, serological and hematological approaches, Parasites and Vectors, 9, 260-267.
Moti, Y., De Deken, R., Thys, E., Van Den Abbeele, J., Duchateau, L. and Delespaux, V., 2015. PCR and microsatellite analysis of diminazene aceturate resistance of bovine trypanosomes correlated to knowledge, attitude and practice of livestock keepers in south-western Ethiopia. Acta Tropica 146, 45–52.
Mshelia, W.P., Sambo, K.W., Adamu, S., Edeh, E.E. and Onoja, I.I., 2016. Persistence of equine piroplasmosis in horses in Nigeria, Journal of Equine Veterinary Science, 39, S104-S105.
Mungube, E.O., Vitouley, H.S., Allegye-Cudjoe, E., Diall, O., Boucoum, Z., Diarra, B., Sanogo, Y., Randolph, T., Bauer, B., Zessin, K.H. and Clausen, P.H., 2012. Detection of multiple drug-resistant trypanosoma congolense populations in village cattle of south-east Mali, Parasite and Vectors, 5, 155-161.
NIH , 2011. Guide for the care and use of laboratory animals. (National Academy Press, Washington DC).
Nugraha, A.B., Cahyaningsih, U., Amrozi, A., Ridwan, Y., Agungpriyono, S., Taher, D.M. and Sivakumar, T., 2018. Serological and molecular prevalence of equine piroplasmosis in Western Java, Indonesia, Veterinary Parasitology: Regional Studies and Reports, 14, 1–6.
Nweze, N.E., Okoro, H.O., Robaian, M.A., Omar R.M.K., Tor-Anyiin, T.A., Watson D.G. and Igoli, J. O., 2017. Effects of Nigerian red propolis in rats infected with Trypanosoma brucei brucei, Comparative Clinical Pathology, 26, 1129-1133.
Okoh, D., Eze, A., Adedoja, O., Okere, B. and Okeke, P.N., 2012. A comparison of IRI-TEC predictions with GPS-TEC measurements over Nsukka, Nigeria, Space Weather, 10, 1-6.
Okolo, C.C., Ezeh, I.O., Uju, C.N. and Nweze, N.E., 2019. Combination of a probiotic mix and diminazene aceturate in treatment of Trypanosoma brucei infection in sprague dawley rats, Veterinary Sciences: Research and Reviews, 5, 43-52.
Okolo, C.C., Eze, J.I. and Nweze, N.E., 2020. Hematobiochemical and immunological responses of rats treated with multi-strain probiotics and infected with Trypanosoma brucei, Probiotics and Antimicrobial Proteins, 12, 952-960
Oladipo, T.A., Adekunle, O.F., Okuneye, O.J., Adebayo, M.D., Adeoye, A.T. and Banjoko, O. J, 2015. Pre and Post Polo Competition Prevalence of Equine Babesiosis in Stable Horses in Ibadan, Oyo state, Nigeria, International Journal of Research in Agriculture and Forestry, 2, 6-9.
Onyiche, T.E., Suganuma, K., Igarashi, Ikuo., Yokoyama, N., Xuan, X. and Thekisoe, O., 2019. A Review on Equine Piroplasmosis: Epidemiology, Vector Ecology, Risk Factors, Host Immunity, Diagnosis and Control, International Journal of Environmental Research and Public Health, 16, 1736-1759.
Soulsby, E.J.L., 1982. Helminthes, Arithropods and Protozoa of Domesticated Animals, 7th ed. (Bailliere Tindall, London).
Sumbria, D., Moudgil, A.D. and Singla, L.D., 2014. Equine Piroplasmosis: Current status, Veterinaria, 1, 9–14.
Sumbria, D., Singla, L.D. and Sharma, A., 2016. Theileria equi and Babesia caballi infection of equids in Punjab, India: a serological and molecular survey, Tropical Animal Health and Production, 48, 45-52.
Taylor, M.A., Coop, R.L. and Wall, R.L. 2016. Veterinary Parasitology, 4th ed. (Wiley-Blackwell, West Susssex).
Thrall, M. A. and Weiser, M. G., 2002. Haematology: Laboratory Procedures for Veterinary Technicians, 4th ed. (Mosby Incorporated, Missouri).
Thrusfield M., 2007. Veterinary Epidemiology, 2nd ed. (Blackwell, USA).
Turaki, U.A., Kumsha, H.A., Biu, A.A. and Bokko, P.B., 2014. Prevalence of piroplasmosis amongst local horses in Northeastern Nigeria, IOSR Journal of Agriculture and Veterinary Science, 7, 4-7.
Uilenberg, G., 1998. A field guide for the diagnosis, treatment, and prevention of African animal trypanosomosis. (FAO, Italy).
Wise, L.N., Kappmeyer, L.S., Mealey, R.H. and Knowles, D.P., 2013. Review of equine piroplasmosis, Journal of Veterinary Internal Medicine, 27, 1334–1346.
Woo, P.T.K., 1969. The haematocrit centrifuge for the detection of trypanosomes in blood, Canadian Journal of Zoology, 47, 921-923.
Acknowledgements
We are grateful to the staff of Veterinary Medicine and Veterinary Parasitology laboratories at the University of Nigeria for their technical support towards completing this work.
Funding
The research is a self-funded project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of animal rights
The study does not contain any animal or human clinical trial. Ethical considerations for the use of laboratory animal models in this study were based on procedures of the Animal Use and Care Committee of Faculty of Veterinary Medicine University of Nigeria, which agree with the National Institutes of Health (NIH) guidelines (NIH 2011).
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Emeto, U.E., Okolo, C.C. & Nweze, N.E. Occurrence of Trypanosoma spp. and piroplasm infections of horses at Obollo-Afor southeastern Nigeria and resistance profiles of trypanosomes to isometamidium and diminazene salts. Trop Anim Health Prod 52, 3745–3753 (2020). https://doi.org/10.1007/s11250-020-02412-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-020-02412-5