Abstract
African animal trypanosomosis (AAT) affects the livestock industry, impacting protein intake and herders’ livelihoods. Despite the presence of vector flies, livestock owners often rely solely on chemotherapy, with diminazene aceturate being a common choice, particularly among non-elite herders. Cattle herds were sampled during an outbreak and re-sampled 8 weeks post-treatment with diminazene aceturate (Nonazin®) in Ogun and Osun states, Nigeria. Initial sampling was conducted in October 2019 and January 2020 for farms A and B, respectively. A total of 316 cattle, comprising 20 males and 296 females, were sampled through convenient sampling of the entire herd. Nzi traps were deployed to capture Trypanosoma-transmitting vectors. Haematological parameters, microscopic, and molecular assessments were conducted. The total transmitting vectors captured averaged 3.74 fly/trap/day, with Stomoxys niger being the most captured biting fly. Post-treatment prevalence rates were 21.5% and 2.5% with PCR and thin blood smear, respectively, with Trypanosoma congolense and T. vivax being the species detected by both diagnostic methods. The average parasitaemia before treatment was recorded at 1.95 × 104 ± 1.3 × 104, signifying a high parasitic infection, while post-treatment parasitaemia was 1.5 × 102 ± 1.4 × 102. The PCV, Hb, and RBC counts decreased, causing macrocytic normocytic anaemia before treatment, while post-treatment values suggested lower haemoglobin and MCH values with less-characteristic anaemia. Post-treatment trypanosome prevalence suggests the existence of Trypanosoma-resistant strains, potentially widespread in southwest Nigeria. The situation may be exacerbated by the abundance of biting flies during the dry season, leading to a high T. vivax prevalence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trypanosomes, known as extracellular protozoan parasites affecting mammals, are caused by various Trypanosoma species and transmitted through insect vectors—either biologically (tsetse fly) or mechanically (reduviid, tabanids, and Stomoxys)—and even via coitus, depending on the specific organism involved. Common trypanosome species causing substantial issues in the livestock industry include Trypanosoma congolense, T. vivax, and T. brucei (Odeniran et al. 2019a, b, c).
In Africa, it has been estimated that around 50 million cattle and 70 million sheep and goats are at risk of contracting animal African trypanosomiasis, leading to an annual loss of three million cattle (Geerts et al. 2001). Controlling trypanosomosis involves managing either the vector or the parasite, or a combination of both. Currently, only a limited number of chemoprophylactic and chemotherapeutic trypanocidal compounds are in use, and the development of new compounds is not expected soon (Barrett et al. 2004). Holmes et al. (2004) approximated that 35 million doses of veterinary trypanocidal drugs are administered annually in Africa. Isometamidium chloride, ethidium bromide, and diminazene aceturate are estimated to represent 40%, 26%, and 33%, respectively, of the total trypanocidal drug market by value (Bengaly et al. 2018).
Various methodologies highlight substantial regional variations in resistance problems (Sinyangwe et al. 2004). Increased usage of substandard veterinary preparations has been identified as a risk factor for drug resistance (Teko-agbo et al. 2008). Chemotherapy appears to be the most prevalent measure in sub-Saharan Africa, with Kingsley (2015) reporting a concise survey on substandard veterinary drugs as a major contributor to drug resistance in Nigeria. Political instability, weak regulatory structures, and influential financial market forces have made combating counterfeit drugs a formidable task, leading to the persistent spread of resistant genes in organisms. There is a possibility that the transmitting vectors and reservoir hosts now carry Trypanosoma-resistant parasites that do not respond to conventional trypanocides, posing a significant threat to the livestock industry (Odeniran et al. 2019a).
The use of trypanocides for the control of trypanosomosis is well established, and the concept is widely disseminated. Nevertheless, studies have reported the potential for trypanocide resistance in regions with high tsetse fly populations, even when using standard trypanocide doses (Geerts and Holmes 1998). Similarly, recent studies have suggested the presence of high prevalence of Trypanosoma species in areas with low tsetse flies but high biting flies in southwest Nigeria (Odeniran and Ademola 2018; Odeniran et al. 2019c, 2020, 2021). Multiple drug resistance has been reported in Nigeria (Ilemobade 1979), while recent investigation on the presence of trypanocide resistance isolates has been reported (Odeniran and Ademola 2018). Novel studies are needed to explore the issue of AAT drug resistance in Nigeria. The problem of drug resistance for AAT has been attributed to weak legislation, which allows farmers and unskilled workers to administer trypanocides without consequences, rather than relying on veterinarians (Matovu et al. 1997). Therefore, this study investigates the efficacy of common trypanocide in the market, considering haematological parameters and other risk factors to ascertain the presence of resistant Trypanosoma species.
Materials and methods
Study area and sampling size
The research was carried out in two Nigerian states: Osun and Ogun. Both states are situated in the tropical rainforest zone in the southwest region of Nigeria, with geographic coordinates between latitude 07° 30′ N and longitude 4° 30′ E for Osun state and latitude 7° 00′ N and longitude 3° 35′ E for Ogun state. These locations experience two distinct seasons—the dry season (November to March) and the wet season (April to October). However, for this study, sample collection was limited to the dry season due to constraints imposed by the study design, specifically focusing on outbreaks identified on farms during that period. The cattle population in the region is approximately 400,000, with approximately 97% of the cattle being designated as trade cattle in markets or prepared for slaughter at abattoirs. The remaining 3% are in farm settlements, which are in the range of 12,000–15,000. Two cattle farms were selected conveniently due to reported cases of a trypanosome outbreak. Whole-herd sampling of cattle was carried out in cattle farms managed by veterinarians. The initial sampling took place during the trypanosome outbreak. For farm A (Ogun state), the sampling occurred in early October 2019, while for farm B (Osun state), the first sampling was performed in late January 2020. The second sampling for both farms occurred exactly 8 weeks after treatment with trypanocides, with farm A being sampled in early December and farm B in late March. The sample sizes were determined based on herd sizes, resulting in 201 and 115 samples for farms A and B, respectively, with a total sample size of 316 (Fig. 1).
Fly collection
A total of 16 sampling days were used for this study; in other words, 4 days/month of collection for 2 months/site. A total of eight Nzi traps (four per site) were used to capture Trypanosoma-transmitting vectors, and collection was done at 12 h interval to prevent mortality and desiccation of flies. Traps were site bound for 8 days during the experimental period (8 weeks)/farm location. All trapped flies were preserved in absolute ethanol and transported to the laboratory for assessment under the stereomicroscope. Morphological identification was done using identification keys (Austen 1912; Zumpt 1973), and species were compared with those deposited in Systema Directory.
Blood collection and assessment
Blood samples, measuring 5 ml, were obtained from the jugular vein of each cattle and placed into 5-ml tubes containing ethylene diamine tetra acetic acid (EDTA) as an anticoagulant (1 mg/ml of blood). Additionally, two drops were applied to Whatman FTA cards for molecular analysis. The samples in tubes were transported to the laboratory on ice packs. Haematological parameters were conducted using Veterinary Haematology Autoanalyzer specifically designed for animals (Vetscan® HM5 analyser). This provides information on the complete blood count pre- and post-treatment.
Microscopic examination of parasites
A drop of blood was dropped on glass slide and cover with a slip to examine under the light microscope. The presence or absence of parasites was observed carefully using different objective lens. Blood from each anticoagulant-containing tube was transferred into three capillary tubes, each sealed at one end with plasticine. These capillary tubes underwent centrifugation in a microhematocrit centrifuge at 3000 rpm for 5 min. After centrifugation, the packed cell volume (PCV) was determined using the haematocrit centrifugation technique. Subsequently, the buffy coat and the uppermost layer of red blood cells from one capillary tube were extruded onto a microscope slide and examined under a light microscope at 40 objective lens (×400 magnification) to identify the presence of motile trypanosomes. A blood drop from the 5-ml tubes with anticoagulant was applied to glass slides to create thin and thick smears. After air-drying, the slides were stained with Giemsa stain. Labelled with a lead pen, these stained slides were examined under a light microscope using a ×100 oil immersion objective lens (×1000 magnification) at the Department of Veterinary Parasitology and Entomology, University of Ibadan, Nigeria. Morphological keys for Trypanosoma species, as per FAO guidelines (2023), were applied to identify the observed Trypanosoma species.
Parasitaemia quantification
Blood films were evaluated for parasitaemia following a well-established method (Herbert and Lumsden 1976). Examination of the blood film was conducted under a ×40 objective lens, and parasite counts were determined using the anti-log of values, in accordance with the methodology.
Molecular assessment sampling technique
To identify the Trypanosoma species in bovine hosts, blood samples were collected from the middle ear vein by venipuncture. Blood spots containing 2 to 3 drops of blood were made on Whatman FTA™ cards (Sigma-Aldrich, USA) for DNA extraction. The cards were properly labelled with identification details of the animals, including age, sex, breed, body condition, and location. This is a longitudinal survey (sampling occurred during outbreak, and another sampling occurred after treatment with trypanocide). All cattle in the herd were sampled. Subsequently, the cards were air-dried and stored in sealed pouches (Sigma-Aldrich, USA) following the established procedure (Ahmed et al. 2011).
Extraction of DNA from FTA cards
A comprehensive four-stage extraction process was implemented, encompassing punching, washing, fishing, and eluting. All equipment, including punches, pipette tips, Eppendorf tubes, and PCR tubes, underwent sterilization through a 20-min exposure to ultraviolet rays. Sterilized hole punches were used to generate five discs (3 mm/disc) from each FTA card, depositing them into 1.5-ml Eppendorf tubes. Following the initial punching, an additional set of five discs was punched from a clean Whatman® filter paper to cleanse the punch before sampling from another card. Additionally, five discs from a plain Whatman® filter paper were punched to serve as a negative control. In the subsequent washing phase, 1 ml of FTA purification reagent was added to each Eppendorf tube for a 15-min interval on a rocking platform shaker. Subsequently, the FTA reagent was removed, and a fresh batch of FTA purification reagent was introduced to enhance nucleic acid purification on the cards. The punched discs were then transferred to PCR tubes for the fishing step, allowing the samples to incubate overnight at room temperature. For the elution stage, a 5% w/v Chelex solution was prepared, and 100 µl of this solution was added to the PCR tubes containing the discs. The tubes were then subjected to heating at 90 °C for 30 min in a Peltier thermal cycler.
Amplification of DNA using PCR
To amplify the DNA, PCR analysis targeting the internal transcribed spacer (ITS-1 rDNA) was performed. The master-mix was prepared, which included 1 µl of 50 mM MgCl2, 5 µl of 5 × Mango Taq buffer, 1 µl of both forward 5′-CCG-GAA-GTT-CAC-CGA-TAT-TG-3′ and reverse 5′-TTG-CTG-CGT-TCT-TCA-ACG-AA-3′ primers (as described by Njiru et al. 2005), 0.2 µl of 5 U/µl Taq DNA polymerase, 0.2 µl of 25 mM dNTPs, and 12.6 µl of distilled water. The PCR cycling was carried out using a Bio-Rad Dyad system (MJ Research Inc., USA). The total reaction volume was 25 µl, which included 5 µl of genomic DNA. The PCR cycling conditions involved an initial denaturation step at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 40 s, annealing at 58 °C for 40 s, extension at 72 °C for 90 s, and a final extension at 72 °C for 5 min. The expected base pair sizes, which varied depending on the species or subspecies, ranged from 250 to 700 bp.
Gel electrophoresis of amplified DNA from cattle blood
To examine the DNA amplified by PCR, agarose gel electrophoresis was conducted. A mixture of 1.5 g of agarose and 100 ml of 1 × Tris-borate-EDTA (TBE) buffer was prepared. The mixture was heated and gently agitated every 15 s in a Micro Chef SM 11 until a clear solution was achieved. The gel solution was poured into a gel container and allowed to solidify on a plate stirrer for 3 min. GelRed™ nucleic acid stain (10 µl) from Biotium Inc., USA, was added to the gel and thoroughly mixed. Gel combs were utilized to create wells in the gel, and 10 µl of the PCR amplicon from each sample was loaded into the wells. Additionally, an 8 µl portion of a 100-bp molecular ladder (exACTGene™, Fisher Scientific, UK) was added on either side of the gel to serve as a reference for determining PCR fragment sizes. The gel container was filled with 1 × TBE buffer, and electrophoresis was carried out at 100 V for 60 min. Subsequently, the gel was visualized under a UV transilluminator (Gel-Doc™ 2000) using the Quantity One software from Bio-Rad Laboratories, Inc.
Risk factor assessment
A total of 316 cattle blood samples were conveniently gathered, comprising 20 males and 296 females from diverse breeds, including Sokoto Gudali, White Fulani, N’dama, and Red Bororo. The samples were categorized based on the sampling location, and all collections took place during the dry season. The assessment of cattle body condition considered physical fitness and the presence or absence of clinical signs. Cattle were labelled as ‘good’ if they exhibited proper conformation, gait, and healthy skin without discharges. Those with mild clinical signs, unaffected nutrition, but good conformation were classified as ‘fair’. Cattle displaying obvious signs such as recumbency, severe lacrimation, profuse salivation, anaemia, and anorexia fell into the ‘poor’ category.
Treatment strategies
Diminazene aceturate (Nonazin®) was purchased from local market in southwest Nigeria. Trypanocides are prepared at 3.5 mg/kg, and the herd treatment was done after blood collection. Dosage was calculated based on the weight of the cattle treated.
Statistical analysis
Tukey’s multiple comparison ANOVA test was used for pairwise comparisons, especially in the case of cattle breeds. Pearson chi-square analyses were used for age, body condition, sex, and farm locations to produce the significance and odd ratios. Variations in species distribution based on breeds and farm locations, before and after treatment, were analysed using frequency distribution table. The mean and standard deviation of haematological parameters were also calculated, and significance against standard values was obtained. The percentage of relative apparent density was calculated by dividing the total number of flies collected by the number of traps and the number of trapping days, resulting in flies per trap per day (F/T/D). The qGIS is used to develop a map showing study locations by states.
Results
Total transmitting vector captured during the entire study period was 3.74 F/T/D. Of the transmitting vectors of importance, only mechanical vectors were trapped, namely, Stomoxys niger (Macquart) (1.77 F/T/D), Stomoxys calcitrans (Linnaeus) (1.53 F/T/D), Tabanus taeniola (Pal. Beauvois) (0.16 F/T/D), Tabanus par (Walker) (0.14 F/T/D), Ancala fasciata (Fabricus) (0.08 F/T/D), Tabanus thoracinus (Pal. Beauvois) (0.05 F/T/D), and Tabanus subangustus (Ricardo) (0.01 F/T/D) (Suppl. 1.1). No Glossina species was captured during the study period.
Trypanosome prevalence was evaluated through five methods, with PCR being the most sensitive, displaying a prevalence of 53.3% before trypanocide treatment, which subsequently decreased to 21.5% at 8 weeks post-treatment (Suppl. 1.2 and 1.3). The gold standard method for trypanosome detection in Nigeria, the HCT (buffy coat assessment), exhibited the highest detection under the microscope, indicating a prevalence of 21.8% during the outbreak and 5.4% 8 weeks after herd treatment. The order of sensitivity was PCR > HCT > wet mount > thick smear > thin smear, with the latter being the least sensitive (Table 1). However, the prevalence after treatment with trypanocides showed a significantly higher prevalence for PCR compared to the other parasitological methods employed.
Considering all the methods employed, the average parasitaemia before treatment was recorded at 1.95 × 104 ± 1.3 × 104, signifying a high parasitic infection requiring immediate intervention. However, even after 8 weeks of treatment, parasitaemia persists, albeit with the least sensitive method. The average post-treatment parasitaemia is measured at 1.5 × 102 ± 1.4 × 102, indicating a moderate infection that suggests the need for additional treatment. Based on species, the parasitaemia levels were most elevated for T. congolense (5.1 × 105 ± 2.7 × 105), followed by T. brucei (3.5 × 104 ± 1.8 × 104), and lowest in T. vivax (4.0 × 103 ± 1.1 × 103) before treatment was initiated. Post-treatment, no T. brucei was detected, while T. congolense and T. vivax exhibited parasitaemia levels of 2.5 × 102 ± 1.3 × 102 and 1.5 × 102 ± 1.0 × 102, respectively.
On farm A, before treatment, the overall prevalence based on blood smear was found 10.5% (95% CI 6.6–15.5). This prevalence was notably higher for T. congolense (3.5%) and T. vivax (7.0%). Conversely, on farm B, the prevalence of T. congolense (8.7%) was higher than that of T. vivax (5.2%), resulting in an overall prevalence of 13.9% (95% CI 8.2–21.6). After 8 weeks of treatment, trypanosomes were identified in the blood of cattle that were previously examined. Nevertheless, the overall prevalence remained at 3.5% for both farms A and B. Analysis of datasets from both farms indicated a higher prevalence of T. vivax (3.0% vs. 2.6%) compared to T. congolense (0.5% vs. 0.9%). Notably, there was no detection of T. brucei either before or after the administration of trypanocides with microscopic diagnostic technique. The Pearson chi-square test indicates a significant difference (\(X\)2 = 7.524; P = 0.006) in the total prevalence between before and after treatment in farm A. Likewise, a significant difference (\(X\) 2 = 7.886; P = 0.005) was observed in farm B regarding the total prevalence before and after treatment.
The overall prevalence of trypanosomes on farm A, before treatment as determined by ITS-PCR, stood at 56.7% (95% CI 49.8–63.4). Notably, this prevalence was higher for T. vivax, followed by T. congolense, while T. brucei was the least observed. Meanwhile, mixed infection of T. congolense and T. vivax was detected with prevalence of 0.5% (95% CI 0.1–2.8). In contrast, on farm B, the prevalence of T. congolense was higher than that of T. vivax and T. brucei, and mixed infection was not detected, resulting in an overall prevalence of 47.8% (95% CI 38.9–56.9) (Table 2). After trypanocide treatment, overall prevalence of same set of cattle in farm A revealed 21.4% (16.3–27.6), while farm B showed 21.7% (15.2–30.1), with species detection higher for T. vivax compared to T. congolense in both farms. There were no cases for T. brucei (Table 2). Based on the PCR results, the chi-square analysis reveals a significant difference (\(X\)2 = 52.684; P < 0.0001) in the overall prevalence between before and after treatment with trypanocide in farm A. Likewise, a significant difference (\(X\)2 = 17.25; P < 0.0001) was observed in farm B concerning the overall prevalence before and after treatment.
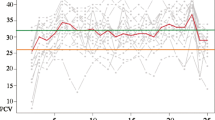
The comprehensive haematological analysis unveiled the presence of anaemia, as evidenced by a significant decrease in PCV, haemoglobin (Hb), and red blood cell (RBC) counts in both herds. During the outbreak, the trypanosome-infected cattle exhibited significantly higher mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH) values, while the mean corpuscular haemoglobin concentration (MCHC) value remained within the normal standard range. Conversely, all parameters measured in the uninfected cattle fell within the standard range values. Consequently, the observed anaemia in the infected cattle before treatment is characterised as macrocytic normochromic anaemia. Post-treatment analysis showed that the PCV, RBC, MCHC, and MCV values in those that tested positive for trypanosomes once again conformed to the normal standard parameters for cattle blood. However, Hb and MCH values were lower than the standard values, indicating the presence of normocytic hypochromic anaemia, albeit to a mild extent. This condition could be attributed to a combination of factors (Table 3).
The mean total WBC count in the infected groups was significantly lower (P < 0.001) compared to the non-infected group both before and after treatment with trypanocides. Although the total WBC count increased after treatment, the difference was not statistically significant. The mean differential WBC count showed that lymphocyte, neutrophil, and eosinophil levels were lower in the infected cattle (P < 0.001). Monocytosis was observed in the infected groups before and after treatment, but the increase was not significant. After treatment, it was observed that basophil levels were normalized, and there were improved levels in other differentials as well, although total WBC counts remained lower than those in the uninfected cattle assessed (Table 4).
Comparing the prevalence rates before and after treatment, the results showed that the drug was most effective in the White Fulani breed (WF = X2: 55.6, P < 0.0001), followed by Red Bororo breed (RB = X2: 1.69, P = 0.193). Meanwhile, the results suggest that treatment does not show significant effectiveness in Red Bororo and Sokoto Gudali breeds before and after treatment. In farm A before treatment, T. congolense was predominantly found in the White Fulani breed with a prevalence of 24.1%, while T. vivax had the highest occurrence in the Red Bororo breed with a prevalence of 63.6%. T. brucei was only identified in White Fulani (1.1%), and mixed infections of T. congolense and T. vivax were exclusively detected in the White Fulani breed (Fig. 2).
After treatment, only T. congolense and T. vivax were detected, with the highest prevalence observed in the Sokoto Gudali breed for both Trypanosoma species. In farm B, before treatment, T. congolense (41.9%) and T. vivax (29.0%) were predominantly identified in the Sokoto Gudali breed. After treatment, the N’dama breed remained infected with T. congolense, showing the highest prevalence (1/3 = 33.3%), while T. vivax was mostly detected in the White Fulani breed (19.5%) (Fig. 3).
The distribution of Trypanosoma spp. indicated that T. congolense was exclusively present in female cattle at a prevalence of 7.4% (95% CI 7.5–12.1) in farm A. However, in farm B, both male 12.5% (2.2–47.1) and female 8.4% (4.5–15.2) cattle were infected with T. congolense. Notably, in farm A, the prevalence of T. vivax was 16.7% (4.7–44.8) in males and 14.3% (10.0–19.9) in females. In farm B, the prevalence was 12.5% (2.2–47.1) in males and 13.1% (7.9–20.8) in females. The overall prevalence demonstrated a higher occurrence of T. congolense (7.8% vs. 5.0%) in females, while T. vivax (15.0% vs. 13.9%) had a higher prevalence in males. However, these differences did not reach statistical significance in either sex. Mixed infections of T. congolense and T. vivax were only observed in females 0.3% (0.1–1.9).
Prevalence based on body condition score showed significant difference (P < 0.001) between good and poor body score. There was no significance between good and fair, likewise between fair and poor based on Tukey multiple comparison test. There is no significance between cattle greater than 1 year 30.4% (25.4–35.8) and those less than 1 year 23.1% (18.6–28.2).
Discussions
The abundance of Stomoxys and tabanids and detection of trypanosomes in cattle blood post-treatment with trypanocides indicate the effectiveness of mechanical vectors in AAT disease epidemiology. These vectors have been incriminated in trypanosome infection in southwest region of Nigeria (Odeniran and Ademola 2018; Odeniran et al. 2019c, 2020, 2021; Omonona et al. 2021).
The outcomes of wet mount, thick, and thin smears from this study underscore the importance of employing multiple techniques in trypanosome detection to confirm trypanocidal resistance when animals are treated (Delespaux et al. 2008; Tchamdja et al. 2017). The persistent presence of trypanosomes in the bloodstream 8 weeks post-treatment suggests either re-infection from biting flies that could not be eliminated due to low plasma levels of trypanocides, or relapse due to the ineffectiveness of the drug. This possibility is in accordance with a previous suggestion of trypanocidal resistance in Nigeria (Delespaux et al. 2008; Geerts et al. 2001; Holmes et al. 2004). The study reports from both study sites yielded comparable results from both farms concerning blood smears, indicating a persistent prevalence of 3.5% post-treatment with trypanocide. This implies the existence of Trypanosoma-resistant strains in the environment, as both T. congolense and T. vivax were identified. This aligns with a previous report where both species were molecularly isolated as resistant strains in southwest Nigeria (Odeniran et al. 2019a). The prevalence in blood smear was low because of the less sensitivity of microscopy and resistance may be quite difficult to detect. The high prevalence of trypanosomes using PCR post-treatment raises significant concerns for livestock farmers in this region, suggesting potential resistance to commonly available trypanocidal drugs in the market.
PCR results revealed a significantly high prevalence, particularly for T. congolense and T. vivax. Overall, T. vivax exhibited the highest prevalence both before and after treatment with trypanocide. This could be attributed to the prevailing vector flies and the trypanosomes harboured. The presence of biting flies in both study sites likely contributed to the high T. vivax infection, as suggested by previous studies (Majekodunmi et al. 2013; Odeniran et al. 2019b). It appears that there is an abundance of biting flies, particularly during the dry season. T. brucei and mixed infections were exclusively detected by PCR, possibly due to the absence of the biological vector, Glossina, and the high sensitivity of the PCR technique. Mixed infections were generally not prevalent, with the exception being farm A. This observation may suggest that the transmitting vectors possess specific species infectivity.
The significant decrease in mean PCV, Hb concentration, RBC, and WBC counts was observed in all infected groups before treatment compared to the non-infected cattle group. Leukopenia and pancytopenia, directly associated with trypanosome infections, were evident. Previous reports suggest a similar effect of lower cell counts in T. congolense and T. vivax infections (Maxie et al. 1979; Dagnachew et al. 2015). Macrocytic normochromic anaemia, attributed to a compensatory mechanism in response to erythrocyte and haemoglobin loss, was observed (Sharma et al. 2000). The significant leukopenia may be due to the host’s increased production of leukocytes against invading pathogens during infection and subsequent reduction (Obi et al. 2013). Macrocytosis results from erythropoiesis occurring after the onset of infection, releasing immature erythrocytes into the bloodstream (Dagnachew et al. 2015, 2017).
This anaemia could be due to trypanosome-induced haemolysis (Longstaffe 1984) or haemodilution as the fluid content of blood increases. Red cell count decrease could be due to increased erythrophagocytosis, a crucial mechanism in the pathophysiology of T. congolense infection (Witola and Lovelace 2001), or other immunological factors and dyshaemopoiesis in which the bone marrow fails to produce red blood cells (Anosa and Isoun 1980; Murray and Dexter 1988).
Post-treatment analyses of examined cattle revealed either recovery or relapse, with some differentials not showing significance. For instance, PCV, Hb, RBC, MCH, MCHC, and MCV were within the normal range, although lower than the non-infected group. Hence, in the case of slight anaemia for post-treatment infected cattle group, it can be characterised as normocytic hypochromic anaemia. White blood cell differentials remained significantly lower than the non-infected group, except for basophils, which were within range. There was no pronounced anaemia in post-treatment values, as most values were within the normal range. The observed reduction in total WBC count in this study, both before and after treatment, aligns with findings in other trypanosome studies (Bengaly et al. 2002; Ekanem and Yusuf 2008; Dagnachew et al. 2015). Post-treatment total WBC counts showed an increase compared to the time of outbreak, but the change was not significant. This suggests that the impact of trypanosomes on the cells of the immune system remained substantial even after treatment.
Considering risk factors, in both study sites, Sokoto Gudali cattle breed was predominantly affected by trypanosome infection before and after treatment. This suggests that this breed is highly trypanosusceptible, emphasizing the need for proper care when breeding in trypanosome-infested areas. While the overall study did reveal significance in the analyses conducted before and after treatment, it is worth noting that certain cattle breeds (Sokoto Gudali and Red Bororo) exhibited no significant changes after treatment. This suggests that these breeds may be susceptible to reinfection or quick relapses.
T. vivax was highest detected species in both farms. This could be associated to infectivity of Stomoxys species observed in both farms. The prominence of T. congolense in females and T. vivax in male may not have any significance, as both sexes have been established to be susceptible to infection (Takeet et al. 2013; Odeniran et al. 2019b; Ikenna-Ezeh et al. 2020).
Body condition scores revealed a positive correlation between poor scores and trypanosome parasites in cattle. This suggests that the body condition of trypanosome-infected cattle could serve as a determinant in physical examination and diagnosis. This finding is in accordance with the report by Takeet et al. (2013). Similarly, animals younger than 1 year exhibited a higher prevalence, although not significantly, compared to those older than 1 year. Hence, age may not play a definitive role in the diagnosis of trypanosomosis in cattle (Majekodunmi et al. 2013).
Conclusions
The study indicates the presence of trypanocidal resistance, resulting in widespread resistance among Trypanosoma species in numerous cattle herds in southwest Nigeria. The situation may have been exacerbated by the abundance of biting flies during the dry season, leading to a high prevalence of T. vivax. It is crucial to isolate these Trypanosoma species using molecular tools, and further investigations on their genomes are warranted. There is an urgent necessity to reassess trypanocidal drugs available in the Nigerian market, and recommendations should be submitted to national authorities.
Data availability
The datasets generated during the current study are available under request to the corresponding author.
References
Ahmed HA, MacLeod ET, Hide G et al (2011) The best practice for preparation of samples from FTA(R)cards for diagnosis of blood borne infections using African trypanosomes as a model system. Parasit Vectors 4:68. https://doi.org/10.1186/1756-3305-4-68
Anosa VO, Isoun TT (1980) Haematological studies on Trypanosoma vivax infection of goats and intact and splenectomized sheep. J Comp Pathol 90:155–168. https://doi.org/10.1016/0021-9975(80)90036-5
Austen EE (1912) New African species of Tabanus—Part I. Bull Entomol Res 2:279. https://doi.org/10.1017/S0007485300001516
Barrett MP, Coombs GH, Mottram JC (2004) Future prospects in chemotherapy for trypanosomiasis. Trypanosomiases. https://doi.org/10.1079/9780851994758.0445
Bengaly Z, Sidibe I, Ganaba R et al (2002) Comparative pathogenicity of three genetically distinct types of Trypanosoma congolense in cattle: clinical observations and haematological changes. Vet Parasitol 108:1–19. https://doi.org/10.1016/S0304-4017(02)00164-4
Bengaly Z, Vitouley SH, Somda MB et al (2018) Drug quality analysis of isometamidium chloride hydrochloride and diminazene diaceturate used for the treatment of African animal trypanosomosis in West Africa. BMC Vet Res 14:361. https://doi.org/10.1186/s12917-018-1633-7
Dagnachew S, Terefe G, Abebe G et al (2015) In vivo experimental drug resistance study in Trypanosoma vivax isolates from tsetse infested and non-tsetse infested areas of Northwest Ethiopia. Acta Trop 146:95–100. https://doi.org/10.1016/j.actatropica.2015.03.014
Dagnachew S, Tsegaye B, Awukew A et al (2017) Prevalence of bovine trypanosomosis and assessment of trypanocidal drug resistance in tsetse infested and non-tsetse infested areas of Northwest Ethiopia. Parasite Epidemiol Control 2:40–49. https://doi.org/10.1016/j.parepi.2017.02.002
Delespaux V, Geysen D, Van den Bossche P, Geerts S (2008) Molecular tools for the rapid detection of drug resistance in animal trypanosomes. Trends Parasitol 24:236–242. https://doi.org/10.1016/j.pt.2008.02.006
Ekanem JT, Yusuf OK (2008) Some biochemical and haematological effects of black seed (Nigella sativa) oil on T. brucei-infected rats. Afr J Biomed Res 11:79–85. https://doi.org/10.4314/AJBR.V11I1.50671
Geerts S, Holmes PH (1998) Drug management and parasite resistance in bovine trypanosomiasis in Africa. (PAAT Technical and Scientific Series; 1). Food and Agriculture Organization of the United Nations (FAO). http://lib.itg.be/pdf/itg/1998/1998paat0001.pdf
Geerts S, Holmes PH, Diall O, Eisler MC (2001) African bovine trypanosomiasis: the problem of drug resistance. Parasitol Today 17:25–28. https://doi.org/10.1016/S0169-4758(00)01827-5
Herbert WJ, Lumsden WHR (1976) Trypanosoma brucei: a rapid “matching” method for estimating the host’s parasitemia. Exp Parasitol 40:427–431. https://doi.org/10.1016/0014-4894(76)90110-7
Holmes PH, Eisler MC, Geerts S (2004) Current chemotherapy of animal trypanosomiasis. Trypanosomiases doi. https://doi.org/10.1079/9780851994758.0431
Ikenna-Ezeh NH, Ezeh IO, Obi CF et al (2020) Prevalence and hematological parameters of bovine trypanosomosis in Nsukka municipal abattoir, Southeastern Nigeria. Comp Clin Path 29:1173–1177. https://doi.org/10.1007/S00580-020-03168-Y
Ilemobade AA (1979) Drug sensitivity of mouse infective Trypanosoma vivax isolates in cattle and sheep. Proceedings of the 16th Meeting of the International Scientific Council for Trypanosomiasis Research and Control. Yaounde, Cameroon, pp 251–253
Kingsley P (2015) Inscrutable medicines and marginal markets: tackling substandard veterinary drugs in Nigeria. Pastoralism 5:2. https://doi.org/10.1186/s13570-014-0021-6
Longstaffe JA (1984) Helminths, arthropods and protozoa of domesticated animals (7th edition). Trans R Soc Trop Med Hyg 78:329. https://doi.org/10.1016/0035-9203(84)90110-X/2/78-3-329.PDF.GIF
Majekodunmi AO, Fajinmi A, Dongkum C et al (2013) A longitudinal survey of African animal trypanosomiasis in domestic cattle on the Jos Plateau, Nigeria: prevalence, distribution and risk factors. Parasit Vectors 6:239. https://doi.org/10.1186/1756-3305-6-239
Matovu E, Iten M, Enyaru JCK et al (1997) Susceptibility of Ugandan Trypanosoma brucei rhodesiense isolated from man and animal reservoirs to diminazene, isometamidium and melarsoprol. Trop Med Int Health 2:13–18. https://doi.org/10.1046/J.1365-3156.1997.D01-122.X
Maxie MG, Losos GJ, Tabel H (1979) Experimental bovine trypanosomiasis (Trypanosoma vivax and T. congolense). I. Sumptomatology and Clinical Pathology. Tropenmed Parasitol 30:274–282
Murray M, Dexter TM (1988) Anaemia in bovine African trypanosomiasis. A review. Acta Trop 45:389–432
Njiru ZK, Constantine CC, Guya S, Crowther J, Kiragu JM, Thompson RC, Dávila AM (2005) The use of ITS1 rDNA PCR in detecting pathogenic African trypanosomes. Parasitol Res 95(3):186–92. https://doi.org/10.1007/s00436-004-1267-5
Obi CF, Obidike RI, Ezeh IO et al (2013) Effects of Trypanosoma brucei infection and diminazene aceturate therapy on testicular morphology and function of Nigerian local dogs. Vet Parasitol 196:283–288. https://doi.org/10.1016/J.VETPAR.2013.03.023
Odeniran PO, Ademola IO (2018) A meta-analysis of the prevalence of African animal trypanosomiasis in Nigeria from 1960 to 2017. Parasit Vectors 11:280. https://doi.org/10.1186/s13071-018-2801-0
Odeniran PO, Macleod ET, Ademola IO et al (2021) Morphological, molecular identification and distribution of trypanosome-transmitting dipterans from cattle settlements in southwest Nigeria. Acta Parasitol 66:116–128. https://doi.org/10.1007/S11686-020-00260-9/TABLES/3
Odeniran PO, Macleod ET, Ademola IO, Welburn SC (2019a) Suspected resistance of Trypanosoma species to diminazene aceturate on a cattle farm in Nigeria. Trop Anim Health Prod 51:2091–2094. https://doi.org/10.1007/S11250-019-01902-5/TABLES/1
Odeniran PO, Macleod ET, Ademola IO, Welburn SC (2019b) Molecular identification of bovine trypanosomes in relation to cattle sources in southwest Nigeria. Parasitol Int 68:1–8. https://doi.org/10.1016/j.parint.2018.09.005
Odeniran PO, Macleod ET, Ademola IO, Welburn SC (2019c) Molecular identification of bloodmeal sources and trypanosomes in Glossina spp., Tabanus spp. and Stomoxys spp. trapped on cattle farm settlements in southwest Nigeria. Med Vet Entomol. https://doi.org/10.1111/mve.12358
Odeniran PO, Onifade AA, MacLeod ET et al (2020) Mathematical modelling and control of African animal trypanosomosis with interacting populations in West Africa-could biting flies be important in main taining the disease endemicity? PLoS ONE 15:e0242435. https://doi.org/10.1371/JOURNAL.PONE.0242435
Omonona AO, Abioye SA, Odeniran PO, Ademola IO (2021) Catch composition of dipteran flies in old oyo national park, Nigeria. Niger J Parasitol 42:41–48. https://doi.org/10.4314/NJPAR.V42I1.6
Sharma DK, Chauhan SVK, Agrawal RD (2000) Haematological changes in experimental trypanosomiasis in Barbari goats. Small Rumin Res 38:145–149
Sinyangwe L, Delespaux V, Brandt J et al (2004) Trypanocidal drug resistance in eastern province of Zambia. Vet Parasitol 119:125–135. https://doi.org/10.1016/j.vetpar.2003.11.007
Takeet MI, Fagbemi BO, De DM et al (2013) Molecular survey of pathogenic trypanosomes in naturally infected Nigerian cattle. Res Vet Sci 94:555–561. https://doi.org/10.1016/j.rvsc.2012.10.018
Tchamdja E, Kulo AE, Vitouley HS et al (2017) Cattle breeding, trypanosomosis prevalence and drug resistance in Northern Togo. Vet Parasitol 236:86–92. https://doi.org/10.1016/J.VETPAR.2017.02.008
Têko-Agbo A, Messomo FN, Walbadet L, Akoda K, Niang ELH, Abiola FA (2008) Quality of veterinary medicinal products in circulation in Cameroon and Senegal. In OIE Conference on Veterinary Medicinal Products in Africa. Dakar, pp 25–27. http://web.oie.int/eng/VETMED08/pdf_manuscrits/pdf_anglais/TEKO_fr_2603_Session2_Manuscript.pdf. Accessed 1 Mar 2017
Witola WH, Lovelace CEA (2001) Demonstration of erythrophagocytosis in Trypanosoma congolense-infected goats. Vet Parasitol 96:115–126. https://doi.org/10.1016/S0304-4017(00)00430-1
Zumpt F (1973) The Stomoxyine biting flies of the world. Diptera: Muscidae. taxonomy, biology, economic importance and control measures. Fischer, Stuttgart, p 175
Acknowledgements
We are grateful to the staff of Veterinary Parasitology Laboratory, University of Ibadan, for their kind assistance. We appreciate Dr. Folu of University of Nottingham for her contributions.
Author information
Authors and Affiliations
Contributions
POO and IOA conceptualized the study. All authors designed the study. POO participated in fieldwork and data collection. All authors performed the data analysis; POO interpreted the data. POO prepared the first draft of the manuscript and reviewed by IOA and JOA. All authors contributed to the development of the final manuscript and approved its submission.
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All protocols and procedures used in the field work were reviewed and approved by the University of Ibadan Animal Ethics Committee with approval number (UI-ACUREC/App/12/2016/05).
Informed consent
For this type of study informed consent is not required.
Consent for publication
Consent for publication was obtained for every individual person’s data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Odeniran, P.O., Ademola, I.O. & Adejinmi, J.O. Trypanocidal resistance in two cattle farms using varying diagnostic techniques in southwest Nigeria. Comp Clin Pathol (2024). https://doi.org/10.1007/s00580-024-03589-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00580-024-03589-z