Abstract
The need for energy efficiency is leading to the growing use of additives that reduce friction in thin film boundary and mixed lubrication conditions. Several classes of such friction modifier additive exist, the main ones being organic friction modifiers, functionalised polymers, soluble organo-molybdenum additives and dispersed nanoparticles. All work in different ways. This paper reviews these four main types of lubricant friction modifier additive and outlines their history, research and the mechanisms by which they are currently believed to function. Aspects of their behaviour that are still not yet fully understood are highlighted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In order to reduce energy consumption and thus CO2 emissions, it is necessary to increase the energy efficiency of mechanical systems and an important way to achieve this is to design lubricants that give low friction in machine components. One design approach is to optimise liquid lubricant rheology so as to minimise hydrodynamic shear, churning and pumping losses. In practice, this often means reducing lubricant viscosity to the lowest possible value consonant with maintaining fluid or mixed film lubrication. Another approach is to add small proportions of friction modifier additives to the lubricant in order to reduce friction in the boundary and mixed lubrication regimes. In practice, both of these approaches are best applied concurrently since, as lubricant viscosity is progressively reduced, contacts are separated by thinner and thinner hydrodynamic films and so operate increasingly the regimes where friction modifier additives are effective.
Although in practice many polar organic species and solid particles dissolved or dispersed in base oils produce measurable reductions in boundary friction, there are four main classes of material that have been deliberately developed and applied as additives in liquid lubricants to reduce friction and may thus be formally termed friction modifier additives.
One class is the group of compounds based on amphiphilic surfactants now usually called organic friction modifiers (OFMs). The first of these were free fatty acids derived from fats and vegetable oils, discovered almost exactly a century ago. OFMs are key additives in modern engine oils and are also employed in fuels.
The second class is the oil-soluble, organo-molybdenum compounds, developed initially as antiwear additives but recognised in the 1980s to be very effective in reducing boundary friction. They are currently used in many engine oils and, more recently, in gear oils.
In the last few years, attention has focused on functionalised polymers tailored to adsorb specifically on polar tribological surfaces and these have been shown to markedly reduce friction in some contact conditions. The fourth class of friction modifier additive encompasses dispersed nanoparticles. Some such particles have been shown to reduce boundary friction but, so far as the author is aware, they have not yet found widespread employment in this role in practical applications. However, they have the potential to become a valuable class of friction modifier additives. Figure 1 shows a timeline to indicate when each of these main classes of friction modifier was identified, as well as showing the first introduction of the other main classes of lubricant additive.
This paper outlines both the history and our current understanding of the mechanisms of action of all four of the above classes of friction modifier additive. It is inevitably imbalanced given that the quantities of research carried out on the four are very different, so consideration of adsorbing polymers is much shorter than that of OFMs. It should be noted that this review does not attempt to examine other ways to reduce friction, such as the use of low-friction solid coatings or of micron-sized dispersed particles as presently employed in many greases. Nor does it attempt to provide a complete account of all suggested possible friction modifier structures—such an account can be found in a very comprehensive review of friction modifier additives in the recent literature [1]. The principle objective of the current paper is to provide an assessment of the state of our understanding of the mechanisms by which friction modifier additives work.
2 Organic Friction Modifiers
2.1 History of OFMs
In 1886, Osborne Reynolds published his seminal paper on hydrodynamic lubrication [2]. This demonstrated the key role of lubricant viscosity in forming a separating lubricant film between rubbing surfaces and, since viscosity is the only lubricant property that appears in Reynolds’ equation, it implied at the time that only viscosity mattered in lubrication—i.e. all lubricants having the same viscosity should be equally effective. However, this was contrary to the experience of many practising engineers of the day who knew that natural oils based on plant and animal oils and fats generally gave lower friction and wear than corresponding mineral oils. To explain this difference, a mysterious lubricant property known as oiliness was invoked.
It is clear that oiliness was a significant issue in the period and aroused considerable passion. Thus, in 1918, upon being shown evidence that the difference in lubrication properties between natural and mineral oils had chemical origins, the famous automotive engineer Lanchester wrote [3]:
I am sure that to-day it is impossible to dispute the existence of a property of oiliness apart from the ordinary physical properties of a lubricant and, so far as this view is only of recent acceptance amongst scientific men, so far we may take the period from 1886 until today as a measure of the time it has taken to recover from an overdose of Osborne Reynolds.
In the same year of 1918, Wells and Southcombe submitted a patent which showed that a low concentration of vegetable oil-derived free fatty acid when dissolved in mineral oil was able to impart the latter with oiliness, i.e. to make it as effective as a vegetable oil in reducing friction and wear [4]. They ascribed this effect to an improvement in wetting properties of the oil. Other researchers proposed alternative mechanisms including “an intensified viscosity in that part of the fluid within the region of the surface molecules of the metal” [5] and “the unsaturated molecules of the lubricant enter into a firm physicochemical union with the metallic surfaces, thus forming a friction surface which is a compound of metal and oil” [6].
However, the early 1900s was the heyday of research on monolayer surfactant films at air–liquid interfaces, and this concept was readily transferred to the solid–liquid interface so that by the 1920s, many scientists believed that oiliness resulted from single layers of surfactant adsorbed on solid surfaces. Langmuir in 1920 described the deposition of a monolayer of oleic acid on a glass surface and its dramatic influence in reducing friction [7], while in 1922 Hardy showed that fatty acids and alcohols produced a progressively lower friction on glass and steel surfaces as their chain length was increased [8]. Hardy proposed that friction reduction was produced by vertically oriented, single monolayers of these surfactants on each surface and coined the term “boundary lubrication”.
Thus by the early 1920s, both the concept and a mechanism of behaviour of OFMs had been developed—that they adsorb from solution or “self-assemble” on solid surfaces and that sliding occurs within the low-shear-strength plane between methyl groups on opposing, adsorbed, vertically oriented monolayer films. Hardy’s very simple model of boundary lubrication is shown schematically in Fig. 2a, while Fig. 2b summarises a later development of this model by Bowden and Tabor, who measured localised solid–solid adhesion events during sliding and suggested that OFMs only partially separate opposing surfaces, but that as a result they inhibit metallic junction growth and thus reduce the extent of solid adhesion [9].
It is important to note, however, that these models based on the concept of vertically oriented monolayers were (and still are) by no means universally accepted. In 1936, Hersey listed six general hypotheses that might explain the origins of oiliness [10], while, as indicated by Allen and Drauglis [11], there was still considerable debate even in the 1970s as to whether the boundary lubricating films formed by OFMs consisted of single monolayers or were much thicker.
2.2 Main Types of OFM
The first OFMs were fatty acids, but by the 1930s these were found to cause high levels of corrosion of some bearing metals such as copper, cadmium, lead and tin [12, 13] and they were gradually replaced in most applications by less corrosive amphiphiles such as amides, amines and esters [14–16] or by more complex organo-acid-based compounds that gave insoluble metal salts and thus formed passivating layers [17].
Figure 3 illustrates three OFMs in use today. These are examples with relatively well-defined molecular structures although it should be noted that the glyceryl monooleate (GMO) in practical use as a lubricant and fuel additive generally contains considerable proportions of other esters. All are based on amphiphiles having a predominantly linear alkyl chain with one or more functional groups at one end.
In practice, many commercial OFMs derive from natural fats and oils and thus contain complex mixtures of mainly unsaturated alkyl chains, while others are prepared via oligimerisation of unsaturated surfactants to give quite poorly defined mixtures of products such as the dimer acids. A recent theme is the design of OFMs with multiple functional groups to promote chelation and thus adsorption, for example tartrate and citrate derivatives [18, 19].
In addition to the corrosive action of some carboxylic acids, there are other constraints on the polar head group. For example, primary amines may inhibit the film-forming behaviour of antiwear films and also promote degradation of elastomers. It should be noted that there is considerable concurrence between the structures of OFMs and rust inhibitor additives, especially in terms of head group type; thus, GMO was patented as a rust inhibitor considerably before its friction-reducing properties were recognised [20].
Although most OFMs are based on molecules containing only C, H, O and N atoms, some researchers categorise organo-phosphorus compounds such as dialkylphosphites as OFMs, especially when they are used in automatic transmission fluids [21, 22], and even some sulphur and boron-based amphiphiles [1, 23]. However, these also show antiwear and EP properties and are rarely used solely for their friction-reducing properties. For the sake of brevity, they will not be discussed in the current paper.
2.3 Early Research on OFMs
Throughout the 1930s–1980s, there were many studies of the nature and properties of the friction-reducing properties of model OFMs and in particular of saturated fatty acids. A useful review up to about 1950 is given by Bowden and Tabor [9]. Two key problems in understanding the films formed by this type of friction modifier are that they are (1) extremely thin and (2) in many cases only very weakly bound to the surfaces and thus fragile when extracted from the liquid phase. In practice, this has meant that until recently it was generally not possible to study them within rubbing contacts or even ex situ on rubbed surfaces. The vast majority of early research was therefore limited to measuring the friction behaviour of solutions of OFMs and observing how this varied with operating conditions such as applied load, temperature and sliding speed, with concentration of additive, nature of solvent, additive molecular structure and substrate material. The presence and nature of the self-assembled OFM films were then inferred from the observed friction behaviour.
In a classical study in 1940, Bowden and Leben [24] investigated the ability of stearic acid monolayers deposited by the Langmuir–Blodgett technique to reduce friction of a steel/steel contact. They found that a single monolayer of stearic acid deposited on the steel was able to reduce friction down to the same level as that produced by immersion of the contact in a solution of the same fatty acid in paraffin oil. However, this friction-reducing effect was lost after only 2–3 passes and was durable only when multilayers were deposited, indicating the importance of there being a supply of stearic acid to repair the initial monolayer. This reflects what is now recognised to be a key feature of friction modifier, and indeed other surface-active additives such as antiwear and extreme pressure additives, that they must be able to replenish surface films as these are worn away or otherwise damaged during rubbing.
In general, it was found that OFMs start to produce a reduction in friction between a wide range of metal–metal tribopairs when their concentration reaches about 0.00001 M, above which friction decreases progressively to level out when concentration reaches ca. 0.01 M [25–27]. This range is equivalent to approximately 2–2000 ppm. Figure 4 shows friction measurements for hexadecylamine solutions with stainless steel [25].
Change in friction coefficient with concentration of hexadecylamine in hexadecane on stainless steel [25]
Several researchers have explored the impact of chain length on friction for homologous series of amphiphiles such as carboxylic acids and alcohols [28–33]. All found that significant friction reduction only occurs for alkyl chains of more than four carbon atoms and that friction then decreases progressively as chain length increases. Jahanmir [31] found that friction continued to decrease to the maximum chain length tested of 16 carbon atoms, but most other researchers observed a levelling out in friction above 10 carbon atoms.
The effect of head group has been studied extensively to show that on ferrous substrates, carboxylic acid and amine groups are generally more effective (i.e. their amphiphiles reduce friction at lower additive concentrations and/or reach lower friction values at high concentrations) than alcohol, ester, nitrile or halide groups [26, 34, 35]. Studies have shown friction being proportional to load, i.e. constant friction coefficient, except at very low loads when friction coefficient increases [28, 36]. This was later explained by Deryaguin et al. [37] as resulting from friction depending on a two-term expression, the first pressure independent and the second varying linearly with pressure. This was subsequently confirmed from friction measurements on Langmuir–Blodgett films in a mica–mica contact by Briscoe and Evans [38] who derived an expression for the shear strength, τ, of the film:
where τ ο is a pressure-independent shear stress term, p the pressure, and α is a constant. Since τ ο is quite small, of order 1 MPa, at high pressures the second term dominates and the friction coefficient is simply α.
The effect of sliding speed will be discussed later in this review. Friction may increase or decrease with temperature at moderate temperatures, but a number of studies have indicated that the effectiveness of OFM films can be lost quite suddenly, as evinced by a rapid rise in friction or the onset of severe stick slip, when a critical temperature is reached [39, 40]. In some cases, for example for alcohols, this temperature corresponds approximately to the melting point of the OFM, but in others, in particular for fatty acids, it is higher, suggestive of a chemical reaction between additive and substrate to form a metal soap [9]. It should be noted that much of this early work used pure OFMs as lubricants rather than dilute solutions [39, 40] although more recent work has confirmed similar behaviour for additive solutions [31].
Although the head group is critical in determining the effectiveness of OFMs, it was soon recognised that the strength of a monolayer OFM film, i.e. its ability to support load, is determined at least for saturated chain additives by the cumulative, short range van der Waals forces between the methylene groups of neighbouring close-packed alkyl chains [41, 42]. The importance of these lateral side chain interactions may explain why short-chain OFMs are less effective than long chains, especially on hard substrates where high contact pressures are reached, and also why linear chains are more effective than branched ones or ones whose polar group is not at the end of the molecule [27, 43].
The above friction studies were accompanied by numerous studies of the adsorption of OFMs on solids. Due to a lack of suitable surface analysis methods most of these used radio-tagged OFMs [44–47], ellipsometry [48] or estimated adsorption from the depletion of solution concentration as OFM adsorbed [25, 32, 49–51]. The last approach required powdered solids to provide enough surface area to give a measurable concentration change.
Such studies showed that saturated fatty acids generally gave Langmuir-type, monolayer adsorption isotherms to reach levels indicative of vertical orientation on many metals (or more accurately metal oxides) including iron, steel, aluminium and nickel, but the slow formation of more than a single monolayer on others such as copper, zinc and cadmium [44, 50–52]. This difference in behaviour may be related to whether metals form wholly insoluble and thus passivating soap films, or soaps that are weakly soluble. The presence of water led to much thicker films on many metal oxides [52]. Adsorption of alcohols, esters and amines gave monolayers on all metals studied, but the adsorption was weaker than for the acids and did not always increase monotonically with concentration [50]. Desorption studies showed only partial loss of adsorbed films of acids and amines on iron and steel, suggesting a mix of physical and chemical adsorption [25, 47]. Few studies have examined systematically the rate of adsorption, but ellipsometry has been used to show that dilute solutions of fatty acid can take several days to form complete monolayers on aluminium oxide, while radiotracing has shown the rate of monolayer replacement by a deuterated fatty acid to be equally slow [48].
One aspect of OFM behaviour that has appeared intermittently in the literature is that of “chain matching”. This is improved performance in terms of friction reduction, wear and seizure when the number of carbon atoms and thus the chain length are similar for both the OFM and solvent; for example, it was found that palmitic (C16) acid can give lower friction than C14 or C18 acid in hexadecane. This phenomenon was originally highlighted in the 1960s by Cameron and co-workers [53, 54], but has also been reported in later studies [55, 56], although other research has found no such effect [31]. It is conjectured to arise from the presence of intercalated solvent molecules aligned within the vertically oriented OFM monolayer such that chain matching maximises lateral side chain bonding while also giving a smooth plane of methyl groups that promotes easy slip. It should be noted that adsorption studies thus far carried out have not directly detected solvent molecules within adsorbed monolayers of OFMs, though it has been shown by pressure–area measurements that mixed stearic acid/hexadecane films on water form stable, incompressible monolayers even when there are three times as many hexadecane as stearic acid molecules present [45].
2.4 OFM Research 1990s to Present
In the 1980s, new experimental techniques were developed with sufficient resolution to enable the very thin films formed by OFMs to be studied directly, both on surfaces ex situ and within contacts. These include the surface forces apparatus (SFA) [57–60], atomic/lateral force microscope (AFM/LFM) [61–64], quartz crystal microbalance (QCM) [65], ultrathin film interferometry (UTFI) [66], neutron reflectometry [67, 68] and various surface-specific vibrational spectroscopies [69–71]. Methods were also developed to generate model close-packed monolayers by chemical grafting of self-assembled alkylthiols and alkyl siloxanes on solid surfaces [72, 73].
These “nanotribology” techniques have been applied quite extensively to study films formed by model OFMs. SFA has confirmed that saturated alkyl chain OFMs adsorb from solution to form vertically oriented monolayers on mica [58, 60] and also cobalt surfaces [57, 59], while QCM has shown that oleic acid molecules appear to adsorb parallel to the surface on iron [65]. In AFM/LFM, a sphere or sharp tip is rubbed against a substrate to provide a reasonably close analogue of a high-pressure sliding asperity contact. Most work has used very smooth mica or silicon wafer substrates and examined three types of surface film: deposited Langmuir–Blodgett films [74], grafted alkylthiol and siloxane monolayers [75, 76] and films of OFMs adsorbed from hydrocarbon solution [77]. Clearly the last of these is most relevant to understanding OFMs, but the first two provide better-characterised, close-packed monolayers. These studies have confirmed the important influence of chain length and packing density on friction and also provided some estimates of film strength [78–80]. Most AFM work has studied films on substrates withdrawn from the liquid phase (i.e. ex situ), but recently liquid cell AFM, in which the whole AFM contact is immersed in liquid, has been carried out to provide a closer approximation to an actual lubricated contact. Figure 5 compares a topography map of a mica surface immersed in hexadecane with the same surface immediately after injection of stearic acid solution in hexadecane [81]. The surface immersed in hexadecane is almost featureless, but upon injection of the acid solution irregular islands, which are about 1.6 nm thick (corresponding to tilted, vertically oriented monolayers) and have low lateral force (the paler regions) compared to the interisland regions, form very rapidly. Interestingly the islands were progressively removed by successive scanning, suggesting that they are too weak to withstand the very high pressure present in the AFM contact. The liquid cell can be heated, and this showed reversible loss of the adsorbed islands above about 40 °C.
AFM height images of mica in hexadecane before (a) and after (b) injection of 0.001 M stearic acid solution. Also profile of surface after injection [81]
The technique of ultrathin film interferometry can study boundary films in actual rubbing contacts, and this confirmed that stearic, oleic and isostearic acid solutions form 2-nm-thick films in dry conditions [66, 82]. However, exposure to water vapour for an extended period resulted in stearic acid forming a significantly thicker film (Fig. 6) [66]. This thicker film was formed only when a bearing steel ball was employed, not a stainless steel ball, suggesting a chemical reaction to form ferrous or ferric stearate. A limitation of the ultrathin film interferometry method is that it cannot be used to study OFM films in high-pressure, high-sliding contacts since the coatings used for interferometry are easily damaged under these conditions.
Film-forming behaviour of 0.1 wt% stearic acid solutions in hexadecane at 25 °C. Adapted from [66]
A variety of reflection-based vibrational spectroscopies including IR, Raman and sum frequency generation (SFG) have been applied to study adsorbed or reacted surfactant films on surfaces [83, 84]. Most work has focused simply on exploring the structure of these films on immersed surfaces, to map features such as molecular tilt or the linearity of the alkyl chain [85], but a few studies have been applied to observe film behaviour within rubbing contacts [86, 87]. Beattie and co-workers studied a Langmuir–Blodgett film of zinc arachidate in sliding sapphire/quartz contacts and found that film order was maintained during sliding, but some film was transferred from the monolayer to the opposing surface [86]. In a recent study by Koshima et al. [71], the friction coefficients of solutions of a range of model OFMs were correlated with the SFG spectral characteristics of their self-assembled films on iron, suggesting that OFMs that give the most ordered surface films also show the lowest friction.
Another quite new technique is neutron reflectometry in which a low-angle beam of neutrons is bounced off a surface and analysed to calculate the location of the various interfaces from which it is reflected and thus the thickness of any layers present. This has been used to determine the thickness of acetic acid films adsorbed on iron and copper from polyalphaolefin (PAO) [68] and of palmitic acid adsorbed on iron oxide [67] and DLC [88] from hexadecane and PAO, respectively. Films indicative of tilted monolayers were detected on the metals and most DLCs. An advantage of the approach is that it can measure very thin films on surfaces covered in oil. Two disadvantages are (1) to distinguish the base oil from additive one of these has to be deuterated and (2) it appears to require extremely smooth surfaces of sub-nanometre roughness—for example of iron deposited on silicon wafers. To date, the method has been used only to study adsorbed films on surfaces and not those within rubbing contacts. In principle, the latter is possible, but the need for very smooth surfaces may limit the amount of rubbing that can be applied.
From the above, it should be evident that while some of these new techniques provide useful insights, few are able to probe contacts operating under the conditions experienced by OFM films in real rubbing systems. QCM and neutron reflectometry have been used only to study films on surfaces out-of-contact, although immersed in liquid. SFA, vibrational spectroscopies and interferometry can look into actual lubricated contacts but only in “mild” contact conditions involving low pressure or very little sliding. AFM can match the pressures experienced within contacts, but to date has been used mainly on mica and very rarely on metal or metal oxide surfaces.
The last few years have also seen new work on OFM films using the more conventional approach of measuring friction in macro-scale contacts under very controlled conditions. An interesting recent observation is that many OFMs require some rubbing action in order to initiate or complete their friction reduction. This has been seen using optical interferometry [89] and also slow-speed ball on disc friction tests [35]. Figure 7 shows friction traces for three concentrations of stearic and oleic acid in hexadecane [35]. It is evident that friction reduces in two stages, one immediately and the second only after the disc has rotated once against the ball. Similar, though less pronounced behaviour was seen with amine solutions. Three possible reasons for this behaviour are (1) that rubbing “activates” the adsorption/reaction process in some way, (2) that it accelerates adsorption by forcing convection or (3) that it helps “order” the film in a similar fashion to the “ironing” process used to form liquid crystal display surfaces [90].
Friction during first ten rotations in a slow-speed ball on disc rig; solutions in hexadecane at 35 °C [35] (Color figure online)
There is considerable research in the literature which suggests that stearic acid can give greater friction reduction than oleic acid when the two are compared at similar concentrations [27, 60], even though SFA shows that both form similar film thickness on mica surfaces [60], while optical interferometry indicates that they also both form monolayers in steel/glass contacts [66]. Recent work has compared friction coefficients of three model OFMs, stearic acid, oleic acid and elaidic acid [91]. The last of these is a geometrical isomer of oleic acid where the carbon atoms on the single double bond are arranged in a trans-configuration rather than the cis one of oleic acid. Although a very small difference, this has a profound effect on the shape of the molecule in that elaidic acid can adopt a linear arrangement, while the oleic acid molecule always has a kink in the middle. As a result, elaidic acid can form closer packing, as evidenced by its higher melting point than oleic acid. Figure 8 compares friction/sliding speed behaviour for 0.01 M solutions of stearic, oleic and elaidic acid solutions in hexadecane using a ball on disc apparatus [91].
Friction versus sliding speed for three 0.01 M fatty acid solutions in hexadecane at 100 °C [91]
It can be seen that stearic and elaidic acids give very low friction at low speeds that increases approximately linearly with log(speed). However, oleic acid gives very different behaviour—the friction is still reasonably low, but is almost constant with speed. These results suggest that the types of film, and thus the friction behaviour, seen with molecules that can adopt linear configurations and thus close-pack easily are very different from those seen with molecules than cannot so easily close-pack.
Most research on OFMs has studied their behaviour on metals or ceramics, but in the last few years it has been recognised that OFMs are generally used in lubricants that contain other tribofilm-forming additives including antiwear additives. These are known to react with rubbed surfaces to form layers of zinc and iron phosphate, so to be effective in a formulated oil an OFM must form a low-friction film on such a surface [92]. As well as their effectiveness in reducing friction of tribofilms, there has also recently been considerable interest in the ability of OFMs to lubricate diamond-like carbon (DLC) surfaces. Some DLCs give inherently low boundary friction, and these are not significantly improved by addition of OFM to the lubricant [93]. However, one specific type of DLC, non-hydrogenated and having mainly sp3 C–C bonding (ta:C), was found to give particularly low friction with GMO. It has been suggested that glycerol in GMO reacts with the ta:C surface to form OH groups and that the low energy of these, combined with the high hardness of ta:C, gives very low friction [94]. Recently the use of GMO containing the stable isotope 13C in combination with TOF–SIMS has been used to confirm that molecules of GMO (and not just oleic acid formed from it) adsorb on DLC surfaces [95].
Another area of growing research in the last few years has been the application of molecular dynamics simulation to model OFM behaviour. Initial work focused mainly on the sliding of opposing, densely packed monolayers to explore factors such packing density, chain length, types of energy dissipation, mixed alkyl chain lengths and fluorination [96–100]. Most of these studies used coarse grain approaches where methyl and methylene groups were simulated by individual point masses even though early work suggested that it was important to include the contribution of hydrogen atoms [101]. Relatively little attention was generally paid to the way the head group bonded to the substrate. More recently simulations of specific OFMs have been carried out to study both how their packing on surfaces influences friction [102] and also how monolayers behave when separated by a high-pressure/high-shear-rate film of base oil [103]. A limitation of all work to date is that because of the very small time steps needed to model molecular motion accurately and the relatively slow rate of molecular diffusion [104], it is not yet possible to simulate the process of self-assembly to form an adsorbed film or the equilibrium between solution and surface film. Instead, at present molecules have to be positioned on or adjacent to surfaces such that the outcome depends to a considerable extent on the assumptions made by the modeller.
2.5 Origins of Monolayer–Monolayer Friction
Various models have been suggested to explain the origins of friction between opposing, vertically oriented monolayers. Cameron [105] and Drauglis et al. [106] estimated friction as the force required to move alkyl chains on a vertically oriented monolayer from one stable equilibrium position to an intermediate minimum position against an opposing monolayer. They calculated the potentials involved from the van der Waals and repulsive interactions of individual methyl groups with all the neighbouring and opposing methyl and methylene groups. Sutcliffe et al. [107] extended this approach to allow for rocking and tilting of the chains and add a second term to represent the work done in lifting methyl end groups over those on the opposing surface.
In 1981, Tabor simplified Sutcliffe’s approach and obtained a two-term expression for shear stress similar to Eq. 1 [108]. The first term was pressure independent and resulting from the tangential force required to displace a methyl end group to its next equilibrium position and the second, proportional to pressure, represented the work required to lift the plane of methyl groups over the opposing plane.
Briscoe and Evans adapted a stress-induced, thermal-activated slip model due to Eyring to explain why friction increased linearly with log(sliding speed) for Langmuir–Blodgett films of saturated fatty acids [38]. This behaviour is shown for stearic and elaidic acid solutions in Fig. 8 and is often seen in boundary lubrication [109–111]. In this model, the energy required for methyl groups to traverse the potential energy barriers created by the methyl groups on the opposing surface is provided by a combination of the work done and thermal energy. Briscoe and Evans also used this model to explain their observed linear increase in friction with pressure for monolayer sliding against monolayer [38].
Based on AFM measurements, Drummond et al. [112] developed a model of boundary friction based on the local forming and breaking of bonds between opposing monolayers. This results in four stages of friction-speed dependence. At very low sliding velocities, friction is proportional to sliding speed and then it increases logarithmically with speed, in response to a thermal activation model very similar to Briscoe and Evans. At still higher speed, friction becomes independent of speed since there is no longer time for any thermal activation to occur; applied stress provides all the energy needed to break the bonds. Finally, at very high velocities, bonds do not have time to reform so friction reduces.
Yoshizawa et al. [113] have suggested that friction correlates with and is fundamentally related to adhesion hysteresis since both originate from the same energy-dissipating processes between interdigitated chain molecules, while Salmeron has used a combination of AFM, SFA and SFG to explore the generation of defects such as chain tilt and terminal and gauche distortions in monolayer films during sliding and the contribution of this to friction [80].
2.6 OFMs: Slip at the Wall
Although the monolayer model is the most widely used to describe the behaviour of OFMs in full boundary lubrication conditions, i.e. when hydrodynamic film formation is negligible, there is growing interest in the possibility that OFMs can also reduce friction in full film hydrodynamic conditions by promoting fluid slip and thus reducing hydrodynamic friction [114, 115]. It is suggested that the planes of methyl groups formed by vertically adsorbed monolayers of OFMs provide smooth, low-wetting surfaces against which liquids can slip [116]. OFMs films are known to form oleophobic monolayers which are not easily wetted by alkanes [117]. Research has shown that the formation of self-assembled monolayers on very smooth surfaces can markedly reduce Couette friction in low-pressure, hydrodynamic contacts and that a similar response is seen when stearic acid is added to a hydrocarbon base fluid [118]. What is still not yet known is whether this mechanism plays a significant role in reducing friction in actual engineering components such as transmissions and engines, where surfaces are generally not so smooth.
2.7 Current State of Knowledge on OFMs
As described above, the classical mechanism proposed to explain the friction-reducing properties of OFMs is that their amphiphilic molecules self-assemble on polar solid surfaces to form vertically oriented, close-packed monolayers. These reduce friction since there is easy slip between the resultant, opposing methyl end groups. The films are strong and able to withstand high applied pressure because of cumulative van der Waals forces between the methylene groups on the closely packed alkyl chains. The polar group serves to locate the molecules of the solid surfaces, and its bonding can be reversible or, especially with carboxylic acids, involve soap formation and is thus irreversible.
This simple model is certainly present in the idealised conditions generally used in research and has been supported by numerous experiments using Langmuir–Blodgett films, thiolate and siloxane monolayers and the adsorbed films formed by model, saturated chain OFM solutions. Of particular value in this context has been the studies carried out using atomic and lateral force microscopy which are able to measure simultaneously the thickness and friction of the film present. However, there still are some issues with the close-packed monolayer model even for saturated chain amphiphiles. Thus, Briscoe et al. have shown considerable differences between the friction properties of monolayers of some fatty acid soaps and their corresponding fatty acids, including quite different friction-speed behaviour [119] and, at high speeds much lower friction for the soaps than the acids [120].
More importantly it has not yet been demonstrated definitively that monolayers are actually present and are solely responsible for reducing friction in real engineering contacts. There is some evidence that when metal is rubbed against metal in heated, damp conditions, thicker, possibly soap-based films are formed by carboxylic acids. But it is not known whether these thicker films influence performance or whether they are displaced under moderate conditions so that friction is eventually determined by the final monolayers present.
A significant limitation of our current research methods is that while it is possible to determine either film thickness or film composition, it is not yet possible to do both at once. Optical- and mechanical-based techniques—for example UTFI, AFM, ellipsometry and SFA—can measure film thickness, while techniques that detect molecules such as solution depletion, radiotracer methods and vibrational spectroscopy can measure film composition. But at present none can reliably do both and thus monitor, for example the mixed solute and solvent composition of films as they form. In principle, it would be possible to combine two measurement techniques to achieve this.
Apart from the major question of whether OFM monolayers actually control boundary friction in real engineering components, there remain two outstanding research issues concerning OFMs that have not been fully addressed.
One is that the vast majority of research has studied saturated chain OFM; much less work has looked at unsaturated chain ones. The limited research published on the latter suggests that they form quite different and possible more disordered films than saturated chain compounds and also show different friction behaviour, as indicated in Fig. 8. Since the majority of commercial OFMs are based, for cost and solubility reasons, on mainly unsaturated alkyl chain compounds we need to understand much more about their boundary film-forming and friction properties. Another complication is that with many OFMs, it is not clear whether friction reduction is produced by the original material or by a breakdown product. Long-chain fatty acids are effective at reducing friction, but their use as additives is constrained by their corrosivity to bearing metals. It has been suggested that some OFMs such as glyceryl monooleate act by releasing fatty acid rather than adsorbing to form protective films in their own right [1]. However, an immediate reduction in friction by GMO has also been noted [35]. Both of these issues are further discussed later in this paper.
3 Oil-Soluble Organo-molybdenum Friction Modifiers
3.1 History of Molybdenum Additives
Colloidal molybdenum disulphide (MoS2) was first patented as a lubricant additive to reduce friction in 1939 [121], though it was only produced commercially in the late 1940s [122]. After this, the use of colloidal MoS2 in oils and particularly greases grew rapidly, both to reduce friction [123] and to help control wear and scuffing [124, 125]. Throughout the 1960s and 1970s, there was also considerable effort made to use colloidal MoS2 in crankcase lubricants to reduce engine friction, with variable degrees of success [122].
To overcome the problem of keeping MoS2 in dispersion in liquid lubricants, oil-soluble organo-molybdenum additives were first proposed in 1955, initially in the role of extreme pressure additives [126, 127]. Their use to reduce friction was reported in 1959 [128] and 1963 [129]. Initially the most widely studied organo-molybdenum friction modifier (FM) was molybdenum dialkyldithiophosphate (MoDDP), the molybdenum analogue of ZDDP, and in 1969 its ability to control scuffing, wear and friction was compared with colloidal MoS2 [130].
Until the late 1970s, oil-soluble molybdenum FMs were envisaged primarily as ep, antiwear and antioxidant additives, but fuel shortages following conflict in the Middle East region in 1973 focused attention on fuel economy and thus friction reduction throughout the 1970s. By the end of this decade, these additives were widely recognised as enhancing vehicle efficiency by reducing friction in transmission [131] and engine oils [132, 133].
3.2 Main Types of Oil-Soluble Molybdenum FM
Some typical organo-molybdenum FMs are shown in Fig. 9. The two most widely studied and used types are molybdenum dialkyldithiophosphate (MoDDP) and molybdenum dialkyldithiocarbamate (MoDTC). In MoDTC the atoms shown as X can be either O or S. Most commonly the bridging X atoms are S and the double bonded ones O, but the additive is generally more reactive when the latter are S [134]. The MoDTC shown in Fig. 8 is a dimer, and this is its most widely used form, but its trimer analogue is also produced.
As well as those shown in Fig. 9, a wide range of other organo-molybdenum additives have been developed, many of which have been reviewed by Mitchell [135]. The majority contain sulphur but some, primarily those produced by reacting amines or fatty oils with molybdic acid, are sulphur free; examples are shown in Fig. 9. Typical concentrations of organo-molybdenum FMs used in formulated oils are in the range 200–1000 ppm Mo.
3.3 Research on Oil-Soluble Molybdenum FMs
The characteristic form of friction reduction seen with MoDTC and MoDDP is high initial friction followed by a sudden drop of friction coefficient to a value typically in the range 0.03–0.08, as shown in Fig. 10a for solutions of three different MoDDPs in engine oil [136] and Fig. 10b for two MoDTCs [137]. In Fig. 10a the open circles show friction of the engine oil without MoDDP, while in Fig. 10b MoDTC1 and MoDTC2 are both based on 2-ethylhexyl alkyl groups, but are from different suppliers and with different levels of impurity [137]. Friction appears to drop only when solid–solid rubbing occurs and is particularly clearly seen in reciprocating contacts, where contact conditions during reversal are severe. In unidirectional sliding and mixed sliding–rolling conditions, it only takes place at high pressures or with rough surfaces [137, 138]. Friction reduction also occurs only above a critical concentration and temperature that both depend on the MODTC structure and purity [137].
The original rationale of using organo-molybdenum additives was that they would form MoS2 during rubbing and thus offer the scuffing and wear protection and friction reduction seen with colloidal MoS2. However, it was some years after their introduction before sufficiently sensitive surface analysis became available to demonstrate this convincingly. In 1963, Feng et al. [129] showed that the use of a combination of a molybdic phosphonate complex with ZDDP gave low friction and identified MoS2 on the surfaces using electron diffraction. In the 1980s, Yamamoto et al. used XPS to study the films formed by MoDTC and MoDDP during rubbing and concluded that the films formed when friction was reduced contained MoS2 [136, 139]. They also showed that Mo-containing additives that did not contain sulphur atoms and thus, theoretically, could not form MoS2 were able to reduce friction so long as the base oil contained sulphur [140].
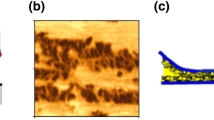
In 1998, Grossiord et al. [141] combined XPS to analyse wear scars with high-resolution TEM to study wear debris from friction tests using MoDTC solution. Like Yamamoto et al., they identified MoS2 in the wear scars with XPS. TEM revealed the presence of individual sheets of MoS2 just a few atomic layers thick dispersed in the tribofilm wear material, as shown in Fig. 11. More recently, similar sheets have been seen in tribofilms on rubbed surfaces using focused ion beam TEM [142].
HRTEM image of wear debris containing chevron-like MoS2 sheets [141]
In 2001, Topolovec Miklozic et al. [143] applied both AFM and Raman spectroscopy to analyse steel surfaces rubbed in MoDTC solutions. Whenever a marked friction drop was observed, Raman detected MoS2 on the surfaces and AFM showed the presence of tiny regions of very low lateral force on the rubbed surface high spots. The latter are shown in Fig. 12 and are typically about 10–40 nm across and 1–2 nm thick, suggesting that they are the MoS2 nanosheets identified by Grossiord. XPS and Raman have subsequently become widely used tools to study organo-molybdenum additive tribofilms [144, 145].
AFM lateral force map of surface rubbed in MoDTC solution showing low (dark)-lateral-force nanosheets [143]
A recurrent theme in the literature is the existence of a “synergy” between MoDTC and ZDDP, where a combination of both types of additive in solution gives lower friction than MoDTC alone and/or lower wear than ZDDP alone [136, 146–150]. It is certainly the case that the rate of onset of friction reduction and in particular the longevity of friction reduction by MoDTC appears to be increased when ZDDP is present, although the actual occurrence of additive synergy, a quantitative concept based on the combined concentrations of pairs of additives [151], does not appear to have been demonstrated. The origins of the beneficial effect of ZDDP on MoDTC response have variously attributed to ZDDP promoting the formation of MoS2 from MoDTC [144, 146], ZDDP suppressing of the formation of a high-friction sulphate [147], digestion of the high-friction MoO3 component of the tribofilm by the ZDDP [148] and ZDDP extending the useful life of the film [149, 150].
It is noteworthy that MoDTCs and presumably other Mo-based additives can form MoS2 on ZDDP films just as they do on steel surfaces. AFM and nanoscratch methods have shown tiny, low lateral regions formed by MoDTC at or close to the surface of the ZDDP tribofilm when ZDDP and MoDTC are used together, very similar to those formed by MoDTC on steel [152–154]. Topolovec-Miklozic et al. [92] pre-formed ZDDP tribofilms on surfaces in a minitraction machine (MTM) and then replaced the lubricant by one containing only MoDTC. Subsequent rubbing gave a drop in friction characteristic of Mo additives, showing that MoDTC was able to form MoS2 on the zinc phosphate and polyphosphate surfaces generated by ZDDP.
A significant practical problem associated with the use of organo-molybdenum FMs in engine lubricants is that their ability to reduce friction can be quite rapidly lost when they operate in oxidising environments at elevated temperature, such as in crankcase engines [155]. This has been variously attributed to ligand exchange between ZDDP and MoDTC resulting in loss of MoDTC [156], oxidation of the MoS2 tribofilm to MoO3 [157] or MoDTC in solution being used up as it acts as a peroxide-decomposer antioxidant [149, 156]. Hoshino et al. [157] found that the addition of a heterocyclic sulphur compound extended the longevity of friction reduction by MoDTC and suggested that it prevented conversion of MoS2 to MoO3 under oxidising conditions. Graham et al. [149] subjected MoDTC solutions to an air/NOx oxidising environment and monitored both MoDTC concentration and friction performance. They found that its friction-reducing capability ceased when the additive in solution fell below a critical concentration. Mo additives are effective peroxide-decomposer antioxidants, and Graham showed that the addition of other peroxide-decomposer antioxidants slowed the consumption of MoDTC and thus friction reduction [149].
One area of considerable interest in recent years has been the use of organo-molybdenum FMs with DLC-coated surfaces. It has been shown that MoDTC solution reduces friction in both DLC–steel and DLC–DLC contacts [158, 159]. AFM has shown the formation of tiny low-friction regions just as seen with MoDTC on steel [159], while TEM has also revealed the characteristic nanosheets of MoS2 previously seen with steel–steel contacts [158]. Unfortunately MoDTC also appears to promote very high wear of most types of DLC coatings [160–164]. The mechanism of this harmful effect is not yet clear and has been variously attributed to MoO3 promoting the oxidation of DLC [160], abrasion of the DLC by MoO3 particles [163] or wear via graphitisation of the DLC surface [162]. Interestingly it only occurs with DLC/steel rubbing pairs and not DLC/DLC ones [162]. Fortunately this high wear can be suppressed, at least to some extent, by the presence of antiwear additives [161] and appears to be less pronounced for other organo-molybdenum additives compared to MoDTC [164].
Despite considerable research, the precise chemical reaction mechanism of MoS2 formation from MoDTC is still unclear. Sakurai et al. suggested that MoDTC forms MoS2 by a process involving homolytic fission of the Mo–S bonds linking the dithiocarbamate with the Mo atoms [134], and a very similar reaction scheme was proposed later by Grossiord as shown in Fig. 13 [141]. An alternative mechanism is cleavage of pairs of C–S bonds within the dithiocarbamate, leaving the sulphur atoms attached to Mo. Coffey et al. [165] have reported the facile double cleavage of these bonds and the application of this cleavage to synthesis.
Proposed reaction scheme for the formation of MoS2 from MoDTC [141]
MoDTCs often show an initial rubbing period in which there is negligible change in friction followed by a very rapid fall to a stable, low value, as shown in Fig. 10b, and it has been suggested that this may indicate that the reaction process to form MoS2 is autocatalytic [137].
Sarin et al. [166] have shown that for MoDDPs the alkyl group size has relatively little effect on their friction or wear-reducing properties, but there has been little systematic research on the effect of alkyl group size on MoDTC performance. An intriguing recent observation is that asymmetric MoDTCs, i.e. those having several different alkyl groups (the R groups in Fig. 9) in a molecule, give lower friction than those in which the alkyl groups are all the same [167, 168]. Best of all are MoDTCs made from asymmetric amines, i.e. having two different alkyl groups on each nitrogen atom [168].
3.4 Current State of Knowledge of Oil-Soluble Molybdenum FMs
Based on the above, we are currently considerably more confident about how organo-molybdenum FMs work than we are about OFMs. Under rubbing conditions, they form nanosheets of MoS2 on rubbing surfaces. These form only on the load-bearing asperity tips and thus have a disproportionate effect on reducing friction. Grossiord suggests that the MoS2 single sheets form by degradation of the MoDTC molecule by electron transfer mechanisms activated by the friction process, but this reaction scheme is not yet confirmed. One striking observation is that MoDTC appears to form films and reduce friction on a very wide range of surfaces, including DLCs, the tribofilms formed by ZDDP [92] and ceramic coatings [169]. This suggests that the formation of the friction-reducing MoS2 sheets does not involve a chemically specific reaction with metal or metal oxide, but may rather be an intramolecular reaction stimulated by applied stress.
There remain several quite important aspects of organo-molybdenum FMs that are not well understood. One is why an asymmetric arrangement of alkyl groups in MoDTC appears to improve performance: one conjecture is that this asymmetry may influence the stress experienced by the bonds that must break in order to form MoS2, or perhaps it may influence adsorption. Nor has the mechanism by which MoDTC promotes DLC wear been convincingly proved.
On a more general note, there has been a great deal of research on the mechanism of action of MoDTC but far less in recent years on other Mo-based additives. Recent work showed that one S-free Mo additive reduced friction only in the presence of ZDDP and formed MoS2 [170, 171], but it would be helpful both in understanding the mechanisms of Mo additive behaviour and from the practical point of view to explore precisely how non-S containing organo-molybdenum additives extract sulphur from other additives present.
4 Functionalised Polymers
4.1 Early Functionalised VM Research
Polymeric additives are routinely added to engine and transmission oils as “viscosity modifiers” (VMs) in order to increase viscosity index. More recently it has been recognised that the temporary shear thinning displayed by VM solutions plays an important role in reducing friction in hydrodynamic conditions. Although these are considered to be their two main roles, in the early 1960s Okrent also found that some VM solutions reduced friction and wear of engines in a fashion that could not be explained solely by their impact on viscosity [172, 173]. This effect was especially evident for a “multifunctional” polymer, and one explanation suggested by Okrent was that the polymers interacted with surfaces to give anomalous rheological properties in thin film conditions.
In 1994, ultrathin film interferometry was applied to measure the film thickness of dilute solutions of polyisoprene in PAO in a rolling ball on flat contact. This showed the presence of thicker than expected elastohydrodynamic (EHD) films at low speeds [174]. The study indicated the presence of boundary films of thickness ranging from 2 to 20 nm depending on the polymer molecular weight. These thicknesses corresponded to approximately twice the diameter of the polymers in their random coil arrangements in solution and were similar to the thicknesses of immobile films formed by the corresponding polymer melts in a surface forces apparatus [175].
This research was extended in 1995 to study solutions of a range of commercial VMs in mineral oil using optical interferometry. It was found that most VMs showed conventional EHD behaviour down to film thicknesses of <2 nm, but that three formed boundary films of thickness ca 15–30 nm, similar to those previously seen with polyisoprene [176, 177]. These films persisted at temperatures up to 150 °C as shown in Fig. 14 for one of the polymers. It was noted that while the non-boundary film-forming VMs were based on non-polar monomers, the three boundary film-forming polymers contained some polar monomers, likely to adsorb on polar surfaces. For example, the one shown in Fig. 14a was a copolymer of olefin and a nitrogen-containing monomer having dispersant properties. The proposed mechanism of boundary film formation is shown in Fig. 14b. It was suggested that the polymer molecules adsorb on the two solid surfaces to give surface layers that have a higher polymer concentration than the bulk solution and thus are much more viscous. At low speeds, this viscous surface film fills the contact inlet and thus controls the amount of fluid entrained. However, at higher speeds, when the EHD film thickness formed by the bulk solution is significantly greater than the viscous layer thickness, the adsorbed polymer no longer enhances fluid entrainment.
a Boundary film formation of a functionalised polymer solution and b the proposed mechanism [177]
Further research showed that these thick VM boundary films were also formed and markedly reduced friction in mixed rolling/sliding conditions [178]. The dependence of friction on entrainment speed was measured and correlated with film thickness measurements. This showed that the viscous surface films formed by the VMs had the effect of delaying to slower speeds the onset of mixed and boundary lubrication since all friction results collapsed on the same curve when friction coefficient was plotted against film thickness [178]. A high-pressure SFA technique was also applied to compare the rheology of thin adsorbed films of functionalised and non-functionalised polymers and showed that the latter formed much more compact and uniform films in a confined contact [179].
4.2 Recent Research on Functionalised Polymer Additives
A considerable limitation of most early research was that it was based on commercial viscosity modifiers whose precise composition was not available for proprietary reasons and whose molecular weight distribution was very broad. This made it difficult to explore the relationship between polymer structure and boundary film-forming ability. In subsequent research, a series of polymethacrylate polymers (PMAs) were synthesised using controlled radical polymerisation methods to give very narrow molecular weight distribution copolymers of alkylmethacrylate with various functionalised methacrylates [180, 181]. For each polymer, 10 % of the monomers were functionalised with a variety of structures including aminic, acidic, hydroxyl and ethoxy groups. Figure 15 shows some of these structures, together with non-functionalised alkylmethacrylate.
Polymethacrylate monomers used to make functionalised PAMs [180]
It was found that the functionalised PMAs only formed boundary films when they had block copolymer architecture, i.e. all of the functionalised monomers were linked together as a block at one end of the overall polymer rather than being randomly distributed along the whole polymer chain. This is probably because several functional monomers grouped together in a block copolymer molecule adsorb much more rapidly than when the functional groups are scattered throughout the polymer chain in random copolymers and also give higher coverage since the non-polar block segment can stretch away from the surface eventually to form a “brush” configuration [182, 183].
Research has shown that linear polymer molecules can adsorb on surfaces in three configurations: “pancake”, “mushroom and “brush”, as illustrated in Fig. 16 [184]. In the pancake arrangement, the polymer molecules are arranged across the surface and this type of arrangement might be expected when there are several bonding positions along the chain—for example with statistically distributed functionality. When polymers have all their functionality located at one end of their molecules, as with functionalised block copolymers, in good solvents they initially adsorb to form mushrooms, but at higher adsorption densities they elongate to form brushes. Good solvents are ones in which the non-functionalised polymer chain segments are fully soluble; in poor solvents, all adsorbed polymers will tend to form the collapsed, pancake configuration.
Figure 17 compares the friction behaviour of two statistically distributed functionalised copolymer solutions with two corresponding block copolymer structures at 120 °C. The reduction in friction of the block copolymers is evident.
Comparison of friction coefficient versus entrainment speed for statistically distributed and block copolymers. Test temperature 150 °C [181]
One limitation of these adsorbing polymers was that while they reduced friction very effectively in unidirectional rolling–sliding where mixed lubrication was present, they appeared to be less effective in the more severe boundary lubrication conditions present in reciprocating contact [178, 185]. The behaviour of the copolymers with other additives commonly present in lubricants was also studied [186]. In general, the functionalised PAMAs combined in a complementary fashion with other additives, but were less effective at reducing friction when amine-based dispersants or corrosion inhibitors were present. This probably results from competition for surface adsorption sites by the latter additives.
The above polymers generally combine VM and often dispersancy properties with a friction modifier response. It is interesting to note, however, that commercial functionalised polymer additives have recently been designed which are intended solely as friction modifiers [187].
In addition to the research on oil-soluble polymers described above, there has also been research interest concerning the use of adsorbing polymers in aqueous systems to reduce friction, both with respect to bio-lubrication and also with a view to designing improved water-based lubricants. Spencer and co-workers have explored the boundary friction properties of one water soluble copolymer system, poly(l-lysine)-g-poly(ethylene glycol) (PLL-g-PEG), in some detail [188–190]. This polymer has a backbone of polylysine in which some of the monomers have a free primary amine group. This means that this backbone adsorbs horizontally and strongly on polar surfaces. However, some of the monomers are bonded to polyethylene glycol chains, and these extend out into the supernatant water to form a brush configuration. Aqueous solutions of the polymer markedly reduced friction in rolling–sliding conditions, but were less effective in slow-speed, pure sliding conditions [191].
The frictional properties of adsorbed polymer films have also been studied quite extensively by Klein and co-workers using a SFA to measure the thickness and friction of end-functionalised polymers adsorbed on mica from various solvents [192–194]. In good solvents, very low friction was observed. Molecular dynamics simulation suggested that this was because the polymers brushes on the two opposing surfaces showed very little interpenetration even in static conditions due to osmotic pressure within the brush system; the surfaces were therefore held apart by the extended polymer chains [192]. When sliding occurs, the chains stretched along the sliding direction to reduce this overlap still further and in consequence friction was very low. At higher pressures, friction was found to increase as greater interpenetration occurred and eventually the polymers were detached from the surfaces [195]. Electrolytic polymers in aqueous solution have also been studied, and these showed low friction up to higher contact pressures than uncharged ones. This is because hydration forces between the water molecules and the ions of the polymer strongly resist collapse of the polymer brushes [194].
4.3 Current State of Knowledge of Polymer-Based Friction Modifiers
It is clear that functionalised VM polymers can adsorb on solid surfaces to markedly reduce friction and that they appear to do this by separating the surfaces with a pressurised fluid film at much lower entrainment speeds than is possible by the base oil alone. This behaviour has been interpreted as resulting from an increase in viscosity close to the surface, though it is not known whether there is also a viscoelastic contribution. Nor do we know the tethered density of these films or whether they have mushroom or brush configuration. There is evidence that the polymers fail to reduce friction effectively in severe reciprocated contact conditions, but the friction and film thickness of functionalised VMs have not yet been studied at slow enough entrainment speeds to explore the true boundary lubrication properties of the adsorbed films. Some insights may be gained from SFA studies of end-functionalised polymers which suggest that these polymers adsorb to form quite stiff, brush-like adsorbed films that tilt and stretch under shear, resulting in very little interpenetration and thus very low friction at low pressure, but give higher friction and eventual tearing from the surface at high pressure. Adsorbed VM polymers may follow a similar progression in low entrainment speed conditions, though the contact pressures in the SFA are much lower than those normally found in engineering components.
One limitation of adsorbing polymers is that unlike OFMs, it is very difficult for them to form very dense packing. This is because, as the polymer molecules adsorb, they sterically hinder further polymer molecules from reaching the surface. This problem increases with molecular weight and means that the VM functionalised polymers may actually operate in the mushroom rather than brush regime. One solution is to grow polymers on surfaces by polymerisation from initiators pre-attached the surface, so-called grafting-from. Very dense polymer films can be formed in this way, and these show low friction in both high- and low-speed conditions [196, 197]. Unfortunately this approach does not provide the replenishment process generally required of lubricant additives and is thus a surface treatment rather than being delivered and maintained by the lubricant.
Almost of the work described above is based on amphiphilic polymers having a polar segment at one end and oil-soluble segment at the other and thus able to adsorb on surfaces to form a mushroom or brush configuration. However, it should be noted that some of the earliest research outlined above showed that polyisoprene forms thick boundary lubricating films [174, 175] and this has been confirmed by other research [198]. In polyisoprene, the functional C=C double bonds are distributed throughout the polymer chain, and it is of interest to question how such polymers bond to the metal oxide surfaces present and how the resulting adsorbed, presumably pancake configuration can form a separating lubricating film.
5 Nanoparticles
5.1 Rationale and Early Research
In recent years, there has been growing interest in the possibility of using colloidal solid particles in the size range 1 to 500 nm as friction modifier and also antiwear additives. In principle, such particle sizes are small enough both to remain dispersed in liquids by Brownian motion and to pass undisturbed through filters used in oil systems. This interest has been fuelled in particular by the recent development of fullerenes and inorganic fullerenes, whose graphitic-like structures resonate with the concept that layer-lattice materials can reduce friction. However, as will be described below many other types of nanoparticle have also been suggested as friction-reducing additives, including metals and inorganic salts. The main potential advantages of using nanoparticles as lubricant additives are envisaged as: (1) the ability to use chemistries that are insoluble in non-polar base oils; (2) since their activity is limited to their surfaces, they should show less interaction with other additives present in a lubricant; (3) since their film formation is largely mechanical, they may form films on many different types of surface; (4) because their films form mechanically, they need to be less chemically reactive than normal additives and so will be more durable and less likely to react with other additives; and (5) they are likely to be highly non-volatile and thus not lost in high-temperature conditions.
Nanoparticles have in fact been used as additives in lubricants for many years in the form of overbased detergents [199, 200]. These are tiny particles of calcium or magnesium carbonate (typically 5–50 nm diameter) stabilised by a sulphonate, salicylate or phenate salt with the matching cation. In these additives, the organic salt acts as a detergent to solubilise polar oxidation material and prevent deposit/varnish formation, while the carbonate core neutralises inorganic acids formed during fuel combustion. It has long been recognised that the overbased detergents possess some antiwear properties [201], and recent work using optical interferometry and AFM has shown that they form thick (100 nm+) films of CaCO3 or MgCO3 on rubbing surfaces [202]. The films are much thicker than the original size of the nanoparticles, so the particles must have fused together on the surface to form a thick layer of calcium carbonate. The effect of these particles on boundary friction is controlled by the type of surfactant employed, with long, linear alkyl chains giving low friction and branched ones high friction, as would be the case with OFMs. Thus, with the appropriate stabilising surfactants, overbased detergents can reduce boundary friction. Unfortunately, because their thick surface films are rough, they show enhanced friction in mixed lubrication conditions [202].
Apart from the overbased detergents, it is difficult to chart the precise introduction of nanoparticle additives in lubricants since dispersed micron-sized particulate additives have been in use since the 1900s and it is not always clear whether these included a proportion of smaller particles. The use of colloidal MoS2 was described earlier in this paper. In 1977, Bennington et al. [203] showed that three unidentified colloidal additives reduced friction in engines, while Reick [204, 205] describes the preparation and use of dispersed 50-nm-sized PTFE in oil in the early 1980s.
5.2 Nanoparticle Additive Research; 1990 to Present
Reviews of the use of nanoparticles in lubricants can be found in [206–208]. Many different types of material have been examined as colloidal additives to reduce both friction and wear, but the most common are metals, metallic oxides, borates and phosphates, fullerenes, inorganic fullerenes, boron oxide and nitride and recently graphene.
Considerable research on metal nanoparticles has been carried out. It has been shown that both copper [209] and nickel [210] nanodispersions reduce friction as well as improve antiwear and seizure performance in sliding contact conditions. Optical interferometry has been used to investigate the film-forming properties of dispersions of gold and silver nanoparticles [211, 212]. The particles formed monolayer films on rolling–sliding surfaces, but these were quite easily dislodged under high-speed conditions.
Metal oxide nanoparticles have also been quite widely investigated as possible boundary lubricating additives. Titanium and iron(III) oxides were found to be only moderately effective [213, 214], but La(OH)3 showed quite good friction and wear-reducing performance [215]. Very recently it was shown that CuO and ZrO2 nanoparticles dispersed in a formulated engine oil were effective in reducing both friction and wear [216].
A recent area of great interest is the possible use of fullerenes and related carbon structures dispersed in lubricants as potential boundary lubricating additives. Gupta et al. [217] studied the influence of dispersed fullerene (C60-rich) powder on friction and wear and found similar performance to dispersed graphite or MoS2. The authors reported that the improvement in wear was due either to the formation of a transfer film in the contact area or to the C60 clusters acting as “tiny ball bearings”. Ginzburg et al. [218] analysed the tribofilm formed by fullerene using mass spectrometry, wide angle X-ray diffraction and TEM. They proposed that the film consists of a network of fullerene molecules linked by polyolefin chains formed during rubbing. Joly-Pottuz et al. [219] have studied dispersed carbon nanoonions and found these to reduce friction at least as effectively as graphite particles. Based on TEM–EELS analysis of the rubbed surfaces, they suggested that the carbon particles may stabilise maghemite, a low friction form of iron oxide. Carbon nanotubes have also been investigated as potential boundary lubricant additives and found to reduce significantly both friction and wear [220, 221]. In the last few years, there has been considerable focus on graphene as a potential lubricant, including its use as an additive dispersed in oil. Graphene particles do not disperse in hydrocarbon, but they can be stabilised by treatment with a surfactant such as a fatty acid. Several studies have shown that such dispersed particles reduce friction in boundary lubricating conditions [222–225].
A very widely studied class of nanoparticulate additives are the “inorganic fullerenes” (IFs). These are lamellar, inorganic compounds which have a similar spherical or tubular structure to fullerenes and carbon nanotubes. They have an onion-like structure built up of concentric layers of material and are usually solid although sometimes hollow [226]. The IFs of main interest in tribology to date have been molybdenum and tungsten disulphides (IF-MoS2, IF-WS2). Both of these have been shown to have friction-reducing and antiwear properties [227, 228]. In situ high-resolution SEM has identified three different mechanisms by which they reduce friction, as summarised in Fig. 18 [229]. At low pressures, particle rolling can occur depending on the particles’ shape and hardness. At intermediate pressures, the particles remain intact but slide against the bounding surfaces. At still higher pressures, the particles are crushed to form a low-shear-strength layer-lattice film. The onset of exfoliation of IF-MoS2 has also been observed using in situ TEM [230]. Because of this pressure-dependent exfoliation, IF particles give lower friction coefficient at high than at low pressure [228].
Three friction mechanisms of IF nanoparticles, a rolling, b sliding, c crushing/exfoliation. Red dot is gold marker reference point [229] (Color figure online)
The inorganic fullerenes have a layer-lattice structure which, once exfoliation occurs, is believed to provide low-shear-strength sheets that adhere to the surfaces to reduce sliding friction in a similar fashion. Other nanoparticulate solids also having layer-lattice structure, such as hexagonal boron nitride [231, 232] and boric acid [233], have also been shown to reduce friction, probably by a similar mechanism.
5.3 Discussion: Nanoparticle Additives
The friction-reducing properties of nanoparticles are disparate and still quite poorly understood. One problem in interpreting their effectiveness as additives lies in distinguishing the benefits of the particles themselves from those of their stabilising surfactant since in much of the published work, performance is compared to a nanoparticle-free lubricant rather than a blend containing only stabiliser. Another problem is that unlike dissolved additives, for nanoparticles the delivery and adhesion of particles to the rubbing surfaces are strongly dependent on the mechanics of the rubbing contact and the presence of any separating fluid films. This means that the performance of nanoparticles as additives is likely to be very system-specific and in consequence, very different performance is likely to be seen in different tests and test conditions. Figure 19 shows two friction traces for a dispersion of IF-MoS2 in a reciprocating test rig. In one test, the oil in the test holder was stirred for the first and third hours. The unstirred sample showed almost complete loss of friction reduction after about half an hour, while in the other stirring enabled friction to remain low and even to recover friction reduction after this was lost during an unstirred period [234].
Loss of IF-MoS2 friction reduction during rubbing and its recovery by stirring [234]
For a nanoparticle to be effective in controlling friction, three requirements must be fulfilled: (1) the dispersed particles must enter the contact in sufficient quantities; (2) once between the surfaces, the particles must impart low friction; (3) some adhesion of the particles to the surface is probably necessary.
The need to ensure that dispersed solid particles enter a lubricated contact to be effective is an issue with all colloidal lubricants. As liquid lubricant approaches a sliding or sliding–rolling contact, almost all undergoes reverse flow or is deflected round the sides; very little passes through the contact and solid particles may be deflected [235]. It is possible that carbon nanotubes may be more effective than spherical fullerene particles simply because their shape makes them more likely to be trapped in the contact inlet [236]. It has also been shown that external mechanical excitation of the contact can increase the access of nanoparticles in a lubricated contact [237].
How the particles reduce friction once they are in the contact is more problematic. Particles may roll or slide as individuals. But based on the number of particles present, this may not reduce friction significantly. Consider a circular contact of radius R lubricated by a dispersion of concentration C vol% of nanoparticles of radius r. Suppose the film thickness is 2r (just enough to be bridged by the undeformed nanoparticles). Assuming that all particles enter the contact unhindered by flow, a simple calculation yields the number of particles present in the contact at any one moment to be 1.5CR 2/r 2 and the fraction of the contact area covered by particles to be simply 1.5C. For a quite high nanoparticle concentration of 1 vol%, this implies that only 1.5 % of the contact area will be covered by particles at any given time, probably insufficient to hold the surface apart. This suggests that to separate the surfaces effectively, either particles must become more concentrated in the contact inlet or some adhesion of particles to surface the rubbing is required to accumulate the requisite film.
To date, although there has been a great deal of research on nanoadditives, this has generally not been translated into practical use in commercial liquid lubricants. This is probably because there have to be clear performance and/or cost–benefits in introducing such additives, especially since there are likely to be some concerns about the long-term stability of nanoparticulate dispersions. It is possible that such additives will only become widely used if they can address problems that current, soluble additives cannot solve.
A final important question when considering nanoparticle additives is whether they might harm other aspects of the lubricant performance. One type of nanoparticle that cannot be avoided in engine oils is soot, formed by incomplete combustion of hydrocarbon fuel, and some of which accumulates in the lubricant. This soot is known to be promote wear of engine parts, and it has recently been shown that one mechanism by which this occurs is that the carbon particles abrade the tribofilms normally formed by antiwear additives [238]. In consequence, lubricants that contain both antiwear additive and carbon nanoparticles actually give faster wear than when the antiwear additive is left out. The key to this phenomenon is that the hardness of the nanoparticles, although less than the steel substrate, is greater than that of the antiwear film. It has been shown that other hard nanoparticles also remove antiwear films, while nanoparticles that are softer than antiwear films such as CuO do not [238]. It has also recently been reported that carbon nanoparticles can prevent MoDTC from reducing friction to an extent dependent on the particle size [239]. Clearly it is very important to ensure that nanoparticle additives do not damage beneficial tribofilms formed by other additives present in the lubricant.
6 General Discussion
This paper has reviewed the four main classes of friction modifier additive in current use or under consideration, focusing on research that investigates the mechanisms by which they reduce friction. It must be stressed that it does not cover all possible types of friction modifier additive; for example, it has also been suggested that ionic liquid additives, boron and phosphorus compounds may also reduce friction [1].
Two of the friction modifier classes, OFMs and functionalised polymers, act by adsorbing on polar solid surfaces to form low-shear-strength films that separate the solid sliding surfaces. However, the thickness and strength of their adsorbed films are very different.
OFMs are low molecular weight amphiphiles that, at least when their alkyl structure permits a linear configuration, form close-packed, vertically oriented monolayers with strong, interchain van der Waals bonding. This bonding confers strength and stiffness to the monolayers, while the opposing alkyl groups provide a plane of low shear strength. However, such strong, close-packed monolayers have been seen almost exclusively with long, straight, saturated chain molecules with small head groups—molecules that are often insufficiently soluble or too expensive for commercial application. A key practical as well as scientific question is thus how much unsaturation and/or branching and/or larger head group can be tolerated before molecules no longer pack sufficiently closely to support load and reduce friction effectively. A larger head group leads to a lower maximum adsorption coverage, but this can be accommodated to some extent by tilting of the alkyl chains to increase their packing density, as indicated by molecular simulation [240]. Recently it has been shown that in mixtures of saturated and unsaturated chain acids, the unsaturated chains reach the surface first and effectively block it from saturated chains, to the detriment of low friction [104].
It has also been suggested that OFMs may form relatively thick viscous or paste-like metal soaps on rubbed steel surfaces, especially when water is present and that these may contribute to friction reduction. Such soaps would, however, only be expected to result from OFMs based on fatty acids. This raises the important issue of whether OFMs such as esters and amides hydrolyse or otherwise break down during use to give free fatty acids and whether these species are actually responsible for some or all of the friction reduction seen with these additives. There appears to be no published research to measure the persistence of molecules of ester and amide OFM additives in actual use in machines. In a recent study, the friction properties of 0.01 M solutions in hexadecane of the two esters, ethyl stearate and stearyl acetate, have been compared at 100 °C in a high-frequency reciprocating rig (HFRR). If ethyl stearate decomposes, it should form stearic acid, which ought to be a very effective OFM, while the structurally quite similar stearyl acetate should give stearyl alcohol, a relatively poor OFM. The results are shown in Fig. 20, together with the friction of 0.01 M stearic acid and 0.01 M stearyl alcohol.
Both solutions give modest friction reduction, typical of an ester or alcohol but much less effective than stearic acid, suggesting no significant free acid production during the 3-h tests. This approach is not conclusive since it does not allow for the presence of water and strong acid that might promote hydrolysis in some machine components. But it does show that fatty esters do adsorb on steel surfaces to reduce friction and also that rubbing alone does not promote ester breakdown.
Functionalised polymers also adsorb on polar surfaces so long as the functionality is located at one end of the molecule. This can give rise to adsorbed films that are tens of nanometres thick. Steric constraints mean that polymer packing density is relatively low, so there is no strong interchain bonding as is present in OFM monolayers. This means that the boundary lubrication properties of these polymers in high-pressure conditions may be limited, though how limited has not been fully explored. However, the adsorbing polymers do create thick surface layers of high effective viscosity which help separate the surfaces and thus give low friction in less severe, mixed lubrication conditions.
From the scientific point of view, it might be of interest to bridge the gap between the boundary film-forming and friction properties of OFMs and functionalised polymers by exploring the impact on adsorption and friction of progressively increasing the OFM chain length.
The friction-reducing properties of the organo-molybdenum FMs and especially MoDTC are quite well understood in that they form the low-shear-strength, layer-lattice compound MoS2 on rubbing surfaces. The main practical interest is performance longevity, how long the additives survive in solution and under what conditions does the surface film transform to high-friction MoO3. Unfortunately, the many oxidation states of molybdenum make its compounds susceptible to oxidation. The philosopher’s stone would be an additive that reacted to form a layer lattice on rubbed surfaces similar to MoS2 and the other molybdenum and tungsten chalcogenides, but which was not susceptible to oxidation. There is no obvious candidate, though boric acid may be a possibility.
As outlined above, many types of nanoparticle have been studied as potential lubricant additives. Of these, most are more suited to reducing wear than friction since the films that they form do not have very low shear strength. In terms of friction reduction, the most promising have 2D sheet structures, the various carbon-based graphitic materials and the inorganic fullerenes. These show low friction in boundary lubrication conditions in laboratory tests. The challenge is to transfer this behaviour reliably to operating machine component lubricants and to demonstrate better performance than alternative friction modifier classes.
A final question is whether the different classes of friction modifier additive work well together, whether combinations give the advantages of both types of additive or whether they are antagonistic. There is relatively little published work on this. Solutions containing both functionalised PMA and organo-molybdenum additives were found to provide the low mixed friction of the PMA and the low boundary friction of the molybdenum compounds, the best of both worlds [186]. However, functionalised PMA reduced the boundary friction-reducing properties of an aminic OFM. Very recently it was shown that the addition of small amounts of MoDTC to an OFM solution reduced boundary friction to lower values than either additive alone in a ZDDP-containing lubricant [241].
7 Conclusions
The mechanisms of action of the four main classes of friction modifier additive have been reviewed. Considerable research over many years has provided a quite clear understanding the conditions under which these additives work and, in the cases of organo-molybdenum and functionalised polymer additives, how they work. Organo-molybdenum additives react on rubbing asperities to form low-shear-strength MoS2 nanosheets, though the precise driver and mechanisms of this is not yet clear. Functionalised polymer molecules adsorb to form high-viscosity surface films that enable surfaces to remain separated in low entrainment speed conditions, thus postponing the onset of mixed lubrication. The mechanism of nanoparticle friction is more problematic since there are many types of nanoparticles and they may work in different ways. Here a critical issue is to ensure that the particles enter rubbing contacts and adhere to surfaces. Remarkably, the class of friction modifiers about which we know least in terms of mechanisms is the OFMs, even though they have been in use for almost a century. Much academic research has shown how model OFMs can behave in model conditions—by forming vertically oriented absorbed monolayers on polar surfaces. But it is not yet proven that this is the actual mechanism that prevails in real machine components such as found in engines. There is also a mismatch between OFMs studied in academia, mostly straight, saturated alkyl chain, small head group amphiphiles, and the largely unsaturated and/or larger head group ones more commonly employed in formulated lubricants.
References
Tang, Z., Li, S.: A review of recent developments of friction modifiers for liquid lubricants (2007-present). Curr. Opin. Solid State Mater. Sci. 18, 119–139 (2014)
Reynolds, O.: On the theory of lubrication and its application to Mr. Beauchamp Tower’s experiments, including an experimental determination of the viscosity of olive oil. Proc. R. Soc. Lond. 40, 191–203 (1886)
Lanchester, F.W.: Communicated remarks to discussion on lubrication. Proc. Phys. Soc. Lond. 32, 29s–33s (1919)
Wells, H.M., Southcombe, J.E.: The theory and practice of lubrication: the “Germ” process. J. Soc. Chem. Ind. 39, 51T–60T (1920)
Kingsbury, A.A.: New type of oil-testing machine and some of its results. Trans. ASME 24, 143–160 (1903)
Deeley, M.: Oiliness and lubrication; Discussion on lubrication. Proc. Phys. Soc. Lond. 32, 1s–11s (1919)
Langmuir, I.: The mechanism of the surface phenomena of flotation. Trans. Faraday Soc. 1, 62–74 (1920)
Hardy, H.B., Doubleday, I.: Boundary lubrication—the paraffin series. Proc. R. Soc. Lond. A100, 550–557 (1922)
Bowden, F.P., Tabor, D.: The Friction and Lubrication of Solids. Clarendon Press, Oxford (1971)
Hersey, M.D.: Theory of lubrication. In: The problem of oiliness. Wiley, New York (1936)
Allen, C.M., Drauglis, E.: Boundary layer lubrication: monolayer or multilayer. Wear 14, 363–384 (1969)
Bray, U.B., Moore, C.C., Merrill, D.R.: Improvements in diesel-engine lubricating oils. SAE Technical Paper No. 390125 (1939)
Prutton, C.F., Frey, D.R., Turnbull, D., Dlouhy, G.: Corrosion of metals by organic acids in hydrocarbon solvents. Ind. Eng. Chem. 37, 90–100 (1945)
Cantrell, T.L., Peters, J.G., Smith, H.G.: Mineral oil compositions containing amidic acids or salts thereof. US Patent No. 2,699,427, 11 Jan 1955
Eckert, G.W.: Adducts of aliphatic monocarboxylic acids and aliphatic amines in gasoline. US Patent No. 3,055,746, 25 Sept 1962
Schick, J.W., Kaminski, J.M.: Lubricant composition for reduction of fuel consumption in internal combustion engines. US Patent No. 4,304,678, 8 Dec 1981
Puddington, I.E., Sirianni, A.F.: Friction reducing additives for lubricants. US Patent No. 2,689,224, 14 Sept 1954
Hiebert, J., Rowe, C.N., Rudnick, L.R.: Alkylated citric acid adducts as antiwear and friction modifying additives. US Patent No. 5,338,470, 16 Aug 1994
Kocsis, J., Vilardo, J.S., Brown, J.R., Barrer, D.E., Vickerman, R.J., Mosier, P.E.: Tartaric acid derivatives in fuel compositions. US Patent 8,133,290, 13 March 2012
Heinz, W.E., Schiermeier, K.F.: Corrosion inhibiting composition. US Patent No. 2,482,517, 20 Sept 1949
Haviland, M.L., Goodwin, M.C.: Fuel economy improvements with friction-modified engine oils in Environmental Protection Agency and road tests. SAE Technical Paper No. 790945 (1979)
Dasai, M.: Lubricating oil composition. US Patent 5064546 (1991)
Horodysky, A.G., Gemmill, Jr. R.M.: Mixed borate esters and their use as lubricant and fuel additives. US Patent No. 4,472,289, 18 Sept 1984
Bowden, F.P., Leben, L.: The friction of lubricated metals. Philos. Trans. R. Soc. Lond. A239, 1–27 (1940)
Spikes, H.A., Cameron, A.: A comparison of adsorption and boundary lubricant failure. Proc. R. Soc. Lond. A336, 407–419 (1974)
Okabe, H., Masuko, M., Sakurai, K.: Dynamic behavior of surface-adsorbed molecules under boundary lubrication. ASLE Trans. 24, 467–473 (1981)
Jahanmir, S., Beltzer, M.: An adsorption model for friction in boundary lubrication. ASLE Trans. 29, 423–430 (1986)
Hardy, W., Bircumshaw, I.: Bakerian lecture. Boundary lubrication. Plane surfaces and the limitations of Amontons’ law. Proc. Roy. Soc. Lond. A108, 1–27 (1925)
Beare, W.G., Bowden, F.P.: Physical properties of surfaces. I. Kinetic friction. Philos. Trans. R. Soc. Lond. A234, 329–354 (1935)
Bowden, F.P., Gregory, J.N., Tabor, D.: Lubrication of metal surfaces by fatty acids. Nature 15, 97–101 (1945)
Jahanmir, S.: Chain length effects in boundary lubrication. Wear 102, 331–349 (1985)
Studt, P.: Boundary lubrication: adsorption of oil additives on steel and ceramic surfaces and its influence on friction and wear. Tribol. Int. 22, 111–119 (1989)
Cottington, R.L., Shafrin, E.G., Zisman, W.A.: Physical properties of monolayers at the solid/air interface. III. Friction and durability of films on stainless steel. J. Phys. Chem. 62, 513–518 (1958)
Jahanmir, S., Beltzer, M.: Effect of additive molecular structure on friction coefficient and adsorption. Trans. ASME J. Tribol. 108, 109–116 (1986)
Campen, S.M.: Fundamentals of organic friction modifier behaviour. PhD Thesis, Imperial College London (2012)
Whitehead, J.R.: Surface deformation and friction of metals at light loads. Proc. R. Soc. Lond. A201, 109–124 (1950)
Deryaguin, B.V., Karassev, V.V., Zakhavaeva, N.N., Lazarev, V.P.: The mechanism of boundary lubrication and the properties of the lubricating film: short- and long-range action in the theory of boundary lubrication. Wear 1, 277–290 (1958)
Briscoe, B.J., Evans, D.C.B.: The shear properties of Langmuir–Blodgett layers. Proc. R. Soc. Lond. A 380, 389–407 (1982)
Frewing, J.J.: The influence of temperature on boundary lubrication. Proc. R. Soc. Lond. A181, 23–42 (1942)
Bowden, F.P., Gregory, J.N., Tabor, D.: Lubrication of metal surfaces by fatty acids. Nature 156, 97–101 (1945)
Salem, L.: Attractive forces between long saturated chains at short distances. J. Chem. Phys. 37, 2100–2113 (1962)
Beltzer, M., Jahanmir, S.: Role of dispersion interactions between hydrocarbon chains in boundary lubrication. ASLE Trans. 30, 47–54 (1987)
Studt, P.: The influence of the structure of isomeric octadecanols on their adsorption from solution on iron and their lubricating properties. Wear 70, 329–334 (1981)
Bowden, F.P., Moore, A.C.: Physical and chemical adsorption of long chain compounds on radioactive metals. Trans. Faraday Soc. 47, 900–908 (1951)
Cook, H.D., Ries Jr, H.: Adsorption of radiostearic acid and radiostearyl alcohol from n-hexadecane onto solid surfaces. J. Phys. Chem. 63, 226–230 (1959)
Gaines, G.L.: Some observations on monolayers of carbon-14 labeled stearic acid. J. Colloid Sci. 15, 321–339 (1960)
Block, A., Simms, B.B.: Desorption and exchange of adsorbed octadecylamine and stearic acid on steel and glass. J. Colloid Interface Sci. 25, 514–518 (1967)
Allara, D.L., Nuzzo, R.G.: Spontaneously organized molecular assemblies. 1. Formation, dynamics, and physical properties of n-alkanoic acids adsorbed from solution on an oxidized aluminum surface. Langmuir 1, 45–52 (1985)
Hutchinson, E.: On adsorption and lubrication at crystal surfaces. Part II. On the adsorption of paraffin chain compounds on sodium nitrate. Trans. Faraday Soc. 43, 439–442 (1947)
Greenhill, E.B.: The adsorption of long chain polar compounds from solution on metal surfaces. Trans. Faraday Soc. 45, 625–631 (1949)
Daniel, S.G.: The adsorption on metal surfaces of long chain polar compounds from hydrocarbon solutions. Trans. Faraday Soc. 47, 1345–1359 (1951)
Hirst, W., Lancaster, J.K.: Effect of water on the interaction between stearic acid and fine powders. Trans. Faraday Soc. 47, 315–322 (1951)
Askwith, T.C., Cameron, A., Crouch, R.F.: Chain length of additives in relation to lubricants in thin film and boundary lubrication. Proc. R. Soc. Lond. A291, 500–519 (1966)
Grew, W., Cameron, A.: Friction transition temperature effect of matching surfactant and carrier. Nature 214, 429–430 (1967)
Okabe, H., Kanno, T.: Behavior of polar compounds in lubricating-oil films. ASLE Trans. 24, 459–466 (1981)
Hirano, F., Sakai, T., Kuwano, N., Ohno, N.: Chain matching between hydrocarbon and fatty acid as interfacial phenomena. Tribol. Int. 20, 186–204 (1987)
Georges, J.M., Tonck, A., Mazuyer, D.: Interfacial friction of wetted monolayers. Wear 175, 59–62 (1994)
Zhu, Y., Ohtani, H., Greenfield, M.L., Ruths, M., Granick, S.: Modification of boundary lubrication by oil-soluble friction modifier additives. Tribol. Lett. 15, 127–134 (2003)
Mazuyer, D., Cayer-Barrioz, J., Tonck, A., Jarnias, F.: Friction dynamics of confined weakly adhering boundary layers. Langmuir 24, 3857–3866 (2008)
Lundgren, S.M., Ruths, M., Danerlöv, K., Persson, K.: Effects of unsaturation on film structure and friction of fatty acids in a model base oil. J. Colloid Interface Sci. 326, 530–536 (2008)
Slough, C.G., Ohtani, H., Everson, M.P., Melotik, D.J.: The effect of friction modifiers on the low-speed friction characteristics of automatic transmission fluids observed with scanning force microscopy. SAE Technical Paper No. 981099 (1998)
Ruths, M., Lundgren, S., Danerlöv, K., Persson, K.: Friction of fatty acids in nanometer-sized contacts of different adhesive strength. Langmuir 24, 1509–1516 (2008)
Cheng, H., Hu, Y.: Influence of chain ordering on frictional properties of self-assembled monolayers (SAMS) in nano-lubrication. Adv. Colloid Interface Sci. 171–172, 53–65 (2012)
Campen, S., Green, J.H., Lamb, G.D., Spikes, H.A.: In situ study of model organic friction modifiers using liquid cell AFM: self-assembly of octadecylamine. Tribol. Lett. 58, 1–15 (2015)
Lundgren, S.M., Persson, K., Kronberg, B., Claesson, P.M.: Adsorption of fatty acids from alkane solution studied with quartz crystal microbalance. Tribol. Lett. 22, 15–20 (2006)
Ratoi, M., Anghel, V., Bovington, C., Spikes, H.A.: Mechanisms of oiliness additives. Tribol. Int. 33, 241–247 (2000)
Campana, M., Teichert, A., Clarke, S., Steitz, R., Webster, J.R., Zarbakhsh, A.: Surfactant adsorption at the metal–oil interface. Langmuir 27, 6085–6090 (2011)
Hirayama, T., Torii, T., Konishi, Y., Maeda, M., Matsuoka, T., Inoue, K., Takeda, M.: Thickness and density of adsorbed additive layer on metal surface in lubricant by neutron reflectometry. Tribol. Int. 54, 100–105 (2012)
Allara, D.L., Nuzzo, R.G.: Spontaneously organized molecular assemblies. 2. Quantitative infrared spectroscopic determination of equilibrium structures of solution-adsorbed n-alkanoic acids on an oxidized aluminum surface. Langmuir 1, 52–66 (1985)
Marrucci, L., Paparo, D., Cerrone, G., Solimeno, S., Russo, R., Lenza, T.L., Siano, P.: Optical analysis of surfaces by second-harmonic generation: possible applications to tribology. Tribotest 8, 329–337 (2002)
Koshima, H., Kamano, H., Hisaeda, Y., Liu, H., Ye, S.: Analyses of the adsorption structures of friction modifiers by means of quantitative structure-property relationship method and sum frequency generation spectroscopy. Tribol. Online 5, 165–172 (2010)
Porter, M.D., Bright, T.B., Allara, D.L., Chidseyi, C.E.D.: Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry. J. Am. Chem. Soc. 109, 3559–3568 (1987)
Maoz, R., Sagiv, J.: On the formation and structure of self-assembling monolayers I A comparative ATR-wettability study of Langmuir-Blodgett and adsorbed films on flat substrates and glass microbeads. J. Colloid Interface Sci. 100, 465–496 (1984)
Tsukruk, V.V., Bliznyuk, V.N., Hazel, J., Visser, D., Everson, M.P.: Organic molecular films under shear forces: fluid and solid Langmuir monolayers. Langmuir 12, 4840–4849 (1996)
Bhushan, B., Liu, H.: Nanotribological properties and mechanisms of alkylthiol and biphenyl thiol self-assembled monolayers studied by AFM. Phys. Rev. B 63, 245412 (2001)
Xiao, X., Hu, J., Charych, D.H., Salmeron, M.: Chain length dependence of the frictional properties of alkylsilane molecules self-assembled on mica studied by atomic force microscopy. Langmuir 1, 235–237 (1996)
Benitez, J.J., Kopta, S., Ogletree, D.F., Salmeron, M.: Preparation and characterization of self-assembled monolayers of octadecylamine on mica using hydrophobic solvents. Langmuir 18, 6096–6100 (2002)
Brewer, N.J., Beake, B.D., Leggett, G.J.: Friction force microscopy of self-assembled monolayers: influence of adsorbate alkyl chain length, terminal group chemistry, and scan velocity. Langmuir 17, 1970–1974 (2001)
Lee, S., Shon, Y.S., Colorado, R., Guenard, R.L., Lee, T.R., Perry, S.S.: The influence of packing densities and surface order on the frictional properties of alkanethiol self-assembled monolayers (SAMs) on gold: a comparison of SAMs derived from normal and spiroalkanedithiols. Langmuir 16, 2220–2224 (2000)
Salmeron, M.: Generation of defects in model lubricant monolayers and their contribution to energy dissipation in friction. Tribol. Lett. 10, 69–79 (2001)
Campen, S., Green, J.H., Lamb, G.D., Spikes, H.A.: In situ study of model organic friction modifiers using liquid cell AFM; saturated and mono-unsaturated carboxylic acids. Tribol. Lett. 57, 1–20 (2015)
Glovnea, R.P., Forrest, A.K., Olver, A.V., Spikes, H.A.: Measurement of sub-nanometer lubricant films using ultra-thin film interferometry. Tribol. Lett. 15, 217–230 (2003)
Fraenkel, R., Butterworth, G.E., Bain, C.D.: In situ vibrational spectroscopy of an organic monolayer at the sapphire–quartz interface. J. Am. Chem. Soc. 120, 203–204 (1998)
Beattie, D.A., Haydock, S., Bain, C.D.: A comparative study of confined organic monolayers by Raman scattering and sum-frequency spectroscopy. Vib. Spectrosc. 24, 109–123 (2000)
Jacob, J.D.C., Rittikulsittichai, S., Lee, T.R., Baldelli, S.: Characterization of SAMs derived from octadecyloxyphenylethanethiols by sum frequency generation. J. Phys. Chem. C 117, 9355–9365 (2013)
Beattie, D.A., Fraenkel, R., Winget, S.A., Petersen, A., Bain, C.D.: Sum-frequency spectroscopy of a monolayer of zinc arachidate at the solid-solid interface. J. Phys. Chem. B 110, 2278–2292 (2006)
Miyake, K., Kume, T., Nakano, M., Korenaga, A., Takiwatari, K., Tsuboi, R., Sasaki, S.: Effects of surface chemical properties on the frictional properties of self-assembled monolayers lubricated with oleic acid. Tribol. Online 7, 218–224 (2012)
Simič, R., Kalin, M., Hirayama, T., Korelis, P., Geue, T.: Fatty acid adsorption on several DLC coatings studied by neutron reflectometry. Tribol. Lett. 53, 199–206 (2014)
Nakano, K., Spikes, H.A.: Initial process of boundary film formation with fatty acid solution. Tribol. Online 7, 1–7 (2012)
Walba, D.M., Liberko, C.A.: Self-assembled monolayers for liquid crystal alignment. US Patent No. 5,596,434, 21 Jan 1997
Campen, S., Green, J.H., Lamb, G.D., Atkinson, D., Spikes, H.A.: On the increase in boundary friction with sliding speed. Tribol. Lett. 48, 237–248 (2012)
Topolovec-Miklozic, K., Forbus, T.R., Spikes, H.A.: Performance of friction modifiers on ZDDP-generated surfaces. Tribol. Trans. 50, 328–335 (2007)
Vengudusamy, B.: Behaviour of lubricant additives on DLC coatings. PhD Thesis, Imperial College London (2011)
Kano, M., Yasuda, Y., Okamoto, Y., Mabuchi, Y., Hamada, T., Ueno, T., Yec, J., Konishi, S., Takeshima, S., Martin, J.M., De Barros Bouchet, M.I., Le Mogne, T.: Ultralow friction of DLC in presence of glycerol mono-oleate (GMO). Tribol. Lett. 18, 245–251 (2005)
Minami, I., Kubo, T., Nanao, H., Mori, S., Sagawa, T., Okuda, S.: Investigation of tribo-chemistry by means of stable isotopic tracers, Part 2: lubrication mechanism of friction modifiers on diamond-like carbon. Tribol. Trans. 50, 477–487 (2007)
Glosli, J.N., McClelland, G.M.: Molecular dynamics study of sliding friction of ordered organic monolayers. Phys. Rev. Lett. 70, 1960–1963 (1993)
Koike, A., Yoneya, M.: Molecular dynamics simulations of sliding friction of Langmuir–Blodgett monolayers. J. Chem. Phys. 105, 6060–6067 (1996)
Koike, A., Yoneya, M.: Effects of molecular structure on frictional properties of Langmuir–Blodgett monolayers. Langmuir 13, 1718–1722 (1997)
Mikulski, P.T., Herman, L.A., Harrison, J.A.: Odd and even model self-assembled monolayers: links between friction and structure. Langmuir 21, 12197–12206 (2005)
Wu, C.D., Lin, J.F., Fang, T.H.: Molecular dynamic simulation and characterization of self-assembled monolayer under sliding friction. Comput. Mater. Sci. 39, 808–816 (2007)
Moller, M.A., Tildesley, D.J., Kim, K.S., Quirke, N.: Molecular dynamics simulation of a Langmuir–Blodgett film. J. Chem. Phys. 94, 8390–8401 (1991)
Doig, M., Camp, P.J.: The structures of hexadecylamine films adsorbed on iron-oxide surfaces in dodecane and hexadecane. Phys. Chem. Chem. Phys. 17, 5248–5255 (2015)
Doig, M., Warrens, C.P., Camp, P.J.: Structure and friction of stearic acid and oleic acid films adsorbed on iron oxide surfaces in squalane. Langmuir 30, 186–195 (2013)
Loehle, S., Matta, C., Minfray, C., Le Mogne, T., Martin, J.M., Iovine, R., Obara, Y., Miura, R., Miyamoto, A.: Mixed lubrication with C18 fatty acids: effect of unsaturation. Tribol. Lett. 53, 319–328 (2014)
Cameron, A.: A theory of boundary lubrication. ASLE Trans. 2, 195–198 (1959)
Drauglis, E., Lucas, A.A., Allen, C.M.: Smectic model for liquid films on solid surfaces. Part 1—application to monolayer boundary lubrication. Spec. Discuss. Faraday Soc. 1, 251–256 (1970)
Sutcliffe, M.J., Taylor, S.R., Cameron, A.: Molecular asperity theory of boundary friction. Wear 51, 181–192 (1978)
Tabor, D.: The role of surface and intermolecular forces in thin film lubrication. Tribology series. In: Georges, J.M. (ed.) Microscopic Aspects of Adhesion and Lubrication, vol. 7, pp. 651–682. Elsevier, Amsterdam (1982)
Dorinson, A.: The slow speed frictional behavior of some lubricant additive type-substances. ASLE Trans. 13, 215–224 (1970)
Chugg, K.J., Chaudhri, M.M.: Boundary lubrication and shear properties of thin solid films of dioctadecyl dimethyl ammonium chloride (TA 100). J. Phys. D Appl. Phys. 26, 1993–2000 (1993)
Ingram, M., Noles, J., Watts, R., Harris, S., Spikes, H.A.: Frictional properties of automatic transmission fluids: Part 1: measurement of friction-sliding speed behaviour. Tribol. Trans. 54, 145–153 (2011)
Drummond, C., Israelachvili, J., Richetti, Ph: Friction between two weakly adhering boundary lubricated surfaces in water. Phys. Rev. E 67, 066110 (2003)
Yoshizawa, H., Chen, Y.L., Israelachvili, J.: Fundamental mechanisms of interfacial friction. 1. Relation between adhesion and friction. J. Phys. Chem. 97, 4128–4140 (1993)
Spikes, H.A.: The half-wetted bearing. Part 2: potential application in low load contacts. Proc. Inst. Mech. Eng. J. 217, 15–26 (2003)
Spikes, H.A.: Slip at the wall—evidence and tribological implications. In: Dowson, D., et al. (ed.) Proceedings of 29th Leeds/Lyon Symposium, Tribological Research and Design for Engineering Systems, pp. 525–535. Elsevier, Amsterdam (2003)
Choo, J.H., Spikes, H.A., Ratoi, M., Glovnea, R., Forrest, A.: Friction reduction in low-load hydrodynamic lubrication with a hydrophobic surface. Tribol. Int. 40, 154–159 (2007)
Hare, E.F., Zisman, W.A.: Autophobic liquids and the properties of their adsorbed films. J. Phys. Chem. 59, 335–340 (1954)
Choo, J.-H., Forrest, A.K., Spikes, H.A.: Influence of organic friction modifier on liquid slip: a new mechanism of organic friction modifier action. Tribol. Lett. 27, 239–244 (2007)
Briscoe, B.J., Evans, D.C.B., Tabor, D.: The influence of contact pressure and saponification on the sliding behavior of stearic acid monolayers. J. Colloid Interface Sci. 61, 9–13 (1977)
Briscoe, B.J., Tabor, D.: Rheology of thin organic films. ASLE Trans. 17, 158–165 (1974)
Cooper, H.S., Damerell, V.R.: Lubricants suitable for various uses. US Patent 2156803 (1939)
Risdon, T.J., Gresty, D.A.: An historical review of reductions in fuel consumption of United States and European engines with MoS2. SAE Technical Paper 750674 (1975)
White, H.S., Zei, D.: Static friction tests with various metal combinations and special lubricants. J. Res. Nat. Bur. Stand. 46, 292–298 (1951)
Gansheimer, J., Holinski, R.: Molybdenum disulfide in oils and greases under boundary conditions. Trans. ASME J. Tribol. 95, 242–246 (1973)
Gänsheimer, J., Holinski, R.: A study of solid lubricants in oils and greases under boundary conditions. Wear 19, 439–449 (1972)
Hugel, G.: Fragen der Schmierőlforschung. Erdol und Kohle 8, 651–655 (1955)
Hugel, G.: Bleu de molybène soluble dans les hydrocarbures. French Patent FR1099953A (1955)
Spengler, G., Weber, A.: Über die Schmierfähigkeit organischer Molybdänverbindungen. Chem. Ber. 92, 2163–2171 (1959)
Feng, I.M., Perilstein, W.L., Adams, M.R.: Solid film deposition and non-sacrificial boundary lubrication. ASLE Trans. 6, 60–66 (1963)
Black, A.L., Dunster, R.W.: Comparative study of surface deposits and behaviour of MoS2 particles and molybdenum dialkyl-dithio-phosphate. Wear 13, 119–132 (1969)
Gresty, D.A., Kunz, E.J., Risdon, T.J.: The effect of MoS2 based lubricants on automotive gear efficiency and operating temperatures. SAE Technical Paper 770834 (1977)
Braithwaite, E.R., Greene, A.B.: A critical analysis of the performance of molybdenum compounds in motor vehicles. Wear 46, 405–432 (1978)
Greene, A.B., Risdon, T.J.: The effect of molybdenum-containing, oil-soluble friction modifiers on engine fuel economy and gear oil efficiency. SAE Technical Paper 811187 (1981)
Sakurai, T., Okabe, H., Isoyama, H.: The synthesis of di-μ-thio-dithio-bis (dialkyldithiocarbamates) dimolybdenum (V) and their effects on boundary lubrication. Bull. Jpn. Pet. Inst. 13, 243–249 (1971)
Mitchell, P.C.: Oil-soluble Mo–S compounds as lubricant additives. Wear 100, 281–300 (1984)
Yamamoto, Y., Gondo, S., Kamakura, T., Tanaka, N.: Frictional characteristics of molybdenum dithiophosphates. Wear 112, 79–87 (1986)
Graham, J., Korcek, S., Spikes, H.A.: The friction-reducing properties of molybdenum dialkyldithiocarbamate additives. Part 1. Factors influencing friction reduction. Tribol. Trans. 44, 626–636 (2001)
Graham, J.C.H.: The behaviour of molybdenum dialkyldithiocarbamate friction modifier additives. PhD Thesis, University of London (2001)
Yamamoto, Y., Gondo, S.: Friction and wear characteristics of molybdenum dithiocarbamate and molybdenum dithiophosphate. Tribol. Trans. 32, 251–257 (1989)
Gondo, S., Konishi, M.: Organoamine and organophosphate molybdenum complexes as lubricant additives. Wear 12, 51–60 (1987)
Grossiord, C., Varlot, K., Martin, J.M., Le Mogne, Th, Esnouf, C., Inoue, K.: MoS2 Single sheet lubrication by molybdenum dithiocarbamate. Tribol. Int. 31, 737–743 (1998)
Evans, R.D., Doll, G.L., Hager, C.H., Howe, J.Y.: Influence of steel type on the propensity for tribochemical wear in boundary lubrication with a wind turbine gear oil. Tribol. Lett. 38, 25–32 (2010)
Topolovec Miklozic, K., Graham, J., Spikes, H.: Chemical and physical analysis of reaction films formed by molybdenum dialkyldithiocarbamate friction modifier additive using Raman and atomic force microscopy. Tribol. Lett. 11, 71–81 (2001)
Unnikrishnan, R., Jain, M.C., Harinarayan, A.K., Mehta, A.K.: Additive–additive interaction: an XPS study of the effect of ZDDP on the AW/EP characteristics of molybdenum based additives. Wear 252, 240–249 (2002)
Windom, B.C., Sawyer, W.G., Hahn, D.W.: A Raman spectroscopic study of MoS2 and MoO3: applications to tribological systems. Tribol. Lett. 42, 301–310 (2011)
Muraki, M., Yanagi, Y., Sakaguchi, K.: Synergistic effect on frictional characteristics under rolling-sliding conditions due to a combination of molybdenum dialkyldithiocarbamate and zinc dialkyldithiophosphate. Tribol. Int. 30, 69–75 (1997)
Kasrai, M., Cutler, J.N., Gore, K., Canning, G., Bancroft, G.M., Tan, K.H.: The chemistry of antiwear films generated by the combination of ZDDP and MoDTC examined by X-ray absorption spectroscopy. Tribol. Trans. 41, 69–77 (1998)
Martin, J.M., Grossiord, C., Varlot, K., Vacher, B., Igarashi, J.: Synergistic effects in binary systems of lubricant additives: a chemical hardness approach. Tribol. Lett. 8, 193–201 (2000)
Graham, J., Jensen, R., Spikes, H.A.: The friction-reducing properties of molybdenum dialkyldithiocarbamate additives. Part 2. Durability of friction reducing capability. Tribol. Trans. 44, 637–646 (2001)
Morina, A., Neville, A.: Understanding the composition and low friction tribofilm formation/removal in boundary lubrication. Tribol. Int. 40, 1696–1704 (2007)
Spikes, H.A.: Additive-additive and additive-surface interactions in lubrication. Lubr. Sci. 2, 3–23 (1989)
Topolovec-Miklozic, K., Cann, P.M., Spikes, H.A.: The use of AFM to study lubricant films. In: Franek, F., et al. (ed.) Plenary and Session Key Papers. 2nd WTC Conference, Vienna (2001)
Ye, J., Kano, M., Yasuda, Y.: Determination of nanostructures and mechanical properties on the surface of molybdenum dithiocarbamate and zinc dialkyl-dithiophosphate tribochemical reacted films using atomic force microscope phase imaging technique. J. Appl. Phys. 93, 5113–5117 (2003)
Ye, J., Kano, M., Yasuda, Y.: Evaluation of nanoscale friction depth distribution in ZDDP and MoDTC tribochemical reacted films using a nanoscratch method. Tribol. Lett. 16, 107–112 (2004)
Johnson, M.D., Jensen, R.K., Clausing, E.M., Schriewer, K., Korcek, S.: Effects of aging on frictional properties of fuel efficient engine oils. SAE Technical Paper 952532 (1995)
Johnson, M.D., Jensen, R.K., Korcek, S.: Base oil effects on friction reducing capabilities of molybdenum dialkyldithiocarbamate containing engine oils. SAE Technical Paper 972860 (1997)
Hoshino, K., Kawai, H., Akiyama, K.: Fuel efficiency of SAE 5 W-20 friction modified gasoline engine oil. SAE Technical Paper 982506 (1998)
de Barros’ Bouchet, M.I., Martin, J.M., Le-Mogne, T., Vacher, B.: Boundary lubrication mechanisms of carbon coatings by MoDTC and ZDDP additives. Tribol. Int. 38, 257–264 (2005)
Topolovec-Miklozic, K., Lockwood, F., Spikes, H.: Behaviour of boundary lubricating additives on DLC coatings. Wear 265, 1893–1901 (2008)
Shinyoshi, T., Fuwa, Y., Ozaki, Y.: Wear analysis of DLC coating in oil containing Mo-DTC. SAE Technical Paper 2007-01-1969 (2007)
Haque, T., Morina, A., Neville, A., Kapadi, R., Arrowsmith, S.: Effect of oil additives on the durability of hydrogenated DLC coating under boundary lubrication conditions. Wear 266, 147–157 (2009)
Vengudusamy, B., Green, J.H., Lamb, G.D., Spikes, H.A.: Behaviour of MoDTC in DLC/DLC and DLC/steel contacts. Tribol. Int. 54, 68–76 (2012)
Sugimoto, I., Honda, F., Inoue, K.: Analysis of wear behavior and graphitization of hydrogenated DLC under boundary lubricant with MoDTC. Wear 305, 124–128 (2013)
Masuko, M., Ono, T., Aoki, S., Suzuki, A., Ito, H.: Friction and wear characteristics of DLC coatings with different hydrogen content lubricated with several Mo-containing compounds and their related compounds. Tribol. Int. 82, 350–357 (2015)
Coffey, T.A., Forster, G.D., Hogarth, G.: Molybdenum(VI) imidodisulfur complexes formed via double sulfur–carbon bond cleavage of dithiocarbamates. J. Chem. Soc., Dalton Trans. 2, 183–193 (1996)
Sarin, R., Tuli, D.K., Sureshbabu, A.V., Misra, A.K., Rai, M.M., Bhatnagar, A.K.: Molybdenum dialkylphosphorodithioates: synthesis and performance evaluation as multifunctional additives for lubricants. Tribol. Int. 27, 379–386 (1994)
Tanaka, N., Fukushima, A., Tatsumi, Y., Saito, Y.: A molybdenum dithiocarbamate, improved stability and solubility. US Patent 5,627,146, 6 May 1997
Tynik, R.J., Donnelly, S.G., Karol, T.J: Additive for lubricating oil compositions, comprising the reaction product of: at least one asymmetrical dialkylamine, carbon disulfide, and a molybdenum source. US Patent 7,763,744, 27 July 2010
Yajun, M., Wancheng, Z., Shenghua, L., Yuansheng, J., Yucong, W., Simon, T.: Tribological performance of three advanced piston rings in the presence of MoDTC-modified GF-3 oils. Tribol. Lett. 18, 75–83 (2005)
Hu, J.Q., Wei, X.Y., Dai, G.L., Fei, Y.W., Xie, F., Zong, Z.M.: Tribological behaviors and mechanism of sulfur- and phosphorus-free organic molybdate ester with zinc dialkyldithiophosphate. Tribol. Intern. 41, 549–555 (2008)
Yan, L., Yue, W., Wang, C., Wei, D., Xu, B.: Comparing tribological behaviors of sulfur- and phosphorus-free organomolybdenum additive with ZDDP and MoDTC. Tribol. Int. 53, 150–158 (2012)
Okrent, E.H.: The effect of lubricant viscosity and composition on engine friction and bearing wear. ASLE Trans. 4, 97–108 (1961)
Okrent, E.H.: The effect of lubricant viscosity and composition on engine friction and bearing wear. II. ASLE Trans. 4, 257–262 (1961)
Cann, P.M., Spikes, H.A.: The behavior of polymer solutions in concentrated contacts: immobile surface layer formation. Tribol. Trans. 37, 580–586 (1994)
Georges, J.M., Millot, S., Loubet, J.L., Tonck, A.: Drainage of thin liquid films between relatively smooth surfaces. J. Chem. Phys. 98, 7345–7360 (1993)
Smeeth, M., Gunsel, S., Spikes, H.A.: Boundary film formation by viscosity index improvers. Tribol. Trans. 39, 726–734 (1996)
Guangteng, G., Smeeth, M., Cann, P.M., Spikes, H.A.: Measurement and modelling of boundary film properties of polymeric lubricant additives. Proc. Inst. Mech. Eng. J. 210, 1–15 (1996)
Smeeth, M., Gunsel, S., Spikes, H.A.: Friction and wear reduction by boundary film-forming viscosity index improvers. SAE Technical Paper 962037 (1996)
Georges, E., Georges, J.M., Diraison, C.: Rheology of olefinic copolymer layers adsorbed on solid surfaces. Tribol. Trans. 39, 563–570 (1996)
Müller, M., Topolovec-Miklozic, K., Dardin, A., Spikes, H.A.: The design of boundary film-forming PMA viscosity modifiers. Tribol. Trans. 49, 225–232 (2006)
Fan, J., Stohr, T., Muller, M., Spikes, H.A.: Reduction of friction by functionalized viscosity index improvers. Tribol. Lett. 28, 287–298 (2007)
Munch, M.R., Gast, A.P.: A study of block copolymer adsorption kinetics via internal reflection interferometry. J. Chem. Soc., Faraday Trans. 86, 1341–1348 (1990)
Chevalier, Y., Fixari, B., Brunel, S., Marie, E., De Guio, P.: Review: the adsorption of functional polymers from their organic solutions: applications to fuel additives. Polym. Int. 53, 475–483 (2004)
Brittain, W.J., Minko, S.: A structural definition of polymer brushes. J. Polym. Sci. A Polym. Chem. 45, 3505–3512 (2007)
Muller, M., Fan, J., Spikes, H.A.: Design of functionalised PAMA viscosity modifiers to reduce friction and wear in lubricating oils. ASTM Int. 4, Paper ID JAI100956 (2007)
Muller, M., Fan, J., Spikes, H.A.: Influence of polymethacrylate viscosity index improvers on friction and wear of lubricant formulations. SAE Technical Paper 2007-01-1985 (2007)
Thompson, L, Randles, S.J., Boyde, S., Gamwell, J., Readman, N.: Friction reducing additive. US Patent Appl. 13/582,589, 3 March 2011
Lee, S., Müller, M., Ratoi-Salagean, M., Vörös, J., Pasche, S., De Paul, S.M., Spikes, H.A., Textor, M., Spencer, N.D.: Boundary lubrication of oxide surfaces by poly(l-lysine)-g-poly (ethylene glycol)(PLL-g-PEG) in aqueous media. Tribol. Lett. 15, 231–239 (2003)
Müller, M., Lee, S., Spikes, H.A., Spencer, N.D.: The influence of molecular architecture on the macroscopic lubrication properties of the brush-like co-polyelectrolyte poly(l-lysine)-g-poly (ethylene glycol)(PLL-g-PEG) adsorbed on oxide surfaces. Tribol. Lett. 15, 395–405 (2003)
Yan, X., Perry, S.S., Spencer, N.D., Pasche, S., De Paul, S.M., Textor, M., Lim, M.S.: Reduction of friction at oxide interfaces upon polymer adsorption from aqueous solutions. Langmuir 20, 423–428 (2004)
Müller, M.T., Yan, X., Lee, S., Perry, S.S., Spencer, N.D.: Lubrication properties of a brushlike copolymer as a function of the amount of solvent absorbed within the brush. Macromolecules 38, 5706–5713 (2005)
Klein, J., Kumacheva, E., Mahalu, D., Perahia, D., Fetters, L.J.: Reduction of frictional forces between solid surfaces bearing polymer brushes. Nature 370, 634–636 (1994)
Kreer, T., Müser, M.H., Binder, K., Klein, J.: Frictional drag mechanisms between polymer-bearing surfaces. Langmuir 17, 7804–7813 (2001)
Klein, J.: Molecular mechanisms of synovial joint lubrication. Proc. Inst. Mech. Eng. J. 220, 691–710 (2006)
Klein, J., Kumacheva, E., Perahia, D., Fetters, L.J.: Shear forces between sliding surfaces coated with polymer brushes: the high friction regime. Acta Polym. 49, 617–625 (1998)
Nomura, A., Okayasu, K., Ohno, K., Fukuda, T., Tsujii, Y.: Lubrication mechanism of concentrated polymer brushes in solvents: effect of solvent quality and thereby swelling state. Macromolecules 44, 5013–5019 (2011)
Bielecki, R.M., Benetti, E.M., Kumar, D., Spencer, N.D.: Lubrication with oil-compatible polymer brushes. Tribol. Lett. 45, 477–487 (2012)
Mitsui, H., Spikes, H.A.: Predicting EHD film thickness of lubricant polymer solutions. Tribol. Trans. 41, 1–10 (1998)
Mertes, R.W.: Modified lubricating oil. US Patent No. 2,501,731, 28 March 1950
Peri, J.B.: The state of dispersion of detergent additives in lubricating oil and other hydrocarbons. J. Am. Oil Chem. Soc. 35, 110–117 (1958)
Mansot, J.L., Hallouis, M., Martin, J.M.: Colloidal antiwear additives. 2. Tribological behaviour of colloidal additives in mild wear regime. Colloids Surf. A 75, 25–31 (1993)
Topolovec-Miklozic, K., Forbus, T.R., Spikes, H.A.: The film-forming and friction properties of overbased calcium sulphonate detergents. Tribol. Lett. 29, 33–44 (2008)
Bennington, J.E., Cole, D.E., Ghirla, P.J., Smith, R.K.: Stable colloid additives for engine oils—potential improvement in fuel economy. SAE Technical Paper 750677 (1975)
Reick, F.G.: Lubricant oil containing polytetrafluoroethylene and fluorochemical surfactant. US Patent No. 4,224,173, 23 Sept 1980
Reick, F.G.: Energy saving lubricants containing colloidal PTFE. Lubr. Eng. 38, 635–646 (1982)
Chinas, F.C.: The behaviour of colloids in lubricated contacts. PhD Thesis, University of London (2000)
Bakunin, V.N., Suslov, AYu., Kuzmina, G.N., Parenago, O.P.: Recent achievements in the synthesis and application of inorganic nanoparticles as lubricant components. Lubr. Sci. 17, 127–145 (2005)
Martin, J.M., Ohmae, N.: Nanolubricants, vol. 13. Wiley, New York (2008)
Zhou, J., Wu, Z., Zhang, Z., Liu, W., Xue, Q.: Tribological behaviour and lubricating mechanism of Cu nanoparticles in oil. Tribol. Lett. 8, 213–218 (2000)
Qiu, S., Zhou, Z., Dong, J., Chen, G.: Preparation of Ni nanoparticles and evaluation of their tribological performance as potential antiwear additives in oils. Trans. ASME. J. Tribol. 123, 441–443 (2001)
Chinas-Castillo, F., Spikes, H.A.: The behavior of colloidal solid particles in elastohydrodynamic contacts. Tribol. Trans. 43, 387–394 (2000)
Chinas-Castillo, F., Spikes, H.A.: The lubricating properties of dilute colloidal solid dispersions. In: Proceedings of ITC Conference, Nagasaki, Oct. 2000, vol. 1, pp. 649–654. Publ. JST 2001, Tokyo
Xue, Q., Liu, W., Zhang, Z.: Friction and wear properties of a surface-modified TiO2 nanoparticle as an additive in liquid paraffin. Wear 213, 29–32 (1997)
Hu, Z.S., Dong, J.X., Chen, G.X.: Study of antiwear and reducing friction additive of nanometer ferric oxide. Tribol. Int. 31, 355–360 (1998)
Zhang, Z., Liu, W., Xue, Q.: Study of lubricating mechanism of La(OH)3 nanocluster modified by compound containing nitrogen in liquid paraffin. Wear 218, 139–144 (1998)
Battez, A., Hernández, R., González, J.L., Viesca, J.L., Fernández, J.E., Díaz Fernández, J.M., Machado, A., Chou, R., Riba, J.: CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 265, 422–428 (2008)
Gupta, B.K., Bhushan, B.: Fullerene particles as an additive to liquid lubricants and greases for low friction and wear. Lubr. Eng. 50, 524–528 (1994)
Ginzburg, B.M., Kireenko, O.F., Shepelevskii, A.A., Shibaev, L.A., Tochilnikov, D.G., Leksovskii, A.M.: Thermal and tribological; properties of fullerene-containing composite systems. Part 2. Formation of tribo-polymer films during boundary sliding friction in the presence of fullerene C60. J. Macromol. Sci. B 44, 93–115 (2005)
Joly-Pottuz, L., Vacher, B., Ohmae, N., Martin, J.M., Epicier, T.: Anti-wear and friction reducing mechanisms of carbon nano-onions as lubricant additives. Tribol. Lett. 30, 69–80 (2008)
Chen, C.S., Chen, X.H., Xu, L.S., Yang, Z., Li, W.H.: Modification of multi-walled carbon nanotubes with fatty acid and their tribological properties as lubricant additive. Carbon 43, 1660–1666 (2005)
Peng, Y., Hu, Y., Wang, H.: Tribological behaviors of surfactant-functionalized carbon nanotubes as lubricant additives in water. Tribol. Lett. 25, 247–253 (2006)
Zhang, W., Zhou, M., Zhu, H., Tian, Y., Wang, K., Wei, J., Ji, F., Li, X., Zhang, P., Wu, D.: Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 44, 205303 (2011)
Lin, J., Wang, L., Chen, G.: Modification of graphene platelets and their tribological properties as a lubricant additive. Tribol. Lett. 41, 209–215 (2011)
Eswaraiah, V., Sankaranarayanan, V., Ramaprabhu, S.: Graphene-based engine oil nanofluids for tribological applications. ACS Appl. Mater. Interface 3, 4221–4227 (2011)
Choudhary, S., Mungse, H.P., Khatri, O.P.: Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications. J. Mater. Chem. 22, 21032–21039 (2012)
Cizaire, L., Vacher, B., Le Mogne, T., Martin, J.M., Rapoport, L., Margolin, A., Tenne, R.: Mechanisms of ultra-low friction by hollow inorganic fullerene-like MoS2 nanoparticles. Surf. Coat. 160, 282–287 (2002)
Tenne, R.: Inorganic nanotubes and fullerene-like nanoparticles. Nat. Nanotech. 1, 103–111 (2006)
Joly-Pottuz, L., Dassenoy, F., Belin, M., Vacher, B., Martin, J.M., Fleischer, N.: Ultra-low friction and wear properties of IF-WS2 under boundary lubrication. Tribol. Lett. 18, 477–485 (2005)
Tevet, O., Von-Huth, P., Popovitz-Biro, R., Rosentsveig, R., Wagner, H.D., Tenne, R.: Friction mechanism of individual multilayered nanoparticles. Proc. Natl. Acad. Sci. 108, 19901–19906 (2011)
Lahouij, I., Dassenoy, F., de Knoop, L., Martin, J.M., Vacher, B.: In situ TEM observation of the behavior of an individual fullerene-like MoS2 nanoparticle in a dynamic contact. Tribol. Lett. 42, 133–140 (2011)
Kimura, Y., Wakabayashi, T., Okada, K., Wada, T., Nishikawa, H.: Boron nitride as a lubricant additive. Wear 232, 199–206 (1999)
Reeves, C.J., Menezes, P.L., Lovell, M.R., Jen, T.-C.: The size effect of boron nitride particles on the tribological performance of biolubricants for energy conservation and sustainability. Tribol. Lett. 51, 437–452 (2013)
Lovell, M.R., Kabir, M.A., Menezes, P.L., Higgs, C.F.: Influence of boric acid additive size on green lubricant performance. Philos. Trans. R. Soc. Lond. A368, 4851–4868 (2010)
Rabaso, P., Ville, F., Dassenoy, F., Diaby, M., Afanasiev, P., Cavoret, J., Vacher, B., Le Mogne, T.: Boundary lubrication: influence of the size and structure of inorganic fullerene-like MoS2 nanoparticles on friction and wear reduction. Wear 320, 161–178 (2014)
Chinas Castillo, F., Spikes, H.A.: Mechanism of action of colloidal solid dispersions. Trans. ASME J. Tribol. 125, 552–557 (2003)
Chauveau, V., Mazuyer, D., Dassenoy, F., Cayer-Barrioz, J.: In situ film-forming and friction-reduction mechanisms for carbon-nanotube dispersions in lubrication. Tribol. Lett. 47, 467–480 (2012)
Perfiliev, V., Moshkovith, A., Verdyan, A., Tenne, R., Rapoport, L.: A new way to feed nanoparticles to friction interfaces. Tribol. Lett. 21, 89–93 (2006)
Olomolehin, Y., Kapadia, R.G., Spikes, H.A.: Antagonistic interaction of antiwear additives and carbon black. Tribol. Lett. 37, 49–58 (2009)
Yamamoto, K., Kotaka, A., Umehara, K.: Additives for improving the fuel economy of diesel engine systems. Tribol. Online 5, 195–198 (2010)
Ramachandran, S., Tsai, B.L., Blanco, M., Chen, H., Tang, Y., Goddard, W.A.: Self-assembled monolayer mechanism for corrosion inhibition of iron by imidazolines. Langmuir 12, 6419–6428 (1996)
Lundgren, S.M., Eriksson, K., Rossenaar, B.: Boosting the friction performance of amine friction modifiers with MoDTC. SAE Int. J. Fuels Lubr. 8, 827–830 (2015)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spikes, H. Friction Modifier Additives. Tribol Lett 60, 5 (2015). https://doi.org/10.1007/s11249-015-0589-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-015-0589-z