Abstract
Fe3O4 nanoparticles were prepared by co-precipitation and coated with SiO2 following the Stöber process. N-Amidinoglycine amino acid was then covalently connected to provide an excellent ligand for the immobilization of Pd nanoparticles. The resulting material was characterized by FE-SEM, TEM, EDX, XRD, VSM and ICP-AES analysis. The Fe3O4@SiO2@N-amidinoglycine@Pd0 proved to be a highly active catalyst for the Suzuki coupling reactions of various aryl halides with substituted phenylboronic acids in water, giving the desired products in excellent yields for short reaction times. Moreover, this catalyst can be easily recovered by using an external magnet and directly reused for several times without significant loss of activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic nanoparticles are of great interest in the field of catalysis [1,2,3], biotechnology and biomedical applications such as drug delivery, MRI contrast agents and cancer therapy [4,5,6,7,8]. Magnetic nanoparticles with good stability and ready availability can effectively improve the loading and catalytic efficiency of immobilized catalysts, due to their high surface-to-volume ratios. In terms of recycling expensive catalysts, immobilization of these active species on magnetic nanoparticles leads to facile and quick magnetic separation without the need for filtration, centrifugation or other workup processes [9, 10]. However, surface oxidation and agglomeration of chemically highly active naked magnetic nanoparticles can result in a significant decrease in their magnetic properties, dispersibility, surface area and catalytic activity [11]. Given the strong surface affinity of magnetic nanoparticles toward silica as a chemically inert material with high surface area and good stability, it is often employed as coating layer over the surface of magnetic nanoparticles. This surface modification can weaken the particle–particle magnetic bipolar interactions and protect the nanoparticles from aggregation and oxidation processes. Moreover, silica coating not only stabilizes the nanoparticles, but can also be readily functionalized with amines, carboxyls, thiols and biological species [12, 13]. The Stöber method and sol–gel processes are the preferred choices for the coating of magnetic nanoparticles with silica [14,15,16].
The low cost, good biocompatibility and range of available functional groups of amino acids as capping ligands for magnetic nanoparticles make them an attractive candidate for both coating and surface functionalization. Amino acids interact with the nanoparticle’s surface through their carboxyl groups, while their side chains containing various surfaced-exposed functional groups are available. Available amino acid functional groups include guanidine, thiol and phenolic hydroxyl groups, which are potential reaction sites for further modification [17,18,19].
The palladium-catalyzed Suzuki cross-coupling of organic halides with boronic acids is one of the most versatile methods for the synthesis of biaryls [20]. Biaryls are of significant current interest for the synthesis of biologically active compounds, natural products, macrocycles, liquid-crystal materials and asymmetric catalysts [21,22,23,24]. The increasing need for greener synthetic methodologies has led to a growing interest in the development of catalysis that can operate in aqueous medium. The stability of boronic acids in aqueous solvent is viewed as an advantage of Suzuki coupling compared to other cross-coupling reactions. Recent reviews have covered the literature on palladium-catalyzed Suzuki cross-coupling reactions in water [25, 26].
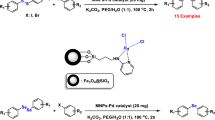
In this paper, we report the synthesis and characterization of a palladium nanocatalyst based on Fe3O4@SiO2 functionalized with N-amidinoglycine amino acid, and its activity in Suzuki cross-coupling reactions.
Results and discussion
Preparation and characterization of the nanocatalyst
The magnetic nanoparticle-supported Pd catalyst designated as Fe3O4@SiO2@N-amidinoglycine@Pd0 was synthesized via multiple steps. The first step was to prepare Fe3O4 nanoparticles from FeCl3·6H2O to FeCl2·4H2O [27]. The magnetic nanoparticles (MNPs) so obtained were subsequently coated with silica (Fe3O4@SiO2) through the well-known Stöber method, using tetraethylorthosilicate (TEOS) [14]. The Fe3O4@SiO2 core/shell structure was then sequentially treated with N-amidinoglycine to form Fe3O4@SiO2@N-amidinoglycine. Finally, Pd nanoparticles were immobilized on this support by reaction with Pd(OAc)2 in ethanol, such that PdII was reduced to Pd0 without the addition of any external reducing agent (Scheme 1).
The catalyst was subjected to microanalysis, and the proportions of carbon, hydrogen and nitrogen by weight were found to be 1.90, 0.73 and 0.79%, respectively. From the N content, the loading of N-amidinoglycine was calculated as 0.19 mmol/g. The amount of palladium in the catalyst, as determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES), was 1.7 mmol/g.
Figure S1 shows the FT IR spectra of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2@N-amidinoglycine, palladium supported on this functionalized support, and pure N-amidinoglycine. In the spectrum of Fe3O4 nanoparticles, a band at 584 cm−1 is assigned to the Fe–O bond stretches, and a peak at 3421 cm−1 is attributed to the –OH groups. The spectrum of Fe3O4@SiO2 showed a strong absorption band at 1091 cm−1, related to Si–O–Si antisymmetric stretching vibrations; peaks at 799 and 464 cm−1 are assigned to the Si–O–Si symmetric stretching and Si–O–Si and or O–Si–O bending modes, respectively. These data confirm the successful coating of silica layers on the Fe3O4. Comparisons of the spectra of Fe3O4@SiO2@N-amidinoglycine with those of pure amidinoglycine (3384 cm−1 (νN–H of the guanidino group), 3303 and 3172 cm−1 (νas and νs of primary amines), 1670 cm−1 (νC=N of the guanidino group), 1581 and 1411 cm−1 (νas and νs of the carboxylate group)) and Fe3O4@SiO2 all indicate successful functionalization of the surface of Fe3O4@SiO2 nanoparticles with N-amidinoglycine (Fig. S1d).
In the powder XRD pattern of the catalyst (Fig. S2b), the diffraction peaks observed at 2θ = 30.14, 35.50, 43.07, 53.03, 57.11 and 62.74° correspond, respectively, to the crystalline planes (220), (311), (400), (422), (511) and (440) of Fe3O4, with a cubic spinel structure. The XRD pattern of the nanocatalyst also showed additional peaks at 39.10, 44.57 and 66.29° which are indexed, respectively, to the (111), (200) and (220) crystalline planes of Pd nanocrystals, suggesting the formation of metallic Pd NPs (Pd0). Comparison of the spectrum of Fe3O4@SiO2@N-amidinoglycine@Pd0 with that of pure N-amidinoglycine (Fig S2a) indicates successful immobilization of this amino acid derivative on the surface of the Fe3O4@SiO2 nanoparticles.
To evaluate the chemical oxidation state of palladium in the catalyst, X-ray photoelectron spectroscopy (XPS) was performed (Fig. S3). The resulting spectrum showed an intense doublet at a binding energy (BE) of 335.7 and 340.8 eV related to Pd0, assigned to the Pd 3d5/2 and Pd 3d3/2 peaks, respectively. This observation confirmed the successful reduction of PdII to Pd0 in the process of catalyst preparation. An XPS elemental survey scan of the surface of the nanocatalyst also revealed the presence of Fe (Fe 2p at binding energies of 711.08 and 724.08 eV), O (O 1s at binding energies of 530.58 and 532.98 eV), Si (Si 2p at binding energy of 103.08 eV and Si 2s at 154.08 eV), N (N 1s at 399.88 eV) and C (C 1s at 284.78 and 288.48 eV).
The magnetic properties of the nanocatalyst were investigated with a vibrating sample magnetometer (VSM) at room temperature in an applied magnetic field sweeping from − 10 to 10 kOe (Fig. S4). The magnetization curves of the prepared catalyst exhibit no hysteresis loop, indicating superparamagnetic behavior. The saturation magnetization (Ms) value for the Fe3O4@SiO2@N-amidinoglycine@Pd0 catalyst (23.35 emu g−1) is lower than that of bare Fe3O4 due to the successful grafting of SiO2 and N-amidinoglycine@Pd0 on the surface of the Fe3O4 nanoparticles. The magnetic properties of the silica coated magnetic samples depend on the Fe3O4 content. The non-magnetic coating layer on the surface of magnetic core changed the uniformity or magnitude of magnetization due to quenching of surface moments. However, the magnetization of the catalyst was still sufficient to respond to an external magnetic field.
The morphology and size of the nanoparticles were obtained from field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM) analysis (Fig. S5). The images obtained at different magnifications demonstrated that the prepared magnetic nanoparticles are spherical in shape, with slight agglomeration.
In the EDX spectrum of the nanocatalyst (Fig. S6), the presence of Fe, Si and O signals indicates that the Fe3O4 nanoparticles are loaded with silica. Figure S6 also reveals the presence of the elemental composition of N-amidinoglycine (C, N and O) and palladium on the surface of the nanocatalyst.
Catalysis of Suzuki cross-coupling
In order to explore the catalytic activity of the present Pd nanocatalyst in the Suzuki reaction, we employed the coupling of 4-bromoacetophenone with phenylboronic acid as a model system to optimize the reaction conditions. The best results were obtained using water as the solvent and NaHCO3 as the base, with 0.001 g of nanocatalyst (0.17 mmol% palladium) at 90 °C. As this catalyst is not sensitive to oxygen, the reactions were carried out under air (Table 1).
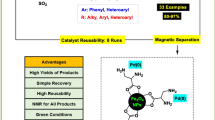
The scope and efficiency of this approach were then investigated for the synthesis of a wide variety of biaryl derivatives under the optimized conditions. The results are summarized in Table 2. Various aryl halides were tested, including either electron-donating or electron-withdrawing substituents such as Me, OMe, CN, NO2, CHO, COMe and Cl. Also, both electron-rich and electron-deficient aryl boronic acids with Me, OMe, COMe and F substituents were utilized. As evident from Table 2, all the examined aryl iodides and bromides were successfully coupled with various phenylboronic acids, affording the corresponding products with good to excellent yields in short reaction times. Prolonged reaction times were required for the less active aryl chlorides to obtain the desired products in moderate yields.
A hot filtration test was also carried out, in order to demonstrate the absence of homogeneous catalytic species which is produced via leaching phenomena during the reaction. An mixture of 0.001 g of the Fe3O4@SiO2@N-amidinoglycine@Pd0 catalyst and NaHCO3 (2 mmol) in 3 mL water was prepared and left to stir at 90 °C for 30 min, and then, the catalyst was magnetically removed. The filtrate so obtained showed negligible catalytic activity for Suzuki cross-coupling under the optimized reaction conditions (Table 3).
To study the recyclability of this palladium catalyst, we carried out a set of experiments using the recycled catalyst for the coupling of 4-bromoacetophenone with phenylboronic acid under the optimized conditions. After the completion of the first reaction, the catalyst was recovered magnetically, washed with ethanol, and dried for the next run. Then, a further reaction was carried out with fresh reactants and solvent under the same conditions. Based on the results, the catalyst retained its activity quite well over four successive catalytic runs, albeit with slightly longer reaction times.
We have compared the efficiency of Fe3O4@SiO2@N-amidinoglycine@Pd0 with previously reported catalysts for the synthesis of biphenyls to show the merits of the present work (Table 4). The results obtained with this catalytic system are superior to those for the other systems, giving better yields in shorter times with lower loading of palladium catalyst under mild reaction conditions.
Experimental
All reagents and solvents were purchased from commercial suppliers (Acros, Merck and Aldrich) and used without any additional purification. FT IR spectra were recorded using a Bruker Tensor 27 spectrometer within the 400–4000 cm−1 range using KBr disks. 1H and 13C NMR spectra were recorded with a Bruker Avance 400 and 100 MHz spectrometer using CDCl3 as solvent and TMS as standard. XRD patterns were recorded with a PW 3710 X-ray diffractometer (Philips) at room temperature using monochromatic Cu Kα radiation with a wavelength of λ = 0.15418 nm. The peak positions and intensities were obtained between 5° and 80° with a rate of 0.04° s−1. The morphology of the catalyst was observed with FE-SEM (HITACHI (S4160) Japan) and TEM (PHILIPS CM30) instruments. EDX patterns were recorded using a TESCAN, VEGA 3 LMU instrument. Magnetic susceptibility measurements were taken using a vibrating sample magnetometer (VSM, MDK Co. Ltd, Iran) with a magnetic field range of + 10,000 to − 10,000 Oe at room temperature. Elemental analyses were obtained on an Eager 300 for EA1112 CHNS instrument. X-ray photoelectron spectroscopy (XPS) measurements were taken with a Thermo Scientific, ESCALAB 250 Xi Mg X-ray instrument.
Synthesis of Fe3O4@SiO2
Magnetic nanoparticles (MNPs) were prepared by a conventional co-precipitation method. A mixture of FeCl3·6H2O (5.84 g, 22 mmol) and FeCl2·4H2O (2.15 g, 11 mmol) was dissolved in deionized water (100 mL) under a nitrogen atmosphere. The mixture was stirred vigorously at 85 °C for 30 min; then, ammonia solution (25 wt%, 10 mL) was added. The color of the solution changed immediately from orange to black. The black magnetite precipitate was collected with a magnet, washed twice with deionized water and then with 0.02 M NaCl solution and finally dried at 80 °C for 10 h.
The obtained Fe3O4 MNPs were subsequently coated with silica through the well-known Stöber method. Fe3O4 MNPs (1.0 g) were dispersed by ultrasonic vibration in a mixture of ethanol (40 mL), deionized water (6 mL) and aqueous ammonia solution (NH3, 25 wt%, 3 mL), followed by the addition of tetraethylorthosilicate (1.4 mL). After stirring for 12 h at room temperature under an N2 atmosphere, the black precipitate was collected using a magnet. The resulting Fe3O4@SiO2 nanoparticles were washed several times with water and ethanol, and then dried at 80 °C for 10 h.
Synthesis of the nanocatalyst
Fe3O4@SiO2 (1 g) was suspended in deionized water (40 mL) and sonicated until highly dispersed. N-Amidinoglycine (1.35 g) was then added to the mixture, which was stirred at 90 °C for 15 h. The resulting Fe3O4@SiO2@N-amidinoglycine nanoparticles were separated by applying an external magnet, washed with distilled water several times and then dried in an oven overnight. Finally, incorporation of palladium onto the synthesized nanocomposite was carried out by mixing Fe3O4@SiO2@N-amidinoglycine (0.5 g) and Pd(OAc)2 (0.03 g) in absolute ethanol (30 mL). The mixture was refluxed for 24 h. The resulting Fe3O4@SiO2@N-amidinoglycine@Pd0 nanocatalyst was separated magnetically, washed with absolute ethanol and dried at room temperature.
General Suzuki coupling procedure
To a round-bottomed flask containing a mixture of aryl halide (1 mmol), arylboronic acid (1 mmol) and NaHCO3 (2 mmol) in H2O (3 ml), Fe3O4@SiO2@N-amidinoglycine@Pd0 catalyst (0.001 g, 0.017 mmol% Pd) was added, and the mixture was stirred at 90 °C for the time specified in Table 2. After completion of the reaction [monitored by TLC (n-hexane:EtOAc, 9:1) or GC], the mixture was cooled to room temperature and the catalyst was separated with an external magnet. The decantate was diluted with water and extracted with n-hexane to isolate the products. The combined organic layers were dried over CaCl2, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (n-hexane:EtOAc, 9:1).
Conclusion
In conclusion, we have demonstrated the Suzuki cross-coupling reaction in water using a highly efficient and green Fe3O4@SiO2@N-amidinoglycine@Pd0 catalyst. The generation of palladium nanoparticles without the addition of any external reducing agent in the catalyst synthesis process, stability toward air and humidity, use of low catalyst loadings, easy handling and recyclability are the main advantages of this catalytic system. This catalyst was found to tolerate a broad range of functional groups. We hope that this heterogeneous magnetic nanocatalyst will find applications in palladium-catalyzed reactions.
References
Lim CW, Lee IS (2010) Nano Today 5:412–434
Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset JM (2011) Chem Rev 111:3036–3075
Shylesh S, Schünemann V, Thiel WR (2010) Angew Chem Int Ed 49:3428–3459
Tang T, Fan H, Ai S, Han R, Qiu Y (2011) Chemosphere 83:255–264
Hu B, Pan J, Yu HL, Liu JW, Xu JH (2009) Process Biochem 44:1019–1024
Gupta AK, Gupta M (2005) Biomaterials 26:3995–4021
Ln Wu, Yang CY, Lv Z, Cui FW, Zhao L, Yang P (2015) RSC Adv 5:50557–50564
Ahmadi R, Ranjbarnodeh E, Gu N (2012) Mater Sci Poland 30:382–389
Balu AM, Baruwati B, Serrano E, Cot J, Garcia-Martinez J, Varma RS, Luque R (2011) Green Chem 13:2750–2758
Deng Y, Cai Y, Sun Z, Zhao D (2011) Chem Phys Lett 510:1–13
Lu AH, Salabas EL, Schüth F (2007) Angew Chem Int Ed 46:1222–1244
Chekina N, Horak D, Jendelova P, Trchova M, Benes MJ, Hruby M, Herynek V, Turnovcova K, Sykova E (2011) J Mater Chem 21:7630–7639
Yang J, Lee J, Kang J, Chung CH, Lee K, Suh JS, Yoon HG, Huh YM, Haam S (2008) Nanotechnology 19:075610
Stöber W, Fink A, Bohn EJ (1968) J Colloid Interface Sci 26:62–69
Lu Y, Yin Y, Mayers BT, Xia Y (2002) Nano Lett 2:183–186
Graf C, Vossen DLJ, Imhof A, Van Blaaderen A (2003) Langmuir 19:6693–6700
Qu H, Maa H, Zhou W, O’Connor CJ (2012) Inorg Chim Acta 389:60–65
Ebrahiminezhad A, Ghasemi Y, Rasoul-Amini S, Barar J, Davaran S (2013) Colloids Surf B Biointerfaces 102:534–539
Wang Z, Zhu H, Wang X, Yang F, Yang X (2009) Nanotechnology 20:465606–465615
Miyaura N, Suzuki A (1995) Chem Rev 95:2457–2483
Bringmann G, Gulder T, Gulder TAM, Breuning M (2011) Chem Rev 111:563–639
Wencel-Delord J, Panossian A, Leroux FR, Colobert F (2015) Chem Soc Rev 44:3418–3430
Meyer FM, Collins JC, Borin B, Bradow J, Liras S, Limberakis C, Mathiowetz AM, Philippe L, Price D, Song K, James K (2012) J Org Chem 7:3099–3114
Kappaun S, Slugovc C, List EJW (2008) Int J Mol Sci 9:1527–1547
Polshettiwar V, Decottignies A, Len C, Fihri A (2010) Chem Sus Chem 3:502–522
Chatterjee A, Ward TR (2016) Catal Lett 146:820–840
Liu X, Ma Z, Xing J, Liu H (2004) J Magn Magn Mater 270:1–6
Sobhani S, Ghasemzadeh MS, Honarmand M, Zarifi F (2014) RSC Adv 4:44166–44174
Ghorbani-Choghamarani A, Azadi G (2016) Appl Organomet Chem 30:247–252
Veisi H, Ghadermazi M, Naderi A (2016) Appl Organomet Chem 30:341–345
Yang P, Ma R, Bian F (2016) ChemCatChem 18:3746–3754
Hajipour AR, Tadayoni NS, Mohammadsaleh F (2016) Appl Organomet Chem 30:777–782
Acknowledgements
We gratefully acknowledge the partial financial support received from the research council of Alzahra University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rafiee, F., Mehdizadeh, N. A green synthesis of biaryls in water catalyzed by palladium nanoparticles immobilized on N-amidinoglycine-functionalized iron oxide nanoparticles. Transit Met Chem 43, 295–300 (2018). https://doi.org/10.1007/s11243-018-0215-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0215-7