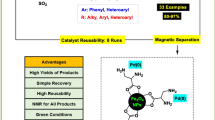
Abstract
A simple, efficient and less expensive protocol for the phosphine-free C–C coupling reactions and synthesis of anilines in the presence of 2-aminobenzamide complex of palladium supported on Fe3O4 magnetic nanoparticles (Pd(0)-ABA-Fe3O4) has been reported. The Suzuki reaction was carried out in water or PEG using phenylboronic acid (PhB(OH)2) or sodium tetraphenyl borate (NaBPh4). Pd(0)-ABA-Fe3O4 has been found promising for Heck reaction of butyl acrylate, styrene or acrylonitrile with aryl halides (including Cl, Br and I). Also, Pd(0)-ABA-Fe3O4 has been found as efficient catalyst for the amination of aryl halides using aqueous ammonia. The products have been obtained in short reaction times and high yields. The catalyst was easily separated using an external magnet from the reaction mixture and reused for several runs without significant loss of its catalytic efficiency or palladium leaching. The leaching of catalyst has been examined by hot filtration and ICP-OES technique. The nanomagnetical catalyst was characterized by FTIR, TGA, XRD, VSM, TEM, SEM, EDS, DLS and ICP-OES techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The formation of carbon–carbon bond via Heck and Suzuki reaction has been frequently employed in the synthesis of natural products, agrochemicals, pharmaceuticals, biologically active compounds and advanced materials [1, 2]. The Suzuki cross-coupling reaction is one of the most general and powerful tools for the synthesis of pharmaceuticals, herbicides, polymers, liquid crystals, natural products, ligands for catalysis and advanced materials [3, 4]. Traditionally, the carbon–carbon bond forming reactions are carried out in organic solvents in the presence of palladium complexes including organic ligands at high temperatures [5]. However, there are some disadvantages accompany with these procedures. First of all, the employed organic solvents may cause serious environmental problems due to their toxicity, and the reactions carried out at higher temperatures definitely consume more energy [6]. Secondly, the most common catalytic system employed for C–C coupling reaction is promoted by homogeneous palladium complexes containing ligands such as phosphine, dibenzylideneacetone and carbenes, which the preparation of most of the homogeneous Pd complexes with air- and moisture-sensitive phosphine and expensive ligands, leading to high production cost, reuse of these catalysts is difficult; moreover, they are environmentally malignant [7, 8]. Therefore, the immobilization of homogeneous catalysts on various support materials to combine the advantages of both homogeneous and heterogeneous catalysis such as high catalytic activity, easily recyclable, moisture- and air-stable catalyst is of great importance in catalyst science [9–11]. Meanwhile, some of the previously heterogeneous supports such as MCM-41 [28], SBA-15 [12] or some nanoparticles such as TiO2 NPs [13] require high temperature for calcination or a lot of time and tedious condition to prepare. Also, some of these reported catalysts such as heteropolyacids [14], carbon nanotubes [10], ionic liquids [15] or some polymers [16] are more expensive. In addition, most common heterogeneous catalyst can be separated from products by conventional techniques, which has some disadvantages such as time-consuming and expensive separation of fine particles from a reaction mixture [17]. Fe3O4 MNPs were prepared in water using commercially available materials such as FeCl3·4H2O and FeCl2·4H2O. Furthermore, magnetic nanoparticles (MNPs) can be easily and rapidly separated from the reaction mixture with the assistance of an external magnet [18, 19]. More important, magnetic separation of the MNPs is more effective and easier than filtration or centrifugation. In the other hand, MNPs are readily available, insoluble in organic and aqueous solvents and high surface area resulting in high catalysts loading capacity and outstanding stability heterogeneous supports for catalyst [20–22]. Recently, much attention has been focused on the surface modification with appropriate capping agents onto the MNPs surface to anchor the catalytically active complexes [9, 20, 23]. The Pd/H2N/Fe3O4 catalyst provides excellent reactivity and reusability in the C–C coupling reactions [23]. Typically, Suzuki reactions involve palladium catalyzed cross-coupling between aryl halides and organoboronic compounds such as trifluoroborates, organoboranes, boronic esters and boronic acid derivatives [24]. These advantages are based on the properties of the air- and moisture-stable organoboron nucleophiles, which can be easily obtained by a variety of synthetic routes and have low toxicity [25]. Also, the Heck reaction is one of the most powerful and versatile method for the coupling of aryl halides with olefins. Heck reaction is normally catalyzed by Pd complexes in the presence of base for the formation of C–C bonds [16].
Amination of aryl halides is a very important transformation and a method for the synthesis of anilines. Anilines are important building blocks for the constructing natural products, pharmaceutical and medicinal compounds, as well as in polymers and materials [26, 27]. Recently, few groups have reported several catalytic systems for the direct conversion of aryl halides into anilines [27–29]. Among them, ammonia is used as one of the most attractive sources of nitrogen for the synthesis of anilines because of its inexpensive cost and wide availability [30]. Thus, the development of efficient method for the amination of aryl halides is highly desirable. Owing to the inherent advantages of recovery, in the context of reuse of palladium, herein a new Pd-based heterogeneous catalyst has been reported for the C–C coupling reactions and synthesis of anilines from aryl halides.
Experimental
Preparation of the catalyst
Fe3O4 MNPs were prepared using chemical precipitation of ions Fe3+ and Fe2+ with a molar ratio of 2:1. Typically, FeCl3·6H2O (5.838 g) and FeCl2·4H2O (2.147 g) were dissolved in 100 mL deionized water at 80 °C under N2 atmosphere and vigorous mechanical stirring conditions. Then, 10 mL of 25% NH4OH was added to the reaction mixture. After 30 min, the mixture of reaction was cooled to room temperature. After completion of the reaction, nanoparticles (Fe3O4) were washed two times with distilled water and 0.02 M solution of NaCl, and each time was decanted with external magnet.
Fe3O4 nanoparticles (1.5 g) were dispersed in 50 mL ethanol/water (volume ratio, 1:1) solution by sonication for 30 min, and then, APTES (2.5 mL) was added to the mixture reaction. The reaction mixture was stirred under N2 atmosphere at 40 °C for 8 h. Then, the nanoparticles was re-dispersed in ethanol by sonication for 5 times and separated through magnetic decantation. The nanoparticles (NH2–MNPs) were dried at room temperature. The obtained NH2–Fe3O4 nanoparticles (1 g) were dispersed in 50 mL ethanol by sonication for 30 min, and then, isatoic anhydride (2.5 mmol) was added to the reaction mixture. The reaction mixture was stirred under N2 atmosphere at 80 °C for 8 h. Then, the reaction mixture was cooled down to room temperature, and the resulting nanoparticles washed with ethanol for several times and separated using magnetic decantation and dried at room temperature. The obtained ABA-Fe3O4 (0.5 g) was dispersed in 25 mL ethanol by sonication for 30 min, and then, palladium acetate (0.25 mmol) was added to the reaction mixture. The reaction mixture was stirred under N2 atmosphere at 80 °C for 20 h. Then, the NaBH4 (0.3 mmol) was added to the reaction mixture and stirred for 2 h. Then, the final product was separated by magnetic decantation and washed by ethanol to remove the unattached substrates. The nanoparticles product (Pd(0)-ABA-Fe3O4) was dried at room temperature.
General procedure for C–C coupling reaction using sodium tetraphenyl borate
A mixture of aryl halide (1 mmol), sodium tetraphenyl borate (0.5 mmol), Na2CO3 (3 mmol) and Pd(0)-ABA-Fe3O4 (0.01 g, 1.85 mol%) was stirred in PEG at 80 °C and the progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature and catalyst was separated by an external magnet and washed with diethyl ether and the reaction mixture was extracted with H2O and diethyl ether. The organic layer was dried over Na2SO4 (1.5 g). Then, the solvent was evaporated and pure biphenyl derivatives were obtained in good to excellent yields.
General procedure for coupling of aryl halides with phenylboronic acid (Suzuki reaction)
A mixture of aryl halide (1 mmol), phenylboronic acid (1 mmol), Et3N (3 mmol) and Pd(0)-ABA-Fe3O4 (0.003 g, 0.56 mol%) was added to a reaction vessel. The resulting mixture was stirred in H2O at room temperature, and the progress of the reaction was monitored by TLC. After completion of the reaction, catalyst was separated by an external magnet and washed with ethylacetate and the reaction mixture was extracted with H2O and ethylacetate and dried over anhydrous Na2SO4 (1.5 g). Then, the solvent was evaporated and pure biphenyl derivatives were obtained in good to excellent yields.
General procedure for coupling of aryl halides with butyl acrylate, acrylonitrile or styrene (Heck reaction)
A mixture of aryl halide (1 mmol), alkene (1.2 mmol), K2CO3 (3 mmol), and Pd(0)-ABA-Fe3O4 (0.008–0.016 g, 1.48–2.96 mol%) was stirred in DMF at 120 °C, and the progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature and catalyst was separated by an external magnet and washed with diethyl ether and the reaction mixture was extracted with H2O and diethyl ether. The organic layer was dried over Na2SO4 (1.5 g). Then, the solvent was evaporated and pure products were obtained in 95–98% yields.
General procedure for amination of aryl halides
Aryl halide (1 mmol) was added to a mixture of the catalyst (10 mg, 1.85 mol%), K2CO3 (3 mmol) and NH4OH (1 mL) and stirred at 60 °C. The progress of the reaction was monitored by TLC. After the completion of the reaction, catalyst was separated using an external magnet. Then, gathered aqueous phases were extracted with ethyl acetate. The organic layer was dried over Na2SO4 and then evaporated under reduced pressure, aniline derivatives have been obtained in good yields.
Results and discussion
Catalyst preparation
The N-propylamine supported on Fe3O4 MNPs (NH2–Fe3O4) core shell was synthesized by a modified new reported procedure [31], and then, 2-aminobenzamide immobilized on Fe3O4 (ABA-Fe3O4) has been prepared by reaction of NH2–Fe3O4 with isatoic anhydride. Finally, 2-aminobenzamide complex of Pd supported on Fe3O4 (Pd(0)-ABA-Fe3O4) has been obtained via reaction of palladium(II) acetate and ABA-Fe3O4 (Scheme 1). This catalyst has been characterized by transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), thermogravimetric analysis (TGA), vibrating sample magnetometer (VSM), Fourier transform infrared spectroscopy (FTIR), dynamic laser scattering (DLS) and inductively coupled plasma atomic emission spectroscopy (ICP-OES).
Catalyst characterizations
The morphology and size of the catalyst was evaluated by SEM and TEM analysis (Fig. 1). Transmission electron microscopy was used to obtain direct information about the size and morphology of the prepared nanoparticles. It can be seen that most of the particles are quasi-spherical with an average diameter about 15–25 nm. Also, the size and morphology of Pd(0)-ABA-Fe3O4 is quite homogeneous with obtained diameter of 15–20 nm. To investigate the size distribution of these nanoparticles, particles size was calculated by SEM analysis, which the catalyst was formed of nanometer-sized particles as shown in SEM image. A typical EDS spectrum taken from the Pd(0)-ABA-Fe3O4 is shown in Fig. 2. The EDS spectra at different points of the image confirm the presence of Pd in the prepared modified nanoparticles. As shown in this figure, EDS spectrum of catalyst showed the presence of C, N, O, Si, Fe and Pd species in the catalyst (Fig. 2).
Also, to extend the scope of catalysts characterization, we have determined the exact amount of Pd on MNPs by ICP-OES technique. The Pd amount of the immobilized catalyst on MNPs was found to be 1.85 × 10−3 mol g−1 based on inductively coupled plasma atomic emission spectroscopy (ICP-OES).
The XRD pattern of Pd(0)-ABA-Fe3O4 is shown in Fig. 3. As it is shown in Fig. 3, Pd(0)-ABA-Fe3O4 displays six characteristic peaks at the 2θ values of 30.18, 35.64, 43.62, 54.02, 57.35 and 63.09 which could be attributed for the (220), (311), (400), (422), (511) and (440) reflections, respectively, which were in agreement with magnetite standard data. Weak broad bands (2θ < 20°) appeared in XRD pattern which could be attributed to the amorphous silane shell formed around the magnetic cores [32]. Also, the XRD pattern of Pd(0)-ABA-Fe3O4 contains a series of characteristic diffraction peaks (39.8°, 46.3° and 67.8°) which are indexed to the Pd indicating the presence of Pd on surface of magnetic nanoparticles [33].
The average size of Pd(0)-ABA-Fe3O4 nanocatalyst diameter was calculated to be 21 ± 1.2 nm from the XRD results by Scherrer’s equation:
where D is the average particle size of the phase under investigation, k is Scherrer’s constant (0.9), λ is the applied wavelength (1.5405 Å), β is the corrected diffraction line full width at half-maximum (FWHM), applied to the principal diffraction peak corresponding to the plane (3 1 1) and θ is the angle of diffraction.
One indication of bond formation between the nanoparticles and the complex can be inferred from TGA. The TGA curve of the Pd(0)-ABA-Fe3O4 shows the mass loss of the organic functional groups as it decomposes upon heating. Figure 4 shows the TGA curves for bare Fe3O4 nanoparticles (black curve), NH2–Fe3O4 (red curve), 2-aminobenzamide supported on Fe3O4 magnetic nanoparticle (ABA-Fe3O4) (blue curve) and the catalyst treated nanoparticles (green curve). The TGA curve of the all samples shows the small amount of weight loss below 200 °C is due to desorption of physically adsorbed solvents and surface hydroxyl groups. In TGA curve of the catalyst, a weight loss about of 20% from 200 to 500 °C, resulting from the decomposition of immobilized organic spaces on the Fe3O4 surface. Meanwhile, weight loss about 5 and 10% from 200 to 500 °C is occurred for NH2–Fe3O4 and ABA-Fe3O4, respectively. On the basis of this result, the well grafting of organic groups including palladium complex on the Fe3O4 is verified.
The dynamic laser scattering measurement of Pd(0)-ABA-Fe3O4 nanoparticle is shown in Fig. 5. The hydrodynamic diameter of the nanoparticles in water was found to be monodisperse with mean values of 48.8 nm, slightly larger than observed from TEM and XRD report due to the effect of agglomeration of nanoparticles, solvation and the presence of organic layers and Pd complex immobilized on the surface of Fe3O4 nanoparticles.
The magnetic property of Fe3O4 nanoparticles and Pd(0)-ABA-Fe3O4 was characterized by VSM. The room-temperature magnetization curves of Fe3O4 MNPs and Pd(0)-ABA-Fe3O4 are shown in Fig. 6. The magnetic measurement shows that the Pd(0)-ABA-Fe3O4 has a saturated magnetization value of 38.9 emu g−1. As expected, the Fe3O4 nanoparticles showed the higher magnetic value (saturation magnetization, Ms) of 74.1 emu g−1 [18], and the Ms value of Pd(0)-ABA-Fe3O4 is decreased due to the silica coating and the layer of the grafted catalyst (39.1 emu g−1).
Successful functionalization of the Fe3O4 MNPs can be inferred from FTIR technique. The FTIR spectrum for bare Fe3O4 nanoparticles (a), NH2–Fe3O4 (b), ABA-Fe3O4 (c) and Pd(0)-ABA-Fe3O4 (d) is shown in Fig. 7. The FTIR spectrum of Pd(0)-ABA-Fe3O4 shows several peaks that are characteristic of a functionalized palladium complex, which clearly differs from that of the unfunctionalized bare Fe3O4, NH2–Fe3O4 and ABA-Fe3O4 nanoparticles. The FTIR spectrum for the Fe3O4 shows a stretching vibration at 3393 cm−1 which incorporates the contributions from both symmetrical and asymmetrical modes of the O–H bonds which are attached to the surface magnetic nanoparticles. The strong band at low wavenumbers (562 cm−1) comes from the vibrations of Fe–O bonds of iron oxide [6]. In the FTIR spectra of NH2–Fe3O4, the presence of the anchored N-propylamine group is confirmed by C–H stretching vibrations that appear at 2924 and 2856 cm−1 and also N–H stretching vibration modes as a broad band that appear at 3440 cm−1 [1]. More importance, the Fe–O–Si stretching vibrations and both symmetrical and asymmetrical modes of the O–Si as a broad band appear between 1000 and 1100 cm−1, which indicates that the silica organic group has successfully coated on the surface of Fe3O4 nanoparticles [19, 34]. Reaction of isatoic anhydride with N-propylamine produces ABA-Fe3O4 in which the presence of carbonyl group is asserted with 1625 cm−1 bands in FTIR spectra (Fig. 7c). Also, vibrations at 1450 cm−1 and vibration at 1525 cm−1 were probably attributed to the terminal amine groups and ring attached C–N, respectively [7]. In addition, in the spectrum of Pd(0)-ABA-Fe3O4, the broadening in the range of 1400–1650 cm−1 is attributed to the formation of palladium complex. All of those bands reveal that the surface of Fe3O4 nanoparticles is successfully modified with organic layers.
Catalytic studies
As a part of our ongoing program directed toward the catalytic activity of modified nanoparticles in organic reactions [35–37], we were interested in finding a simple and efficient method for the carbon–carbon coupling reaction in the presence of magnetic nanoparticles bonded palladium complex (Pd(0)-ABA-Fe3O4) as a magnetically recoverable nanocatalyst (Scheme 2).
To illustrate the catalytic activity of Pd(0)-ABA-Fe3O4 in the C–C coupling reaction, we first examined the reaction between 4-nitrobromobenzene and sodium tetraphenyl borate, and the results are summarized in Table 1. In order to optimize reaction conditions, we examined different parameters such as solvent, base, reaction temperature and different amounts of Pd(0)-ABA-Fe3O4, on the outcome of C–C coupling reaction of 4-nitrobromobenzene and sodium tetraphenyl borate. The catalyst is not thermal, air or moisture sensitive, and hence, inert atmosphere was not employed. It is very important that the reaction did not proceed in the absence of Pd(0)-ABA-Fe3O4 even after long time (Table 1, entry 1). In order to choose the reaction media, different solvents such as PEG, DMF, DMSO, H2O and dioxane were used (Table 1, entries 6–9) and the best results were obtained in PEG using 0.01 g (1.85 mol%) of Pd(0)-ABA-Fe3O4 (Table 1, entry 4). Also, the effect of base was studied in which inferior results were obtained using NaOH, NaOEt, KOH and Et3N (Table 1, entries 10–13). But, when Na2CO3 was used (Table 1, entry 4), excellent biphenyl yields were obtained. Also, we found that the coupling reaction yields were susceptible to temperature changes. Therefore, the effect of temperature was described (Table 1, entries 14–16) and the best results were obtained at 80 °C. As listed in Table 1, 4-nitrobromobenzene (1 mmol) in the presence of catalytic amount of Pd(0)-ABA-Fe3O4 (0.01 g, 1.85 mol%) using sodium tetraphenyl borate (0.5 mmol) and Na2CO3 (3 mmol) in PEG at 80 °C was found to be ideal in the reaction conditions for the formation of corresponding biphenyl.
With the above-mentioned reaction conditions in hand, we examined the catalytic activity of Pd(0)-ABA-Fe3O4 for various substrates, and the results are given in Table 2. Thus, various ortho-, metha- and para-substituted aryl iodides (Table 2, entries 1–6) and bromides (Table 2, entries 7–13) were coupled into corresponding biphenyls. We also examined the reaction with several substituted aryl chlorides (Table 2, entries 14–16) and sodium tetraphenyl borate under the optimized reaction conditions. Therefore, the Pd(0)-ABA-Fe3O4 catalyst was also capable of chlorobenzene derivatives. However, the completing reaction including aryl chlorides was slower and required more amounts of catalyst (20 mg, 3.7 mol%) than aryl iodides and bromides. In order to show the chemoselectivity of the presented protocol, 1-chloro-4-bromobenzene was subjected to the C–C coupling reaction. Interestingly, chloro-group remained intact during the coupling reaction, while the bromo-group was coupled successfully (Table 2, entry 12). The experimental procedure is very simple and convenient and has the ability to tolerate a variety of other functional groups such as OH, CN, NO2, alkyl and OCH3 under the reaction conditions. Therefore, the results revealed that this methodology is effective for a wide range of aryl halide including Cl, Br and I; however, aryl chlorides showed less reactivity toward the coupling reaction than their aryl iodides and bromides analogs.
Also, we report the application of Pd(0)-ABA-Fe3O4 as catalyst for the Suzuki reaction using the coupling of various aryl halides with phenylboronic acid (Scheme 3). In order to optimize the reaction conditions, the coupling of iodobenzene (1 mmol) with PhB(OH)2 (1 mmol) was optimized in the presence of different amounts of catalyst (Table 3, entries 1–5) and in the various solvents such as H2O, EtOH, DMSO, PEG and DMF (Table 3, entries 5–9). The reaction was significantly affected by the nature of base and the additive used. Therefore, to found the best reaction condition, the effect and amount of base were described (Table 3, entries 9–14) and the best results were obtained using 3 mmol of triethylamine (Et3N). When the amount of Et3N was reduced to 1.5 mmol, the yield of the reaction decreased to 54%. Therefore, the best results were obtained in water at room temperature for 45 min in the presence of 3 mg (0.56 mol%) of catalyst using 3 mmol of Et3N (Table 3, entry 4).
After the optimization of the reaction condition, the various aryl halides including several of functional groups have been described in optimum condition and the corresponding biphenyl were obtained in short reaction times with good to excellent yields (Table 4). The experimental procedure is very simple, and also a variety of aryl halides (involving of Cl, Br and I) possessing of electron-donor and electron-withdrawing substituents were successfully employed to prepare the corresponding biphenyl derivatives in excellent yields at room temperature. Aryl bromides and aryl iodides show lower reaction times compared to those corresponding aryl chlorides (Table 4, entries 5–8).
To extend the scope of our work, we next investigated the Heck reaction using coupling of various aryl halides with butyl acrylate, styrene and acrylonitrile (Scheme 4).
The reaction conditions, such as solvent (DMF, DMSO or PEG), base (Et3N, Na2CO3 or K2CO3) and amount of catalyst, were optimized in the coupling of iodobenzene with butyl acrylate as model reaction (Table 5). Also, the effect of temperature was studied, in which inferior results were obtained in 80 and 100 °C (Table 5, entries 9, 10).
As given in Table 5, the best result was obtained with the use of K2CO3 as the base and DMF as the solvent in the presence of 8 mg (1.48 mol%) of Pd(0)-ABA-Fe3O4 at 120 °C (Table 5, entry 3). When the same reaction was tested at 100 °C, the reaction yield increased to 38% (Table 5, entry 9).
The best conditions were then applied to coupling of other aryl halides (including Cl, Br and I) with butyl acrylate in a short reaction times (Table 6). As listed in Table 6, aryl bromides and aryl iodides underwent the Heck reaction with butyl acrylate under similar conditions to afford the corresponding products in 95–98% yields (Table 6, entries 1–9), whereas aryl chlorides such as chlorobenzene and 4-chlorobenzonitrile have been coupled with butyl acrylate in the presence of 16 mg (2.96 mol%) of Pd(0)-ABA-Fe3O4 in 97 and 96% yields, respectively (Table 6, entries 10, 11). Aryl halides with electron-donor (Table 6, entries 2–5) and electron-withdrawing (Table 6, entries 7–10) functional groups reacted with butyl acrylate to afford the corresponding products and the all products were obtained in good to excellent yields.
We also applied optimized reaction conditions to coupling aryl halides with acrylonitrile and styrene (Table 6, entries 12–22). The electron-neutral, electron-rich and electron-poor aryl halides reacted with acrylonitrile and styrene efficiently to produce the corresponding cross-coupling products in good to excellent yields, whereas styrene has been coupled with aryl halides in the presence of 16 mg (2.96 mol%) of Pd(0)-ABA-Fe3O4 in 68–80% yields. Therefore, these results revealed that this methodology is effective for a wide range of alkenes and aryl halides.
In order to extend applications of the catalytic activity of Pd(0)-ABA-Fe3O4, this catalyst was investigated in the direct amination of aryl iodides and aryl bromides using aqueous ammonia (Scheme 5).
The Pd(0)-ABA-Fe3O4 has been tested as catalyst in the amination of iodobenzene using ammonia solution to ascertain the most optimum conditions. The effect of solvent, base, temperature and amounts of catalyst on the outcome of the amination of iodobenzene was examined. A summary of the results is given in Table 7. The reaction did not perform in the absence of Pd(0)-ABA-Fe3O4 or base (Table 7, entries 4, 10). As given in Table 7, the best result was obtained with the use of K2CO3 (3 mmol) in the presence of 10 mg (1.85 mol%) of Pd(0)-ABA-Fe3O4 at 60 °C under solvent-free conditions.
This optimized reaction conditions were then applied to direct amination of electron-poor or electron-rich aryl iodides or bromides using aqueous ammonia to give aniline derivatives (Table 8). In all cases, the products were resulted in good yield.
Reusability of the catalyst
The Pd(0)-ABA-Fe3O4 as magnetically nanocatalyst can be easily recycled for repeatedly C–C coupling reaction. To investigate this issue, the recyclability of the catalyst was examined for the coupling reaction of 4-nitrobromobenzene with phenylboronic acid and sodium tetraphenyl borate. We found that this catalyst demonstrated remarkably excellent reusability; after the completion of the reaction, the catalyst was easily and rapidly recovered from the reaction mixture using an external magnet, the remaining magnetic nanocatalyst was washed with diethyl ether to remove residual product and the reaction mixture decanted. Then, the reaction vessel was charged with fresh substrates and subjected to the next run. As shown in Fig. 8, the catalyst was used over five runs without any significant loss of its activity. The average isolated yield for five successive runs was 93 and 95%, which clearly demonstrates the practical recyclability of this catalyst. In addition, one of the attractive features of this catalytic system is the rapid and efficient separation of the catalyst using an appropriate external magnet, which minimizes the loss of catalyst during separation.
Catalyst leaching study
In order to examine leaching of palladium in reaction mixture and heterogeneity of described catalyst, we performed hot filtration for the synthesis of biphenyl with tetraphenyl borate. In this experiment, we obtained the yield of product in half time of the reaction that it was 56%. Then, the reaction was repeated and in half time of the reaction, the catalyst separated and allowed the filtrate to react further. We found that, after this hot filtration, no further reaction was observed. The yield of reaction in this stage was 57% that confirmed the leaching of palladium is negligible.
Also, to extend the analysis of leaching of catalyst, amount of Pd in Pd(0)-ABA-Fe3O4 was determined by ICP-OES after five times recycled. The amount of Pd in catalysts was found to be 1.81 × 10−3 mol g−1 based on ICP-OES for catalyst after five runs reused. Therefore, the catalyst can be recovered and reused without any significant loss of amount of Pd leaching. Based on ICP-OES results, amount of Pd in the catalyst after 5 times reused is comparable with fresh catalyst (1.85 × 10−3 mol g−1 for fresh Pd(0)-ABA-Fe3O4). Therefore, only 2% leaching of catalyst was observed after five runs.
Comparison of the catalyst
In order to examine the efficiency of these procedures, we compared the results of the coupling of iodobenzene with phenylboronic acid (Table 9) with the previous methods in the literature. This catalyst showed short reaction time and good yield than other catalysts reported in the previous literatures. Also, this new catalyst is comparable in terms of price, non-toxicity, stability and easy separation. In addition, the recovered and recycled of this catalyst is more rapid and easier than the other catalysts.
Conclusions
In conclusion, an efficient heterogeneous catalyst (Pd(0)-ABA-Fe3O4) was synthesized from loading palladium acetate onto functionalized Fe3O4 nanoparticles. The Pd(0)-ABA-Fe3O4 nanoparticles exhibit an excellent catalytic activity, high reusability and air or moisture stability for the Heck and Suzuki reactions also amination of aryl halides. This methodology is effective for a wide range of aryl halide including Cl, Br and I. A high conversion of the substrates was obtained in C–C coupling reaction using sodium tetraphenyl borate, phenylboronic acid, styrene, acrylonitrile and butyl acrylate in the presence of this catalyst. The advantages of this protocol are the use of a commercially available, eco-friendly, cheap, chemically stable materials, the operational simplicity, practicability and good to high yields, and more importance the catalyst can be synthesized readily from inexpensive and commercially available starting materials. Also, the all reactions were carried out in air atmosphere.
References
S. Das, S. Bhunia, T. Maity, S. Koner, J. Mol. Catal. A Chem. 394, 188 (2014)
M. Wena, S. Takakura, K. Fuku, K. Moria, H. Yamashita, Catal. Today 242, 381 (2015)
S. Kazemi Movahed, R. Esmatpoursalmani, A. Bazgir, RSC Adv. 4, 14586 (2014)
N. Miyaura, A. Suzuki, Chem. Rev. 95, 2457 (1995)
G. Herve, G. Sartori, G. Enderlin, G. Mackenzie, C. Len, RSC Adv. 4, 18558 (2014)
D.A. Alonso, C. Najera, Chem. Soc. Rev. 39, 2891 (2010)
X. Le, Z. Dong, Z. Jin, Q. Wang, J. Ma, Catal. Commun. 53, 47 (2014)
D. Zhang, Q. Wang, Coord. Chem. Rev. 286, 1 (2015)
A. Ghorbani-Choghamarani, P. Moradi, B. Tahmasbi, RSC Adv. 6, 56638 (2016)
A. Ghorbani-Choghamarani, B. Tahmasbi, P. Moradi, N. Havasi, Appl. Organomet. Chem. 30, 619 (2016)
M. Opanasenko, P. Stepnicka, J. Cejka, RSC Adv. 4, 65137 (2014)
P. Sharma, A.P. Singh, Catal. Sci. Technol. 4, 2978 (2014)
S.S. Soni, D.A. Kotadia, Catal. Sci. Technol. 4, 510 (2014)
M.M. Heravi, S. Sadjadi, J. Iran. Chem. Soc. 6, 1 (2009)
P. Nehra, B. Khungar, K. Pericherla, S.C. Sivasubramanian, A. Kumar, Green Chem. 16, 4266 (2014)
P.M. Uberman, L.A. Perez, S.E. Martın, G.I. Lacconi, RSC Adv. 4, 12330 (2014)
M. Gholinejad, B. Karimi, F. Mansouri, J. Mol. Catal. A Chem. 386, 20 (2014)
B. Atashkar, A. Rostami, B. Tahmasbi, Catal. Sci. Technol. 3, 2140 (2013)
R.K. Sharma, M. Yadav, R. Gaur, Y. Monga, A. Adholeya, Catal. Sci. Technol. 5, 2728 (2015)
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Chem. Rev. 111, 3036 (2011)
B. Atashkar, A. Rostami, H. Gholami, B. Tahmasbi, Res. Chem. Intermed. 41, 3675 (2015)
A. Rostami, B. Tahmasbi, A. Yari, Bull. Korean Chem. Soc. 34, 1521 (2013)
C.W. Lim, I.S. Lee, Nano Today 5, 412 (2010)
K. Kulkarni, J. Friend, L. Yeo, P. Perlmutter, Ultrason. Sonochem. 21, 1305 (2014)
T. Yu, X.Y. Wu, J. Yang, Tetrahedron Lett. 55, 4071 (2014)
R.B. Nasir Baig, R.S. Varma, RSC Adv. 4, 6568 (2014)
F. Havasi, A. Ghorbani-Choghamarani, F. Nikpour, N. J. Chem. 39, 6504 (2015)
R.A. Green, J.F. Hartwig, Angew. Chem. Int. Ed. 54, 1 (2015)
B. Yang, L. Liao, Y. Zeng, X. Zhu, Y. Wan, Catal. Commun. 45, 100 (2014)
J. Li, L. Liu, RSC Adv. 2, 10485 (2012)
A. Ghorbani-Choghamarani, Z. Darvishnejad, B. Tahmasbi, Inorg. Chim. Acta 435, 223 (2015)
M. Hajjami, B. Tahmasbi, RSC Adv. 5, 59194 (2015)
W. Zhang, X. Chen, T. Tang, E. Mijowska, Nanoscale 6, 12884 (2014)
M. Nikoorazm, A. Ghorbani-Choghamarani, M. Khanmoradi, Appl. Organomet. Chem. 30, 236 (2016)
A. Ghorbani-Choghamarani, M. Hajjami, B. Tahmasbi, N. Noori, J. Iran. Chem. Soc. 13, 2193 (2016)
A. Ghorbani-Choghamarani, B. Tahmasbi, F. Arghand, S. Faryadi, RSC Adv. 5, 92174 (2015)
A. Ghorbani-Choghamarani, B. Tahmasbi, N. J. Chem. 40, 1205 (2016)
Y.Y. Peng, J. Liu, X. Lei, Z. Yin, Green Chem. 12, 1072 (2010)
L. Baia, J.X. Wanga, Adv. Synth. Catal. 350, 315 (2007)
X. Zheng, Q. Yang, Z. Li, Z. Zhu, X. Cui, H. Fu, H. Chen, R. Li, Catal. Commun. 57, 143 (2014)
M. Nasrollahzadeh, S.M. Sajadi, M. Maham, J. Mol. Catal. A: Chem. 396, 297 (2015)
V.W. Faria, D.G.M. Oliveira, M.H.S. Kurz, F.F. Goncalves, C.W. Scheeren, G.R. Rosa, RSC Adv. 4, 13446 (2014)
S.J. Sabounchei, A. Hashemi, Inorg. Chem. Commun. 47, 123 (2014)
M. Nasrollahzadeh, A. Azarian, M. Maham, A. Ehsani, J. Ind. Eng. Chem. 21, 746 (2015)
T. Chen, J. Gao, M. Shi, Tetrahedron 62, 6289 (2006)
N. Iranpoor, H. Firouzabadi, A. Tarassoli, M. Fereidoonnezhad, Tetrahedron 66, 2415 (2010)
Q. Xu, W.L. Duan, Z.Y. Lei, Z.B. Zhu, M. Shi, Tetrahedron 61, 11225 (2005)
Y.P. Wang, H.M. Lee, J. Organomet. Chem. 791, 90 (2015)
Q. Du, W. Zhang, H. Ma, J. Zheng, B. Zhou, Y. Li, Tetrahedron 68, 3577 (2012)
Y. Leng, F. Yang, K. Wei, Y. Wu, Tetrahedron 66, 1244 (2010)
L. Wang, H. Li, P. Li, Tetrahedron 65, 364 (2009)
A. Ghorbani-Choghamarani, B. Tahmasbi, P. Moradi, RSC Adv. 6, 43205 (2016)
A. Ghorbani-Choghamarani, B. Tahmasbi, P. Moradi, Appl. Organomet. Chem. 30, 422 (2016)
K.G. Thakur, K.S. Srinivas, K. Chiranjeevi, G. Sekar, Green Chem. 13, 2326 (2011)
M. Nikoorazm, A. Ghorbani-Choghamarani, N. Noori, B. Tahmasbi, Appl. Organomet. Chem. 30, 843 (2016)
M. Gholinejad, H.R. Shahsavari, Inorg. Chim. Acta 421, 433 (2014)
H.A. Patel, A.L. Patel, A.V. Bedekar, Appl. Organomet. Chem. 29, 1 (2015)
Acknowledgements
This work was supported by the research facilities of Ilam University, Ilam, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbani-Choghamarani, A., Tahmasbi, B., Noori, N. et al. A new palladium complex supported on magnetic nanoparticles and applied as an catalyst in amination of aryl halides, Heck and Suzuki reactions. J IRAN CHEM SOC 14, 681–693 (2017). https://doi.org/10.1007/s13738-016-1020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-1020-x