Abstract
Plants synthesize a variety of metabolites in response to biotic elicitors. To comprehend how the digested cell wall of Piriformospora indica affects the response of ROS burst, antioxidant enzymes, amino acids profiling, and phenylpropanoid compounds such as lignans, phenolic acids, and flavonoids in Linum album hairy roots; we accomplished a time-course analysis of metabolite production and enzyme activities in response to CDCW and evaluated the metabolic profiles. The results confirms that CDCW accelerates the H2O2 burst and increases SOD and GPX activity in hairy roots. The HPLC analysis of metabolic profiles shows that the H2O2 burst shifts the amino acids, especially Phe and Tyr, fluxes toward a pool of lignans, phenolic acids, and flavonoids through alterations in the behavior of the necessary enzymes of the phenylpropanoid pathway. CDCW changes PAL, CCR, CAD, and PLR gene expression and transiently induces PTOX and 6MPROX as the main-specific products of PAL and PLR genes expression. The production of phenolic acids (e.g., cinnamic, coumaric, caffeic, and salicylic acid) and flavonoids (e.g., catechin, diosmin, kaempferol, luteolin, naringenin, daidzein, and myricetin) show different behaviors in response to CDCW. In conclusion, our observations show that CDCW elicitation can generate H2O2 molecules in L. album hairy roots and consequently changes physiological, biochemical, and molecular responses such as antioxidant system and the specific active compounds such as lignans. Quantification of metabolic contents in response to CDCW suggests enzyme and non-enzyme defense mechanisms play a crucial role in L. album hairy root adaptation to CDCW.
Graphical abstract
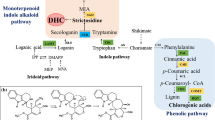
A summary revealed that the correlation between H2O2 generation and L. album hairy root defense system under CDCW. Increase of H2O2 generation led plant to response against oxidative conditions. SOD, and GPX modulated H2O2 content, Phe, and Tyr shifted to the phenylpropanoid compounds as a precursor of PAL and TAL enzyme, the predominant phenylpropanoid compounds controlled oxidative conditions, and the other amino acids responsible for amino acid synthesis and development stages.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Linum album Kotschy ex Boiss is an essential source of phenylpropanoid metabolites, especially PTOX and 6MPTOX, growing in Iran (Fuss 2003; Ahmadian Chashmi et al. 2013). L. album has anti-tumor, anti-cancer, and anti-inflammatory effects, which may be relevant to specific secondary metabolites such as lignans, which are highly accumulated in this species (Westcott and Muir 2003; Javadian et al. 2017). Lignans are the main group of phenolic compounds, which are generally synthesized by the phenylpropanoid pathway (Samari et al. 2020). The phenylpropanoid pathway is among the most commonly considered for metabolite production in plants. This pathway can produce various phenolic acids, flavonoids, anthocyanins, lignin, and lignans (Hano et al. 2006a).
In association with the phenylpropanoid pathway, PAL first converts Phe and Tyr as the main precursor constituents of the phenylpropanoid pathway to cinnamic acid (Mur et al. 2017; Sagharyan et al. 2020). Also, the phenylpropanoid pathway could be modified by several genes, including CCR, CAD, and PLR, which convert cinnamic acid into monolignol (Corbin et al. 2017). The phenylpropanoid compounds are an important group of secondary metabolites synthesized by plants at a low molecular weight (Samari et al. 2020). Eliciting plants is considered an effective strategy for induction of the plant defense system and increasing secondary metabolite production (Delaunois et al. 2014).
Despite a considerable number of reports for the increase of secondary metabolites for pharmaceutical applications, gene expression studies on plant-endophytic interactions and their bioactive compounds production are not yet fully understood (Esmaeilzadeh et al. 2011, 2014; Kamalipourazad et al. 2016). It is well-known that Piriformospora indica, a root endophytic fungus, and its derivatives elicitation such as cell wall and mycelium could induce phenylpropanoid metabolites such as lignans and phenolic metabolites (Baldi 2008, 2010; Tashackori et al. 2016, 2018).
Therefore, the current study was performed on hairy root culture of L. album treated with Chitinase Digested Cell Wall (CDCW) derived from P. indica cell wall to understand how L. album respond to CDCW. This study aims to elucidate the inducing effects of CDCW on the reprogramming of amino acids and gene expression in connection with lignans and other phenolic metabolite accumulation in L. album hairy roots.
Materials and methods
Plant material and hairy root culture
Mature seeds of L. album were collected from wild-grown plants in the Sohanak region (35°480′N, 51°32′E, and altitude of 1900 m), Tehran province Iran. This plant species was identified by Dr. Shahrokh Kazmepour-Osaloo, a professor of systematic and evolutionary botany. A voucher sample (No. 2450) of L. album was stored at the Herbarium, plant biology department, biological sciences faculty, Tarbiat Modares University, Tehran, Iran. The hairy root cultivation was established based on the previous study (Ahmadian Chashmi et al. 2013). Briefly, Agrobacterium rhizogenes strain LBA9402 was used to transform the hairy roots. These roots were cultivated into a 200 mL flask with a 50 mL liquid MS medium (Murashige and Skoog 1962) and incubated at dark conditions on a shaker (100 rpm) and 25 °C. Two grams of hairy roots were subsequently transferred into the fresh medium every three weeks.
Elicitor preparation
The cell wall of P. indica was provided according to Tashackori et al. (2018). Chitinase treatment of P. indica cell wall was performed based on the manufacture's instruction using chitinase (EC 3.2.1.14) lyophilized powder from Trichoderma viride (ref C8241, Sigma Aldrich). 100 mg of the fine cell wall P. indica was mixed with 100 µL of chitinase enzyme powder and 1 mL phosphate buffer (pH = 6.1) for 12 h. Finally, HCl (0.5 M) and hot water were used to deactivate the chitinase enzyme permanently. Then, the CDCW elicitor was obtained and used as a biotic elicitor at 1 mg mL−1.
A representative growth curve revealed that root growth reached the log phase after the 10th day (data not shown).
2 g of hairy roots were treated with different concentrations of sterilized CDCW (0.5, 1, 2.5, and 5% v/v). Each sample was collected after 5 days of elicitation. After optimizing CDCW concentration, we performed a time-course test to study the elicitation effect of CDCW on growth efficiency and SM production by hairy roots of L. album.
For this purpose, 2 g of hairy roots were poured into 30 mL of the medium with CDCW (1% (v/v)) and incubated in dark conditions at 25 ± 2 °C on a rotary shaker (110 rpm). Samples were harvested after 12, 24, 48, 72, and 120 h cultivation.
H2O2 content assay
H2O2 content in treated hairy roots was determined according to Velikova et al. (2000), as described by Zafari et al. (2017). 200 mg of the fresh hairy root was extracted with 5 mL of Trichloroacetic acid (TCA; 0.1%, w/v) in an ice bath. The samples were centrifuged at 12,000 rpm for 15 min and then thoroughly added 0.5 mL of supernatant to 0.5 mL of phosphate buffer (pH = 7.0) and 1 mL of KI (1 M). The absorbance of these mixtures was evaluated at 390 nm. Finally, H2O2 content was analyzed by comparison with a standard curve and described as µmol g−1 FW.
Antioxidant enzyme, and total protein assay
For the enzyme assay, hairy roots were extracted based on Garden (2003) method. Protein content was assayed by the Bradford (1976) protocol. SOD (E.C.1.15.1.1) activity was quantified using the nitroblue tetrazolium (NBT) reduction method as described by Giannopotitis and Ries (1977). SOD activity was measured by the IC50 rate (the amount of SOD that inhibits the NBT reduction at 560 nm). Also, GPX activity (E.C.1.11.1.7) was estimated based on the measurement of guaiacol oxidation by H2O2 at 470 nm (Lin and Wang 2002).
HPLC analysis of lignans
2 g of dried hairy roots was crushed to fine powder. The fine powder was subjected to lignan extraction with methanol/dichloromethane (Ahmadian Chashmi et al. 2013). Lignan content was analyzed by an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA). The HPLC system was equipped with a stationary phase, a C18 column (Perfectsil Target ODS-3 (5 μm), 250 × 4.6 mm, MZ-Analysentechnik GmbH, Mainz, Germany), with a mobile phase consisting of acetonitrile and water. A UV detector was applied to detect lignans at 290 nm (Yousefzadi et al. 2010; Ahmadian Chashmi et al. 2013). The lignan contents were determined according to an authentic standard (LARI, PINO, PTOX, and 6MPTOX standards) purchased from Sigma-Aldrich (Taufkirchen, Germany).
Lignin content assay
Lignin assay was performed according to Iiyama and Wallis (1990) method. For this reason, 0.5 mg of the fresh roots were mixed with distilled water and centrifuged (1200×g for 20 min). The pellet was washed with 10 volumes (v/w) EtOH and added CHCl3–MeOH to the reaction mixture (1:2, v/v). The mixtures were incubated at 25 °C for 24 h and washed with acetone. Then, 6 mg of fine powder was dissolved to 0.1 mL of HClO4 70% and 25% acetyl bromide (v/v in glacial acetic acid). The reaction mixture was blended with NaOH and glacial acetic acid and incubated at 70 °C for 30 min. Finally, the lignin content was measured based on the calibration curve (20 g L−1 cm−1).
Determination of phenolic acids, flavonoids, and amino acid profiling using HPLC
We used the Owen's method with a slight modification through HPLC (Agilent Technologies, a UV diode array detector at 280 and 300 nm) for phenolic acid quantification (Owen et al. 2003).
Measurement of amino acids and flavonoids by HPLC was clarified in detail in our previous study (Tashackori et al. 2018). The description of sample peaks was attained by comparison of the observed retention time with reference standards.
All phenolic acids standards (e.g., Cinnamic acid, coumaric acid, caffeic acid, ferulic acid, and SA) and flavonoids (e.g., catechin, myricetin, kaempferol, diosmin, luteolin, daidzein, and naringenin), and amino acids standards (e.g., Glu: Glutamine, Asp: Asparagine, Arg: Arginine, Met: Methionine, Gly, Glycine, Ile, Isoleucine, Leu, Leucine, Phe: Phenylalanine, Tyr, Tyrosine) were obtained from Sigma-Aldrich.
PAL and CAD activity assay
PAL (EC 4.3.1.24) activity was assayed based on the rate of cinnamic acid production, and the absorbance of samples was estimated at 290 nm (Wang et al. 2006). Measurement of CAD (EC 1.1.1.195) activity was accomplished according to Garden (2003) method with a slight modification. Coniferyl aldehyde formation was evaluated by measuring the absorbance at 390 nm.
Gene expression analyses
PAL, CAD, CCR, and PLR were chosen to analyze gene expression through qPCR in hairy roots of L. album (Applied Biosystem/MDS SCIEX, Foster City, CA, USA). First, total RNA was extracted from frozen hairy roots using the RNX™-Plus solution kit (CinnaGen Inc, Tehran, Iran) based on the manufacturer's recommendation. Total RNA (2 µg) was used for cDNA preparation (RevertAid™ M-MuLV) (Fermentas, Burlington, ON, Canada) using RT primers listed in Table 1 (Esmaeilzadeh Bahabadi et al. 2011). The PCR reaction was done at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 15 s. The visualization of data was done by using the light cycler 7500 software, version 2.0.1.
Statistical analysis
At first, the raw data were normalized at log2, and subsequently, Pearson’s correlation coefficient was applied by Hierarchical cluster analysis (HCA) via the algorithms inserted in the web-based MetaboAnalyst (http://www.metaboanalyst.ca). All data were analyzed using SPSS 24 software based on a completely randomized design with three replications. Duncan’s multiple range test (p value ≤ 0.05) was used to compare the significant difference between means. In this study, we also employed Excel 2019 and GraphPad Prism 8 plotting diagrams.
Results
Hairy root growth and lignans production
As shown in Table 2, we observed that different concentrations of CDCW had a negative effect on DW after 5 days of elicitation. The growth rate of hairy roots was remarkably declined by about 40.02% compared to the control when the CDCW concentration was 5% (v/v).
According to Table 2, the results exhibited that the highest level of LARI and PINO was achieved at 1% CDCW, respectively. PTOX content attained the highest value at 1% CDCW, whereas 6MPTOX content showed the highest level at both 1 and 2% elicitor. According to hairy roots' lignans, we selected CDCW (1%) for the subsequent experiments. The alterations of Lignans were significantly dependent on CDCW (1%), where it was able to induce the highest lignans production with a minimal decrease in hairy root DW.
Time-course hairy roots growth
No significant changes in root growth were realized up to 72 h after CDCW (1%) elicitation compared to the control (Fig. 1). However, our results indicated that the root growth was considerably decreased 120 h after elicitation.
H2O2 content and antioxidant enzyme activity
Data mining showed that H2O2 content significantly generated after 12 and 24 h treatment with CDCW elicitation (Fig. 2a). H2O2 content attained highest level after 120 h of treatment, which was 1.89-times compared to control.
Also, an increasing generation of H2O2 was coordinated by SOD activity, which peaked at 48 h after CDCW treatment by 1.41-times higher compared to control (Fig. 2b). In addition, another antioxidant enzyme activity analysis showed that the time-dependent CDCW elicitation enhanced GPX activity after 12 h. The highest GPX activity was recorded at 48 and 72 h after elicitation, respectively (Fig. 2c).
Lignans and lignin content
Eliciting of hairy roots at all times had a significant effect on lignans accumulations and lignin production (Fig. 3). Results showed that PINO, LARI, and PTOX accumulation were improved after 24 h of elicitation. PINO content peaked at 48 h after treatment (compared to control), which was unchanged up to 72 h (Fig. 3a). The highest accumulation of LARI was recorded 72 h after elicitation, which was 2.6 times more than the untreated roots (Fig. 3b). A time-course study has shown that PTOX content was enhanced in response to CDCW and reached the highest level after 48 and 120 h compared to their respective controls (Fig. 3c).
The effect of P. indica CDCW (1% v/v) on PINO (a), LARI (b), PTOX (c), 6MPTOX (d) and lignin (e) in L. album hairy root. Means from 3 separate experiments ± SD. Duncan’s multiple range test was applied to reveal differences at p value ≤ 5%. The same letters represent no significant difference among means
6MPTOX content reached the highest level in the treated hairy roots 120 h after elicitation (Fig. 3d). Moreover, results showed that lignin content slightly increased in elicited hairy roots and peaked at 120 h after elicitation (Fig. 3e).
Phenolic acid and flavonoid contents
The HPLC analysis revealed the presence of 12 main phenolic compounds: 5 phenolic acids (e.g., cinnamic acid, coumaric acid, caffeic acid, and SA) and 7 flavonoids (catechin, diosmin, kaempferol, luteolin, naringenin, daidzein, and myricetin), listed in Tables 3 and 4, respectively.
Due to the treatment of CDCW, significant differences were observed in the profile of phenolic acids between the treated and control hairy roots (Table 3). The highest amount of cinnamic acid was detected 24 h after elicitation. Coumaric acid content was enhanced after 24 h in treated roots and reached a peak at 48 and 72 h after elicitation, respectively. Also, we observed highest level of caffeic acid and ferulic acid at 48 h after elicitation. The highest amount of SA was at 48 and 72 h after elicitation, respectively.
The production of flavonoids as antioxidant compounds was mainly increased during elicitation with CDCW (1%) (Table 4). Catechin and naringenin peaked at a maximum level of 72 and 120 h after elicitation, respectively. Myricetin content showed no significant difference in treated and control hairy roots. Also, the accumulation of kaempferol increased 48 h after elicitation. Ultimately, we observed that the flavonoid profile (e.g., diosmin, luteolin, and daidzein) increased and reached the highest at 120 h after elicitation.
PAL and CAD activity assay
To dissect whether PAL and CAD are involved in response to CDCW elicitation, their activity was assayed in L. album hairy roots (Fig. 4). CDCW treatment had an extremely positive effect on the PAL and CAD activity. The PAL activity sharply peaked at 48 and 72 h after elicitation, respectively. The lowest PAL activity was detected in elicited hairy roots 48 h after elicitation compared to the control (Fig. 4a). Also, the CAD activity-induced within a considerable period of 120 h, reaching the highest level at 72 h after elicitation (Fig. 4b).
Gene expression assay
We were interested in understanding the mechanism of CDCW treatment modifying upstream and downstream gene expression of the phenylpropanoid pathway. For this purpose, we selected PAL, PLR, CCR, and CAD genes expression for the qRT-PCR experiment (Table 1). Further analysis revealed that PAL and CAD genes' expression was significantly up-regulated 48 h after elicitation, 4.39 and 2.128-fold, respectively (Fig. 5a, b). The expression of the PLR gene showed higher expression values 48 h after elicitation when compared to the control (Fig. 5c). PLR gene plays a vital role in the production of lignans compounds in L. album hairy roots. Additionally, the significant up-regulation of CCR was revealed 72 h after elicitation (Fig. 5d).
The effect of P. indica CDCW (1% v/v) on PAL (a), CAD (b), PLR (c), and CCR (d) gene expression in L. album hairy root. Means from 3 separate experiments ± SD. Duncan’s multiple range test was applied to reveal differences at p value ≤ 5%. The same letters represent no significant difference among mean
Free amino acid assay
The amino acid profiling of hairy roots during elicitation is shown in Fig. 6. The increased Glu and Asp accumulations content was observed only 72 and 120 h after elicitation (Fig. 6a, b). Also, the highest value of Arg was observed 72 h after elicitation (76.98% higher compared to the control) (Fig. 6c). The induction of Met production was observed 48 and 72 h after elicitation, respectively (Fig. 6d). Gly content was significantly induced 72 and 120 h after elicitation compared to control (Fig. 6e). At 72 h, Leu content reached the highest (18.53 μg.g −1 FW), which was 76.54% higher compared to control (Fig. 6g). The highest accumulation of Ile in treated roots was obtained 48 h after elicitation compared to control (Fig. 6f). Phe content peaked 24 and 48 h after elicitation, 1.81 and 1.69-fold more than the same point control (Fig. 6h). Tyr production reached a maximum of 48 h after elicitation, where it was 1.83 times compared to the control (Fig. 6i).
The effect of P. indica CDCW (1% v/v) on amino acids in L. album hairy root. Glu (a), Asp (b), Arg (c), Met (d) Gly (e), Ile (f), Lue (g), Phe (h) and Tyr (i). Means from 3 separate experiments ± SD. Duncan’s multiple range test was applied to reveal differences at p value ≤ 5%. The same letters represent no significant difference among means
Time-course metabolic profiling of CDCW-elicited hairy roots
To address how the effects of time-course of CDCW elicitor influence the performance of L. album hairy roots, we analyzed the correlation pattern between metabolites through Hierarchical Cluster Analysis (HCA) based on the Pearson coefficient (Fig. 7a). Here, we described the correlation analysis of changes in H2O2 generation, antioxidant enzymes, and primary and SM content in L. album hairy roots under CDCW elicitation. We designed a heatmap that indicated the relative frequency of metabolites based on each metabolite's Pearson correlation coefficient (positive and negative correlation were shown by red and green color, respectively) (Fig. 7a). These results implied that the different change patterns in primary and secondary metabolites at a set of time course after treatment. At the first, the metabolic accumulation was chiefly shifted to the enhancement of constituents for defensive mechanisms such as amino acids (Phe, Tyr, Arg, Met, Glu and Asp), regulatory molecules as H2O2 and SA, and the activities of antioxidant enzymes such as SOD. On other hand, some phenylpropanoid metabolites such as PINO, LARI, Kaempferol, Caffeic acid, Naringenin and Catechin apparated an induction level at the latest hours of treatment.
a A Correlation between primary and secondary metabolites (phenylpropanoid and the predominant amino acids). Cluster analysis of metabolites involved in metabolic pathways is shown as a heatmap (distance measure using Pearson, and clustering algorithm using Ward.D). We identified five distinct clusters; 1: Lignin to Myricetin, 2: Ferulic acid to PLR, 3: H2O2 content to PAL, 4: SOD activity to PAL activity, 5:Glu to Leu. The intensity of the red and blue colorants indicates the high and low relative abundance of metabolites, respectively (color figure online). b H2O2 burst acts as a secondary messenger involved in oxidative response in L. album hairy roots under P. indica CDCW elicitation. There is a close relationship between H2O2 and the others compounds such as, phenolic acids, flavonoids, and amino acids. Also, the phenylpropanoid pathway starts with the aromatic amino acid phenylalanine, which is converted to cinnamic acid by the phenylalanine ammonia-lyase (PAL)
Ultimately, we observed five main clusters (1–5) with similar fold-change metabolite profiles (displayed on the dendrogram with a dash in Fig. 7a. Cluster's category was assigned to various response models of H2O2 generation, antioxidant enzyme activity, primary and SM accumulation to CDCW elicitation. Cluster 1 (Lignin to Myrecin) described a weak correlation, cluster 2 (Ferulic acid to PLR expression), cluster 3 (H2O2 to PAL expression), cluster 4 (SOD activity to PAL activity) implied positively co-regulated metabolites and enzymes activity. Finally, cluster 5 expressed a positive correlation (Glu to Leu). Also, there was a significant correlation between Phe and other phenylpropanoid compounds (Fig. 7b).
Discussion
In the current study, we tried to understand how CDCW derived from P. indica, endophytic fungi, can increase the phenylpropanoid metabolites, especially lignans. Presented results showed that H2O2 generation was raised in CDCW (1%) at early time points (Fig. 2a). Consistent with our observations, a study has suggested that ROS generation, mainly H2O2, is considered one of the earliest events that have a regulatory role in plants to adapt to various stress conditions (Shen et al. 2018). Also, there was a reverse correlation between H2O2 accumulation and hairy root growth (Fig. 1). Our results are supported by Samari et al. (2020) who reported that plants could tightly control H2O2 content since an increase in H2O2 accumulation can lead to the cell fate toward programmed cell death (PCD) or other adaptative responses such as antioxidant enzymes and SM production.
Also, we recorded an enhancement in the activities of SOD and GPX enzymes in response to CDCW exposure (Fig. 2b, c). Based on our results, alteration in the antioxidant enzyme activities such as SOD, GPX which could play a critical role in mitigating H2O2 accumulation. These results agreed with the observations of Glombitza et al. (2004) and Chakraborty and Tongden (2005). Our observations here implied that the activation of SOD and consequently increasing the H2O2 generation could serve as a defensive mechanism to avoid damages induced by free radicals. The same phenomenon was reported by Tanou et al. (2009) in citrus plants.
On the other hand, we observed a close correlation between free amino acid profiles and H2O2 content in CDCW-elicited hairy roots. Thus, as Zafari et al. (2016) suggested, our results highlighted the importance of reconfiguring amino acids to improve the plant defense system and mitigate ROS content.
In response to CDCW, L. album hairy roots illustrated an improvement in PAL and TAL activities at 72 h, while the concentration of Phe and Tyr generally decreased. These observations give evidence that the enhancement of H2O2 content in the elicited hairy roots of L. album would induce PAL and TAL activities at the early stage of elicitation, which could deplete Phe and Tyr concentrations (Fig. 6). Our findings are in line with those studies that reported Phe/Tyr act as nutrition elements in plants and served as essential precursors for producing SM (Mur et al. 2017; Yang et al. 2020).
Therefore, PAL gene expression was closely associated with the induction of PAL-specific activity. Suzuki et al. (2004) and Hano et al. (2006b) previously reported a mutual correlation between PAL gene expression and the beginning of the phenylpropanoid pathway that can consume Phe. According to increased PAL gene expression, metabolic profiles were analyzed in different branches of the phenylpropanoid pathway response to CDCW. It has been discussed that biotic elicitors could stimulate the accumulation of phenolic compounds (Hano et al. 2006a; Tahsili et al. 2014), which serve as potent antioxidants versus oxidative stress by mitigating ROS (Michalak 2006). In general, all analyzed phenolic acids (cinnamic acid, coumaric, ferulic, caffeic, and salicylic acid) were induced after CDCW elicitation (Table 3). Upon exposure to CDCW, SA content enhancement could improve the defense system as a necessary signaling molecule in plants. Also, this result was in line with the fact that SA had a critical role in controlling H2O2 content through reversible inhibition of antioxidant enzymes such as SOD (Clark et al. 2000; Samari et al. 2020).
Pearson correlation analysis implies a positive correlation between SA content and PAL genes expression. The increase of SA during the first hours of elicitation (Table 3) can partially confirm this hypothesis. Also, CDCW resulted in shifting the phenylpropanoid pathway towards lignans production. We also illustrated the behavior pattern of lignans (Fig. 3). These observations show that LARI is a precursor for PTOX and 6MPTOX decreasing during elicitation, while PTOX and 6MPTOX promote lignan production. It can be argued that in elicited hairy roots of L. album, the phenylpropanoid pathway allocates the amino acids and energy for lignan production rather than other phenolic metabolites in response to CDCW elicitation. The presented data were in line with those studies that reported the accumulation of lignans might be related to responding to various biotic elicitors in L. album such as Fusarium graminearum (Tahsili et al. 2014) and chitosan (Samari et al. 2020). Our results explained that the fungal elicitor could induce the production of phenylpropanoid metabolites by the provision of amino acids and regulation of genes expression.
Conclusion
In conclusion, L. album hairy roots can activate a complicated metabolic network by sensing CDCW derived from P. indica at a set of time-course. The application of CDCW presumably modifies the reprogramming of amino acids toward the phenylpropanoid metabolites. Regarding the bioactive metabolites of L. album hairy roots, the CDCW can change the defensive hairy root response towards the phenylpropanoid production, especially PTOX and 6MPTOX. Our results declare an exploitable strategy for cultivating L. album hairy roots with higher accumulations of its bioactive metabolites. Since the materials and the time constraints limit the study, the molecular mechanisms underlying these processes were not defined. Therefore, more profound research on signaling molecules network (e.g., Ca 2+) and epigenetic markers (e.g., miRNAs) of the phenylpropanoid metabolites is needed. This hypothesis can be performed for finding details in lignan production in this species.
Abbreviations
- 6MPTOX:
-
6-Methoxy podophyllotoxin
- Arg:
-
Arginine
- Asp:
-
Asparagine
- CAD:
-
Cinnamyl alcohol dehydrogenase
- CCR:
-
Cinnamoyl-CoA reductase
- DW:
-
Dry weight
- FW:
-
Fresh weight
- Glu:
-
Glutamine
- Gly:
-
Glycine
- GPX:
-
Guaiacol peroxidase
- H2O2 :
-
Hydrogen peroxide
- Ile:
-
Isoleucine
- LARI:
-
Laricinol, Leu, Leucine
- Met:
-
Methionine
- PAL:
-
Phenylalanine ammonia-lyase
- Phe:
-
Phenylalanine
- PINO:
-
Pinoresinol
- PLR:
-
Pinoresinol-laricinol reductase
- PTOX:
-
Podophyllotoxin
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SM:
-
Secondary metabolite
- SOD:
-
Superoxide dismutase
- Tyr:
-
Tyrosine
References
Ahmadian Chashmi N, Sharifi M, Yousefzadi M, Behmanesh M, Rezadoost H, Cardillo A, Palazon J (2013) Analysis of 6-methoxy podophyllotoxin and podophyllotoxin in hairy root cultures of Linum album Kotschy ex Boiss. Med Chem Res 22:745–752. https://doi.org/10.1007/s00044-012-0067-1
Baldi A, Jain A, Gupta N, Srivastava AK, Bisaria VS (2008) Co-culture of arbuscular mycorrhiza-like fungi (Piriformospora indica and Sebacina vermifera) with plant cells of Linum album for enhanced production of podophyllotoxins: a first report. Biotechnol Lett 30(9):1671. https://doi.org/10.1007/s10529-008-9736-z
Baldi A, Farkya S, Jain A, Gupta N, Mehra R, Datta V, Srivastava AK, Bisaria VS (2010) Enhanced production of podophyllotoxins by co-culture of transformed Linum album cells with plant growth promoting fungi. Pure Appl Chem 82(1):227–241. https://doi.org/10.1351/PAC-CON-09-02-09
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Chakraborty U, Tongden C (2005) Evaluation of heat acclimation and salicylic acid treatments as potent inducers of thermotolerance in Cicer arietinum L. Curr Sci 89:384–389
Clark D, Durner J, Navarre DA, Klessig DF (2000) Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microbe Interact 13:1380–1384
Corbin C, Drouet S, Mateljaki I, Markulin L, Decourtill C, Renouard S, Lopez T, Doussot J, Lamblin F, Auguin D, Laine E, Fuss E, Hano C (2017) Functional characterization of the pinoresinol–lariciresinol reductase-2 gene reveals its roles in yatein biosynthesis and flax defense response. Planta 246:405–420. https://doi.org/10.1007/s00425-017-2701-0
Delaunois B, Farace G, Jeandet P, Clément C, Baillieul F, Dorey S, Cordelier S (2014) Elicitors as alternative strategy to pesticides in grapevine Current knowledge on their mode of action from controlled conditions to vineyard. Environ Sci Pollut Res 21:4837–4846
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097. https://doi.org/10.1105/tpc.7.7.1085
Esmaeilzadeh S, Sharifi M, Ahmadian CN, Murata J, Satake H (2014) Significant enhancement of lignan accumulation in hairy root cultures of Linum album using biotic elicitors. Acta Physiol Plant 36:3325–3331. https://doi.org/10.1007/s11738-014-1700-z
Esmaeilzadeh Bahabadi S, Sharifi M, Safaie N, Murata J, Yamagaki T, Satake H (2011) Increased lignan biosynthesis in the suspension cultures of Linum album by fungal extracts. Plant Biotechnol Rep 5:367–373. https://doi.org/10.1007/s11816-011-0190-3
Fuss E (2003) Lignans in plant cell and organ cultures: an overview. Phytochem Rev 2:307–320
Garden HJ (2003) Biotechnological production of podophyllotoxin by Linum album suspension cultures. Doctoral thesis, Heinrich-Heine Universität, Düsseldorf
Giannopotitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309e314. https://doi.org/10.1104/pp.59.2.309
Glombitza S, Dubuis PH, Thulke O, Welzl G, Bovet L, Götz M, Affenzeller M, Geist B, Hehn A, Asnaghi C (2004) Crosstalk and differential response to abiotic and biotic stressors reflected at the transcriptional level of effector genes from secondary metabolism. Plant Mol Biol 54:817–835
Hano C, Addi M, Bensaddek L, Cronier D, Baltora-Rosset S, Doussot J, Maury S, Mesnard F, Chabbert B, Hawkins S, Laine E, Lamblin F (2006a) Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223:975–989. https://doi.org/10.1007/s00425-005-0156-1
Hano C, Martin I, Fliniaux O, Legrand B, Gutierrez L, Arroo RRJ, Mesnard F, Lmablin E (2006b) Pinoresinol–lariciresinol reductase gene expression and secoisolariciresinol diglucoside accumulation in developing flax (Linum usitatissimum) seeds. Planta 224:1291–1301. https://doi.org/10.1007/s00425-006-0308-y
Iiyama K, Wallis FA (1990) Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agric 51:145–161. https://doi.org/10.1002/jsfa.2740510202
Javadian N, Karimzadeh G, Sharifi M, Moieni A, Behmanesh M (2017) In vitro polyploidy induction: changes in morphology, podophyllotoxin biosynthesis, and expression of the related genes in Linum album (Linaceae). Planta 245:1165–1178. https://doi.org/10.1007/s00425-017-2671-2
Kamalipourazad M, Sharifi M, Zare Maivan H, Behmanesh M, Ahmadian Chashmi N (2016) Induction of aromatic amino acids and phenylpropanoid compounds in Scrophularia striata Boiss. cell culture in response to chitosan-induced oxidative stress. PPB 107:374–384. https://doi.org/10.1016/j.plaphy.2016.06.034
Lin JS, Wang GX (2002) Doubled CO2 could improve the drought tolerance better in sensitive cultivars than in tolerant cultivars in spring wheat. Plant Sci 163:627e637. https://doi.org/10.1016/s0168-9452(02)00173-5
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Mur LA, Simpson C, Kumari A, Gupta AK, Gupta KJ (2017) Moving nitrogen to the center of plant defense against pathogens. Ann Bot 119:703–709. https://doi.org/10.1093/aob/mcw179
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay of tobacco tissue culture. Physiol Plant 15:473–497
Owen RW, Haubner R, Mier W, Giacosa A, Hull WE, Spiegelhalder B, Bartsch H (2003) Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes. Food Chem Toxicol 41:703–717. https://doi.org/10.1016/S0278-6915(03)00011-5
Sagharyan M, Ganjeali A, Cheniyani M, Mousavi-Kouhi M (2020) Optimization of callus induction with enhancing production of phenolic compounds production and antioxidants activity in callus cultures of Nepeta binaloudensis Jamzad (Lamiaceae). Iran J Biotechnol 18(4):47–55. https://doi.org/10.30498/IJB.2020.2621
Samari E, Sharifi M, Ghanati F, Fuss E, Ahmadian Chashmi N (2020) Chitosan-induced phenolics production is mediated by nitrogenous regulatory molecules: NO and PAs in Linum album hairy roots. PCTOC 140:63–576. https://doi.org/10.1007/s11240-019-01753-w
Shen Y, Li J, Gu R, You L, Wang H, Zhan X, Xing B (2018) Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere 197:513–525. https://doi.org/10.1016/j.chemosphere.2018.01.036
Suzuki H, Reddy MSS, Naoumkina M, Aziz N, May GD, Huhman DV, Summer LW, Blount JW, Mendes P, Dixon RA (2004) Methyljasmonate and yeast elicitor induce differential transcriptional and metabolic reprogramming in cell suspension cultures of the model legume Medicago sativa. Planta 220:696–707. https://doi.org/10.1007/s00425-004-1387-2
Tahsili J, Sharifi M, Safaie N, Esmaeilzadeh-Bahabadi S, Behmanesh M (2014) Induction of lignans and phenolic compounds in cell culture of Linum album by culture filtrate of Fusarium graminearum. J Plant Interact 9(1):412–417. https://doi.org/10.1080/17429145.2013.846419
Tanou G, Molassiotis A, Diamantidis G (2009) Hydrogen peroxideand nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants. J Plant Physiol 166:1904–1913
Tashackori H, Sharifi M, Ahmadian Chashmi N, Safaie N, Behmanesh M (2016) Induced-differential changes on lignan and phenolic acid compounds in Linum album hairy roots by fungal extract of Piriformospora indica. PCTOC 127:187–194. https://doi.org/10.1007/s11240-016-1041-2
Tashackori H, Sharifi M, Ahmadian Chashmi N, Safaie N, Behmanesh M (2018) Piriformospora indica cell wall modulates gene expression and metabolite profile in Linum album hairy roots. Planta 248:1289–1306. https://doi.org/10.1007/s00425-018-2973-z
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 151:59–66. https://doi.org/10.1016/s0168-9452(99)00197-1
Wang J, Zheng L, Wu J, Tan R (2006) Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide 15:351–358. https://doi.org/10.1016/j.niox.2006.04.261
Westcott ND, Muir AD (2003) Flax seed lignan in disease prevention and health promotion. Phytochem Rev 2:401–417. https://doi.org/10.1023/B:PHYT.0000046174.97809.b6
Yang Q, Zhao D, Liu Q (2020) Connection between amino acid metabolisms in plants: lysine as an example. Front Plant Sci 11:928. https://doi.org/10.3389/fpls.2020.00928
Yousefzadi M, Sharifi M, Chashmi NA, Behmanesh M, Ghasempour A (2010) Optimization of podophyllotoxin extraction method from Linum album cell cultures. Pharm Biol 48(12):1421–1425. https://doi.org/10.3109/13880209.2010.489564
Yousefzadi M, Sharifi M, Behmanesh M, Ghasempour A, Moyano E, Palazon J (2012) The effect of light on gene expression and podophyllotoxin biosynthesis in Linum album cell culture. PPB 56:41–46. https://doi.org/10.1016/j.plaphy.2012.04.010
Zafari S, Sharifi M, Ahmadian Chashmi N, Mur LAJ (2016) Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds, and amino acids. PPB 99:11–20. https://doi.org/10.1016/j.plaphy.2015.12.004
Zafari S, Sharifi M, Mur LAJ, Ahmadian Chashmi N (2017) Favouring NO over H2O2 production will increase Pb tolerance in Prosopis farcta via altered primary metabolism. Ecotoxicol Environ Saf 142:293–302. https://doi.org/10.1016/j.ecoenv.2017.04.036
Acknowledgements
This work was fiscally supported by Tarbiat Modares University.
Author information
Authors and Affiliations
Contributions
This research paper was accomplished with the collaboration of authors. HT performed the experiments, analyzed data and wrote the manuscript. MSH designed and supervised the study and edited the manuscript. NAC advised the study and established hairy root cultures. MB advised the study and supported the molecular experiments. NS advised the study and provided fungus Piriformospora indica. MS analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tashackori, H., Sharifi, M., Ahmadian Chashmi, N. et al. Physiological, biochemical, and molecular responses of Linum album to digested cell wall of Piriformospora indica. Physiol Mol Biol Plants 27, 2695–2708 (2021). https://doi.org/10.1007/s12298-021-01106-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-01106-y