Abstract
Plant secondary metabolites are produced naturally in the plant system as a defense mechanism to combat environmental stress factors. These metabolites are extensively used in food, cosmetics, agrochemicals and pharmaceutical sectors. With the applications of plant tissue culture, any particular organ which is the major site for secondary metabolite production can be targeted and cultured. Recently, a new strategy to increase the metabolite production in plants has been employed with the use of elicitors. These elicitors are the chemical substances that trigger the biosynthetic pathways by activating certain transcriptional factors and upregulating the genes. Hence the secondary metabolite production increases in the plant system due to the stress developed by the introduction of the elicitors. Generally, elicitors may be abiotically derived from non-living sources or biotically derived from the living sources. In the present review, the mechanism of biotic elicitation and the applications of biotic elicitors like bacterial, fungal, algal elicitors and other polysaccharides extracted from them has been discussed extensively. It has been noted that the addition of bacterial elicitors like Rhizobium rhizogenes showed a 94% increase in genistein production while Escherichia coli showed a 9.1-fold increase in diosgenin production. Similarly, fungal elicitors like Aspergillus niger increased thiophene production by 85% and a 26-fold increase in sanguinarine production was seen when the cultures were treated with Botrytis sps. Algal extracts like Haematococcus pluvialis increased the betalain production by 2.28 folds while Botryococcus braunii elicited Vanillin, Vanillylamine and Capsaicin by 3-fold, 6-fold and 2.3-fold respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From centuries plants are playing a major role in fulfilling the needs of all living organisms especially human beings for their food, shelter and medicine due to the presence of various compounds in plants which are referred as metabolites or phytochemicals. Metabolites like carbohydrates, amino acids and lipids are produced in high quantity and make up the ultrastructure of the plant hence referred to as primary metabolites. Apart from these, plants also synthesize a wide range of chemical compounds in trace amounts known as secondary metabolites to fulfill their physiological roles by facilitating the plants to withstand and interact with the environment. These secondary metabolites possess biological activity against pathogens and play a major role in the defense and signaling of the plant system (Bourgaud et al. 2001; Guerriero et al. 2018). The concept of secondary metabolites was first described by Albrecht Kossel who won the noble prize for physiology or medicine in 1910. The secondary metabolites are produced in plants as a defense response and the metabolites are often induced due to stress signals, such as pathogen invasion, environmental factors and nutrient deficits. Some of these metabolites are important as they are used as preventive or curative drugs for certain diseases (Ramachandra Rao and Ravishankar 2002).
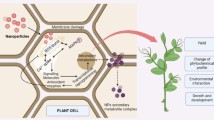
The secondary metabolites of the plants are broadly classified based on their structure as phenolics, alkaloids, saponins and terpenes (Hussein and El-Anssary 2019). Some of the major pathways in biosynthesis of these metabolites are shikimic acid pathway (tannins, phenols and aromatic alkaloids), malonic acid pathway (terpenes, alkaloids and steroids), acetate-malonate pathway (alkaloids, fatty acids and phenols) and pentose phosphate pathway (glycosides) (Kabera et al. 2014). General representation of the biosynthetic pathway of secondary metabolite production is illustrated in Fig. 1.
These wide ranges of secondary metabolites are used in biopesticides, agrochemicals, flavouring agents, essential oils, food additives and most importantly in pharmaceutical industries for medicine and cosmetics (Ramakrishna and Ravishankar 2011). In the global market, many biotic compounds like quinine, vincristine, papervine, ephedrine, caffeine etc. are in high demand and these compounds are often restricted to a particular genus or species of plant (Verpoorte et al. 2002). Naturally, plants produce less than 1% dry weight of secondary metabolites and the production depends on the developmental and physiological stage of the plant along with the environmental conditions (Dixon 2001; Oksman-Caldentey and Inzé 2004). The production of secondary metabolite by plants do not satisfy the need of mankind and the entire plant is utilized for the phytochemical extraction, therefore, the shortage and overuse of the plants are a major limitation. To meet the existing demand, the plants are grown in-vitro using biotechnological techniques as they eliminate the climatic, geographical and edaphic barriers and also promote the plant growth in all seasons and provide a favorable environment for the continuous supply of phytochemicals (Yukimune et al. 1996; Karuppusamy 2009). Depending on the metabolite and its source, different plant tissue culture techniques such as cell suspension, cellular extracts and their culture filtrates (Biswas et al. 2016, 2018), root and shoot multiplication, callus cultures (Awad et al. 2014), adventitious root, hairy root cultures, autoclaved cells etc. are developed for production of useful secondary metabolites (Buitelaar et al. 1992). Production of pure compounds like taxol, morphine, L-DOPA, capsaicin, vinblastine, vincristine and berberine by tissue culture showed only limited success (Vanisree et al. 2004; Vijaya Sree N et al. 2010). To overcome the limitation of the quantity of the bioactive compounds several biotechnological strategies like optimization of the medium, providing suitable culture environments, permeabilization, immobilization, elicitation, precursor feeding, metabolic engineering, biotransformation methods, use of bioreactors, and micropropagation are employed (Naik and Al-Khayri 2016). This review article presents most recent developments in the field of biotic elicitation with equal importance to all the types of biotic elicitors used till date. Apart from this, a simplified tabular column with the plant used, target metabolite, type of biotic elicitor used and results has been tabulated for a quick review for the readers.
Elicitors and their classification
Elicitation is one of the important strategies of biotechnology to enhance the production of secondary metabolites by the addition of certain substances called elicitors (Ramakrishna and Ravishankar 2011; Halder et al. 2019). Elicitor is defined as a substance that is applied in small quantity to a living system to trigger the biosynthesis of a specific compound which plays an important role in the adaptation of plant system and to overcome their stressful conditions (Thakur et al. 2019). Elicitors are considered as signaling molecules that activate transcriptional factors which regulate the expression of genes that are concerned with secondary metabolite production (Yamaner et al. 2013; Zhao 2015). For the enhanced activity of elicitors and maximum production of secondary metabolites in-vitro, elicitor specificity, their concentration, the time course of elicitation with host growth stage and nutrient stage of culture should be optimized (Patel and Krishnamurthy 2013). Elicitors are classified based on their nature as abiotic (non-biological) and biotic (biological) (Radman et al. 2003). Abiotic elicitors are further grouped under three categories such as physical, chemical and hormonal (Naik and Al-Khayri 2016). Biotic elicitors are substances derived from the biological origin and they are further divided into polysaccharides and microorganism based. Polysaccharide elicitors are extracted, isolated and purified from the cell wall of biological organisms like plant-derived or animal-derived which are chitin, lignin, pectin and cellulose. Microorganism-based elicitor includes cell extracts of yeast, bacteria and fungi (Namdeo A. G. 2007).
Mechanism of biotic elicitors
The mechanism is based on elicitor-receptor interaction which leads to a cascade of biochemical events. Though all elicitors start with receptor interaction on the plasma membrane, inside the cell, elicitors trigger various pathways altering biochemical and physiological processes and this leads to the production of different secondary metabolites or defense responsive compounds (Ferrari 2010; Shasmita et al. 2018). The mechanism starts with the binding of the elicitor to the receptor present on the plasma membrane. Elicitor-receptor interaction leads to alteration of ions present across the cell membrane such as the influx of calcium ions (Ca2+) and efflux of the cation (K+) and anions (Cl−) (Jabs et al. 1997; Shabala and Pottosin 2014). In plants, as a response to the environmental variations and pathogen signals, calcium acts as a secondary messenger and it is found that calcium channels are activated within few minutes of elicitor addition into the system. This ion flux leads to cytoplasmic acidification and increases extracellular pH leading to depolarization of plasma membrane (Mathieu et al. 1996; Sakano 2001; Zhao et al. 2005). Few elicitors induce alkalization of apoplast which leads to an influx of protons and some elicitors induce acidification of apoplast which leads to efflux of vacuolar protons (Bolwell et al. 2002; Angelova et al. 2006). As a result of elicitor interaction, there is increased activity of the plant phospholipases and protein phosphorylation (Zhao 2015). This leads to the synthesis of secondary messengers such as D-myo-inositol 1,4,5-triphosphate (InsP3) Diacylglycerol (DAG) and activation of ca2+ mediated secondary messengers (Gillaspy 2011; Aldon et al. 2018). This is followed by activation of the mitogenic protein kinase (MAPK) pathway which leads to a series of events like phosphorylation of MAP kinase etc. These molecules are transported to the nucleus through a nuclear pore complex leading to phosphorylation of specific transcriptional factors (Pitzschke et al. 2009; Sinha et al. 2011; Colcombet et al. 2016). In some plants, as a response to elicitor and G -protein-coupled receptor (GPCR) interaction, NADPH oxidase is activated which is responsible for the generation of Active Oxygen Species (AOS) (Mishra et al. 2012), which leads to acidification of cytoplasm and activation of proteins like chitinases, glucanases, hydroxy proline-rich glycoproteins and protease inhibitors and this leads to activation of transcriptional factors leading to the expression of corresponding defense genes like jasmonates, ethylene, salicylates and later as a defense response plant produces phytoalexins which are secondary metabolites (Zhao et al. 2005; Angelova et al. 2006). The overall mechanism of biotic eliciatation is illustrated in Fig. 2.

Reproduced from Zhao et al. 2005)
Schematic representation of the general mechanism of biotic elicitors (
Bacterial elicitation
Bacteria are single-celled microorganisms found in the environment (Dzhavakhiya and Shcherbakova 2007). The use of bacterial cells, bacterial cellular components and bacterial cellular extracts to elicit a response in plants is called bacterial elicitation. They are also used to induce secondary metabolite production from plants in the in-vitro conditions. Each bacterial species can elicit different secondary metabolites in different quantities. The difference is because of the elicitor-membrane receptor binding, G protein activation, cytoplasmic acidification and reactive oxygen species generation (Zhao et al. 2005; Biswas et al. 2016). Different types of bacterial components such as live cells (Park et al. 2006; Awad et al. 2014), cell homogenate (Buitelaar et al. 1992; Jung et al. 2003), cellular extracts (Gandi et al. 2012; Chodisetti et al. 2013) and culture filtrates (Biswas et al. 2016, 2018) are used to elicit secondary metabolite production. In the environment, plants face huge challenges due to the presence of bacteria in the soil, this stimulates the production of secondary metabolites as a defense mechanism in plants. Similarly, live bacterial cultures can also induce secondary metabolite production in the in-vitro conditions, but very few studies have reported using live bacteria as elicitors (Mañero et al. 2012).
The bacterial cultures of Bacillus aminovorans, Bacillus cereus, Agrobacterium rhizogenes, Agrobacterium tumefaciens and Rhizobium leguminosarum (0.5 ml of culture) were used to elicit glycyrrhizic acid in the root cultures of Taverniera cuneifolia (Roth) Arn. The bacterium R. leguminosarum has shown the highest elicitation (6.37 mg/g) when compared to untreated root cultures (1.48 mg/g). Other bacteria have shown a significant increase in the production whereas A. tumefaciens has shown no significant increase in the production (1.46 mg/g) (Awad et al. 2014). The bacterial cultures of Rhizobium radiobacter and Rhizobium rhizogenes are co-cultured with adventitious root culture of Albizzia kalkora (Roxb.) to elicit the production of isoflavone (daidzein and genistein) in them. Addition of R. rhizogenes showed a 35% increase in daidzein and a 94% increase in genistein production while R. radiobacter showed a 12% increase in daidzein and 89% increase in genistein production on the 6th day of treatment (Park et al. 2006). Atropine production has been significantly reduced to 0.017% from 0.116% (control) and 0.095% from 0.116% when B. cereus and Staphylococcus aureus cultures were used respectively in the hairy root culture of Datura metel L. (Shakeran et al. 2015). Co-culturing of B. cereus with hairy root culture of D. metel L. showed a 13.5-fold increase, the addition of cellular extract showed a 2-fold increase and addition of culture supernatant showed a 4-fold increase of tanshinone production respectively (Wu et al. 2007). Pseudomonas sp. has shown to enhance the production of rosmarinic acid in the shoot-culture of Rosmarinus officinalis L. (Yang et al. 1997). Cell homogenization is a process by which the cell membranes are ruptured and the cellular components are released into the solution which is referred to as cell homogenate. This cell homogenate contains the pathogenic metabolite which triggers the plant defense responses and as a result, the secondary metabolites are produced in the plants. The cell homogenates of Bacillus subtilis, E. coli, Pseudomonas aeruginosa and S. aureus showed a 50, 35, 35 and 20% increase in thiophene production in the hairy root cultures of Tagetes patula L. (Buitelaar et al. 1992). Raw and autoclaved homogenates of P. aeruginosa, B. cereus and S. aureus were used to elicit scopolamine production in the hairy root cultures of Scopolia parviflora (Dunn) Nakai. Raw homogenates enhanced scopolamine production though no significant difference was seen between each of the elicitor used. However, browning of roots was observed after 24 hour of incubation while autoclaved homogenate showed similar scopolamine production as the control (Jung et al. 2003).
In the bacterial cultures, bacteria produce various compounds which are released to the culture media and they are also capable of eliciting defense responses in plants. So, the bacterial cultures are centrifuged and the cells are pelleted, the supernatant is filter sterilized and it is considered as the culture filtrates. The culture filtrates of Pseudomonas monteilii and Bacillus circulans were used to elicit ginsenoside production in cell suspension culture of Panax quinquefolius L., P. monteilii showed a 2.5-fold increase while B. circulans showed a slight decline in total ginsenoside production after five days of treatment. Prolonged exposure of elicitors has shown a 20–30% decline in total ginsenoside production (Biswas et al. 2016). Similarly, Serratia marcescens at 1.25 and 2.5% concentration showed a 1.6 and 2.5-fold increase respectively while B. subtilis at 2.5% concentration showed a 1.8-fold increase in ginsenoside production in cell suspension cultures of Panax sikkimensis R. N. Banerjee., (Biswas et al. 2018). The culture filtrates of gram-negative bacteria like Mesorhizobium huakuii, Mesorhizobium amorphae, Bradyrhizobium ganzhouense, Azotobacter beijerinckii and gram-positive bacteria like Lactobacillus plantarum, Leuconostoc sp., and Bacillus sp. have been used for ginsenoside production in root cultures of Panax ginseng C.A. Meyer. All gram-positive and gram-negative bacteria showed an increase in ginsenoside production but the highest production was seen in gram-negative bacteria (Le et al. 2018). B. subtilis and E. coli culture filtrates were used to elicit diosgenin production in the cell suspension culture of the Helicteres isora L., E. coli (1.5%) showed a 9.1-fold increase while B. subtilis (2%) showed a 6.1-fold increase in diosgenin production (Shaikh et al. 2020). The culture filtrate of E. coli, S. aureus, B. cereus and P. aeruginosa enhanced anthocyanin by 22% in the callus culture of Daucus carota L. (Suvarnalatha et al. 1994). The culture filtrate of Stenotrophomonas maltophilia has shown a 3-fold increase in hypericin production in the shoot culture of Hypericum perforatum L. (Mañero et al. 2012). The cellular extracts of A. rhizogenes resulted in high accumulation (66.12 mg/g) of gymnemic acid and the cellular extracts of non-pathogenic bacteria B. subtilis and E. coli resulted in low accumulation (47.97 mg/g and 33.25 mg/g respectively) of gymnemic acid in the cell suspension cultures of Gymnema sylvestre (Retz.) schult. (Chodisetti et al. 2013). The cell-free cellular extracts of E. coli, B. subtilis, A. rhizogenes and A. tumefaciens have been used to elicit andrographolide production in cell suspension cultures of Andrographis paniculata (Burm.f.) Nees. Out of which E. coli elicited highest (8.3 mg/g) andrographolide production whereas A. tumefaciens showed no significant increase in the production (Gandi et al. 2012). The cellular extracts of Pseudomonas sp. and Enterobacter sp. are used to elicit the alkaloids production in the protocorm-like body cultures of Pinellia ternate (Thumb) breit., Pseudomonas sp. showed 69–166% increase in guanosine, 45–1143% increase in trigonelline and 26% increase in inosine production. While Enterobacter sp. showed 371–1143% increase in guanosine, 114–500% increase in trigonelline and 5–17% increase in inosine production when compared to control (Liu et al. 2010). The cellular extract of Cronobacter sakazakii were used to elicit antioxidants and bactericidal phenolic compounds in the plant tissues of Dionaea muscipula (Makowski et al. 2020). The cellular extract of P. aeruginosa was studied as the elicitor to produce rosmarinic acid, caffeic acid, carnosic acid, carnosol, and rosmanol in the callus culture of R. officinalis. Rosmarinic acid accumulation increased from 3.7 to 3.9 μg/mL, caffeic acid from 0.5 to 2.3 μg/mL, carnosic acid from 2.6 to 3.1 μg/mL and carnosol from 1.7 to 2.4 μg/mL (Rashid et al. 2011). In the callus culture of Ammi majus, Enterobacter sakazakii cellular extract showed a 12% increase in scopoletin, in cell suspension culture, a 9.6% increase in umbelliferone production and in the hairy root culture 2.3 and 0.3% increase in umbelliferone and bergapten production respectively (Staniszewska et al. 2003). The A. tumefaciens and A. rhizogenes cellular extracts significantly increased the production of xanthone to 6.95 and 5.04 mg/g respectively from 3.7 mg/g (untreated) in the cell suspension cultures of H. perforatum L. (Tusevski et al. 2015). Celluar extracts of A. rhizogenes, Pectobacterium carotovorum and E. sakazakii has enhanced the production of acteoside, baicalin, wogonin, scutellarin, and wogonoside while C. sakazakii and Klebsiella pneumonia enhanced wogonin and K. pneumoniae alone enhanced chrysin production in the hairy root cultures of Scutellaria lateriflora L. (Wilczańska-Barska et al. 2012). Common bacterial elicitors used for the production of secondary metabolites have been listed in Table1.
Fungal elicitation
Among the biotic elicitors, fungal elicitors (both free-living and endophytic) are the most important and widely used for the synthesis of commercial compounds. The interaction between the fungi and the plant results in the induction of hypersensitive responses which activates defence pathways in the plant thereby increasing the phytoalexins (Baldi et al. 2009) and inducing secondary metabolite production more effectively (Zhai et al. 2017). The pure fungal cultures are usually obtained from the hyphal tip culture (Salehi et al. 2019).
The aqueous extract of Aspergillus niger, A. flavus, Penicillium notatum and Fusarium oxysporum were used to elicit anthocyanin production in D. carota L. mycelial extracts of A. flavus gave maximum elicitation with a two-fold increase in the anthocyanin production whereas P. notatum and F. oxysporum treatments were not much effective (Rajendran et al. 1994). Similarly, in another study A. niger increased thiophene production by 85% when compared to control in T. patula L. (Buitelaar et al. 1992). A. niger, when used as an elicitor in Mentha piperita L. cell cultures showed enhanced production of menthol (140.8 mg/L) which was higher when compared to other non-biological elicitation (Chakraborty and Chattopadhyay 2008). A. niger showed a 9-fold increase in gymnemic acid production in the cultures of G. sylvestre (Retz.) schult. (Devi and Srinivasan 2011). A. niger and Rhizopus stolonifera showed a 4.9 and 3.8-fold increase respectively in glycyrrhizin production in the cultures of Abrus precatorius L. (Karwasara et al. 2011). Oldenlandia umbellate L. is a commercially important dye yielding plant. Elicitors like A. niger, Mucor prayagensis and Trichoderma viride were used to enhance its growth. Maximum response were observed in A. niger treated cultures which showed 79 shoots and 47 roots (Saranya and Velayutham 2019). The culture filtrates of Trichoderma atroviride and T. harzianum were used to elicit ginsenoside from P. quinquefolius L. cell suspension cultures. T. atroviride produced the highest ginsenoside (3.2 times higher than control) after 5-day treatment (Biswas et al. 2016). They were also used to elicit dual metabolite ginsenoside and anthocyanin in cell suspension cultures of P. sikkimensis R. N. Banerjee (Biswas et al. 2018). The effect of three fungal elicitors was tested on Centella asiatica L. cultures, the addition of T. harzianum filtrate showed a 2.53-fold increase in asiaticoside production while Colletotrichum lindemuthianum decreased asiaticoside production but showed higher biomass and F. oxysporum had an inhibitory effect on shoot growth and poor yield of asiaticosides (Prasad et al. 2013). The H. perforatum L. cultures were treated with the extracts of F. oxysporum, Phomaexigua and Botrytis cinerea resulting in reduced biomass and rapid increase of phenylpropanoid and naptho-dianthrones. Hypericin and pseudohypericin were significantly increased in the early growth phase and later gradually declined (Gadzovska et al. 2015). The culture filtrates of Micromucoris abellina in Catharanthus roseus (L.) G. Don caused a drastic and rapid increase of indole alkaloid biosynthesis leading to the production of 400 µg/L ajmalicine and 600 µg/L catharanthine while only trace amounts of ajmalicine and 5.8 µg/L catharanthine were detected in control (Dicosmo et al. 1987). The cultures of Papaver somniferum L. were incubated with 1 ml of Botrytis sps. which showed a 26-fold increase in sanguinarine production when compared to control while 5 ml of Colletotrichum gloeosporoides elicited 43% of total sanguinarine produced. Other elicitors like Rhodotorula rubra, Helminthosporium gramineum and Sclerotinia sclerotiorum showed a relatively weaker response in sanguinarine production (Eilert et al. 1985). The cell suspension cultures of Corylus avellane L. were treated with endophytic fungi like Chaetomium globosum and Paraconiothyrium brasiliense isolated from Taxus baccata L. and C. avellane L. respectively. Maximum elicitation was seen when 10% (v/v) C. globosum was added which showed 4.1 times higher production of paclitaxel on 17th day (Salehi et al. 2019). Common fungal elicitors used to trigger the metabolite production is shown in Table 2.
Algal Elicitation
Algae and cyanobacteria constitute the major phytoplankton over the earth. The entire plant body or its components can be utilized in various fields of research. One of their most promising applications is in the elicitation studies. Marine algae is an under-utilized bioresource, out of which only a few are used as a source of food, for therapeutic and other biological applications (Vinoth et al. 2012). Seaweed extracts and their cell wall components can also act as a prominent elicitor providing enormous beneficial aspects such as plant protectants, improved yield and increased tolerance to various stresses (Sbaihat et al. 2015). Seaweeds majorly comprises of carbohydrates made up of oligosaccharides and polysaccharides (Arman and Qader 2012). Introduction of these algal polysaccharides such as carrageenans, fucans, laminarans and ulvans into the plant system activates a series of defense cascade leading to the resistance against pathogens and triggers the secondary metabolite production (Stadnik and de Freitas 2014). Chemical analysis of algal extracts reveals the presence of various active compounds, macro and micronutrients, growth regulators which also support the in-vitro growth of the plants apart from acting as an elicitor (Satish et al. 2015). Hence the entire algal mass or their individual components help in in-vitro growth, mass propagation, callus culture etc.
Hot water extracts of 25 strains of cyanobacterium members like Nostoc sp., Anabaena sp., Synechococcus sp. and Xenococcus sp. along with their dialysates and non-dialysates were added to the cell suspension cultures of D. carota L. to study its effect on the embryogenesis. It was observed that the plantlet formation increased on an average by 3.7-fold when these marine cyanobacterial extracts were added. A 4.2 and 3.0-fold increase was seen when dialysates and non-dialysates of the filamentous form were added respectively whereas a 3.2 and 5.2-fold increase was seen when dialysates and non-dialysates of unicellular strains were added respectively. Overall, the maximum number of plantlets was seen when non-dialysate of Synechococcus sp. (240) and Anabaena sp. (211) were added while minimum (32) was seen in control (Wake et al. 1991). Torpedo shaped somatic embryos obtained from the cell suspension cultures of D. carota L. was encapsulated to form artificial seeds with the addition of hot water extract of Synechococcus sp. (50, 200, 400 and 800 mg/L) and their non-dialysates (10, 50, 100, 200 mg/L). High frequency of germination (94%) was seen each in 100 and 200 mg/L of non-dialysate when added followed by 91, 90 and 81% in 400, 800 and 200 mg/L of extract respectively. While 50 and 10 mg/L of non-dialysate showed 77 and 58% respectively. The least of 57 and 35% seed germination were observed in 50 mg/L of extract and control respectively (Wake et al. 1992). Pure cultures of Anabaena sps. and Nostoc carneum belonging to Nostocaceae members cultured in Bold’s Basal medium was used as an elicitor treatment for the neem cell suspension cultures. Two concentrations of Anabaena sps. were taken (265 cells/mL and 530 cells/mL), of which higher concentration of algal cells resulted in a 5-fold increase in biomass in callus line-1 and decreased the biomass in callus line-2 and vice versa was seen in lower concentration. Similarly, addition of N. carneum (1670 cells/mL) initially increased the biomass by 2-fold later its efficiency decreased. N. carneum and Anabaena sp. increased the protein content by a twofold and threefold respectively. Addition of Anabaena sp. (265 cells/mL) in the cell suspension triggered the synthesis of a triterpenoid, azadirachtin (0.32 g/μL) detected through HPLC. Other concentrations of elicitors failed to induce the production of azadirachtin (Poornasri. et al. 2008). Various microalgae were used to elicit the red pigment formation in the cultures of Carthamus tinctorius L. out of which Nostoc linckia, Anabaena cylindrica and A. variabilis showed 8-fold increase in the red pigment formation (5.21 mg/L) while the least was seen in Chlorococcum sp., Chlorella sp. and Scenedesmus sp. (0.61 mg/L) (Hanagata et al. 1994). Phycocyanin, a biliprotein present in Spirulina platensis, a blue-green alga was extracted and introduced into Capsicum frutescens L. and D. carota L. cell cultures in five different concentrations (1.5, 3.0, 6.0, 12 and 24 μg/ml). The addition of phycocyanin in C. frutescens L. cultures showed no significant growth but capsaicin production increased in all the concentrations of phycocyanin, but the highest production was seen in 3.0 – 6.0 μg/ml (190 μg capsaicin/g of fresh weight). There was a 2-fold increase in capsaicin on 3rd day later it reduced to 1.5-fold on the 6th day when compared to control. While the application of phycocyanin in D. carota L. in lower concentrations (3.0 and 6.0 μg/ml) slightly increased the callus growth but at higher concentrations callus growth decreased. Initially, all concentrations of phycocyanin elicited anthocyanin production and the highest was seen in 3.0 μg/ml (24.8 mg anthocyanin /0.1 g of fresh weight) on 3rd day but later on 12th day they declined. Thus, phycocyanin shows early elicitation in both cultures (Ramachandra Rao et al. 1996). Similarly, three-week-old callus of C. frutescens L. developed from leaf and hypocotyl were transferred to the media containing the filter-sterilized acetone extracts of Botryococcus braunii, a colonial Chlorophyceae microalga was taken in four different concentrations (1, 2, 4 and 8 mg/L). 8 mg/L showed a threefold increase in total chlorophyll content, a 2-fold increase in the seed germination rate, a 1.5-fold increase in shoot and leaf length and a one-fold increase in root length. After 15 days maximum carotenoid content (0.18 mg/g) was seen in 8 mg/L followed by 4 mg/L (0.156 mg/g) and 2 mg/L (0.134 mg/g). Also, 8 mg/L showed a 3-fold, 6-fold and 2.3-fold increase while 4 mg/L showed 2-fold, 3-fold and a fold increase in vanillin, vanillylamine and capsaicin respectively (Sharma et al. 2010). Aqueous extracts of Haematococcus pluvialis and Spirulina platensis were used to elicit food color, betalain production from Beta vulgaris L. and an insecticide, thiophene from T. patula L. Addition of H. pluvialis extract to the hairy root cultures of B. vulgaris L. increased the biomass by 1.25-fold and the betalain production by 2.28-fold initially and later its content decreased after 15th day while S. platensis extract showed a 1.13-fold increase in biomass and 1.16-fold increase in betalain synthesis. When the hairy root cultures of T. patula L. were treated with H. pluvialis extract it showed a 1.40-fold increase in the biomass and 1.2 times increase in thiophenes while extract of S. platensis neither showed any significant effect on biomass nor thiophene accumulation (Ramachandra Rao et al. 2001). Aqueous extracts of a red seaweed Kappaphycus alvarezii were added to the cultures of Picrorhiza kurroa Royle ex Benth, an endangered medicinal plant in four different concentrations (0.1, 1.0, 2.0 and 3.0 g/L) to elicit the picroside-I production. Out of which 2.0 g/L was found to be best for the in-vitro growth as there were 3.23, 1.55, 2.41 and 2.42-fold increase in biomass, plant length, number of roots and shoots respectively on 30th day. Hence this concentration remains best for shoot multiplication and root induction. Also, it was noted that with the addition of seaweed extracts picroside-I content increased 3–4 folds at 25 °C and 2–3 folds at 15 °C respectively (Sharma et al. 2015). The various algal elicitors used for the biotic elicitation of secondary metabolites is summarized in Table 3.
Elicitation using polysaccharides
Polysaccharides are biotic elicitors as they are found in all living organisms like plants, animals and microbes. Polysaccharides are biopolymers formed by the bonding of monosaccharides (Fukui et al. 1990). The physical and chemical properties of polysaccharides are characterized by their structural orientation (Nartop 2018). Polysaccharides according to their biological functions in living organisms are categorized into storage polysaccharides (reservoirs of energy like glycogen and starch) and structural polysaccharides (providing structure and support) (Fukui et al. 1990). Polysaccharides play an important role in the cellular communication, shielding the plants from stress conditions etc. Polysaccharide elicitors are categorized into two groups: endogenous (cellulose, pectin etc.) and exogenous (chitin, chitosan etc.) (Nartop 2018). Polysaccharides can be extracted, isolated and purified from living organisms or they can be chemically synthesized. Polysaccharides are very commonly used to elicit antimicrobial metabolites (Paulert et al. 2009; Lu et al. 2019). Yeast extract is one of the elicitors majorly used for the secondary metabolite synthesis as well as to study the plant defense responses (Putalun et al. 2007). Yeast extract stimulated synthesis of several important metabolites in various plants (Funk et al. 1987; Jeong et al. 2005).
Out of all the biotic and abiotic elicitors used in the hairy root cultures of Pueraria candollei, wall.ex Benth., yeast extract (0.5 mg/ml) efficiently induced isoflavonoid 4.5-fold higher than the control (60 mg/g) (Udomsuk et al. 2011). Similar results were also observed in Salvia miltiorrhiza Bunge cell cultures where yeast extract showed 10-fold higher tanshinone production (2.3 mg/g) which was more efficient compared to other biotic and abiotic elicited cell cultures (Zhao et al. 2012). Addition of yeast extract (1.5 mg/L) in germinating embryos of C. roseus (L.) G. Don showed highest yield of vinblastine (22.74%), vincristine (48.49%), and high levels of alkaloids (Maqsood and Abdul 2017). Yeast, as an elicitor also showed an efficient increase on biomass along with metabolite production (Funk et al. 1987; Putalun et al. 2007).
There are several other elicitors sharing origin with yeast extract such as chitosan, chitin, mannan used in the in-vitro induction of pharmaceutically useful metabolites (Baque et al. 2012). Withaferin-A production was stimulated 4.03-fold higher than the control when chitosan was added (100 mg/L) to the hairy root cultures of Withania somnifera (L.) Dunal. (Thilip et al. 2019). Similarly, chitosan (150 mg/L) drastically improved an active antimalarial compound, artemisinin accumulation in the hairy root cultures of Artemisia annua L. (Putalun et al. 2007). Addition of chitosan (200 mg/L) to the immobilized cells of Plumbago rosea L. resulted in 8.2-fold higher accumulation of plumbagin over control (Komaraiah et al. 2003). Chitosan (50 mg/L) along with salicylic acid and jasmonic acid in cell suspension cultures of Azadirachta indica A. Juss induced a 5-fold increase of azadirachtin, an active natural bio pesticide (Prakash and Srivastava 2008). Chitosan along with chitin induces phytoalexin, phenylpropanoid and naphtodianthrone production in plants (Orlita et al. 2008; Gadzovska et al. 2015). Chitin (200 mg/L) induced phenylpropanoid and naphtodianthrone production in cell suspension cultures of H. perforatum L. Pectin and dextran stimulated pseudohypericin 1.7 and 1.5-fold respectively and also increased the phenylalanine ammonia lyase activity in H. perforatum L., cultures (Gadzovska et al. 2015). While 0.01% chitin and 0.1% chitosan induced all the phytoalexins and showed a higher biomass than control in the shoots of Ruta graveolens L. (Orlita et al. 2008).
Certain endophytic fungal derived polysaccharides play an important role in the elicitation process (Cheng et al. 2006; Wiktorowska et al. 2010). These endogenous organisms produce polysaccharides like mannan which are highly active (Fukui et al. 1990). Mannan is a primary polysaccharide majorly extracted from the yeast cell wall. Mannan stimulated pseudohypericin and hypericin 2.8 and 1.7-fold higher respectively in the cultures of H. adenotrichum Spach. This study also reported enhanced production of secondary metabolites in the presence of mannan and pectin (Yamaner et al. 2013). A 3.83-fold increased production of diosgenin was seen when water extracted mycelial polysaccharides (20 mg/L) of F. oxysporum isolated from the rhizome of Dioscorea zingiberensis C.H Wright. was introduced into the hairy root cultures of D. zingiberensis C.H Wright (Li et al. 2011).
Polysaccharides like pectin derived from plants are extensively used for elicitation studies (Veerashree et al. 2012). When agro-pectin was introduced into the cell suspension culture of Lithospermum erythrorhizon Siebold & Zucc., it induced naphthoquinone shikonin metabolite content (Fukui et al. 1983). Different elicitors like pectin, sodium alginate, gum arabic, chitosan and yeast extract were used to elicit anthocyanin and phenolic compounds in cell suspension cultures of Vitis vinifera L. Highest anthocyanin production (1.8-fold times higher than control) was observed in pectin elicited cultures on the 9th day (Cai et al. 2012). An extract from yeast cell wall when added to the hairy root cultures of P. ginseng C.A. Meyer it remarkably increased the saponin content (66.9 mg/g) which was 1.17-fold higher than the control (Jeong et al. 2005).
Dextran, a polysaccharide present in the bacterial cells obtained from sucrose by the action of an enzyme dextran sucrase can be used as an potent elicitor (Nagella and Murthy 2010; Rahpeyma et al. 2015). The wounds on Solanum lycopersicum L. caused by B. cinerea infection when treated with dextran and laminarin induced high phenylpropanoid and flavonoids in them (Lu et al. 2019).
Alginate, a frequently and commonly used elicitor is a polysaccharide extracted from the seaweeds (Paulert et al. 2009). There are several other seaweed polysaccharides such as ulvan, carrageenan, laminarin, etc., which are efficiently involved in secondary metabolite pathway activation (Salah et al. 2018). In recent research, several seaweeds are under study due to their significant ability to enhance the secondary metabolite production (Thilip et al. 2019). Sodium alginate induced a 1.7-fold increase in total phenolics in the cell suspension cultures of V. vinifera L. (Cai et al. 2012). Ulvan when added (2000 mg/L) to the shoot cultures of Olea europae L. stimulated active phenolic compounds and declined the wilt disease (Salah et al. 2018). Similarly, laminarin when added (0.05 mg/L) to the cell suspension cultures of Pueraria candollei Wall.ex Benth induced high accumulation of isoflavonoids (Korsangruang et al. 2010). Polysaccharides of various origin which are used to trigger the metabolite production in the in-vitro cultures is presented in Table 4.
Conclusion
From the above discussion it is very much evident that biotic elicitors play a major role in elicitation of secondary metabolites. The production of secondary metabolites like guanosine and trigonelline is found to be increased to a greater extent by introducing bacterial elicitors like Pseudomonas sp. and Enterobacter sp. Fungal elicitor A. niger has shown 9-fold increase in gymnemic acid production. An 8-fold increase was observed in the formation of red pigment in C. tinctorius L. by the use of microalgal elicitors. Yeast extract showed a significant increase in production of metabolites such as vinblastine and vincristine. From the above works it can be noted that when fungal elicitors were used there was a higher, significant increase in the metabolite produced in certain plants followed by the bacterial elicitation. However, this is not universal as the elicitation depends primarily on the plant species, type of culture, target metabolites and the type of elicitor used. From all the studies that has been reported it is well understood that any form or type of biotic elicitor introduced into the plant system will definitely have its effect over the secondary metabolite production. However, in most cases there has been a positive effect shown. There is a significant increase in the phytochemicals produced due to the introduction of these elicitors which triggers various biochemical pathways.
Though the biotic elicitors enhance the metabolite production there are few disadvantages which needs to be addressed. All biotic elicitors do not impact the secondary metabolite production in a significant manner. Hence, the experiment needs to be designed widely to optimize the concentration and the type of biotic elicitors to be used. Also, the chemical constituents and its concentration in the crude extracts are unknown hence it is very much uncertain to conclude which component from the extract is actually responsible for the elicitation. Apart from these, the biotic elicitors affect the physiological parameters as they induce a stress environment for the plant growth. Nevertheless, though many studies have been reported we are yet to understand the working of biotic elicitors at their molecular level. There are many biological organisms with potent role in elicitation which has not been explored till date. Hence, when such microbes or plant extracts (like weeds) are explored they can be used in elicitation studies which will also help us to curb their unwanted growth and utilize them in a larger scale in an eco-friendly manner. Combination of two or more biotic elicitors for the metabolite production has not been reported in any studies so far. Owing to this as a major lacuna in the field of elicitation, biotic elicitors have opened a plethora of gateways to improve the in-vitro secondary metabolite production for the upcoming researchers.
Data availability
All data generated or analysed during this study are included in this published article.
References
Aldon D, Mbengue M, Mazars C, Galaud J-P (2018) Calcium signalling in plant biotic interactions. Int J Mol Sci 19:665. https://doi.org/10.3390/ijms19030665
Angelova Z, Georgiev S, Roos W (2006) Elicitation of Plants. Biotechnol Biotechnol Equip 20:72–83. https://doi.org/10.1080/13102818.2006.10817345
Arman M, Qader SAU (2012) Structural analysis of kappa-carrageenan isolated from Hypnea musciformis (red algae) and evaluation as an elicitor of plant defense mechanism. Carbohydr Polym 88:1264–1271. https://doi.org/10.1016/j.carbpol.2012.02.003
Awad V, Kuvalekar A, Harsulkar A (2014) Microbial elicitation in root cultures of Taverniera cuneifolia (Roth) Arn. for elevated glycyrrhizic acid production. Ind Crops Prod 54:13–16. https://doi.org/10.1016/j.indcrop.2013.12.036
Baldi A, Srivastava AK, Bisaria VS (2009) Fungal elicitors for enhanced production of secondary metabolites. in plant cell suspension cultures. In: Varma A, Kharkwal AC (eds) Symbiotic Fungi. Springer, Berlin, pp 373–380. https://doi.org/10.1007/978-3-540-95894-9-23
Baque MdA, Shiragi MdHK, Lee E-J, Pack K-Y (2012) Elicitor effect of chitosan and pectin on the biosynthesis of anthraquinones, phenolics and flavonoids in adventitious root suspension cultures of Morinda citrifolia (L.). Aust J Crop Sci 6:1349–1355
Barrientos CH, Pérez C, Zúñiga G, Mahn A (2014) Effect of methyl jasmonate, sodium selenate and chitosan as exogenous elicitors on the phenolic compounds profile of broccoli sprouts. J Sci Food Agric 94:2555–2561. https://doi.org/10.1002/jsfa.6596
Bayraktar M, Naziri E, Akgun IH (2016) Elicitor induced stevioside production, in vitro shoot growth, and biomass accumulation in micropropagated Stevia rebaudiana. Plant Cell Tiss Organ Cult 127:289–300. https://doi.org/10.1007/s11240-016-1049-7
Biswas T, Kalra A, Mathur AK, Lal RK, Singh M, Mathur A (2016) Elicitors influenced differential ginsenoside production and exudation into medium with concurrent Rg3/Rh2 panaxadiol induction in Panax quinquefolius cell suspensions. Appl Microbiol Biotechnol 100:4909–4922. https://doi.org/10.1007/s00253-015-7264-z
Biswas T, Pandey SS, Maji D, Gupta V, Kalra A, Singh M, Mathur A, Mathur AK (2018) Enhanced expression of ginsenoside biosynthetic genes and in vitro ginsenoside production in elicited Panax sikkimensis (Ban) cell suspensions. Protoplasma 255:1147–1160. https://doi.org/10.1007/s00709-018-1219-z
Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53:1367–1376. https://doi.org/10.1093/jexbot/53.372.1367
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851. https://doi.org/10.1016/S0168-9452(01)00490-3
Brodelius P, Funk C, Haner A, Villegas M (1989) A procedure for the determination of optimal chitosan concentrations for elicitation of cultured plant cells. Phytochemistry. 28:2651–2654. https://doi.org/10.1016/S0031-9422(00)98060-9
Buitelaar RM, Casário MT, Tramper J (1992) Elicitation of thiophene production by hairy roots of Tagetes patula. Enzyme Microb Technol 14:2–7. https://doi.org/10.1016/0141-0229(92)90017-I
Cai Z, Kastell A, Mewis I, Knorr D, Smetanska I (2012) Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult 108:401–409. https://doi.org/10.1007/s11240-011-0051-3
Chakraborty A, Chattopadhyay S (2008) Stimulation of menthol production in Mentha piperita cell culture. Vitro Cell Dev Biol - Plant 44:518–524. https://doi.org/10.1007/s11627-008-9145-y
Chen F, Ren CG, Zhou T (2016) A novel exopolysaccharide elicitor from endophytic fungus Gilmaniella sp. AL12 on volatile oils accumulation in Atractylodes lancea. Sci Rep 6: 34735. https://doi.org/10.1038/srep34735
Cheng X-Y, Zhou H-Y, Cui X, Ni W, Liu C-Z (2006) Improvement of phenylethanoid glycosides biosynthesis in Cistanche deserticola cell suspension cultures by chitosan elicitor. J Biotechnol 121:253–260. https://doi.org/10.1016/j.jbiotec.2005.07.012
Cho JS, Kim JY, Kim IH (2003) Effect of polysaccharide elicitors on the production of decursinol angelate in Agelica gigas Nakai root cultures. Biotechnol Bioproc 8:158. https://doi.org/10.1007/BF02940273
Chodisetti B, Rao K, Gandi S, Giri A (2013) Improved gymnemic acid production in the suspension cultures of Gymnema sylvestre through biotic elicitation. Plant Biotechnol Rep 7:519–525. https://doi.org/10.1007/s11816-013-0290-3
Colcombet J, Sözen C, Hirt H (2016) Convergence of multiple MAP3Ks on MKK3 identifies a set of novel stress MAPK modules. Front Plant Sci 7:1–7. https://doi.org/10.3389/fpls.2016.01941
Devi CS, Srinivasan VM (2011) In vitro studies on stimulation of gymnemic acid production using fungal elicitor in suspension and bioreactor based cell cultures of Gymnema sylvestre R.BR. Recent Res Sci Technol 3:101–104
Dicosmo F, Quesnel A, Misawa M, Tallevi SG (1987) Increased synthesis of ajmalicine and catharanthine by cell suspension cultures of Catharanthus roseus in response to fungal culture-filtrates. Appl Biochem Biotechnol 14:101–106. https://doi.org/10.1007/BF02798428
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:843–847. https://doi.org/10.1038/35081178
Doernenburg H, Dietrich K (1994) Effectiveness of plant-derived and microbial polysaccharides as elicitors for aanthraquinone synthesis in Morinda citrifolia cultures. J Agri Food Chem 42:1048–1052. https://doi.org/10.1021/jf00040a040
Dzhavakhiya VG, Shcherbakova LA (2007) Chapter 16 - Creation of disease-resistant plants by gene engineering. In: Dyakov YT, Dzhavakhiya VG, Korpela T (eds) Comprehensive and Molecular Phytopathology. Elsevier, Amsterdam, pp 439–466. https://doi.org/10.1016/B978-044452132-3/50021-3
Ebel J, Ayers AR, Albersheim P (1976) Response of suspension-cultured soybean cells to the elicitor isolated from Phytophthora megasperma var. sojae, A fungal pathogen of soybeans. Plant Physiol 57:775–779. https://doi.org/10.1104/pp.57.5.775
Eilert U, Kurz WGW, Constabel F (1985) Stimulation of sanguinarine accumulation in Papaver somniferum cell cultures by fungal elicitors. J Plant Physiol 119:65–76. https://doi.org/10.1016/S0176-1617(85)80216-9
Ferrari S (2010) Biological elicitors of plant secondary metabolites: Mode of action and use in the production of nutraceutics In: Giardi MT, Rea G, Berra B (eds) Bio-Farms for nutraceuticals. Springer US, Boston, MA, vol 698, pp 152–166. https://doi.org/10.1007/978-1-4419-7347-4_12
Fukui H, Tani M, Tabata M (1990) Induction of shikonin biosynthesis by endogenous polysaccharides in Lithospermum erythvorhizon cell suspension cultures. Plant Cell Rep 9:73–76. https://doi.org/10.1007/BF00231552
Fukui H, Yoshikawa N, Tabata M (1983) Induction of shikonin formation by agar in Lithospermum erythrorhizon cell suspension cultures. Phytochemistry 22:2451–2453. https://doi.org/10.1016/0031-9422(83)80138-1
Funk C, Gügler K, Brodelius P (1987) Increased secondary product formation in plant cell suspension cultures after treatment with a yeast carbohydrate preparation (Elicitor). Phytochemistry 26:401–405. https://doi.org/10.1016/S0031-9422(00)81421-1
Gadzovska Simic S, Tusevski O, Maury S, Delaunay A, Lainé E, Joseph C, Hagège D (2015) Polysaccharide elicitors enhance phenylpropanoid and naphtodianthrone production in cell suspension cultures of Hypericum perforatum. Plant Cell Tissue Organ Cult 122:649–663. https://doi.org/10.1007/s11240-015-0798-z
Gandi S, Rao K, Chodisetti B, Giri A (2012) Elicitation of andrographolide in the suspension cultures of Andrographis paniculata. Appl Biochem Biotechnol 168:1729–1738. https://doi.org/10.1007/s12010-012-9892-4
Gillaspy GE (2011) The cellular language of myo-inositol signaling. New Phytol 192:823–839. https://doi.org/10.1111/j.1469-8137.2011.03939.x
Guerriero G, Berni R, Muñoz-Sanchez J, Apone F, Abdel-Salam E, Qahtan A, Alatar A, Cantini C, Cai G, Hausman J-F, Siddiqui KS, Hernández-Sotomayor SMT, Faisal M (2018) Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 9:309. https://doi.org/10.3390/genes9060309
Halder M, Sarkar S, Jha S (2019) Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci 19:880–895. https://doi.org/10.1002/elsc.201900058
Hanagata N, Uehara H, Ito A, Takeuchi T, Karube I (1994) Elicitor for red pigment formation in Carthamus tinctorius cultured cells. J Biotechnol 34:71–77. https://doi.org/10.1016/0168-1656(94)90167-8
Hao Y-J, An X-L, Sun H-D, Piao X-C, Gao R, Lian M-L (2020) Ginsenoside synthesis of adventitious roots in Panax ginseng is promoted by fungal suspension homogenate of Alternaria panax and regulated by several signaling molecules. Indust Crops Prod 150:112414. https://doi.org/10.1016/j.indcrop.2020.112414
Hussein RA, El-Anssary AA (2019) Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. In: Builders PF (ed) Herbal medicine. Intech Open, pp 11–30. https://doi.org/10.5772/intechopen.76139
Jabs T, Tschope M, Colling C, Hahlbrock K, Scheel D (1997) Elicitor-stimulated ion fluxes and O2- from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci 94:4800–4805. https://doi.org/10.1073/pnas.94.9.4800
Jeong G-T, Park D-H, Ryu H-W, Hwang B, Woo J-C, Kim D, Kim S-W (2005) Production of antioxidant compounds by culture of Panax ginseng CA Meyer hairy roots. Appl Biochem Biotechnol. https://doi.org/10.1007/978-1-59259-991-2_96
Jung H-Y, Kang S-M, Kang Y-M, Kang M-J, Yun D-J, Bahk J-D, Yang J-K, Choi M-S (2003) Enhanced production of scopolamine by bacterial elicitors in adventitious hairy root cultures of Scopolia parviflora. Enzyme Microb Technol 33:987–990. https://doi.org/10.1016/S0141-0229(03)00253-9
Kabera JN, Semana E, Mussa AR, He X (2014) Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol 2:377–392
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3:1222–1239. https://doi.org/10.5897/JMPR.9000026
Karwasara VS, Tomar P, Dixit VK (2011) Influence of fungal elicitation on glycyrrhizin production in transformed cell cultures of Abrus precatorius Linn. Pharmacogn Mag 7:307–314. https://doi.org/10.4103/0973-1296.90411
Khan T, Khan T, Hano C, Abbasi BH (2019) Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Industrial Crops and Products 129:525–535. https://doi.org/10.1016/j.indcrop.2018.12.048
Komaraiah P, Ramakrishna SV, Reddanna P, Kavi Kishor PB (2003) Enhanced production of plumbagin in immobilized cells of Plumbago rosea by elicitation and in situ adsorption. J Biotechnol 101:181–187. https://doi.org/10.1016/S0168-1656(02)00338-3
Korsangruang S, Soonthornchareonnon N, Chintapakorn Y, Saralamp P, Prathanturarug S (2010) Effects of abiotic and biotic elicitors on growth and isoflavonoid accumulation in Pueraria candollei var. candollei and P. candollei var. mirifica cell suspension cultures. Plant Cell Tissue Organ Cult 103:333–342. https://doi.org/10.1007/s11240-010-9785-6
Kundu A, Jawali N, Mitra A (2012) Shikimate pathway modulates the elicitor-stimulated accumulation of fragrant 2-hydroxy-4-methoxybenzaldehyde in Hemidesmus indicus roots. Plant Physiol Biochem 56:104–108. https://doi.org/10.1016/j.plaphy.2012.04.005
Le K-C, Im W-T, Paek K-Y, Park S-Y (2018) Biotic elicitation of ginsenoside metabolism of mutant adventitious root culture in Panax ginseng. Appl Microbiol Biotechnol 102:1687–1697. https://doi.org/10.1007/s00253-018-8751-9
Li P, Mou Y, Shan T, Xu J, Li Y, Lu S, Zhou L (2011) Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgenin production in cell suspension culture of Dioscorea zingiberensis. Molecules 16:9003–9016. https://doi.org/10.3390/molecules16119003
Liu YH, Liang ZS, Chen B, Yang DF, Liu JL (2010) Elicitation of alkaloids in in vitro PLB (protocorm-like body) cultures of Pinellia ternata. Enzyme Microb Technol 46:28–31. https://doi.org/10.1016/j.enzmictec.2009.08.005
Lu L, Ji L, Shi R, Li S, Zhang X, Guo Q, Wang C, Qiao L (2019) Dextran as an elicitor of phenylpropanoid and flavonoid biosynthesis in tomato fruit against gray mold infection. Carbohydr Polym 225:1. https://doi.org/10.1016/j.carbpol.2019.115236
Makowski W, Tokarz KM, Tokarz B, Banasiuk R, Witek K, Królicka A (2020) Elicitation-based method for increasing the production of antioxidant and bactericidal phenolic compounds in Dionaea muscipula. J Ellis Tissue Molecules 25:1794. https://doi.org/10.3390/molecules25081794
Mañero FJG, Algar E, Martín Gómez MS, Saco Sierra MD, Solano BR (2012) Elicitation of secondary metabolism in Hypericum perforatum by rhizosphere bacteria and derived elicitors in seedlings and shoot cultures. Pharm Biol 50:1201–1209. https://doi.org/10.3109/13880209.2012.664150
Maqsood M, Abdul M (2017) Yeast extract elicitation increases vinblastine and vincristine yield in protoplast derived tissues and plantlets in Catharanthus roseus. Braz J Pharmacogn 27:549–556. https://doi.org/10.1016/j.bjp.2017.05.008
Mathieu Y, Lapous D, Thomine S, Lauriere C, Guern J (1996) Cytoplasmic acidification as an early phosphorylation-dependent response of tobacco cells to elicitors. Planta 199:416–424. https://doi.org/10.1007/BF00195734
Mishra AK, Sharma K, Misra RS (2012) Elicitor recognition, signal transduction and induced resistance in plants. J Plant Interact 7:95–120. https://doi.org/10.1080/17429145.2011.597517
Mukundan U, Hjortso MA (1990) Effect of fungal elicitor on thiophene production in hairy root cultures of Tagetes patula. Appl Microbiol Biotechnol 33:145–147. https://doi.org/10.1007/BF00176515
Nagella P, Murthy HN (2010) Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour Technol 101:6735–6739. https://doi.org/10.1016/j.biortech.2010.03.078
Naik PM, Al-Khayri JM (2016) Abiotic and biotic elicitors–Role in secondary metabolites production through in vitro culture of medicinal plants. In: Shanker AK, Shanker C (eds) Abiotic and biotic stress in plants - Recent Advances and Future Perspectives. InTech Open, pp 247–277. https://doi.org/10.5772/61442
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: A Review. Pharmacogn Rev 1:69–79
Nartop P (2018) Engineering of biomass accumulation and secondary metabolite production in plant cell and tissue cultures. In: Plant metabolites and regulation under environmental stress. Elsevier, pp 169–194. https://doi.org/10.1016/B978-0-12-812689-9.00009-1
Oksman-Caldentey K-M, Inzé D (2004) Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9:433–440. https://doi.org/10.1016/j.tplants.2004.07.006
Orlita A, Sidwa-Gorycka M, Paszkiewicz M, Malinski E, Kumirska J, Siedlecka EM, Łojkowska E, Stepnowski P (2008) Application of chitin and chitosan as elicitors of coumarins and furoquinolone alkaloids in Ruta graveolens L. (common rue). Biotechnol Appl Biochem 51:91–96. https://doi.org/10.1042/BA20070200
Park S-Y, Lee W-Y, Park Y, Ahn J-K (2006) Effects of nitrogen source and bacterial elicitor on isoflavone accumulation in root cultures of Albizzia kalkora (Roxb.) Prain. J Integr Plant Biol 48:1108–1114. https://doi.org/10.1111/j.1744-7909.2006.00259.x
Park CH et al (2017) Comparison of different strains of Agrobacterium rhizogenes for hairy root induction and betulin and betulinic acid production in Morus alba. Natural Product Commun 12:1. https://doi.org/10.1177/1934578X1701200403
Patel H, Krishnamurthy R (2013) Elicitors in plant tissue culture. J Pharmacogn Phytochem 2:60–65
Paulert R, Talamini V, Cassolato JEF, Duarte MER, Noseda MD, Smania A, Stadnik MJ (2009) Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L). J Plant Dis Prot 116:263–270. https://doi.org/10.1007/BF03356321
Pitzschke A, Schikora A, Hirt H (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12:421–426. https://doi.org/10.1016/j.pbi.2009.06.008
Poornasri BD, Vimala A, Isha S, Chandra S (2008) Effect of cyanobacterial elicitor on neem cell suspension cultures. Indian J Sci Technol 1:1–5. https://doi.org/10.17485/ijst/2008/v1i7/29601
Prakash G, Srivastava AK (2008) Statistical elicitor optimization studies for the enhancement of azadirachtin production in bioreactor Azadirachta indica cell cultivation. Biochem Eng J 40:218–226. https://doi.org/10.1016/j.bej.2007.12.017
Prasad A, Mathur A, Kalra A, Gupta MM, Lal RK, Mathur AK (2013) Fungal elicitor-mediated enhancement in growth and asiaticoside content of Centella asiatica L. shoot cultures. Plant Growth Regul 69:265–273. https://doi.org/10.1007/s10725-012-9769-0
Putalun W, Luealon W, De-Eknamkul W, Tanaka H, Shoyama Y (2007) Improvement of artemisinin production by chitosan in hairy root cultures of Artemisia annua L. Biotechnol Lett 29:1143–1146. https://doi.org/10.1007/s10529-007-9368-8
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plants and microbial cell systems. Biotechnol Appl Biochem 37:91. https://doi.org/10.1042/BA20020118
Rahpeyma S-A, Moieni A, Jalali Javaran M (2015) Paclitaxel production is enhanced in suspension-cultured hazel (Corylus avellana L.) cells by using a combination of sugar, precursor, and elicitor. Eng Life Sci 15:234–242. https://doi.org/10.1002/elsc.201400115
Rajendran L, Suvarnalatha G, Ravishankar GA, Venkataraman LV (1994) Enhancement of anthocyanin production in callus cultures of Daucus carota L. under the influence of fungal elicitors. Appl Microbiol Biotechnol 42:227–231. https://doi.org/10.1007/BF00902721
Ramachandra Rao S, Sarada R, Ravishankar GA (1996) Phycocyanin, a new elicitor for capsaicin and anthocyanin accumulation in plant cell cultures. Appl Microbiol Biotechnol 46:619–621. https://doi.org/10.1007/s002530050871
Ramachandra Rao S, Tripathi U, Suresh B, Ravishankar GA (2001) Enhancement of secondary metabolite production in hairy root cultures of Beta vulgaris and Tagetes patula under the influence of microalgal elicitors. Food Biotechnol 15:35–46. https://doi.org/10.1081/FBT-100103893
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731. https://doi.org/10.4161/psb.6.11.17613
Ramachandra Rao S, Ravishankar GA (2002) Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol Adv 20:101–153. https://doi.org/10.1016/S0734-9750(02)00007-1
Rashid KI, Ibrahim KM, Hamza SJ (2011) Effect of some biotic and abiotic elicitors on phenolic acids and diterpenes production from rosemary (Rosmarinus officinalis L.) leaf and callus analyzed by high performance liquid chromatography (Hplc). J Al-Nahrain Univ Sci 14:104–109. https://doi.org/10.22401/JNUS.14.3.16
Roja G, Bhangale AS, Juvekar AR, Eapen S, Dapos Souza SF (2005) Enhanced production of the polysaccharide arabinogalactan using immobilized cultures of Tinospora cordifolia by elicitation and in situ adsorption. Biotechnol Progress 21:1688–1691. https://doi.org/10.1021/bp050188w
Sakano K (2001) Metabolic regulation of pH in plant cells: Role of cytoplasmic pH in defense reaction and secondary metabolism. Int Rev Cytol 206:1–44. https://doi.org/10.1016/S0074-7696(01)06018-1
Salah IB, Aghrouss S, Douira A, Aissam S, Alaoui-Talibi ZE, Filali-Maltouf A, Modafar CE (2018) Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J Plant Interact 13:248–255. https://doi.org/10.1080/17429145.2018.1471528
Salehi M, Moieni A, Safaie N, Farhadi S (2019) Elicitors derived from endophytic fungi Chaetomium globosum and Paraconiothyrium brasiliense enhance paclitaxel production in Corylus avellana cell suspension culture. Plant Cell Tissue Organ Cult 136:161–171. https://doi.org/10.1007/s11240-018-1503-9
Saranya S, Velayutham P (2019) Effect of fungus elicitors on plant growth in the in vitro cultures of Oldenlandia umbellata L. Adalya J 8:731–741
Satish L, Rameshkumar R, Rathinapriya P, Pandian S, Rency AS, Sunitha T, Ramesh M (2015) Effect of seaweed liquid extracts and plant growth regulators on in vitro mass propagation of brinjal (Solanum melongena L.) through hypocotyl and leaf disc explants. J Appl Phycol 27:993–1002. https://doi.org/10.1007/s10811-014-0375-6
Sbaihat L, Takeyama K, Koga T, Takemoto D, Kawakita K (2015) Induced resistance in Solanum lycopersicum by algal elicitor extracted from sargassum fusiforme. Sci World J 2015:1–9. https://doi.org/10.1155/2015/870520
Shabala S, Pottosin I (2014) Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol Plant 151:257–279. https://doi.org/10.1111/ppl.12165
Shaikh S, Shriram V, Khare T, Kumar V (2020) Biotic elicitors enhance diosgenin production in Helicteres isora L. suspension cultures via up-regulation of CAS and HMGR genes. Physiol Mol Biol Plants 26:593–604. https://doi.org/10.1007/s12298-020-00774-6
Shakeran Z, Keyhanfar M, Asghari G, Ghanadian M (2015) Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. Turk J Biol 39:111–118. https://doi.org/10.3906/biy-1405-25
Sharma A, Ambati RR, Chandrappa D, Ravi S, Aswathanarayana RG (2010) Botryococcus braunii, a new elicitor for secondary metabolite production in Capsicum frutescens. Func Plant Sci Biotechnol 5:9–13
Sharma N, Chauhan RS, Sood H (2015) Seaweed extract as a novel elicitor and medium for mass propagation and picroside-I production in an endangered medicinal herb Picrorhiza kurroa. Plant Cell Tissue Organ Cult 122:57–65. https://doi.org/10.1007/s11240-015-0749-8
Shasmita, Singh NR, Rath SK, Behera S, Naik SK (2018) In vitro secondary metabolite production through fungal elicitation: An Approach for sustainability. In: Prasad R, Kumar V, Kumar M, Wang S (eds) Fungal Nanobionics: Principles and Applications. Springer Singapore, Singapore, pp 215–242. https://doi.org/10.1007/978-981-10-8666-3_9
Sinha AK, Jaggi M, Raghuram B, Tuteja N (2011) Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal Behav 6:196–203. https://doi.org/10.4161/psb.6.2.14701
Srivastava S, Conlan XA, Cahill DM (2016) Rhizophagus irregularis as an elicitor of rosmarinic acid and antioxidant production by transformed roots of Ocimum basilicum in an in vitro co-culture system. Mycorrhiza 26:919–930. https://doi.org/10.1007/s00572-016-0721-4
Stadnik MJ, de Freitas MB (2014) Algal polysaccharides as source of plant resistance inducers. Trop Plant Pathol 39:111–118. https://doi.org/10.1590/S1982-56762014000200001
Staniszewska I, Królicka A, Maliński E, Łojkowska E, Szafranek J (2003) Elicitation of secondary metabolites in in vitro cultures of Ammi majus L. Enzyme Microb Technol 33:565–568. https://doi.org/10.1016/S0141-0229(03)00180-7
Suvarnalatha G, Rajendran L, Ravishakar GA, Venkataraman LV (1994) Elicitation of anthocyanin production in cell cultures of carrot (Daucus carota L.) by using elicitors and abiotic stress. Biotechnol Lett 16:1275–1280. https://doi.org/10.1007/BF00149631
Thakur M, Bhattacharya S, Khosla PK, Puri S (2019) Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants 12:1–12. https://doi.org/10.1016/j.jarmap.2018.11.004
Thilip C, Mehaboob VM, Varutharaju K, Faizal K, Raja P, Aslam A, Shajahan A (2019) Elicitation of withaferin-A in hairy root culture of Withania somnifera (L.) Dunal using natural polysaccharides. Biologia (bratisl) 74:961–968. https://doi.org/10.2478/s11756-019-00236-9
Tusevski O, Petreska Stanoeva J, Stefova M, Gadzovska Simic S (2015) Agrobacterium enhances xanthone production in Hypericum perforatum cell suspensions. Plant Growth Regul 76:199–210. https://doi.org/10.1007/s10725-014-9989-6
Udomsuk L, Jarukamjorn K, Tanaka H, Putalun W (2011) Improved isoflavonoid production in Pueraria candollei hairy root cultures using elicitation. Biotechnol Lett 33:369–374. https://doi.org/10.1007/s10529-010-0417-3
Vanisree M, Lee C-Y, Lo S-F, Nalawade SM, Lin CY, Tsay H-S (2004) Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot Bull Acad Sin 45:1–22
Veerashree V, Anuradha CM, Kumar V (2012) Elicitor-enhanced production of gymnemic acid in cell suspension cultures of Gymnema sylvestre R. Br Plant Cell Tissue Organ Cult 108:27–35. https://doi.org/10.1007/s11240-011-0008-6
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25. https://doi.org/10.1023/A:1015871916833
Vijaya Sree N, Udayasri P, Kumar VVA, Ravi Babu B, Phani Kumar Y, Vijay Varma M (2010) Advancements in the production of secondary metabolites. J Nat Prod 3:112–123
Vinoth S, Gurusaravanan P, Jayabalan N (2012) Effect of seaweed extracts and plant growth regulators on high-frequency in vitro mass propagation of Lycopersicon esculentum L (tomato) through double cotyledonary nodal explant. J Appl Phycol 24:1329–1337. https://doi.org/10.1007/s10811-011-9717-9
Wake H, Akasaka A, Umetsu H, Ozeki Y, Shimomura K, Matsunaga T (1992) Enhanced germination of artificial seeds by marine cyanobacterial extract. Appl Microbiol Biotechnol 36:684–688. https://doi.org/10.1007/BF00183250
Wake H, Umetsu H, Ozeki Y, Shimomura K, Matsunaga T (1991) Extracts of marine cyanobacteria stimulated somatic embryogenesis of Daucus carota L. Plant Cell Rep 9:655–658. https://doi.org/10.1007/BF00235350
Wiktorowska E, Długosz M, Janiszowska W (2010) Significant enhancement of oleanolic acid accumulation by biotic elicitors in cell suspension cultures of Calendula officinalis L. Enzyme Microb Technol 46:14–20. https://doi.org/10.1016/j.enzmictec.2009.09.002
Wilczańska-Barska A, Królicka A, Głód D, Majdan M, Kawiak A, Krauze-Baranowska M (2012) Enhanced accumulation of secondary metabolites in hairy root cultures of Scutellaria lateriflora following elicitation. Biotechnol Lett 34:1757–1763. https://doi.org/10.1007/s10529-012-0963-y
Wu J-Y, Ng J, Shi M, Wu S-J (2007) Enhanced secondary metabolite (tanshinone) production of Salvia miltiorrhiza hairy roots in a novel root–bacteria coculture process. Appl Microbiol Biotechnol 77:543–550. https://doi.org/10.1007/s00253-007-1192-5
Yamaner Ö, Erdağ B, Gökbulut C (2013) Stimulation of the production of hypericins in in vitro seedlings of Hypericum adenotrichum by some biotic elicitors. Turk J Bot 37:153–159. https://doi.org/10.3906/bot-1202-1
Yang R, Potter TP, Curtis OF, Sherry K (1997) Tissue culture-based Selection of high rosmarinic acid producing clones of Rosemary (Rosmarinus officinalis L.) using Pseudomonas strain F. Food Biotechnol 11:73–88. https://doi.org/10.1080/08905439709549923
Yukimune Y, Tabata H, Higashi Y, Hara Y (1996) Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat Biotechnol 14:1129–1132. https://doi.org/10.1038/nbt0996-1129
Zhai X, Jia M, Chen L, Zheng C, Rahman K, Han T, Qin L (2017) The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Crit Rev Microbiol 43:238–261. https://doi.org/10.1080/1040841X.2016.1201041
Zhao G, Zhao J, Peng L, Zou L, Wang J, Zhong L, Xiang D (2012) Effects of yeast polysaccharide on growth and flavonoid accumulation in Fagopyrum tataricum sprout cultures. Molecules 17:11335–11345. https://doi.org/10.3390/molecules171011335
Zhao J (2015) Phospholipase D and phosphatidic acid in plant defence response: from protein–protein and lipid–protein interactions to hormone signalling. J Exp Bot 66:1721–1736. https://doi.org/10.1093/jxb/eru540
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333. https://doi.org/10.1016/j.biotechadv.2005.01.003
Zhao JL, Zhou LG, Wu JY (2010) Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotechnol 87:137–144. https://doi.org/10.1007/s00253-010-2443-4
Zheng LP, Tian H, Yuan YF (2016) The influence of endophytic Penicillium oxalicum B4 on growth and artemisinin biosynthesis of in vitro propagated plantlets of Artemisia annua L. Plant Growth Regul 80:93–102. https://doi.org/10.1007/s10725-016-0162-2
Acknowledgements
We would sincerely like to express our deep gratitude to Mr. Sunny Kumar, PhD scholar, Dept. of English, CHRIST (Deemed to be University), Mr. Anthony Kenneth, M.Sc. Botany, St. Joseph’s College (Autonomous) and Mr. Praveen, MBA, New horizon college for their invaluable time in proof reading this article. We acknowledge them for their comments and suggestions to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Christophe Hano.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhaskar, R., Xavier, L.S.E., Udayakumaran, G. et al. Biotic elicitors: a boon for the in-vitro production of plant secondary metabolites. Plant Cell Tiss Organ Cult 149, 7–24 (2022). https://doi.org/10.1007/s11240-021-02131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02131-1