Abstract
In an attempt to find an alternative and potent source of diosgenin, a steroidal saponin in great demand for its pharmaceutical importance, Helicteres isora suspension cultures were explored for diosgenin extraction. The effect of biotic elicitors on the biosynthesis of diosgenin, in suspension cultures of H. isora was studied. Bacterial as well as fungal elicitors such as Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus niger were applied at varying concentrations to investigate their effects on diosgenin content. The HPLC based quantification of the treated samples proved that amongst the biotic elicitors, E. coli (1.5%) proved best with a 9.1-fold increase in diosgenin content over respective control cultures. Further, the scaling-up of the suspension culture to shake-flask and ultimately to bioreactor level were carried out for production of diosgenin. During all the scaling-up stages, diosgenin yield obtained was in the range between 7.91 and 8.64 mg l−1, where diosgenin content was increased with volume of the medium. The quantitative real-time PCR (qRT-PCR) analysis showed biotic elicitors induced the expression levels of regulatory genes in diosgenin biosynthetic pathway, the 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and cycloartenol synthase (CAS), which can be positively correlated with elicited diosgenin contents in those cultures. The study holds significance as H. isora represents a cleaner and easy source of diosgenin where unlike other traditional sources, it is not admixed with other steroidal saponins, and the scaled-up levels of diosgenin achieved herein have the potential to be explored commercially.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicinal plants are receiving great attention from pharmaceutical industries for the synthesis of useful bioactive compounds. Plant tissue cultures promise controlled production of bioactive compounds on demand, and is seen as potent method for their commercial-scale production. Diosgenin, one of the most important plant secondary metabolites, is a steroidal sapogenin traditionally derived from the tubers of Dioscorea species (yams). It is a precursor of sex hormones (progesterone), corticosteroids (corticosone) and contraceptives as well as other important steroids (Zhang et al. 2009; Zhu et al. 2010; Wang et al. 2011; Selim and Al Jaouni 2015; Sirotkin et al. 2019). Despite the introduction of new steroidal drug precursors like solasodine, hecogenin and tigogenin, it remains a principal intermediate for the industrial production of steroidal drugs (Mirunalini and Shahira 2011). It has been vastly investigated in recent years and has shown wide-spectrum therapeutic potentials. Diosgenin has shown anti-proliferative and proapoptotic effects on cancerous cells or on rheumatoid arthritis synoviocytes (Liagre et al. 2004; Selim and Al Jaouni 2015; Sethi et al. 2018). It has also showed pharmacological activities such as anti-lipoperoxidative and anti-aging effects, cognitive impairment, hypoglycaemic effect, antifungal and antiviral activities (Jayachandran et al. 2009; Chiu et al. 2011; Wang et al. 2011; Patel et al. 2012; Hao et al. 2015; Sethi et al. 2018).
In India, steroidal drug production is almost 100% based on diosgenin and diosgenin accounts for two-third of the total world consumption of steroids (Chaturvedi et al. 2007). Its annual global demand is 3000 tonnes; while in India, 150 tonnes of diosgenin are required per year, however, total production of diosgenin in India is only 30 tonnes annually and rest is met by imports (Dangi et al. 2014; Deshpande and Bhalsing 2015).
The traditional sources of diosgenin are under threat due to their over-exploitation for extracting diosgenin, consequently, some of the species with high diosgenin content such as Dioscorea zingiberensis and D. deltoidea are fast depleting (Chaturvedi et al. 2007; Li et al. 2012). This necessitates new alternative diosgenin sources and to develop strategies for its maximum, cost-effective production. Helicteres isora L. (Indian screw tree, a plant with traditional medicinal usages) has been reported as a cleaner source of diosgenin, where the compound is not admixed with other steroidal sapogenins (Barik et al. 1998; Deshpande and Bhalsing 2014; Kumar et al 2014). Exploration of this plant for diosgenin production is therefore advantageous. One more advantage of choosing this plant is that it is abundantly found in almost all parts of the country in forests as undergrowth, especially as secondary growth. This makes it a natural choice for exploration as a source of diosgenin. However, the diosgenin content is low in H. isora as compared to other traditional plant sources of diosgenin (Barik et al. 1998), which needs to be enhanced before commercial exploration of this plant as an alternative source of diosgenin. As stated earlier, plant cell cultures have been established as potent alternative sources for the production of high value secondary metabolites of industrial importance in a holistic (without causing destruction to the natural sources) and sustainable way (Rao and Ravishankar 2002; Mulabagal and Tsay 2004; Hussain et al. 2012).
In an attempt to identify an alternative and potent source of diosgenin, present study was therefore focused on exploration of suspension cultures of H. isora for optimal production of diosgenin via biotic elicitation and scaling-up the diosgenin production using shake-flasks and bioreactor techniques. The objectives also include to study and correlate the expression levels of diosgenin biosynthesis pathway genes, to shed a light on the mechanism underlying induced levels of diosgenin in suspension cultures treated with elicitors.
Materials and methods
Collection of plant material
The plant material (mature pods) of H. isora L. was collected from Khopoli region of the state of Maharashtra, India. Due permissions were taken from the Maharashtra Biodiversity Board for sample collections from wild, and the samples were botanically authenticated at Anantrao Pawar College, Pune, with a specimen voucher submitted (APCP/21/2012-13). Seeds were separated from the pods and used for further studies.
Establishment of callus and suspension cultures
Callus cultures were established using nodal explants from in vitro germinated seedlings as described previously by our group (Shaikh et al. 2018). Briefly, seeds were treated with concentrated H2SO4 (3–4 min) to break the seed dormancy, washed thoroughly with sterile distilled water and inoculated on MS (Murashige and Skoog 1962) medium (Himedia, India) supplemented with 3% (w/v) sucrose and 0.8% (w/v) agar (pH 5.8) for germination. Nodal explants (1–1.2 cm) from 25-day-old seedlings were inoculated for callus induction in MS medium containing sucrose (3%), agar (0.8%), supplemented with varying concentrations (0.5 to 3.0 mg l−1) of 2,4-dichlorophenoxyacetic acid (2,4-D), kinetin (Kin) and 6-Benzylaminopurine (BAP). Callus produced on MS medium supplemented with 0.5 mg l−1 of 2,4-D was used further for establishment of suspension cultures.
The suspension cultures were established in liquid MS medium with or without 2,4-D (0.0, 0.5. 1.0 and 2.0 mg l−1) and the cultures were maintained on rotary shaker (Remi, India) with an average speed of 50–60 rpm. The cultures were incubated at 40 µmol m−2 s−1 light intensity and 25 ± 2 °C temperature with a 16/8-h light/dark cycle. Repeated sieving and sub-culturing of callus aggregates after every 7th day was performed. MS medium (without 2,4-D) was used further for the establishment and propagation of suspension cultures.
Time-course analysis for biomass and diosgenin production
The fresh inoculum from the stock suspension cultures was inoculated in 50 ml liquid MS medium with 3% (w/v) sucrose, without any plant growth regulator (PGR) for optimizing the time-duration for highest biomass production. For this purpose, the cultures were harvested after 7, 14, 21, 28 and 35 days of inoculation and analysed for biomass production. The produced biomass was separated from the medium to determine the dry weight (DW). This step provided the fix time-point for inoculum preparation (to obtain highest biomass).
For optimizing the time-point providing highest diosgenin from suspension cultures, inoculum from the stock suspension culture was inoculated in 50 ml liquid MS medium with 3% (w/v) sucrose, without any PGR. The samples were harvested after 5, 10 and 15 days after inoculation and successively processed for diosgenin estimation.
Preparation and application of biotic elicitors
The microbial cultures used as elicitors in this study were obtained from the National Collection of Industrial Microorganisms (NCIM), CSIR-National Chemical Laboratory (NCL), Pune, India and the Institute of Microbial Technology (IMTECH), Chandigarh, India.
Fungal elicitor
Fungal elicitors from cultures of Aspergillus niger (ATCC10578) and Saccharomyces cerevisiae (NCIM3050) were prepared using fresh biomass. Fungal mat-growth was obtained in petri dish containing potato dextrose agar medium. From the obtained mat-growth, disc (5 mm) of the active fungal biomass (mycelial growth with spores) was inoculated aseptically in a 250 ml conical flask containing 50 ml of sterile potato dextrose broth. The cultures were incubated for one week on rotary shaker (90 rpm) at room temperature. After completion of incubation, fungal biomass was filtered-off from the medium aseptically using Whatman filter paper and the filtrate was centrifuged (10,000 rpm, 10 min) to ensure complete removal of fungal biomass. The obtained supernatant was then filter-sterilized (0.22 µm, PVDF filters, Millipore) and stored in glass vials at 4 °C for further use. The prepared fungal elicitor was then aseptically added to the autoclaved liquid MS medium prior to adding the inoculums for getting effective concentration of elicitor as 1%, 1.5% and 2% (v/v). Twenty-one-day old cultures were used for fungal elicitor treatment. The plain potato dextrose broth was used as corresponding control for every elicitation treatment. The suspension cultures were maintained on rotary shaker (Remi, India) with an average speed of 50–60 rpm. The cultures were incubated at 16/8-h light/dark cycle with 40 µmol m−2 s−1 light intensity and 25 ± 2 °C temperature.
Bacterial elicitor
Bacterial elicitors from cultures of Bacillus subtilis (ATCC6051) and Escherichia coli (MTCC837) were prepared using freshly grown bacterial biomass. Freshly grown isolated colony of the bacteria was inoculated aseptically in conical flask (250 ml) containing 50 ml of sterile nutrient broth. The cultures were incubated for 24 h on a rotary shaker (90 rpm) at 37 °C. Further, the bacterial biomass was pellet-out by centrifuging the 24 h old broth at 10,000 rpm for 20 min. The supernatant was then filter-sterilized (0.22 µm, PVDF filters, Millipore) and stored in glass vials at 4 °C for further use. Similar to fungal treatment, 21-day-old cultures were used for bacterial elicitor treatment. The prepared bacterial elicitor was then aseptically added to the autoclaved liquid MS medium to get effective elicitor concentrations as 1%, 1.5% and 2% (v/v) prior to the inoculation of callus. The plain nutrient broth was used as corresponding control for each elicitation treatment. The suspension cultures were maintained on rotary shaker (Remi, India) with an average speed of 50–60 rpm. The cultures were incubated with 16/8-h light/dark cycle at 40 µmol m−2 s−1 light intensity and 25 ± 2 °C temperature.
Bioreactor cultivation
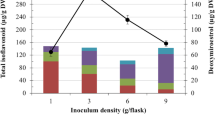
Amongst all the elicited cultures, the best responsive culture (in terms of diosgenin production) was further used for scaling-up via shake-flasks with gradual increment in media volume and finally to the lab-scale bioreactor. The media used for shake flasks and bioreactor contained MS liquid medium treated with E. coli (1.5%) elicitor with increasing volumes from 25 to 300 ml in shake-flask and 3000 ml in bioreactor as described in Table 1. The inoculum from scaled-up shake flasks was further used in bioreactor (ALF05, Napro scientific, India) with following culture conditions: 0.7 min−1 of an air flow rate, 60 rpm sparger agitation speed, at 25 ± 2 °C under 16/8-h light/dark cycle. After 10 days of incubation, all the scaled up cultures of different volumes, were harvested, and analysed for biomass and diosgenin production.
Extraction of diosgenin
The samples (completely dried suspension biomass) were extracted by acid hydrolysis and subsequently checked for presence of diosgenin. The powder of dried filtered suspension biomass (300 mg) was refluxed with 15 ml of 2 N H2SO4 in 70% isopropanol for 2 h. The solution was filtered followed by addition of 15 ml water. The aqueous phase of the solution was extracted four times with hexane (10 ml). The resulting combined hexane extract was pooled together and further neutralized with 2 N NaOH (10 ml). Finally, the hexane fractions were evaporated using a rotary evaporator (Equitron, India) to obtain the concentrated extract. The resulting extract was then reconstituted in 1 ml of 50% methanol in tetrahydrofluran and used for further analysis (Deshpande and Bhalsing 2014).
Estimation of diosgenin by HPLC
High performance liquid chromatography (HPLC) was carried out for quantification of diosgenin from the extracts (Shimadzu HPLC system). The HPLC instrument comprised of variable wavelength programmable UV–visible UV 730 detector (controlled by the LC real time software). Samples were injected by using a rheodyne injector fitted with a 20 μl fixed loop. Standard and sample solutions filtered through 0.45 μm syringe filter (PVDF) before injecting. Diosgenin present in the extracts was quantified by C18 reverse-phase column (4.6 × 250 mm, particle size 5 μm grace C18 column) with elution using an isocratic binary system of acetonitrile:water (90:10, v/v). Flow rate and injection volume was 0.7 ml min−1 and 20 μl respectively with total run time of 30 min. Diosgenin was quantified at 210 nm. The chromatographic peaks of the diosgenin were confirmed by comparing the retention times with those of the standards. All chromatographic operations were carried out at room temperature.
Confirmation of diosgenin by NMR and HRMS
The presence of diosgenin was further confirmed via magnetic resonance properties using nuclear magnetic resonance (NMR) spectroscopy and molecular mass properties using high resolution mass spectroscopy (HRMS). Diosgenin was confirmed in seed extracts by performing 1H NMR and 13C NMR on DRX-200 and 500 MHz (Bruker, Germany). The mass spectra were recorded on a Bruker impact HD Q-TOF spectrometer (Bruker Daltonics, Billerica, MA, USA) at the Central Instrumentation Facility, Savitribai Phule Pune University, Pune, India. HRMS analysis was performed by direct infusion of 5 μl aliquots into the electrospray ionization chamber at the rate 120 μl min−1. Instrument information: Bruker IMPACT HD, Impact II UHR-TOF Mass Spectrometer system, Dionex UHPLC Ultimate 3000 System, HR-MS, U-HPLC-MS. Software: LCMS: HighStar 3.2, HPLC: Chromeleon, Mass: OTOF-Q-control. Acquisition Parameter: Source Type—ESI, Focus—Active, Scan Begin—50 m/z, Scan End—600 m/z, Ion Polarity—Positive, Set Capillary—3500 V, Set End Plate Offset—500 V, Set Charging Voltage—2000 V, Set Corona—0 nA, Set Nebulizer—0.3 Bar, Set Dry Heater—200 °C, Set Dry Gas—4.0 l/min, Set Divert Valve—Waste, Set APCI Heater—0 °C. The molecular mass of diosgenin was confirmed by mass-to-charge ratio of diosgenin with the help of isotopic pattern software package.
Gene expression analyses
Expression levels of two key-genes involved in diosgenin synthesis in plants, namely 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and cycloartenol synthase (CAS) were analysed using specific primers for amplification via quantitative real-time polymerase chain reaction (qRT-PCR) analysis. Two best responsive elicitor treatments from biotic elicitors (E. coli elicitor 1.5%, B. subtilis elicitor 2%) and bioreactor samples were considered for gene expression analysis.
Fresh callus tissue was homogenized using liquid nitrogen and Total RNA isolation was performed using TRIzol method as per the manufacturer’s instructions (Life Technologies, USA). The cDNA synthesis was carried out using oligo dT18 primer and moloney murine leukemia virus reverse transcriptase (MuMLV-RT) following the manufacturer’s instructions using 1 μg of total RNA as a starting material. The cDNA synthesis was performed using 200 U of MuMLV-RT (Invitrogen, USA), 5 mM oligoDT18, 1 mM dNTP solution, and 3 mM Mg2+ in a volume of 20 μl.
For primer designing, reference sequences from GenBank® database for HMGR and CAS gene were used. Using the homologous sequences of species related to Helicteres, primers were designed for amplification of these two genes. The real time quantitative PCR (qRT-PCR) primers specific to gene of interest were used (verified using in silico primer BLAST analysis at NCBI) (Table 2).
The qRT-PCR reaction was performed using the primers specific to test and housekeeping (GAPDH) genes. All PCR reactions were performed with ABI StepOne Real-Time PCR System using DNA-binding SYBR Green dye for detection of the PCR products and results were analysed by ABI StepOne Software version 2 (Applied Biosystems). All the samples were processed in replicate analysis including negative control in each run. The results of the real time ddCT PCR were analysed using Data Assist Software v3.01 (Applied Biosystems).
Statistical analyses
All the experiments were performed in triplicates with at least 10 explants per treatment before conducting the statistical analyses. The results are presented as mean ± standard error. The means were compared using Duncan’s multiple range test (DMRT) at P ≤ 0.05 using MSTAT-C program. The graphs were plotted using Microcal Origin 6.0 software.
Results and discussion
Suspension culture establishment and time-course analyses for biomass and diosgenin production
Time-course analysis of growth in suspension cultures was carried out, and overall, maximum biomass production was obtained in plain MS medium without any supplement. A time-course of H. isora suspension cultures showed a typical growth curve for fresh biomass which exhibited a rapid, linear growth phase followed by a stationary phase. Cell biomass formation was started after 4–7 days of inoculation and was highest in 21-day-old cultures as shown in Supplementary Fig. S1. The suspension cultures had a shorter lag phase of 2–3 days in which the cells were probably adjusting their metabolic machinery to the newly inoculated environment, till 10 to12 days biomass growth was maximum, hence the log phase with 10–12 days of logarithmic growth where the cells are finally adapted to new environment and hence they multiply at their maximum rate was considered. Kumar et al. (2016) reported short lag phase within 4 day and exponential phase till 12th day in Lantana camara suspension culture. Duration of 15–17 days was observed as the late exponential growth phase. In the present investigation, the suspension cultures showed stationary growth phase after 21 days of inoculation with very limited growth apparently due to the exhaustion of growth medium. In stationary phase, growth density remained constant due to equal growth and death rates. Eventually, death phase was started after 28 days, in which reduction in biomass growth was observed, total medium (and intracellular storage) was depleted, as well as decomposition of cells occurred which led to the decline in dry weight.
Time-course analysis of diosgenin production at different time points (5, 10 and 15 days) in suspension cultures of H. isora is shown in the Supplementary Table S1. Maximum production of diosgenin was observed on 10th day of culture with 107 µg g−1 DW of diosgenin i.e. exponential growth phase. The diosgenin content reduced after 10 days, with lowest on 15th day (103 µg g−1 DW), which is late exponential phase indicating a positive correlation between the active growth of suspension and production of diosgenin. Our findings are in accordance with the previous reports, for instance, Gomez et al. (2004) reported maximum diosgenin production in the initial growth phase of Trigonella foenum-graecum suspension cultures. Similarly, Rokem et al. (1985) also observed that accumulation of precursor of diosgenin was found in growing cells of Dioscorea deltoidea cell cultures. Narayani and Srivastava (2017) advocated that elicitation is more effective in the initial growth phase of cell culture. Similarly, maximum saponin content was obtained in the suspension cultures of Panax ginseng treated with the elicitor on the day of inoculation, whereas the same elicitor given at the early-log phase, mid-log phase and stationary phase did not show such remarkable effects on the production of secondary metabolites (Lu et al. 2001). In current study, as the maximum increase in diosgenin level was recorded on 10th day after inoculation, and it was selected as optimal time-period for harvesting elicitor-treated cells of H. isora.
Extraction, qualitative and quantitative analysis of diosgenin
Pure white crystalline diosgenin was isolated from H. isora suspension cultures (un-/treated) powder by acid hydrolysis method. About 50 mg pure diosgenin obtained by this method was then dissolved in CDCl3 solvent for spectroscopic characterization using NMR and HR-MS techniques and the results confirmed the presence of diosgenin (C27H42O3), as follows:
NMR analysis
1H NMR (500 MHz, CDCl3) (Supplementary Fig. S2); 0.88–0.92 (s, C-26 methyl, C-27 methyl, C-28 methyl and C-29 methyl), 5.32–5.43 (t, C-7), 4.98–5.06 (d, C-24), 2.78–2.80 (q C-16), 2.26–2.29 (broad C2-OH), 2.05–2.07(d C-3) 1.24–1.63 (22-H).
13C NMR (126 MHz, CDCl3) (Supplementary Fig. S3); 173.45 130.14, 127.80, 76.78, 67.44, 34.58, 31.89, 31.53, 29.89, 28.83, 27.21, 25.52, 24.87, 22.47, 21.86, 14.46.
1H and 13C-NMR spectroscopies were used to verify the molecular structure of the purified steroidal sapogenin. In 1H-NMR spectra, signals at ~ 0.88–0.92 ppm represents chemical shift due to the terminal four -CH3 methyl protons. The signals at ~ 14.6–22.47 ppm in 13C-NMR spectra are due to the terminal carbons of four methyl groups. The carbon double bond shows cmr signal at 127.80–130.14 ppm. The steroidal sapogenin also contains methylene units, which give proton and carbon signals in the range of ~ 1.24–2.07 and ~ 24.87–76.78 ppm, respectively.
HR-MS analysis
Monoisotopic mass: M + Na—437.3, M + H—415.3 (Supplementary Fig. S4). The peaks at 415.3 [M + H]+ and 437.3 [M + H]+ confirmed the presence of diosgenin with molecular mass 414.3, and was in confirmation of previous report (Ciura et al. 2015).
Quantification of diosgenin by HPLC
Diosgenin from extracts of un-/treated H. isora suspension cultures was estimated using a simple and rapid HPLC method (Deshpande and Bhalsing 2014) with some modifications. Mobile phase comprised of acetonitrile: water (90:10, v/v) and flow rate of 0.7 ml min−1 and elution was observed at 210 nm with 30 min total run time. The separation was achieved using C18 column. The HPLC chromatogram clearly revealed the presence of diosgenin as confirmed by the retention time of diosgenin isolated from H. isora to be 23 min, which was similar to the reference/standard diosgenin (Supplementary Fig. S5).
Elicitation of diosgenin production in suspension cultures using biotic elicitors
Elicitors of biological origin such as polysaccharides, glycoproteins, cell-wall fragments and protein fractions derived from bacterial or fungal source are also widely used for elicitation of secondary metabolites in plant cell cultures. We used bacterial and fungal elicitors with varying concentrations for investigating their possible impacts on diosgenin contents in H. isora suspension cultures.
Fungal and yeast elicitor
In the current investigation, elicitor prepared using Aspergillus niger was responsible for significant increment in biomass production at all the applied concentrations (1%, 1.5% and 2%). Highest DW was observed at 1.5% fungal elicitor-treatment with a twofold increase over control (Fig. 1). The same treatment was found responsible for highest diosgenin production (1.42-fold higher over controls). Fungal elicitors have been previously applied successfully for elicited diosgenin production in cell cultures of Dioscorea zingiberensis using fungal elicitors from Fusarium oxysporum Dzf17, with 2.85-fold higher diosgenin content in treated cultures over their controls (Li et al. 2011a). Regulatory role of oligosaccharides has been correlated with enhanced secondary metabolism/diosgenin production (Li et al. 2011b), which might be a possible cause behind elicited diosgenin production in cell cultures of H. isora.
Effect of varying concentrations (0, 1%, 1.5% and 2%) of A. niger elicitor on biomass and diosgenin production in H. isora suspension cultures. Values represent mean ± S.E. of three replicated experiments. The Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 was applied. Bars with different lower-case and upper-case letters are significantly different from each other for diosgenin and biomass, respectively
Fungal elicitors are considered as surface structures and/or fungal cell-secretions, with fungal mycelia or degraded fungal mycelial-products, and fermentation broth which may also contain fungal secretions. The said fungal elicitor hence may contain sugars (polysaccharides, oligosaccharides), proteins (glycolipid proteins, glycoprotein, and peptides), fatty acids and other substances. Fungal elicitors often result in biomass and secondary metabolite enhancement, as well as improved enzymatic activities in plants (Chen et al. 2015). Applications of non-pathogenic as well as pathogenic fungal elicitors are turning into potent approaches for elevated secondary metabolite production in plant cells growing in vitro (Lattanzio et al. 2006). Early defensive response of plant cells attacked by fungal-borne entities comprises rapid production of reactive oxygen species (ROS) followed by initiation of complex enzymatic and non-enzymatic antioxidant system (Gill and Tuteja 2010).
Biomass enhancement in plant suspension cultures has been observed by investigators on several occasions when plant cell cultures were treated with fungal cell homogenates or filtrates. For instance, application of Piriformospora indica cell homogenate exhibited growth promoting potential, when applied to Withania somnifera suspension cultures (Ahlawat et al. 2016). The addition of 3% (v/v) of P. indica cell homogenate significantly enhanced the cell growth (1.11 times) as compared to the untreated control. Similarly, hairy root lines generated from Linum album were treated with fungal extracts of Piriformospora indica, and a short-term exposure to fungal extract increased the hairy root biomass by 1.5-fold (Tashackori et al. 2016). Fungal elicitors have been explored for plant secondary metabolites enhancement in plant cell suspension cultures. Nine-fold elicited psoralen production was observed in cell cultures of Psoralea corylifolia treated with Aspergillus niger (Ahmed and Baig 2014). However, fungal mycelial extracts (prepared using Fusarium oxysporum, Phoma exigue and Botrytis cinerea) negatively affected the biomass production in cell cultures of Hypericum perforatum (Simic et al. 2015). It indicates the variable degree of effect of fungal elicitors on biomass production.
Extract of common yeast, Saccharomyces cerevisiae, has been utilized in the past for elicitation purposes due to its status as natural source of vitamins, carbohydrates, minerals and other compounds, which shows plant-growth stimulatory effects (Ibraheim 2014). This elicitor has been applied either using preparations from actual yeast (S. cerevisiae) biomass or using purified yeast extracts available commercially. Yeast extracts was found beneficial in eliciting Plumbagin production in P. indica root cultures when treated with 100, 500 and 1000 mg l−1 of yeast extract, where maximum (twofold) enhancement was observed at 100 mg l−1 concentration (Jaisi and Panichayupakaranant 2016). Similarly, yeast extract elicited the production of Tanshinone in hairy root cultures of Salvia miltiorrhiza and Salvia castanea (Yang et al. 2018). However, current investigation showed that after an initial decrease in diosgenin content in cultures treated at 1% yeast, the diosgenin levels were increased with a slightly higher content at 2% S. cerevisiae treatment (Fig. 2). These results hint towards specific plant responses to particular microbial interactions.
Effect of varying concentrations (0, 1%, 1.5% and 2%) of S. cerevisiae elicitor on biomass and diosgenin production in H. isora suspension cultures. Values represent mean ± S.E. of three replicated experiments. The Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 was applied. Bars with different lower-case and upper-case letters are significantly different from each other for diosgenin and biomass, respectively
Bacterial Elicitor
The E. coli (1.5%) treatment proved best for elicitation of diosgenin with a 9.1-fold increase in diosgenin production (Fig. 3). Though, the diosgenin content was reduced in cultures treated with higher (2%) or lower (1%) concentrations of E. coli, indicating 1.5% as the most responsive treatment for elicited diosgenin production. E. coli treatments also exerted positive impacts on cell biomass as indicated by higher DW at all the concentrations, with highest DW at 2% treatment.
Effect of varying concentrations (0, 1%, 1.5% and 2%) of E. coli elicitor on biomass and diosgenin production in H. isora suspension cultures. Values represent mean ± S.E. of three replicated experiments. The Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 was applied. Bars with different lower-case and upper-case letters are significantly different from each other for diosgenin and biomass, respectively
Likewise, B. subtilis elicitor also enhanced the diosgenin contents in treated suspension cultures of H. isora (Fig. 4). Highest elicitation was observed in cultures treated with 2% B. subtilis elicitor, where diosgenin content was enhanced by 6.1-fold (Fig. 4). B. subtilis elicitor showed positive impacts on cell biomass, though highest DW was measured in 1% treatment. Elicitation by both the used bacterial elicitors has been previously achieved by Chodisetti et al. (2013) for enhanced accumulation of gymnemic acid in suspension cultures of Gymnema sylvestre.
Effect of varying concentrations (0, 1%, 1.5% and 2%) of B. subtilis elicitor on biomass and diosgenin production in H. isora suspension cultures. Values represent mean ± S.E. of three replicated experiments. The Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 was applied. Bars with different lower-case and upper-case letters are significantly different from each other for diosgenin and biomass, respectively
It is an established fact that bacterial invasions are responsible for triggering defensive cellular reactions in plants (Le et al. 2018). Using pathogenic/non-pathogenic bacterial strains in the form of their cellular constituents as biotic elicitors for secondary metabolism enhancement have been successfully examined previously, for instance, in Glycine max (Algar et al. 2012) and Hypericum perforatum (Manero et al. 2012). It is presumed that the microbial elicitors comprise high quantities of glucans and chitosans which tend to mimic disease responses. This ultimately results in the activation of plant defence machinery involving secondary metabolism (Biswas et al. 2016).
On different occasions, bacterial treatments however have shown both negative as well as positive impacts on plant cell growth in vitro. Chodisetti et al. (2013) observed a dose-dependent decrease in cell biomass of suspension cultures of G. sylvestre with time, when culture was treated with biotic elicitors from B. subtilis and E. coli. On the other hand, Biswas et al. (2016) observed increase (6% more than the control) in biomass of Panax quinquefolius suspension cultures when the cultures were treated with 1.25% solution of Bacillus circularans. In the current experiment, elicitor treatment with E. coli at 1%, 1.5% and 2% concentration increased the cell biomass production by 1.13-, 1.33- and 1.4-folds over the respective controls, whereas B. subtilis (1%, 1.5% and 2% concentration) treatments enhanced the biomass by 1.3-, 1.1% and 1.09-folds, respectively.
Overall, the biotic elicitors used showed positive impacts on diosgenin production in H. isora suspension cultures, with an exception of S. cerevisiae. Highest diosgenin content with 9.1-fold increase was recorded in suspension cultures treated with 1.5% E. coli. Besides, cultures did not excrete diosgenin in the medium.
Scaling up of cultures via shake-flask and bioreactor approaches
Current study represents a lead for large scale diosgenin production with scaling-up approach from gradual increase in volume of culture medium (25 ml to 3 l). The last leg of scaling-up (bioreactor) provided 23.99 ± 0.016 g of DW from filtered suspension which yielded a total of 23.73 ± 4.12 mg of diosgenin (989 ± 3.89 µg g−1 DW) in a 3 l batch (Table 1).
Application of bioreactor technology for mass production of plant cell-based natural therapeutics is considered as a sustainable, holistic and commercially viable approach, without harming the plants growing in wild conditions. However, in spite of the growing demand and global market for phyto-therapeutics; only a few of them are produced commercially using bioreactors, for instance taxol, shikonin, berberine, rosmarinic acid and ginsenosides (Khanahmadi and Paek 2017; Werner et al. 2017). In the current study, the top responsive treatments in terms of diosgenin elicitation (E. coli at 1.5% concentration) were used for successive scaling-up from shake-flask cultures to lab-based pilot bench top bioreactor.
Bioreactor based large-scale production of secondary metabolites has been achieved successfully using various types of reactors. Notable citations include production of podophyllotoxin in Podophyllum hexandrum cell cultures (Chattopadhyay et al. 2002), artimisinin production in hairy root cultures generated from Artemisia annua (Patra and Srivastava 2016) in gas/liquid phase reactors, saikosaponins from root culture of Bupleurum falcatum (Kusakari et al. 2012) in air-lift reactors and taxane from cell cultures of Taxus cuspidate cell cultures (Son et al. 2000) in balloon type bubble reactors.
Expression patterns of diosgenin biosynthetic pathway genes (HMGR and CAS)
The extensively compartmentalized process of terpenoid biosynthesis (saponins and steroids) primarily takes place in cytosol, using isopentenyl pyrophosphate (IPP) from mevalonate (MVA) pathway. IPP serves as essential building block for all terpenoids, including triterpenoids (C30). In angiosperms, sterols are synthesized via either MVA pathway or methyl-D-erythritol-4-phosphate (MEP) pathway, however MVA pathway is considered as preferred one (Nes 2011). Previous analyses of the biosynthetic pathway for diosgenin have revealed that four enzymes, namely; CAS, squalene synthase (SQS), fernesyl pyrophosphate synthase (FPPS) and HMGR play key roles in synthesis of triterpene diosgenin (Hwang et al. 2015). In the typical pathway, a reaction involving cyclization of 2,3-oxidosqualene is catalysed by CAS, which is considered as rate-limiting step for steroidal sapogenin formation (Zhu et al. 2018). Similarly, HMGR acts as catalyst for first committed reaction of mevalonate pathway (MVA pathway) resulting in isoprenoid biosynthesis. HMGR plays vital regulatory role in MVA pathway which is critical for plant adaptations against harsh environmental conditions (Leivar et al. 2011). In current investigation, we therefore assessed the expression levels of two key-genes of this pathway, the CAS and HMGR, from the samples showing best elicitation responses, to investigate whether there is any link between up-regulation of these genes and diosgenin accumulation. Both these genes are established as key regulators in secondary metabolism (especially MVA pathway) and in plant development. In Trillium govanianum, the HMGR gene has been presented with higher expression levels compared to other genes in the MVA pathway, in positive association with its role in shikonin and aconites formation (Singh et al. 2010; Pal et al. 2015; Sharma et al. 2016). It also tends to regulate MVA pathway by acting as GPP-feeder to influence phyto-sterol biosynthesis (Nogues et al. 2006). CAS is also known for its vital role in plant cell viability and regulation of triterpenoid production. The biosynthesis of sterols in Nicotiana benthamiana was reported to be controlled by CAS (Gas-Pascual et al. 2015), while it’s essential role in plant development has been widely characterized in Arabidopsis thaliana (Babiychuk et al. 2008).
In the present study, all the selected best-responsive treatments (suspension cultures treated with E. coli at 1.5%, B. subtilis at 2%, and bioreactor culture) were used for analyses of expression profiles of selected genes. All these treatments up-regulated the expression levels of HMGR, a gene responsible for formation of mevalonate from HMG-CoA, though with differential degree (Fig. 5). This post-translationally and post-transcriptionally regulated gene has been previously attributed for its role in isoprene biosynthesis (Suzuki et al. 2004). The treatment with elicitors has demonstrated the increased HMGR activities in many medicinal plants, which eventually resulted in up-regulated mevalonate and subsequent target secondary metabolite (diosgenin) biosynthesis. Example includes improved HMGR activities in cell culture of Withania somnifera with elicited withaferin-A biosynthesis under biotic stress (cell filtrates of Piriformospora indica) (Ahlawat et al. 2017), Bacopa monnieri with improved bacoside-A biosynthesis under biotic stress (Chitiniphilus sp. MTN22 and Streptomyces sp. MTN14) (Gupta et al. 2017), Artemisia annua with enhanced artemisinin biosynthesis under cadmium stress (Zhou et al. 2017) and D. zingiberensis cultures treated with ethylene for enhanced levels of diosgenin (Diarra et al. 2013). In the current work, highest fold-increment in the HMGR activity was observed in the samples harvested from bioreactor (10.28-fold increase), followed by suspension cultures treated with 1.5% E. coli (8.67-fold increase). Though the media composition for both the samples was similar, bioreactor conditions proved to be responsible for marginally more expression level of HMGR and this trend is in correlation with the diosgenin content of both samples, indicating the up-regulation of the pathway. B. subtilis elicitor (2%) treatment showed 3.88-fold rise in the HMGR expression levels. HMGR controls mevalonate pathway by acting as GPP-feeder, thus prompts synthesis of phytosterol (Nogues et al. 2006). The induced HMGR activity in all the samples is in accordance with diosgenin content, confirming the key role HMGR plays during elicitor mediated upregulated diosgenin biosynthesis.
Expression pattern of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and cycloartenol synthase (CAS) in top responsive treatments. Values represent mean ± S.E. of three replicated experiments. The Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05 was applied. Bars with different lower-case and upper-case letters are significantly different from each other in terms of expression levels of HMGR and CAS each, respectively. RQ: Relative quantification of gene expression
The similar expression pattern was also observed with CAS; however, the extent of gene upregulation was less than that of HMGR. Highest increment was observed in samples from bioreactor (3.96-fold) followed by E. coli elicitor (1.5%) treated samples (3.07-fold) and then B. subtilis elicitor (2%) showed increment in the same range (~ twofold). Cycloartenol formation (cyclization) from 2,3-oxidosqualene mediated by CAS is first step leading to steroidal saponin generation (Benveniste 2004). Increased CAS expression was observed in cell cultures of Withania somnifera treated with different fungal cell-homogenates (3% P. indica, 5% V. dahlia, 3% A. alternate and 3% F. solani). Along with HMGR, CAS also exhibited higher expression in Dioscorea zingiberensis (Diarra et al. 2013) under the influence of ethylene treatment resulting in elicited diosgenin content. CAS is one of the important branch point involved in triterpenoid biosynthesis (Phillips et al. 2006; Morlacchi et al. 2009; Xue et al. 2012). The increased expression levels of CAS subsequently indicate the higher cycloartenol formation indicating the elicited diosgenin production via induced CAS activities. Overall, HMGR shown higher expression levels than the CAS in each treatment, however both the genes exhibited similar patterns and both were induced under the elicitors treatment, indicating that the elicitors could significantly induce the pathway which ultimately resulted into the enhanced diosgenin accumulation.
Conclusion
The present study revealed that four biotic elicitors namely E. coli, B. subtilis, S. cerevisiae and A. niger exhibited positive impacts on the diosgenin biosynthesis. Among these four elicitors, E. coli (1.5%), was found to have potential effect on diosgenin synthesis when tested on suspension culture of H. isora. Our results also demonstrated that the E. coli elicited the expression of HMGR and CAS genes, thus apparently activated the HMGR-CAS pathway mediated biosynthesis of steroidal sapogenins, especially diosgenin.
References
Ahlawat S, Saxena P, Ali A, Abdin MZ (2016) Piriformospora indica elicitation of withaferin A biosynthesis and biomass accumulation in cell suspension cultures of Withania somnifera. Symbiosis 69:37–46
Ahlawat S, Saxena P, Ali A, Khan S, Abdin MZ (2017) Comparative study of withanolide production and the related transcriptional responses of biosynthetic genes in fungi elicited cell suspension culture of Withania somnifera in shake flask and bioreactor. Plant Physiol Biochem 114:19–28
Ahmed SA, Baig MM (2014) Biotic elicitor enhanced production of psoralen in suspension cultures of Psoralea corylifolia L. Saudi J Biol Sci 21(5):499–504
Algar E, Gutierrez-Mañero FJ, Bonilla A, Lucas JA, Radzki W, Ramos-Solano B (2012) Pseudomonas fluorescens N21.4 metabolites enhance secondary metabolism isoflavones in soybean (Glycine max) calli cultures. J Agric Food Chem 60(44):11080–11087
Babiychuk E, Bouvier-Navé P, Compagnon V, Suzuki M, Muranaka T, Van Montagu M, Kushnir S, Schaller H (2008) Allelic mutant series reveal distinct functions for Arabidopsis cycloartenol synthase 1 in cell viability and plastid biogenesis. Proc Natl Acad Sci USA 105(8):3163–3168
Barik B, Dey AK, Das PC (1998) Helicteres isora Linn, a new source of diosgenin. Indian J Chem 20(B):938
Benveniste P (2004) Biosynthesis and accumulation of sterols. Ann Rev Plant Biol 55:429–457
Biswas T, Kalra A, Mathur AK, Lal RK, Singh M, Mathur A (2016) Elicitors influenced differential ginsenoside production and exudation into medium with concurrent Rg3/Rh2 panaxadiol induction in Panax quinquefolius cell suspensions. Appl Microbiol Biotechnol 100(11):4909–4922
Chattopadhyay S, Srivastava AK, Bhojwani SS, Bisaria VS (2002) Production of podophyllotoxin by plant cell cultures of Podophyllum hexandrum in bioreactor. J Biosci Bioeng 93(2):215–220
Chaturvedi HC, Jain M, Kidwai NR (2007) Cloning of medicinal plants through tissue culture—a review. Indian J Exp Biol 45:937–948
Chen X, Mou Y, Ling J, Wang N, Wang X, Hu J (2015) Cyclic dipeptides produced by fungus Eupenicillium brefeldianum HMP-F96 induced extracellular alkalinization and H2O2 production in tobacco cell suspensions. World J Microbiol Biotechnol 31(1):247–253
Chiu CS, Chiu YJ, Wu LY, Lu TC, Huang TH, Hsieh MT, Lu CY, Peng WH (2011) Diosgenin ameliorates cognition deficit and attenuates oxidative damage in senescent mice induced by D-galactose. Am J Chin Med 39:551–563
Chodisetti B, Rao K, Gandi S, Giri A (2013) Improved gymnemic acid production in the suspension cultures of Gymnema sylvestre through biotic elicitation. Plant Biotechnol Rep 7(4):519–525
Ciura J, Szeliga M, Tyrka M (2015) Optimization of in vitro culture conditions for accumulation of diosgenin by fenugreek. J Med Plants 3(3):22–25
Dangi R, Misar A, Tamhankar S, Rao S (2014) Diosgenin content in some Trigonella species. Indian J Adv Plant Res 1:47–51
Deshpande HA, Bhalsing SR (2014) Isolation and characterization of diosgenin from in vitro cultured tissues of Helicteres isora L. Physiol Mol Biol Plants 20(1):89–94
Deshpande HA, Bhalsing SR (2015) Plant derived novel biomedicinal: diosgenin. Int J Pharmacog Phytochem Res 6:780–784
Diarra ST, He J, Wang J, Li J (2013) Ethylene treatment improves diosgenin accumulation in in vitro cultures of Dioscorea zingiberensis via up-regulation of CAS and HMGR gene expression. Electron J Biotechnol 16(5):6. https://doi.org/10.2225/vol16-issue5-fulltext-9
Gas-Pascual E, Simonovik B, Schaller H, Bach TJ (2015) Inhibition of cycloartenol synthase (CAS) function in tobacco BY-2 cells. Lipids 50(8):761–772
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Gomez P, Ortuno A, Del Río JA (2004) Ultrastructural changes and diosgenin content in cell suspensions of Trigonella foenum-graecum L. by ethylene treatment. Plant Growth Regul 44(2):93–99
Gupta R, Singh A, Srivastava M, Singh V, Gupta MM, Pandey R (2017) Microbial modulation of bacoside A biosynthetic pathway and systemic defense mechanism in Bacopa monnieri under Meloidogyne incognita stress. Scientific Reports 7:41867. https://doi.org/10.1038/srep41867
Hao S, Xu R, Li D, Zhu Z, Wang T, Liu K (2015) Attenuation of streptozotocin-induced lipid profile anomalies in the heart, brain, and mRNA expression of HMG-CoA reductase by diosgenin in rats. Cell Biochem Biophys 72(3):741–749
Hussain MS, Fareed S, Saba Ansari M, Rahman A, Ahmad IZ, Saeed M (2012) Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 4(1):10
Hwang HS, Lee H, Choi YE (2015) Transcriptomic analysis of Siberian ginseng (Eleutherococcus senticosus) to discover genes involved in saponin biosynthesis. BMC Genomics 16(1):180
Ibraheim SK (2014) Effect of foliar spray with some biostimulants on growth, yield and seeds quality of pea plants grown in sandy soil. J Appl Sci Res 10(5):400–407
Jaisi A, Panichayupakaranant P (2016) Increased production of plumbagin in Plumbago indica root cultures by biotic and abiotic elicitors. Biotechnol Lett 38(2):351–355. https://doi.org/10.1007/s10529-015-1969-z
Jayachandran KS, Vasanthi HR, Rajamanickam GV (2009) Antilipoperoxidative and membrane stabilizing effect of diosgenin, in experimentally induced myocardial infarction. Mol Cell Biochem 327:203–210
Khanahmadi M, Paek KY (2017) Bioreactor technology for sustainable production of valuable plant metabolites: challenges and advances. In: Abdullah S, Chai-Ling H, Wagstaff C (eds) Crop improvement. Springer, Berlin, pp 169–189. https://doi.org/10.1007/978-3-319-65079-1_8
Kumar V, Desai D, Shriram V (2014) Hairy root induction in Helicteres isora L. and production of diosgenin in hairy roots. Nat Prod Bioprospect 4:107–112. https://doi.org/10.1007/s13659-014-0011-9
Kumar P, Chaturvedi R, Sundar D, Bisaria VS (2016) Piriformospora indica enhances the production of pentacyclic triterpenoids in Lantana camara L. suspension cultures. Plant Cell Tissue Organ Cult 125(1):23–29
Kusakari K, Yokoyama M, Inomata S, Gozu Y, Katagiri C, Sugimoto Y (2012) Large-scale production of saikosaponins through root culturing of Bupleurum falcatum L. using modified airlift reactors. J Biosci Bioeng 113(1):99–105
Lattanzio V, Lattanzio VMT, Cardinali A (2006) Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Imperato F (ed) Phytochemistry: advances in research. Research Signpost, Kerala, pp 23–67
Le KC, Im WT, Paek KY, Park SY (2018) Biotic elicitation of ginsenoside metabolism of mutant adventitious root culture in Panax ginseng. Appl Microbiol Biotechnol 102(4):1687–1697
Leivar P, Antolín-Llovera M, Ferrero S, Closa M, Arró M, Ferrer A, Boronat A, Campos N (2011) Multilevel control of Arabidopsis 3-hydroxy-3-methylglutaryl coenzyme A reductase by protein phosphatase 2A. Plant Cell 23:1494–1511
Li P, Mao Z, Lou J, Li Y, Mou Y, Lu S, Peng Y, Zhou L (2011a) Enhancement of diosgenin production in Dioscorea zingiberensis cell cultures by oligosaccharides from its endophytic fungus Fusarium oxysporum Dzf17. Molecules 16(12):10631–10644
Li P, Mou Y, Shan T, Xu J, Li Y, Lu S, Zhou L (2011b) Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgenin production in cell suspension culture of Dioscorea zingiberensis. Molecules 16(11):9003–9016
Li P, Mou Y, Lu S, Sun W, Lou J, Yin C, Zhou L (2012) Quantitative determination of diosgenin in Dioscorea zingiberensis cell cultures by microplate-spectrophotometry and high-performance liquid chromatography. Afr J Pharm Pharmacol 6:1186–1193
Liagre B, Vergne-Salle P, Corbiere C, Charissoux JL, Beneytout JL (2004) Diosgenin, a plant steroid, induces apoptosis in human rheumatoid arthritis synoviocytes with cyclooxygenase-2 overexpression. Arthritis Res Ther 6:R373
Lu M, Wong H, Teng W (2001) Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep 20(7):674–677
Manero FJ, Algar E, Martín Gómez MS, Saco Sierra MD, Solano BR (2012) Elicitation of secondary metabolism in Hypericum perforatum by rhizosphere bacteria and derived elicitors in seedlings and shoot cultures. Pharmac Biol 50(10):1201–1209
Mirunalini S, Shahira R (2011) Novel effect of diosgenin—a plant derived steroid: a review. Pharmacologyonline 1:726–736
Morlacchi P, Wilson WK, Xiong Q, Bhaduri A, Sttivend D, Kolesnikova MD, Matsuda SP (2009) Product profile of PEN3: the last unexamined oxidosqualene cyclase in Arabidopsis thaliana. Org Lett 11(12):2627–2630
Mulabagal V, Tsay HS (2004) Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2(1):29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Narayani M, Srivastava S (2017) Elicitation: a stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem Rev 16(6):1227–1252
Nes WD (2011) Biosynthesis of cholesterol and other sterols. Chem Rev 111(10):6423–6451
Nogues I, Brilli F, Loreto F (2006) Dimethylallyl diphosphate and geranyl diphosphate pools of plant species characterized by different isoprenoid emissions. Plant Physiol 141(2):721–730
Pal T, Malhotra N, Chanumolu SK, Chauhan RS (2015) Next-generation sequencing (NGS) transcriptomes reveal association of multiple genes and pathways contributing to secondary metabolites accumulation in tuberous roots of Aconitum heterophyllum Wall. Planta 242(1):239–258
Patel K, Gadewar M, Tahilyani V, Patel DK (2012) A review on pharmacological and analytical aspects of diosgenin: a concise report. Nat Prod Bioprospect 2:46–52
Patra N, Srivastava AK (2016) Artemisinin production by plant hairy root cultures in gas-and liquid-phase bioreactors. Plant Cell Rep 35(1):143–153
Phillips DR, Rasbery JM, Bartel B, Matsuda SP (2006) Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol 9(3):305–314
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20(2):101–153
Rokem JS, Tal B, Goldberg I (1985) Methods for increasing diosgenin production by Dioscorea cells in suspension cultures. J Nat Prod 48(2):210–222
Selim S, Al Jaouni S (2015) Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.) Sm. BMC Compl Altern Med 15:301
Sethi G, Shanmugam M, Warrier S, Merarchi M, Arfuso F, Kumar A, Bishayee A (2018) Pro-apoptotic and anti-cancer properties of diosgenin: a comprehensive and critical review. Nutrients 10:645
Shaikh S, Shriram V, Khare T, Kumar V (2018) Establishment of callus and cell suspension cultures of Helicteres isora L. Res Plant Biol 8:1–4. https://doi.org/10.25081/ripb.2018.v8.3366
Sharma S, Malhotra N, Sood H (2016) Expression analysis of steroid pathway genes revealed positive correlation with diosgenin biosynthesis in Trillium govanianum. Acta Physiol Plant 38(11):272
Simic SG, Tusevski O, Maury S, Hano C, Delaunay A, Chabbert B, Lamblin F, Lainé E, Joseph C, Hagège D (2015) Fungal elicitor-mediated enhancement in phenylpropanoid and naphtodianthrone contents of Hypericum perforatum L. cell cultures. Plant Cell Tissue Organ Cult 122(1):213–226
Singh RS, Gara RK, Bhardwaj PK, Kaachra A, Malik S, Kumar R, Sharma M, Ahuja PS, Kumar S (2010) Expression of 3-hydroxy-3-methylglutaryl-CoA reductase, p-hydroxybenzoate-m-geranyltransferase and genes of phenylpropanoid pathway exhibits positive correlation with shikonins content in arnebia [Arnebia euchroma (Royle) Johnston]. BMC Mol Biol 11(1):88
Sirotkin AV, Alexa R, Alwasel S, Harrath AH (2019) The phytoestrogen, diosgenin, directly stimulates ovarian cell functions in two farm animal species. Domest Animal Endocrinol 69:35–41
Son SH, Choi SM, Lee YH, Choi KB, Yun SR, Kim JK, Park HJ, Kwon OW, Noh EW, Seon JH, Park YG (2000) Large-scale growth and taxane production in cell cultures of Taxus cuspidata (Japanese yew) using a novel bioreactor. Plant Cell Rep 19(6):628–633
Suzuki M, Kamide Y, Nagata N, Seki H, Ohyama K, Kato H, Masuda K, Sato S, Kato T, Tabata S, Yoshida S (2004) Loss of function of 3-hydroxy-3-methylglutaryl coenzyme A reductase 1 (HMG1) in Arabidopsis leads to dwarfing, early senescence and male sterility, and reduced sterol levels. Plant J 37(5):750–761
Tashackori H, Sharifi M, Chashmi NA, Safaie N, Behmanesh M (2016) Induced-differential changes on lignan and phenolic acid compounds in Linum album hairy roots by fungal extract of Piriformospora indica. Plant Cell Tissue Organ Cult 127(1):187–194
Wang YJ, Pan KL, Hsieh TC, Chang TY, Lin WH, Hsu JT (2011) Diosgenin, a plant-derived sapogenin, exhibits antiviral activity in vitro against hepatitis C virus. J Nat Prod 74(4):580–584
Werner S, Maschke RW, Eibl D, Eibl R (2017) Bioreactor technology for sustainable production of plant cell-derived products. In: Pavlov A, Bley T (eds) Bioprocessing of plant in vitro systems. Springer, Berlin, pp 1–20. https://doi.org/10.1007/978-3-319-32004-5_6-1
Xue Z, Duan L, Liu D, Guo J, Ge S, Dicks J, ÓMáille P, Osbourn A, Qi X (2012) Divergent evolution of oxidosqualene cyclases in plants. New Phytol 193(4):1022–1038
Yang D, Fang Y, Xia P, Zhang X, Liang Z (2018) Diverse responses of tanshinone biosynthesis to biotic and abiotic elicitors in hairy root cultures of Salvia miltiorrhiza and Salvia castanea Diels f. tomentosa. Gene 643:61–67
Zhang Y, Tang L, An X, Fu E, Ma C (2009) Modification of cellulase and its application to extraction of diosgenin from Dioscorea zingiberensis CH Wright. Biochem Eng J 47(1–3):80–86
Zhou L, Yang G, Sun H, Tang J, Yang J, Wang Y, Garran TA, Guo L (2017) Effects of different doses of cadmium on secondary metabolites and gene expression in Artemisia annua L. Front Med 11(1):137–146
Zhu YL, Huang W, Ni JR, Liu W, Li H (2010) Production of diosgenin from Dioscorea zingiberensis tubers through enzymatic saccharification and microbial transformation. Appl Microbiol Biotechnol 85(5):1409–1416
Zhu JH, Li HL, Guo D, Wang Y, Dai HF, Mei WL, Peng SQ (2018) Identification, characterization and expression analysis of genes involved in steroidal saponin biosynthesis in Dracaena cambodiana. J Plant Res 131:555–562
Acknowledgements
The financial support for conducting this from Savitribai Phule Pune University, Pune in the form of Research Grant (No. OSD/BCUD/392/132) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shaikh, S., Shriram, V., Khare, T. et al. Biotic elicitors enhance diosgenin production in Helicteres isora L. suspension cultures via up-regulation of CAS and HMGR genes. Physiol Mol Biol Plants 26, 593–604 (2020). https://doi.org/10.1007/s12298-020-00774-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-020-00774-6