Abstract
As a model plant, poplar 84 K (Populus alba × P. glandulosa) plays a key role in fundamental research in forest molecular biology. Poplar 84 K is a suitable plant for in vitro polyploidy induction and to study the consequent trait variation among perennial trees. In this study, the results indicated that the concentration of NAA (naphthaleneacetic acid) and BA (benzyladenine) has substantial effects on the differentiation rate of adventitious shoots, and media consisting of 0.5 mg L−1 BA and 0.05 mg L−1 NAA yielded the greatest number of shoots per explant. A total of 98 tetraploids were successfully obtained by colchicine treatment on diploid leaves in vitro. The rate of tetraploids was substantially affected by pre-culture duration, colchicine concentration and exposure duration. The highest tetraploid induction efficiency was 37.03%, which was achieved by treating leaves with 50 mg L−1 colchicine for 3 days after 4 days of pre-culture. With increasing ploidy, obvious morphological differences were discovered between tetraploids and diploids. Compared with the diploids, the tetraploids had larger and thicker leaves, larger but sparser stomata, fewer and shorter roots, and larger protoplasts. These tetraploids serve to enrich the polyploid germplasm resources of Populus. Moreover, our study lays an important groundwork for polyploid gene function and is important for exploring trait variation among Populus trees with different ploidy levels.
Key message
In vitro induction of tetraploid by colchicine treatment and differences in morphological features between tetraploid and diploid plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyploidization has long been recognized as a prominent force in the evolution of plant species and is an important contributor to speciation (Stebbins 1971; Comai 2005; Wood et al. 2009). Polyploid plants generally present rapid growth, large vegetative organs and high secondary metabolite contents, and polyploidy is being used as an important strategy in tree genetic improvement (Zhu et al. 1995, 1998; Sattler et al. 2016; Liao et al. 2016; Parsons et al. 2019; Guo et al. 2019; Kang 2020). There are many ways to obtain polyploidy trees (Nilsson-Ehle 1936; Johnsson 1945; Weisgerber 1980; Einspahr 1984). Among them, somatic chromosome doubling is one of the quickest methods to obtain polyploidy (Mattila 1961; Thao et al. 2003; Zhang et al. 2008; Nilanthi et al. 2009; Cai and Kang 2011). Polyploidy can also be induced through the application of antimitotic agents to seeds, seedlings, in vivo shoot tips, or in vitro explants (Dermen 1940; Petersen et al. 2003; Talebi et al. 2017; Parsons et al. 2019). Among them, a controlled environment can be provided by inducing polyploids in vitro, and the test results are repeatable, greatly improve the efficiency and effects of somatic chromosome doubling. In recent years, with the development of plant tissue culture technology, inducing polyploids in vitro has been applied in more and more plants. At present, polyploid forest trees have been successfully obtained in vitro, including those of black locust (Ewald et al. 2009), Paulownia australis (Wang et al. 2017), Populus (Xu et al. 2016, 2018; Liu et al. 2018; Zeng et al.2019), sweetgum (Zhang et al. 2017), Ziziphus (Gu et al. 2005; Cui et al. 2017), mulberry (Wang et al. 2011) and Betula (Särkilahti and Valanne 1990).
Poplar 84 K (Populus alba × P. glandulosa) is a well-known hybrid. This hybrid resulted from a breeding programme led by Professor Sin Kyu Hyun (Seoul National University, Korea) and was first introduced into China in 1984 by Professor Qiwen Zhang (The Research Institute of Forestry, Chinese Academy of Forestry, Beijing) (Wang et al. 2005; Qiu et al. 2019). Poplar 84 K is currently popular in China because of its fast growth, high wood quality, strong resistance and broad adaptability. Importantly, poplar 84 K has been widely used by scientists in transgenic experiments as a model of woody species due to its high rate of transformation and differentiation (Shim et al. 2013; Yoon et al. 2014; Zhao et al. 2018; Zhang et al. 2019; Shu et al. 2019). Qiu et al. (2019) reported the whole genome and identified two subgenomes via comparison, opening up broader areas in forest molecular biology research by using 84 K poplar as an important material. At this time, tetraploids of poplar 84 K can be obtained by somatic chromosome doubling. This study not only provides polyploid germplasm resources for Populus alba × P. glandulosa but also plays an important role by exploring the molecular mechanism of forest polyploid trait variation.
In this study, we used diploid 84 K poplars as research objects. We then optimized the differentiation system of poplar 84 K leaves by applying gradients of different concentrations of hormones. On this basis, we have essentially conducted new research on polyploid induction systems via colchicine treatment on diploid poplar 84 K leaves in vitro. In addition, we observed and studied the growth status, leaf morphology and stomatal characteristics of both diploids and tetraploids.
Materials and methods
Plant materials
Poplar 84 K (Populus alba × P. glandulosa) were provided by the Beijing Forestry University greenhouse staff. All the plants were cultivated in a greenhouse whose light, temperature (10–20 °C), and humidity were controlled. The young stems of poplar 84 K, which served as explants, were washed with running water for 30 min. They were subsequently sterilized in a solution of 70% (v/v) ethanol for 30 s and 1% (v/v) sodium hypochlorite for 10 min and then rinsed in sterile distilled water three times. Finally, thoroughly cleaned explants with at least one bud were inoculated onto solid MS media (Murashige and Skoog 1962). After approximately 20 days, new adventitious shoots were transplanted to ½-strength MS media consisting of 0.05 mg L−1 IBA and 0.02 mg L−1 NAA for root formation. The pH of all the media was adjusted to 5.8–6.2, after which the media were autoclaved at 121 °C for 15 min. Rooting media was incubated at 25 °C under white fluorescent lighting (16 h photoperiod, average light intensity of 30–40 μmol m−2 s−1).
Adventitious shoot regeneration from poplar 84 K leaves
After approximately 30 days, fully expanded leaves were harvested from sterile rooted plantlets and wounded with two transverse cuts crossing the main vein without full separation. The explants were placed on the differentiation media [MS media consisting of 0.5% (w/v) agar, 3% (w/v) sucrose, and different concentrations of NAA and BA, as shown in Table 1], with the adaxial side touching the media, to evaluate the effects of plant growth regulators on shoot formation of poplar 84 K. The number of adventitious shoots per explant was recorded after 30 days of culture, and the percentage of regenerated explants was calculated. The experiments were repeated three times, with ten explants per treatment.
Colchicine treatment for inducing polyploidy and plant recovery
Fully expanded leaves were cut twice without separation and cultured on solid adventitious shoot regeneration media [MS media consisting of 3% (w/v) sucrose, 0.2% (w/v) agar, 0.5 mg L−1 BA and 0.05 mg L−1 NAA] for 3, 4, or 5 days. Afterwards, the cultures were transferred to the same liquid media containing filter-sterilized colchicine at concentrations of 40, 50, or 60 mg L−1 for 2, 3, or 4 days of treatment. Each treatment consisted of 10 replicates and was repeated three times. After colchicine treatment, the explants were washed three times with sterile water and then transplanted into shoot regeneration media, after which they were allowed to grow for 4 weeks. The single adventitious shoot was then excised and placed on ½-strength MS media supplemented with 0.05 mg L−1 IBA and 0.02 mg L−1 NAA for root formation. Once the explants had recovered and produced at least three fully expanded leaves, survival rates were recorded, and each plant was sampled for flow cytometric ploidy analysis.
Flow cytometry is a rapid, reliable and simple method to measure the ploidy level and confirm the success of polyploidy induction, allowing the analysis of a large number of target plants in a short period of time (Galbraith et al. 1983; Roy et al. 2001). Young leaves were collected from healthy cultured plants. The leaf samples were chopped with a razor blade in a dish containing 1 mL of modified Galbraith’s buffer [45 mM MgCl2·6H2O, 30 mM sodium citrate, 20 mM MOPS, 0.1% (v/v) Triton X-100 (pH 7.0); Doležel et al. 1989]. The suspension was then passed through a 30 μm nylon mesh filter to isolate the nuclei. The filtrate was subsequently stained with 80 μL of DAPI (5 mg mL−1) for 5 s. The ploidy level was analysed by a Cyflow® Ploidy Analyzer (Partec PAS, Germany). The fluorescence intensity of the diploids was adjusted to 50, and tetraploids were identified as those whose peaks occurred at a relative fluorescence intensity of 100.
Chromosome number
Chromosome counting is considered the most accurate method to detect polyploid variants. The ploidy level of the regenerated tetraploid and diploid shoots was confirmed by chromosome counting. Young, unexpanded leaves were collected and pretreated with a saturated solution of paradichlorobenzene for 4 h, after which the leaves were then fixed in a 3:1 ethanol:acetic acid mixture for 24–48 h at 4 °C. After three rinses with water, the materials were dissociated in 1 N HCl for 20 min at room temperature. The material was subsequently squashed in modified phenol solution (Carbol fuchsin consisting of phenol solution and basic fuchsin) on a microscope slide. Then pressed down with a cover slip and tapped it a few times with pencil. The cells were imaged using an Olympus BX51 microscope and observed under a 100 × oil immersion objective lens.
Stomatal characteristics and phenotypic analysis
Twenty of the fully expanded poplar 84 K leaves of 10 tetraploids and 10 diploids were used to determine stomatal size and density. A few strips of the abaxial epidermis were added to a drop of water on a microscope slide and covered with a cover slip (Sari et al. 1999). Under an Olympus BX51 microscope, stomatal size was calculated on the basis of 100 randomly selected stomata, and thirty randomly selected microscopic fields were analysed to the measure stomatal density (Cui et al. 2017). To compare the differences between the tetraploid and diploid plants, the leaf size and the number and length of the roots of three selected individuals for each ploidy level were recorded.
Comparison of leaf mesophyll protoplast cell size
To analyse the size of mesophyll protoplast cells, fully expanded diploid and tetraploid leaves (1 g in total) were randomly selected and cut into approximately 1 mm thin strips. After pretreatment, the strips were transferred into an enzyme solution consisting of 3.0% (w/v) Cellulase Onozuka R-10, 0.05% (w/v) Pectinase Y-23, 0.2% (w/v) Macerozyme R-10, 109 g/L mannitol, 0.6 g L−1 MES, and 1 g L−1 BSA in CPW wash media at pH 5.8 (Huang 2015). The flask was sealed and then incubated under darkness and oscillation (20 rpm) at 27 °C for 7 h. After enzymolysis, the contents were filtered with a 200 mesh cell strainer and then transferred to a 10 mL centrifuge tube. The filtrate was centrifuged at 1000 rpm for 3 min, and the volume was fixed to 10 mL with CPW solution. Under an Olympus BX51 microscope, 100 protoplasts were randomly selected from diploids and tetraploids.
Data and statistical analysis
Univariate GLM was used to analyse the difference in effects between BA and NAA on the shoot formation rate and the number of adventitious shoots per explant. We also used the same method to analyse the differences in tetraploid induction rates among pre-culture duration, colchicine concentration, and exposure duration. For the experimental data measured as a percentage, arcsine transformation was used before one-way analysis of variance. When treatments differed significantly, LSD multiple comparison tests were performed at the 0.05 level of probability. The data were analysed with the statistical software SPSS version 20.0 (IBM Inc., New York, NY, USA). Stomatal characteristics and the characteristics of the leaves and roots were determined by ImageJ (http://rsb.info.nih.gov/ij/). The results are presented as the means ± SDs.
Results
Shoot regeneration from leaf explants
We studied the effects of BA and NAA on adventitious shoot regeneration from leaf explants of poplar 84 K under 9 different treatments (Table 1). Adventitious buds and calli formed from leaf explants after 20 days of culture (Fig. 1a), and shoots regenerated from leaf explants after 40 days (Fig. 1b). Both the shoot formation rate and the number of shoots per explant were affected significantly by BA (F = 16.498, P = 0.000) and NAA (F = 16.777, P = 0.000) concentration. Furthermore, the interaction between BA and NAA also significantly affected these parameters (F = 5.581, P = 0.004). When the explants were cultured on medium containing 0.50 mg L−1 BA and 0.05 mg L−1 NAA or 0.70 mg L−1 BA and 0.07 mg L−1 NAA, the shoot formation rate reached more than 90%. But the number of shoots per explant was higher on media containing 0.50 mg L−1 BA and 0.05 mg L−1 NAA than on media containing 0.70 mg L−1 BA and 0.07 mg L−1 NAA (Table 1). In general, media consisting of 0.50 mg L−1 BA and 0.05 mg L−1 NAA was the best media for shoot formation in the experiment.
Tetraploid induction by colchicine treatment and ploidy determination
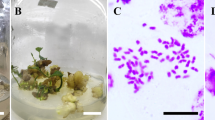
Diploid polar 84 K was subjected to 27 different treatments (Table 2), we obtained 1490 regenerated shoots in total. Flow cytometry analysis revealed 98 tetraploids (Fig. 2). According to the chromosome counting method, we determined that the chromosome number of the diploid plants was 2n = 2 × = 38 (Fig. 3a) and that the chromosome number of tetraploid plants was 2n = 4 × = 76 (Fig. 3b).
The tetraploid induction rates derived from 27 treatments in poplar 84 K are presented in Table 2. As seen from the table, the tetraploid induction rates varied from 0 to 37.03%. The results indicated that the days of pre-culture (F = 25.187, p = 0.000), colchicine concentration (F = 20.889, p = 0.000) and exposure duration (F = 44.149, p = 0.000) caused significant differences in the induction rate of tetraploids. In addition, the frequency of tetraploid induction was significantly affected by the interaction among pre-culture duration, colchicine concentration and exposure duration, suggesting that specific combinations of the three analysed factors are necessary for in vitro tetraploid induction in poplar 84 K (F = 13.147, p = 0.000). In general, When the pre-culture duration and of exposure time were constant, the number of regenerated shoots decreased with the increase of colchicine concentration. When the pre-culture duration and colchicine concentration were constant, the induction rate of tetraploid decreased with the increase of exposure time. The highest tetraploid induction efficiency was 37.03%, which was achieved by treating leaves with 50 mg L−1 colchicine for 3 days after 4 days of pre-culture (Table 2).
Differences in morphological features between tetraploid and diploid plants
To analyse the morphological changes associated with changes in ploidy level, we compared the stomatal length and width and stomatal density between tetraploid and diploid plants. By comparison, we found that there were significant differences between diploid and tetraploid plants in these parameters. The average stomatal length and width of the tetraploid plants were 19.02 µm and 9.00 μm, respectively, but the same parameters were only 8.48 µm and 4.50 μm for the diploids, respectively (Table 3). Compared with those of the diploid leaves, the stomata on the abaxial side of the tetraploid leaves were approximately 50% larger and half as dense (Figs. 4, 5). Moreover, the average diameter of the diploid protoplasts was 15.15 μm, which was smaller than that of the tetraploid protoplasts (25.79 μm) (Table 3).
In addition, there were phenotypic changes resulting from polyploidy. Root formation occurred on regenerated shoots on transfer to ½ MS medium containing 0.05 mg L−1 IBA and 0.02 mg L−1 NAA (Fig. 6). The length of the roots of the tetraploid plants was shorter than that of the diploid plants, and the tetraploid plants presented fewer roots per plant than did the diploid plants (Table 3). In addition, the plant height (Fig. 7a) was lower for the 30-day-old tetraploids than for the diploids of the same age (Table 3). Compared with the diploid leaves, the tetraploid leaves were also larger and more serrated (Fig. 7b).
Rooting of regenerated shoots cultured on ½ MS medium containing 0.05 mg L−1 IBA and 0.02 mg L−1 NAA. a Diploid (left) and tetraploid (right) shoots cultured on the rooting medium for 10 days, b diploid (left) and tetraploid (right)shoots cultured on the rooting medium for 25 days, c diploid (left) and tetraploid (right)shoots cultured on the rooting medium for 35 days
Discussion
Colchicine is an alkaloid extracted from meadow saffron (Colchicum autumnale L.) and is the most widely used antimitotic agent for polyploidy induction (Planchais et al. 2000). Colchicine inhibits chromosome separation during cell division, resulting in chromosome doubling (Wu et al. 2020). Consequently, colchicine has become a common mutagen used in polyploid breeding (Blakeslee and Avery 1937). Numerous tetraploid plant species have been successfully induced by colchicine (Dermen and Henry 1944; Van et al. 1992; Tosca et al. 1995; Dhooghe et al. 2010). However, not all concentrations of colchicine are applicable for producing tetraploids. Low colchicine concentrations or short exposure times are ineffective for inducing somatic chromosome doubling (Allum et al. 2007). In addition, studies have shown that tetraploid induction frequency depends on the interaction between duration and concentration of mitotic inhibitors (Nilanthi et al. 2009; Cai and Kang, 2011). However, high colchicine concentrations or long exposure times are also ineffective because the toxicity of colchicine can lead to death of the tissue (Morejohn et al. 1987; Shao et al. 2003; Wu et al.2020). Therefore, appropriate colchicine concentrations and durations are keys to inducing tetraploids. Xu et al. (2016) found that 30 mg L−1 colchicine applied to explants was the most effective for inducing polyploidization [(P. pseudo-simonii × P. nigra ‘Zheyin #3′) × (P. × beijingensis)]. In this study, we obtained a total of 98 tetraploids, which were achieved by treating leaves with 50 mg L−1 colchicine for 3 days. The results showed that colchicine treatment was effective at inducing tetraploidy in poplar 84 K.
Mixoploids are easily produced from somatic chromosome doubling of multi-cellular organs (Thao et al. 2003; Campos et al. 2009; Ewald et al. 2009; Cai and Kang, 2011; Xu et al. 2018; Liu et al. 2018). Xu et al. (2018) reported that the leaf incision callus developmental status of Populus affects tetraploid production efficiency, which also shows the importance of pre-cultivation. Here, the highest tetraploid induction efficiency was 37.03%, which was achieved by treating leaves with 50 mg L−1 colchicine for 3 days after 4 days of pre-culture. Our findings showed that the tetraploid induction rate was dependent on interactions among pre-culture duration, concentration and duration of colchicine treatment. The findings are concomitant with the results reported by Xie et al. (2015) and Xu et al. (2016). According to another report, mixoploids are considered unstable due to asynchronism in the cell cycle between two types of cells (Dermen and Henry 1944; Mergen and Lester 1971; Wan et al. 1989; Nilanthi et al. 2009). However, several studies have reported the discovery of stable high-performance mixoploids in Populus (Ulrich and Ewald 2014; Xu et al. 2016; Wu et al 2020). In this study, a few mixoploids were also obtained (data not shown). They were stable for six months in subculture, showing that normal and polyploidized cells stably existed in the mixoploid cell cycles. The stability of these hybrids over a long period of time still needs to be studied.
Stomatal characteristics are considered a simple and useful method to determine ploidy level. Changes in stomatal size and density are common among tetraploids (Ascough et al. 2008; Sakhanokho et al. 2009; Rêgo et al. 2011; Talebi et al. 2017). Compared with diploids of Populus, tetraploids of Populus have a lower stomatal density and stomatal guard cells with a larger length and diameter (Lu et al. 2013). In this study, compared with those of the diploids, the stomata of the tetraploids were also approximately 50% larger (length and width) and less than half as dense (50%). Overall, these data suggest that stomatal size and density are reliable phenotypic markers for poplar 84 K polyploids.
Studies have revealed that polyploidy induces distinct phenotypic and morphological changes (Schranz and Osborn.2000) such as differences in leaf size and root architecture as well as alterations in plant physiology, biotic stress tolerance and other developmental processes (Cohen et al. 2013). Polyploidy often causes morphological features and growth development that are distinctly different from those of diploids (Stebbins 1950; Stanys et al. 2006; Allario et al. 2011; Dhooghe et al. 2010; Sattler et al. 2016). Compared with Betula platyphylla diploids, Betula platyphylla tetraploids are generally superior in terms of volume, leaves, fruit and stomata but are inferior in terms of height (Mu et al. 2012). Compared with Ziziphus diploids, Ziziphus tetraploids have larger and thicker leaves, darker green leaves, and shorter internodes (Gu et al. 2005; Cui et al. 2017). In Paulownia australis tetraploid plants, the leaf length and width are greater, the leaf size is larger, and the leaf structures are thicker, including the upper and lower epidermal layers and palisade tissues; however, the spongy parenchyma layer is thinner (Wang et al. 2017). In this study, compared with the diploid plants, the tetraploid plants had larger, thicker and more serrated leaves; larger but sparser stomata; fewer and shorter roots; and larger protoplasts. Poplar has a long growth cycle, so economic traits such as wood yield could not be analyzed in the present study. However, with the increase in ploidy, the morphological changes are obvious and could be used to study trait variations in polyploid poplar germplasm in field conditions. We expect that the finding of the present study could be applied for the production of novel germplasm for Populus breeding efforts.
Roots not only provide structural support to the aerial portion of plants but also acquire nutrients and water, which are vital to plant growth. Plant roots, especially lateral roots (LRs), play crucial roles in adaptation to various conditions (Casimiro et al. 2003; Nibau et al. 2008; Petricka et al. 2012; Lavenus et al. 2013). In this study, the length of the roots of tetraploid plants was shorter than that of diploid plants, and the tetraploid plants presented fewer roots per plant than did the diploid plants, similar to the results reported for induced tetraploids of Thymus persicus (Tavan et al. 2015) and Punica granatum (Shao et al. 2003). Molecular mechanisms involving transcription factors and regulations in production of plant hormone could be the reasons for these differences which need to be studied.
Importantly, poplar 84 K is easily accessible for genetic transformation, and this clone is widely used by scientists as a model for woody species in terms of transgenic experiments. Qiu et al. (2019) described a de novo assembly of the genome sequence of the hybrid poplar (P. alba × P. glandulosa) clone 84 K and identified two subgenomes via comparison of the genomes. The obtained tetraploids in the present study increase genomic resources and contribute to further analysis of gene function and comparative differences in genes across poplar plants of different ploidy levels.
Conclusion
We studied the effects of BA and NAA on adventitious shoot regeneration from leaf explants of poplar 84 K, media consisting of 0.5 mg L−1 BA and 0.05 mg L−1 NAA was the best media for shoot formation. The regeneration system was used for production of colchicine induced polyploids in poplar 84 K, the frequency of tetraploid induction was significantly affected by the interaction among pre-culture duration, colchicine concentration and exposure duration, the highest tetraploid induction efficiency was 37.03%, which was achieved by treating leaves with 50 mg L−1 colchicine for 3 days after 4 days of pre-culture. By comparison, we found that there were significant morphological differences between diploid and tetraploid plants in leaf size, protoplast size, stomatal and root parameters.
Abbreviations
- MS:
-
Murashige and Skoog (1962)
- BA:
-
Benzyladenine
- NAA:
-
1-Naphthaleneacetic acid
- IBA:
-
Indole butyric acid
- DAPI:
-
4,6-Diamidino-2-phenylindole
- MOPS:
-
4-Morpholinepropane sulfonate
- CPW:
-
Cell protoplast washing medium
- MES:
-
4-Morpholine ethane sulfonic acid
- BS:
-
Bovine serum albumin
- LSD:
-
Least significant difference
- SE:
-
Standard error
- SEM:
-
Scanning electron microscopy
References
Allario T, Brumos J, Colmenero-flores JM, Tadeo F, Froelicher Y, Talon M, Navarro L, Ollitrault P, Morillon R (2011) Large changes in anatomy and physiology between diploid Rangpur lime (Citrus limonia) and its autotetraploid are not associated with large changes in leaf gene expression. J Exp Bot 62(8):2507–2519. https://doi.org/10.1093/jxb/erq467
Allum JF, Bringloe DH, Roberts AV (2007) Chromosome doubling in a Rosa rugosa Thunb. hybrid by exposure of in vitro nodes to oryzalin: the effects of node length, oryzalin concentration and exposure time. Plant Cell Rep 26(11):1977–1984. https://doi.org/10.1007/s00299-007-0411-y
Ascough GD, Van SJ, Erwin JE (2008) Effectiveness of cholchicine and oryazlin at inducing polyploidy in Watsonia lepida N.E. Brown. Hortscience Horts 43(7):2248–2251. https://doi.org/10.21273/HORTSCI.43.7.2248
Blakeslee AF, Avery AG (1937) Methods of inducing doubling of chromosomes in plants by treatment with colchicine. J Hered 28:393–411. https://doi.org/10.1093/oxfordjournals.jhered.a104294
Cai X, Kang XY (2011) In vitro tetraploid induction from leaf explants of Populus pseudo-simonii Kitag. Plant Cell Rep 30:1771–1778. https://doi.org/10.1007/s00299-011-1085-z
Campos JMS, Davide LC, Salgado CC, Santos FC, Costa PN, Silva PS, Alves CCS, Viccini LF, Pereira AV (2009) In vitro induction of hexaploid plants from triploid hybrids of Pennisetum purpureum and Pennisetum glaucum. Plant Breed 128:101–104. https://doi.org/10.1111/j.1439-0523.2008.01546.x
Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang HM, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8(4):165–171. https://doi.org/10.1016/S1360-1385(03)00051-7
Cohen H, Fait A, Tel-Zur N (2013) Morphological, cytological and metabolic conseque- nces of autopolyploidization in Hylocereus (Cactaceae) species. BMC Plant Biol 13:173. https://doi.org/10.1186/1471-2229-13-173
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Gen 6:836–846. https://doi.org/10.1038/nrg1711
Cui YH, Hou L, Li X, Huang FY, Pang XM, Li YY (2017) In vitro induction of tetraploid Ziziphus jujuba Mill. var. spinosa plants from leaf explants. Plant Cell Tiss Organ Cult 131:175–182. https://doi.org/10.1007/s11240-017-1274-8
Dermen H (1940) Colchicine polyploidy and technique. Bot Rev 6:599–635. https://doi.org/10.1007/BF02919557
Dermen H, Henry FB (1944) A general cytohistological study of colchicine polyploidy in Cranberry. Am J Bot 31:451–463
Dhooghe E, Laere KV, Eeckhaut T, Leus L, Huylenbroeck JV (2010) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tiss Organ Cult 104(3):359–373. https://doi.org/10.1007/s11240-010-9786-5
Doležel J, Binarová P, Lucretti S (1989) Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31:113–120. https://doi.org/10.1007/BF02907241
Einspahr DW (1984) Production and utilization of triploid hybrid aspen. Iowa State J Res 58:401–409
Ewald D, Ulrich K, Naujoks G, Schrӧder MB (2009) Induction of tetraploid poplar and black locust plants using colchicine: chloroplast number as an early marker for selecting polyploids in vitro. Plant Cell Tiss Organ Cult 99(3):353–357. https://doi.org/10.1007/s11240-009-9601-31
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220(4601):1049–1051. https://doi.org/10.1126/science.220.4601.1049
Gu XF, Yang AF, Meng H, Zhang JR (2005) In vitro induction of tetraploid plants from diploid Zizyphus jujuba Mill. Cv. Zhanhua. Plant Cell Rep 24(11):671–676. https://doi.org/10.1007/s00299-005-0017-1
Guo LQ, Zhang JG, Liu XX, Rao GD (2019) Polyploidy-related differential gene expression between diploid and synthesized allotriploid and allotetraploid hybrids of Populus. Mol Breed 39:69. https://doi.org/10.1007/s11032-019-0975-6
Huang Z (2015) Studies on suspension cell line establishment and protoplast culture of populous spp. (Section Tacamahaca). Dissertation, Beijing Forestry University
Johnsson H (1945) The triploid progeny of the cross diploid 9 tetraploid Populus tremula. Hereditas 31:411–440. https://doi.org/10.1111/j.1601-5223.1945.tb02761.x
Kang XY (2020) Research progress and prospect of triploid breeding of forest trees. Sci Sin Vitae 50:136–143 (in Chinese with English abstract)
Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18(8):450–458. https://doi.org/10.1016/j.tplants.2013.04.006
Liao T, Cheng SP, Zhu XH, Min Y, Kang XY (2016) Effects of triploid status on growth, photosynthesis, and leaf area in Populus. Trees-Struct Funct 30(4):1137–1147. https://doi.org/10.1007/s00468-016-1352-2
Liu WT, Zheng YF, Song SY, Huo BB, Li DL, Wang J (2018) In vitro induction of allohexaploid and resulting phenotypic variation in Populus. Plant Cell Tiss Organ Cult 134:183–192. https://doi.org/10.1007/s11240-018-1411-z
Lu M, Zhang PD, Kang XY (2013) Induction of 2n female gametes in Populus adenopoda Maxim by high temperature exposure during female gametophyte development. Breed Sci 63(1):96–103. https://doi.org/10.1270/jsbbs.63.96
Mattila RE (1961) On the production of the tetraploid hybrid aspen by colchicine treatment. Hereditas 47(3–4):631–640. https://doi.org/10.1111/j.1601-5223.1961tb01792.x
Mergen F, Lester DT (1971) Colchicine induced polyploidy in Abies. For Sci 7:314–319
Morejohn LC, Bureau TE, Molè-Bajer J, Bajer AS, Fosket DE (1987) Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172:252–264. https://doi.org/10.2307/23379101
Mu HZ, Liu ZJ, Lin L, Li HY, Jiang J, Liu GF (2012) Transcriptomic analysis of phenotypic changes in birch (Betula platyphylla) autotetraploids. Int J Mol Sci 13(10):13012–13029. https://doi.org/10.3390/ijms131013012
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tabacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nibau C, Gibbs DJ, Coates JC (2008) Branching out in new directions: the control of root architecture by lateral root formation. New Phytol 179(3):595–614. https://doi.org/10.1111/j.1469-8137.2008.02472.x
Nilanthi D, Chen XL, Zhao FC, Yang YS, Wu H (2009) Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. J Biomed Biotechnol. https://doi.org/10.1155/2009/343485
Nilsson-Ehle H (1936) Note regarding the gigas from of Populus tremula found in nature. Hereditas 21:372–382
Parsons JL, Martin SL, James T, Golenia G, Boudko EA, Hepworth SR (2019) Polyploidization for the Genetic Improvement of Cannabis sativa. Front Plant Sci 10:476. https://doi.org/10.3389/fpls.2019.00476
Petersen KK, Hagberg P, Kristiansen K (2003) Colchicine and oryzalin mediated chro- mosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tiss Organ Cult 73:137–146. https://doi.org/10.1023/A:1022854303371
Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Annu Rev Plant Biol 63:563–590
Planchais S, Glab N, Inzé D, Bergonioux C (2000) Chemical inhibitors: a tool for plant cell cycle studies. FEBS Lett 476(1–2):78–83. https://doi.org/10.1016/s0014-5793(00)01675-6
Qiu DY, Bai SL, Ma JC, Zhang LS, Shao FJ, Zhang KK, Yang YF, Sun T, Huang JL, Zhou Y, Galbraith DW, Wang ZS, Sun GL (2019) The genome of Populus alba x Populus tremula var glandulosa clone 84K. DNA Res 26(5):423–431. https://doi.org/10.1093/dnares/dsz020
Rêgo MD, Rêgo ER, Bruckner CH, Finger FL, Otoni WC (2011) In vitro induction of autotetraploids from diploid yellow passion fruit mediated by colchicine and oryzalin. Plant Cell Tiss Organ Cult 107:451–459. https://doi.org/10.1007/S11240-011-9995-6
Roy AT, Leggett G, Koutoulis A (2001) In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep 20:489–495. https://doi.org/10.1007/s002990100364
Sakhanokho HF, Rajasekaran K, Kelley RY, Islam-Faridi N (2009) Induced polyploidy in diploid ornamental ginger (Hedychium muluense R.M. Smith) using colchicine and oryzalin. HortScience horts 44(7):1809–1814. https://doi.org/10.21273/HO-RTSCI.44.7.1809
Sari N, Abak K, Pitrat M (1999) Comparison of ploidy level screening methods in watermelon: Citrullus lanatus (Thunb.) Matsum. and Nakai. Sci Hort 82:265–277. https://doi.org/10.1016/S0304-4238(99)00077-1
Särkilahti E, Valanne T (1990) Induced polyploidy in Betula. Silva Fenn 24(2):227–234. https://doi.org/10.14214/sf.a15577
Sattler MC, Carvalho CR, Clarindo WR (2016) The polyploidy and its key role in plant breeding. Planta 243(2):281–296. https://doi.org/10.1007/s00425-015-2450-x
Schranz ME, Osborn TC (2000) Novel flowering time variation in the resynthesized polyploid Brassica napus. J Hered 91(3):242–246. https://doi.org/10.1093/jhered/91.3.242
Shao JZ, Chen CL, Deng XX (2003) In vitro induction of tetraploid in pomegranate (Punica granatum). Plant Cell Tiss Organ Cult 75:241–246. https://doi.org/10.1023/A:1025871810813
Shim D, Kim S, Choi YI, Song WY, Park J, Youk ES, Jeong SC, Martinoia E, Noh EW, Lee Y (2013) Transgenic poplar trees expressing yeast cadmium factor 1 exhibit the characteristics necessary for the phytoremediation of mine tailing soil. Chemo- sphere 90(4):1478–1486. https://doi.org/10.1016/j.chemosphere.2012.09.044
Shu WB, Zhou HJ, Jiang C, Zhao ST, Wang LQ, Li QZ, Yang ZQ, Groover A, Lu MZ (2019) The auxin receptor TIR 1 homolog (PagFBL 1) regulates adventitious rooting through interactions with Aux/ IAA 28 in Populus. Plant Biotechnol J 17(2):338–349. https://doi.org/10.1111/pbi.12980
Stanys V, Weckman A, Staniene G, Duchovskis P (2006) In vitro, induction of polyploidy in Japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult 84(3):263–268
Stebbins GL (1950) Variation and evolution in plants. Columbia University Press, New York
Stebbins GL (1971) Chromosomal evolution in higher plants. Addison-Wesley, London
Talebi SF, Saharkhiz MJ, Kermani MJ, Sharafi Y, Raouf Fard F (2017) Effect of different antimitotic agents on polyploid induction of Anise hyssop (Agastache foeniculum L.). Caryologia 70:184–193. https://doi.org/10.1080/00087114.2017.1318502
Tavan M, Mirjalili MH, Karimzadeh G (2015) In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult 122:573–583. https://doi.org/10.1007/s11240-015-0789-0
Thao NTP, Ureshino K, Miyajima I, Ozaki Y, Okubo H (2003) Induction of tetraploids in ornamental Alocasia through colchicine and oryzalin treatments. Plant Cell Tiss Organ Cult 72(1):19–25. https://doi.org/10.1023/A:1021292928295
Tosca A, Pandolfi R, Citterio S, Fasoli A, Sgorbati S (1995) Determination of flow cytometry of the chromosome doubling capacity of oryzalin and colchicine in gynogenetic haploids of gerbera. Plant Cell Rep 14(7):455–458. https://doi.org/10.1007/BF00234054
Ulrich K, Ewald D (2014) Breeding triploid aspen and poplar clones for biomass production. Silvae Genet 63:47–58. https://doi.org/10.1515/sg-2014-0008
van Tuyl JM, Meijer B, van Diën MP (1992) The use of oryzalin as an alternative for colchicine in in vitro chromosome doubling of Lilium and Nerine. Acta Hortic 325:625–630. https://doi.org/10.17660/ActaHortic.1992.325.88
Wan Y, Petolin JF, Widholm JM (1989) Efficient production of doubled haploid plants through colchicine treatment of antherderived maize callus. Theor Appl Genet 77(6):889–892. https://doi.org/10.1007/BF00268344
Wang SY, Chen QJ, Wang WL, Wang XC, Lu MZ (2005) Salt tolerance conferred by over-expression of OsNHX1 gene in Poplar 84K. Chin Sci Bull 50:225–229. https://doi.org/10.1007/BF02897531
Wang XL, Zhou JX, Yu MD, Li ZG, Jin XY, Li QY (2011) Highly efficient plant regeneration and in vitro, polyploid induction using hypocotyl explants from diploid mulberry (Morus multicaulis, Poir.). In Vitro Cell Dev Plant 47(3):434–440. https://doi.org/10.1007/s11627-010-9328-1
Wang Z, Fan G, Dong Y, Zhai X, Deng M, Zhao Z, Liu W, Cao Y (2017) Implications of polyploidy events on the phenotype, microstructure, and proteome of Paulownia Australis. PLoS ONE 12(3):e0172633. https://doi.org/10.1371/journal.pone.0172633
Weisgerber H (1980) 25 years of forest tree breeding in Hesse. Allgemeine Forstzeitsch- rift 26:665–712
Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH (2009) The frequency of polyploid speciation in vascular plants. Proc Natl Acad Sci USA 106:13875–13879. https://doi.org/10.1073/pnas.0811575106
Wu J, Sang YR, Zhou Q, Zhang PD (2020) Colchicine in vitro tetraploid induction of Populus hopeiensis from leaf blades. Plant Cell Tissue Organ Cult 141:339–349. https://doi.org/10.1007/s11240-020-01790-w
Xie XQ, Agüero CB, Wang YJ, Walker MA (2015) In vitro induction of tetraploids in Vitis × Muscadinia hybrids. Plant Cell Tissue Organ Cult 122(3):675–683. https://doi.org/10.1007/s11240-015-0801-8
Xu CP, Huang Z, Liao T, Li Y, Kang XY (2016) In vitro tetraploid plants regeneration from leaf explants of multiple genotypes in Populus. Plant Cell Tissue Organ Cult 125(1):1–9. https://doi.org/10.1007/s11240-015-0922-0
Xu CP, Zhang Y, Huang Z, Yao PQ, Li Y, Kang XY (2018) Impact of the leaf cut callus development stages of Populus on the tetraploid production rate by colchic- ine treatment. J Plant Growth Regul 37(2):635–644. https://doi.org/10.1007/s00344-017-9763-x
Yoon SK, Park EJ, Choi YI, Bae EK, Kim JH, Park SY, Kang KS, Lee H (2014) Response to drought and salt stress in leaves of poplar (Populus alba × P. glandulosa): expression profiling by oligonucleotide microarray analysis. Plant Physiol Biochem 84:158–168. https://doi.org/10.1016/j.plaphy.2014.09.008
Zeng Q, Liu Z, Du K, Kang XY (2019) Oryzalin-induced chromosome doubling in triploid Populus and its effect on plant morphology and anatomy. Plant Cell Tissue Organ Cult 138(3):571–581. https://doi.org/10.1007/s11240-019-01654-y
Zhang ZH, Dai HY, Xiao M, Liu X (2008) In vitro induction of tetraploids in Phlox subulata L. Euphytica 159:59–65. https://doi.org/10.1007/s10681-007-9457-8
Zhang Y, Wang ZW, Qi SZ, Wang XQ, Zhao J, Zhang JF, Li BL, Zhang YD, Liu XZ, Yuan W (2017) In vitro tetraploid induction from leaf and petiole explants of hybrid sweetgum (Liquidambar styraciflua ×Liquidambar formosana). Forests 8(8):264. https://doi.org/10.3390/f8080264
Zhang XM, Cheng ZH, Zhao K, Yao WJ, Sun XM, Jiang TB, Zhou BR (2019) Functional characterization of poplar NAC13 gene in salt tolerance. Plant Sci 281:1–8. https://doi.org/10.1016/j.plantsci.2019.01.003
Zhao Y, Wang D, Zhang YQ, Niu YJ, Zong XJ, Ma Y, Guo XF, Guo J (2018) Cloning and characterization of a mitogen-activated protein kinase gene 84KMPK14 in hybrid poplar (Populus alba × P. glandulosa cv. “84K”). Am J Plant Sci 9:2567–2579. https://doi.org/10.4236/ajps.2018.913186
Zhu ZT, Lin HB, Kang XY (1995) Studies on allotriploid breeding of Populus tomentosa B301 clones. Sci Silvae Sin 31:499–505 (in Chinese with English abstract)
Zhu ZT, Kang XY, Zhang ZY (1998) Studies on selection of natural triploid of Populus tomentosa. Sci Silvae Sin 34:22–31 (in Chinese with English abstract)
Funding
This work was financially supported by National Natural Science Foundation of China (31530012).
Author information
Authors and Affiliations
Contributions
XY and YY conceived and designed the research. YY conducted the experiments, analyzed data and wrote the manuscript. YC processed figures. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Henryk Flachowsky.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, Y., Jing, Y. & Kang, X. In vitro induction of tetraploid and resulting trait variation in Populus alba × Populus glandulosa clone 84 K. Plant Cell Tiss Organ Cult 146, 285–296 (2021). https://doi.org/10.1007/s11240-021-02068-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02068-5