Abstract
Madder (Rubia tinctorum L.) is a perennial plant that its roots and rhizomes have rich anthraquinone (AQ) derivatives including alizarin and purpurin. This study was carried out to determine the effects of salicylic acid (SA) and L-phenylalanine (L-Phe) applications on the root growth parameters and secondary metabolite accumulation in madder adventitious roots derived from internode parts without needing to be collected from nature. For this aim, two different L-Phe (50 and 100 µM) and SA (20 and 40 µM) were added separately and together to the liquid Murashige and Skoog (MS) medium cultured adventitious roots for 7 days. Then roots were evaluated in terms of fresh root weight, root growth index, dry root weight, and contents of total AQ, alizarin, purpurin, total phenolic contents (TPC), and some important phenolic compounds. According to the results, L-Phe stimulated the root growth of madder while the effects of SA on root growth parameters varied depending upon its concentrations. L-Phe had no significant influence on the total AQ, alizarin, and purpurin. Conversely, SA increased the AQs, and 20 µM SA was the most suitable application providing the greatest total AQ, alizarin, and purpurin. TPC and individual phenolic compounds changed according to the applications. Not only L-Phe but also SA had positive effects on the phenolic accumulation in adventitious roots. It was determined that the combinations of 40 µM SA and 100 µM L-Phe were the most effective applications in terms of phenolic accumulation.
Key message
Madder is a plant whose popularity has increased in food, cosmetics, pharmacy and recently medicine. This research was designed in order to produce high amounts of important secondary metabolites in adventitious root cultures of madder without the need to collect plants from nature. In this study, it has been shown that L-phenylalanine and salicylic acid applied to adventitious roots remarkably increase the production of anthraquinones and phenolics when used in appropriate concentrations and combinations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Madder (Rubia tinctorum L.) is an important dye plant due to rich in anthraquinones (AQs) such as lucidine, lucidine ethyl ether, lucidine glucoside, ruberitric acid, alizarin, pseudopurpurin, mycinin, xanthopurpurin, purpurin, quinacrine, 2-hydroxymethyl anthraquinone, anthraquinone, 1,8-dihydroxyanthroquinone, 2,6-dihydroxyanthroquinone in its roots and rhizomes (Derksen and Vanbeek 2002). Madder had lost its importance with the discovery of inexpensive synthetic dyes, but once the harmful effects of synthetic dyes to human health and the environment were understood, it has regained importance (Angelini et al. 1997). Besides being used as a textile dye, madder has become an important source of food dye due to its resistance both to heat and light (Kinnosuko et al. 1991). Apart from dying properties, the understanding of the pharmaceutical efficacy of AQs isolated from madder led to the usage of them for medicinal purposes. Some of the known medical activities of AQs can be listed as; anticancer (Lajkó et al. 2015), antimutagenic (Kawasaki et al. 1992), expelling kidney stones (Westendorf et al. 1990), antimicrobial and antioxidants (Kalyoncu et al. 2006).
Majority of the plant secondary metabolites are derived from plants collected from nature in order to meet industrial demand. Collecting plants from nature causes the number of plants to decrease dramatically and their generation to be endangered (Gaudeul and Till-Bottraud 2004). Valuable metabolites of madder are found in its roots, so collecting plants together with their roots causes their generation to face the threat of extinction (El Houssine Bouiamrine et al. 2017). Moreover, there are also important differences in the quantity and quality of metabolites of plants collected from nature (Dhingra et al. 2000). To eliminate these disadvantages, in vitro secondary metabolite production seems to be as an important alternative that can be used to obtain valuable metabolites in plants (Mulabagal and Hsin-Sheng Tsay 2004). However, the most important problem of in vitro secondary metabolite production is that it generally fails to meet commercial demands due to low efficiency (Karuppusamy 2009). The usage of elicitors acting as signal molecules is one of the most important approaches to increase metabolite efficiency and meet the demand (Han et al. 2001). Salicylic acid (SA) is a signalling molecule that plays a role in regulating stress-related gene expression in plant cells and stimulating secondary metabolite biosynthesis in stress conditions caused by abiotic or biotic factors (Raskin 1992). There are already published studies showing that SA applications increase the accumulation of in vitro secondary metabolites in different plants (Sudha and Ravishankar 2003; Mathur and Yadav 2011; Mahalakshmi et al. 2013).
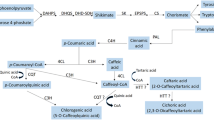
Another important strategy is the addition of precursors into nutrient media to increase the secondary metabolite synthesis. L-phenylalanine (2-amino-3-phenylpropanoic acid, C9H11N02) (L-Phe), known as a hydrophobic amino acid, is a precursor in the plant phenylpropanoid biosynthesis pathway. And L-Phe is known to increase secondary metabolite production (especially phenolics) by triggering phenylpropanoid and flavonoid metabolic pathways in plants (Koca and Karaman 2015). There are several reports of the increased in vitro secondary metabolite levels in plants after L-Phe applications (Al-Gendy et al. 2015; Koca and Karaman 2015).
To the best of our knowledge, there is no study carried out to determine the influences of L-Phe and SA applications on growth and secondary metabolite production in adventitious roots of madder. Therefore, this study aimed to determine the effect of L-Phe and SA applications at different concentrations on both growth parameters and accumulation of alizarin, purpurin and total AQs in madder adventitious root cultures. In addition, it was also determined total phenolic and some phenolics including gallic acid, catechin, chlorogenic acid, caffeic acid, epicatechin, vanillin, p-coumaric acid, ferulic acid, sinapic acid, o-coumaric acid, rutin, cinnamic acid, quercetin, and luteolin, which are very important compounds in areas of cosmetics, food, and pharmaceuticals due to their strong antioxidant impacts.
Material and method
Plant materials
In this study, the internode segments of the shoots taken from 3 years-old madder were used as plant material that collected from the Medical Plants Growing Area of the Field Crops Department of Isparta University of Applied Sciences. The shoots collected by cutting with a garden scissors were brought to the laboratory and used to obtain an adventitious root.
Adventitious root formation
To obtain adventitious roots, the shoots were washed with running tap water and sterilized for 10 min with 20% (v/v) sodium hypochlorite solution (Sigma-Aldrich, Germany) containing 0.1% (v/v) tween 20 (Sigma-Aldrich, Germany). Then shoots were rinsed three times with sterile distilled water each by shaking for 5 min. The internode segments cut approximately 1 cm long were cultured in MS (Duchefa Biochemie, Netherland) (Murashige and Skoog 1962) medium containing 2.5 mg/l 3-indole acetic acid (IAA, Sigma-Aldrich, Germany), 0.1 mg/l kinetin (Sigma-Aldrich, Germany), 20 g/l sucrose (Sigma-Aldrich, Germany), and 2 g/l gelrite agar (Gelzan™ CM, Sigma-Aldrich, Germany), in dark for 28 days (Kubota et al. 1995). The induced adventitious roots (Fig. 1a) were transferred to the liquid MS medium supplemented with 2.5 mg/l IAA, 0.1 mg/l kinetin, and 20 g/l sucrose, in dark for 28 days. Then 250 mg adventitious roots were subcultured twice at 28-days intervals using 30 ml plant growth regulator-free liquid MS medium containing only 20 g/l sucrose (Fig. 2b). the roots were grown at 25 ± 1 °C in the dark, and liquid cultures were agitating on an orbital shaker at 100 rpm.
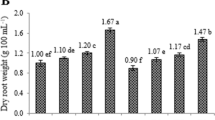
Effects of SA and L-Phe applications on fresh root weight (a), root growth index (b) and dry root weight (c) in adventitious roots of madder (p < 0.05). 1: Control, 2: 20 µM SA, 3: 40 µM SA, 4: 50 µM L-Phe, 5: 100 µM L-Phe, 6: 20 µM SA + 50 µM L-Phe, 7: 20 µM SA + 100 µM L-Phe, 8: 40 µM SA + 50 µM L-Phe, 9: 40 µM SA + 100 µM L-Phe. * Means with the same letters are not statistically significant (p ≤ 0.05)
SA and L-Phe applications
Adventitious roots grown in liquid MS medium were used in the SA (Sigma-Aldrich, Germany) and L-Phe (Sigma-Aldrich, Germany) applications. For this aim, 250 mg adventitious roots were cultured in 100 ml Erlenmeyer flasks containing 30 ml liquid MS medium including 20 g/l sucrose for 7 days. SA and L-Phe were applied to adventitious roots in 9 different combinations (20 µM SA, 40 µM SA, 50 µM L-Phe, 100 µM L-Phe, 20 µM SA + 50 µM L-Phe, 20 µM SA + 100 µM L-Phe, 40 µM SA + 50 µM L-Phe, and 40 µM SA + 100 µM L-Phe]). After filter sterilization, SA and L-Phe were added to 7-days-old roots, and cultured on orbital shakers (100 rpm) at 25 ± 1 °C under dark conditions for another 7 days. Later on, roots were harvested and used in the analyses. Experiments were set up in a completely randomized design and performed in triplicate and four flasks were used for each replication (9 applications x 3 replications x 4 flasks).
Root growth parameters
After the harvest, the adventitious roots were washed 3 times with sterile distilled water. The fresh weights of the roots, which were then removed from the surface water with filter paper, were weighed with an analytical balance and expressed as g/l. Growth index was calculated according to the following formula;
Root Growth Index = (fresh weight of harvested roots (mg) – the fresh weight of inoculated roots (mg)) / fresh weight of inoculated roots (mg).
Then fresh roots were dried at 50 Co for 72 h and weighed. Dry root weight was expressed as g/l.
Extractions of AQs
The extractions of AQs from adventitious roots were performed according to the method of Schulte et al. (1984). Accordingly, the roots harvested from each flask were individually dried at 50 °C for 72 h. Roots were completely powdered with a mortar. Then, 0.1 mg was weighed homogeneously from the powdered roots of each flask, and boiled in a mixture of 10 ml of 80% ethanol (99,5%, Tekkim, Turkey)-water for 30 min. After reaching room temperature, it was filtered at 0.45 µm filter (Millipore Filter Co., Bedford, Mass.) and stored at + 4 °C for determination of total AQ, alizarin, and purpurin.
Determinations of total AQs
The quantitate analysis of total AQs were determined using a PG Instruments spectrophotometer (T70, PG Instruments, UK). The absorbances of the extracts were measured directly at 434 nm and the total amounts of AQs in roots were calculated by using the molar absorption coefficient of alizarin (ε434 = 5.5) and the results were expressed as mg/g dry weight (DW) (Shulte et al. 1984).
Determinations of Alizarin and Purpurin by HPLC
In this study, the method of Derksen et al. (1998) was used to determine the contents of alizarin and purpurin by High Performance Liquid Chromatography (HPLC). The HPLC system (Shimadzu Corporation, Kyoto, Japan) was used during the analyses consisted of Shimadzu SPD-M20 A DAD detector, Shimadzu LC-20 AD pump, Shimadzu DGU-20A degasser, Shimadzu CTO-10 AS VP column oven, C18 column (Inertsil ODS-3, 250 mm X 4.6 mm i.d., 5 µm, GL Sciences Inc., Tokyo, Japan) units. The method was run according to the gradient program at a flow rate of 1 ml/min at 30 C°.
Chromatography was performed by using two solvents: Ultra-pure water (A) and acetonitrile (Sigma Aldrich, Germany) (B) in a gradient program. The gradient program was run as follows; 0 to 6 min, 27% B; 6 to 35 min, 27 to 70% B; and 35 to 45 min, 70 to 27% B. The solvents, extracts, and analytical standards were filtered using 0.45 µm pore size membrane filter. The contents of alizarin and purpurin in the adventitious root extracts were expressed as mg/g, by using external calibration curves of analytical standards. The analytical standards of alizarin and purpurin were purchased from Sigma-Aldrich (for HPLC, Germany).
Extractions of phenolics
For the extractions of phenolics, the powdered roots were weighed to 50 mg, and then 25 ml of a 70% ethanol-water solvent containing 0.2% hydrochloric acid (37%, Sigma Aldrich, Germany) was added thereto. The mixture, which was kept in the ultrasonic water bath for 15 min, was centrifuged at 4,000 rpm for 15 min and then the liquid phase was stored. The process was repeated once more, after adding the same mixture thereto the pellet and vortexing. After filtering with 0.45 µm filters, it was stored at -20 °C for TPC and fourteen individual phenolic compounds analysis.
Determinations of total phenolic contents
The total phenolic contents (TPCs) were determined spectrophotometrically according to the Folin-Ciocalteu colorimetric method (Singleton and Rossi 1965), the absorbances of the samples were measured at 765 nm. TPCs of the roots were calculated by using a calibration curve prepared with standard gallic acid solution and results were expressed as mg gallic acid equivalents (mg GAE/g DW).
Determinations of phenolic compounds by HPLC
The analyses of fourteen individual phenolic contents including gallic acid, catechin, chlorogenic acid, caffeic acid, epicatechin, vanillin, p-coumaric acid, ferulic acid, sinapic acid, o-coumaric acid, rutin, cinnamic acid, quercetin, and luteolin were performed by the HPLC system. All analytical standards were purchased from Sigma Aldrich (Germany) as analytical standards for HPLC. The phenolics analyses were carried out on a C18 (250 × 4.6 mm i.d. 5 µm) column. Acetic acid (2%) (Sigma Aldrich, Germany) ultra-pure water (A) and methanol (Sigma Aldrich, Germany) (B) were used as mobile phases. The method was run according to the gradient program at a flow rate of 0.8 ml/min at 40 °C. The gradient program was as follows: 0 to 12 min, 0 to 12% B; 12 to 13 min, %12 B; 13 to 33 min, 12 to 28% B; 33 to 48 min, 28 to 30% B; 48 to 53 min, 30 to 38% B; 53 to 70 min, 38 to 40% B; 70 to 90 min, 40 to 50% B; 90 to 105 min, 50 to 60% B; 105 to 117 min, 60 to 100% B; 117 to 120 min, 100 to 0% B. The amounts of phenolics in the adventitious root extracts were calculated by using external calibration curves obtained for each standard and results were expressed as mg/g DW.
Statistical Analyses
The presented data are the average of three measurements for each flask (9 applications x 3 replications x 4 flasks each replication x 3 measurements). Statistics were performed by using analysis of variance (ANOVA) with SPSS 16.0 for Windows Software Package (IBM SPSS, Armonk, NY, USA), and the means were separated by Duncan’s multiple range tests.
Results
Effects of SA and L-Phe on Root Growth
Applications of SA and L-Phe to the adventitious root cultures of madder had statistically significant effects on root growth parameters including fresh root weight, root growth index, and dry root weight (Fig. 2). When root growth parameters were examined, it was determined that L-Phe applications were more effective than SA and SA + L-Phe combinations. The greatest fresh root weight, root growth index, and dry root weight values were obtained from the roots applied with 50 and 100 µM L-Phe as 16.75–16.17 mg/l, 1.01–0.94, and 1.56–1.54 g/l, respectively. When SA concentration increased from 20 µM to 40 µM, root growth parameters declined significantly. Compared to the control, 20 µM SA applications increased the root growth parameters while 40 µM SA applications resulted in a significant decrease in them. In the combinations of SA and L-Phe, it was shown that the root growth-promoting effect of L-Phe was suppressed by SA. In particular, the applications of the highest concentration of SA (40 µM) with L-Phe did not cause any increase in root growth parameters compared to controls.
Effects of SA and L-Phe on AQs Productions
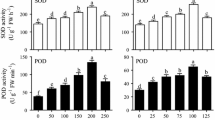
The effects of SA and L-Phe on the total AQs productions of adventitious root cultures of madder were found statistically significant (p < 0.05). The greatest amounts of total AQs were obtained in applications with 20 µM SA, 20 µM SA + 50 µM L-Phe, and 20 µM SA + 100 µM L-Phe (31.47 mg/g, 30.13 mg/g, and 29.39 mg/g, respectively) (Table 1). It may be understood from these results that the greatest total AQs amounts were obtained from the applications where 20 µM SA was irrespective of the presence of L-Phe. But total AQs amounts declined when the SA concentration was increased from 20 µM SA to 40 µM SA. As a result of the HPLC analysis, the amounts of alizarin and purpurin varied according to the SA and L-Phe applications (Table 1). It was determined that SA applications were more effective than L-Phe applications. The greatest amount of alizarin (4.42 mg/g) was obtained from the 20 µM SA application. With this application, approximately two times more alizarin was produced than the control (2.18 mg/g). When the concentration of SA increased to 40 µM, alizarin contents decreased in the roots. On the other hand, the effect of L-Phe applications on alizarine did not make a significant difference compared to the control. In the combinations of SA and L-Phe, it was observed that the L-Phe suppressed the increasing effect of SA on alizarin accumulation. The purpurin accumulations showed similar trends with total AQs. The greatest purpurin amount was obtained from combinations in which 20 µM SA was applied (ranging from 0.73 mg/g to 0.70 mg/g). There was about 1.3 times more purpurin than the control group (0.47 mg/g). 40 µM SA also increased the purpurin compared to the control, but this increase was at lower levels than 20 µM SA applications. On the other hand, L-Phe did not affect purpurin accumulation. When L-Phe applied with SA, it declined the stimulating effect of SA.
Effects of SA and L-Phe on phenolic compounds
In this study, the effects of SA and L-Phe on the accumulation of phenolic compounds were also investigated. All SA and L-Phe applications statistically increased TPC according to the control (p < 0.05). The greatest TPC was obtained from 40 µM SA + 100 µM L-Phe application (35.20 mg/g) followed by 40 µM SA + 50 µM L-Phe application (34.03 mg/g) (Table 2). Although SA and L-Phe increased TPC compared to the control when applied alone, the greatest TPC were obtained in the combinations where the two were applied together. Another remarkable result was the determination that SA applications, especially at the highest concentration, were more effective for increasing TPC than L-Phe applications.
As a result of the analysis of gallic acid, catechin, chlorogenic acid, caffeic acid, epicatechin, vanillin, p-coumaric acid, ferulic acid, sinapic acid, o-coumaric acid, rutin, cinnamic acid, quercetin, and luteolin by HPLC, there were significant differences among the applications (Table 2). The minimum gallic acid, which belongs to the group of hydroxybenzoic acids, was determined in the control roots. In this study, it was understood that there was no significant difference between SA and L-Phe applications when they were applied alone. But the greatest gallic acid amounts were obtained from the roots where 40 µM SA was applied with 50 and 100 µM L-Phe (164.01 and 180.55 µg/g).
Hydroxycinnamic acids including chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, o-coumaric acid, and cinnamic acid were also investigated in this study. The minimum amounts of examined hydroxycinnamic acids were found in the control group. According to the results, it was determined that the amounts of these compounds were significantly changed with SA and L-Phe applications. In particular, the greatest amounts of hydroxycinnamic acid derivatives were obtained from the roots in which 40 µM SA was applied with 50 and 100 µM L-Phe (Table 2). When SA and L-Phe have applied alone, their effectiveness changed depending on the hydroxycinnamic acid derivatives. L-Phe, especially at 100 µM concentration, encouraged the accumulations of chlorogenic acid, caffeic acid, and sinapic acid compared to SA applications while the amount of p-coumaric acid was the greatest with SA applications. On the other hand, there was no significant difference between SA and L-Phe applications in the accumulation of ferulic acid, o-coumaric acid, and cinnamic acid.
The amounts of flavonols including rutin, quercetin, and luteolin were determined and presented in Table 2. Flavonols were also significantly affected by the SA and L-Phe applications. All of the SA and L-Phe applications, except the 40 µM SA + 100 µM L-Phe combination, were included in the same statistical group as the control and did not cause a significant difference for rutin accumulation. On the other hand, it was found that the maximum rutin was obtained from 40 µM SA + 100 µM L-Phe application as 186.16 µg/g. The greatest quercetin and luteolin values were found in the applications where 40 µM SA was used with 50 and 100 µM L-Phe. The minimum luteolin content was obtained from the control group as 37.19 µg/g. In terms of quercetin, 20 µM SA applications and the single applications of L-Phe were found to be included in the same group as the control. It can be understood from these results that when SA and L-Phe were applied together increased the amount of flavonols more than being applied separately.
In this study, the amounts of catechin and epicatechin, as flavanols, were also investigated. Catechin and epicatechin contents in the adventitious root cultures were changed significantly depending on the SA and L-Phe applications (Table 2). The greatest catechin contents were obtained from the combinations of SA and L-Phe. When SA and L-Phe were applied either individually or together, their highest concentrations were found to be more effective in increasing the amounts of catechin. The results of epicatechin accumulation pointed out that 40 µM SA + 100 µM L-Phe combinations were the most effective application in increasing the amount of epicatechin.
Vanillin, another important phenolic substance, showed a similar pattern to the other phenolics. The greatest vanillin accumulations were detected from the roots applied with 40 µM SA + 100 µM L-Phe and 40 µM SA + 50 µM L-Phe as 75.60 µg/g and 68.69 µg/g, respectively (Table 2). On the other hand, there was no statistical difference between control and other applications.
Discussion
In this study, root growth and secondary metabolite accumulation in adventitious root cultures of madder were significantly changed depending on the SA and L-Phe applications. When root growth parameters were evaluated, it was determined that L-Phe applications were more effective than SA and SA + L-Phe combinations and also SA at 40 µM decreased root growth significantly. It was previously demonstrated that the effects of L-Phe on growth and development varied widely. While L-Phe at concentrations of 500, 1000 and 5000 µM increased root growth index compared to the controls in Echinacea purpurea root cultures (Mobin et al. 2015), the increased L-Phe concentrations on strawberry and Larrea divaricata largely inhibited cell growth (Edahiro et al. 2005; Palacio et al. 2011; Demirci et al. 2020) reported that L-Phe had no significant effects on root development. These differences in the effects of L-Phe on growth and development may be due to the L-Phe concentrations, genotypes, and culture type (Giri and Narasu 2000; Jacob and Malpathak 2005; Mobin et al. 2015). However, there are some different studies in which SA affects cell callus and root growth differently in in vitro conditions. Bulgakov et al. (2002) found that the application of 100 µM SA in the Rubia cordifolia callus cultures inhibited growth, but low doses (1 µM and 10 µM) induced cell growth compared to the control. Similarly, it is reported that SA applications have significant reduce effects on root growth in Bacopa monnieri (Largia et al. 2015) and cell growth in Salvia miltiorrhiza (Dong et al. 2010). On the other hand, in the root cultures of Stemona plant at the end of the first week, 0.1 and 0.5 mM SA applications were found to increase the root growth and to decrease the 0.3 and 1.0 mM SA applications. In the second week, all of the SA concentrations caused to decline in root growth (Chaichana and Dheeranupattana 2012). These results show that the effect of SA on growth can vary significantly depending on the culture type, SA concentration, culture duration, and genotype.
The effects of L-Phe and SA applications on AQs accumulation in madder root cultures were also investigated in the present study and it was determined that L-Phe had no significant effect on AQs accumulation compared to controls while SA applications increase the amount of AQs. According to our knowledge, there was no study about the effects of L-Phe on AQs production in madder. But similar to the presented study, it was reported that SA applied to Rubia cordifolia calluses significantly increased total AQs, purpurin, and munjistin productions (Bulgakov et al. 2002; El-Mawla 2012) studied the effects of 5 different SA concentrations from 10 to 50 µM in madder cell suspension cultures and found that 20 µM was the most effective SA concentration on increasing the AQs accumulation. In another study conducted on the effects of SA on cell suspension cultures of madder, 13 mg ml− 1 SA significantly increased the amount of purpurin and lucidine, 100 mg ml− 1 SA significantly enhanced alizarin and total AQs amount (Orban et al. 2008). Increasing the amount of AQs with SA applications is because SA increases the production of secondary metabolites as a signal molecule. Indeed, plant receptors activate the effectors (such as; ion channels, G proteins, protein kinases, NADPH oxidases) and second messenger molecules (hydrogen peroxide, jasmonic acid, SA, internal calcium release, etc.) after detecting the elicitor. As a result, an increase in the expression of related genes increases the accumulation of secondary metabolites (Zhao et al. 2004). When plants are exposed to abiotic stress, they produce SA quickly and thus the produced SA activates the defense mechanisms of the plants (Senaratna et al. 2000; Krantev et al. 2008). The AQs of the madder plant, which is synthesized by the chorismate/succinyl benzoic acid biosynthesis, are significantly affected by the environmental and internal factors acting on this biosynthesis pathway (Orban et al. 2008). According to Govindaraju and Arulselvi (2018), the possible cause of the increased level of secondary metabolites in tissue culture may be due to the use of optimum conditions, nutrient media, and elicitors during the culture period.
Alizarin and purpurin contents in the roots of madder collected from nature vary depending on the age of the plant, climate and cultural conditions. It was found in the previously reported studies that in the madder roots collected from nature, the amount of alizarin and purpurin ranged from 0.4 to 12.3 mg g−1and from 2.6 to 8.1 mg g−1, respectively (Bozan et al. 1999; Derksen et al. 2004; Bányai et al. 2006; Santis and Moresi 2007; Cuoco et al. 2009). Our study showed that only after the 2-week culture period, 4.42 mg g−1 alizarin and 0.73 mg g−1 purpurin can be obtained from the in vitro root cultures of madder by the suitable elicitor application. From all these results, it is shown that in vitro root culture, which allows standard quantity and quality production for a whole year, without destroying the plants in nature and without seasonal restrictions, constitutes an important potential on the production of alizarin, purpurin and also phenolic compounds in madder.
Effects of SA and L-Phe were evaluated not only in terms of root growth parameters and AQ contents but also in phenolic contents in adventitious roots. As a result of the analysis of TPC and fourteen different phenolic substances, there were found significant differences among the applications. The greatest values were obtained from the roots where 40 µM SA was applied with 50 and 100 µM L-Phe. Elicitor applications are effective methods for stimulating the production of secondary metabolites in plant cell and organ cultures. SA, is a signal molecule and plant hormone, plays a major role in tolerance against biotic and abiotic stresses (Khan et al. 2015). This signal molecule promotes the synthesis of defensive compounds such as pathogens-related proteins, alkaloids, or phenolics by affecting certain enzymes that catalyse the biosynthetic reactions with some transmission system signals (Hahlbrock and Scheel 1989; Raskin 1992; Ding et al. 2002; Rivas-San Vicente and Plasencia 2011). Therefore, SA applications used as elicitors increase the accumulation of secondary metabolites, which is an important part of the plant’s defense system, by stimulating related genes.
Although there was no study on the effects of SA on phenolic compounds in madder, it was determined that SA applications in chili fruit, apple, and Achillea gypsicola increased the phenolic compounds (Sánchez-Chávez et al. 2011; Vázquez-Díaz et al. 2016; Açıkgöz et al. 2019; Dong et al. 2010) reported that SA increased the amount of phenolic compounds in Salvia miltiorrhiza, and also made more active some antioxidant enzymes including phenylalanine ammonium lyase (PAL), tyrosine aminotransferase, superoxide dismutase, catalase, and peroxidase. Depending on the concentrations, SA affects the accumulation of phenolic compound synthesis by changing the PAL activity (Dihazi et al. 2003). It was reported that SA stimulates the accumulation of hydrogen peroxide by inhibiting the catalase enzyme (Chen et al. 1993), and increased concentration of hydrogen peroxide stimulates the expression of the PAL gene, resulting in phenolic synthesis (Desikan et al. 1998). Similarly, Hao et al. (2014) also stated that SA stimulates the formation of hydrogen peroxide, which promotes PAL activity responsible for phenolic synthesis. Mora-Herrera et al. (2011) also reported that SA activates secondary metabolism and enhances the production of phytochemical substances. Similarly, it was found that SA applications significantly increased the amount of phenolic compounds in different plant species (Dong et al. 2010; Ali et al. 2007; Thiruvengadam et al. 2016) demonstrated that SA significantly increased the amounts of gallic acid, caffeic acid, p-coumaric acid, ferulic acid, chlorogenic acid, o-coumaric acid, quercetin, routine, and vanillin by applied to the cell suspension cultures of the Polygonum multiflorum. Similar to these results, SA applications increased the accumulation of phenolic compounds in our study.
Most of the natural phenolic compounds in plants are derived from trans-cinnamic acid formed by the deamination of L-Phe by PAL (Boudet 2007). In this study, which investigated the effects of L-Phe applications on phenolic compounds, it was found that although L-Phe did not have an important effect on AQs synthesis; it had a stimulating effect on the accumulation of phenolic compounds. There is no study on the effects of L-Phe on AQs and phenolic compounds in the species belonging to madder and other Rubiaceae families. However, it is determined that the synthesis of these compounds can be increased by stimulating enzymatic pathways in plants, which is a precursor in the synthesis of phenolic compounds in different plants (Koca and Karaman 2015; Al-Gendy et al. 2015).
Conclusions
The results of this study showed that both root growth parameters and the production of secondary metabolites changed significantly depending on the SA and L-Phe applications in the adventitious root of madder. It was concluded that L-Phe promoted root growth directly, on the other hand, the effect of SA on root growth changed depending on its concentration. While L-Phe did not have a significant effect on total AQs, alizarin, and purpurin accumulation, SA significantly increased the accumulation of these metabolites. Especially, the greatest AQ values were obtained from 20 µM SA applications. Both L-Phe and SA were found effective in increasing phenolic compounds; it was determined that the combinations of 40 µM SA and 100 µM L-Phe were the most effective applications to increase the phenolic compounds. Accordingly, it is determined that AQs with the applications of 20 µM SA and phenolic compounds with the combinations of 40 µM SA + 100 µM L-Phe can be successfully produced in adventitious roots of madder in in vitro conditions without a need being collected from nature. To the best of our knowledge, this study reports the use of SA and L-Phe treatments to enhance AQs and phenolics in madder adventitious root cultures for the first time. And results of our study showed that SA and L-Phe applications can be used for enhancing AQs and phenolic compounds in adventitious roots of madder depending on single or dual use of the SA and L-Phe and their concentrations. Additionally, it is shown once again that the important secondary metabolites can be produced successfully by the in vitro techniques.
Abbreviations
- AQ:
-
Anthraquinone
- SA:
-
Salicylic acid
- L-Phe:
-
L-Phenylalanine
- IAA:
-
3-Indole acetic acid
- DW:
-
Dry weight
- TPC:
-
Total phenolic content
References
Açıkgöz MA, Kara ŞM, Aygün A, Özcan MM, Ay EB (2019) Effects of methyl jasmonate and salicylic acid on the production of camphor and phenolic compounds in cell suspension culture of endemic Turkish yarrow (Achillea gypsicola) species. TJAF 43:351–359. https://doi.org/10.3906/tar-1809-54
Al-Gendy AA, Bakr RO, El-Gindi OD (2015) Production of flavonoids and phenolic compounds by elicitation of Iphiona mucronata (Forssk.) Asch. & Schweinf (Asteraceae) callus and suspension cultures. Int J Pharmacogn Phytochem 30:1293–1300
Ali MB, Hahn EJ, Paek KY (2007) Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12:607–621. https://doi.org/10.3390/12030607
Angelini LG, Pistelli L, Belloni P, Bertoli A, Panconesi S (1997) Rubia tinctorum a source of natural dyes: agronomic evaluation, quantitative analysis of alizarin and industrial assays. Ind Crops Prod 6:303–311. https://doi.org/10.1016/S0926-6690(97)00021-6
Bányai P, Kuzovkina IN, Kursinszki L, Szőke É (2006) HPLC analysis of alizarin and purpurin produced by Rubia tinctorum L. hairy root cultures. Chromatographia 63:111–114. https://doi.org/10.1365/s10337-006-0792-z
Boudet AM (2007) Evolution and current status of research in phenolic compounds. Phytochem 68:2722–2735. https://doi.org/10.1016/j.phytochem.2007.06.012
Bozan B, Koşar M, Akyürek C, Ertuğrul K, Başer KHC (1999) Alizarin and purpurin contents of Rubia tinctorum L. roots collected from various regions of Turkey. Acta Pharm Sci 41:187–190
Bulgakov VP, Tchernoded GK, Mischenko NP, Khodakovskaya MV, Glazunov VP, Radchenko SV, Zhuravlev YN (2002) Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on AQ production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotechnol 97:213–221. https://doi.org/10.1016/s0168-1656(02)00067-6
Chaichana N, Dheeranupattana S (2012) Effects of methyl jasmonate and salicylic acid on alkaloid production from in vitro culture of Stemona sp. Int J Biosci Biochem Bioinforma 2:146–150. https://doi.org/10.7763/IJBBB.2012.V2.89
Chen Z, Silva S, Klessig D (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262:1883–1886. https://doi.org/10.1126/science.8266079
Cuoco G, Math C, Archier P, Chemat F, Vieillescazes C (2009) A multivariate study of the performance of an ultrasound-assisted madder dyes extraction and characterization by liquid chromatography-photodiode array detection. Ultrason Sonochem 16:75–82. https://doi.org/10.1016/j.ultsonch.2008.05.014
Demirci T, Çelikkol Akçay U, Göktürk Baydar N (2020) Effects of 24-epibrassinolide and L-phenylalanine on growth and caffeic acid derivatives production in hairy root culture of Echinacea purpurea L. MoenchActa Physiol Plant. https://doi.org/10.1007/s11738-020-03055-7
De Santis D, Moresi M (2007) Production of alizarin extracts from Rubia tinctorum and assessment of their dyeing properties. Ind Crops Prod 26:151–162. https://doi.org/10.1016/j.indcrop.2007.02.002
Derksen GC, Lelyveld GP, van Beek TA, Capelle A, de Groot Æ (2004) Two validated HPLC methods for the quantification of alizarin and other anthraquinones in Rubia tinctorum cultivars. Phytochem Anal 15:397–406. https://doi.org/10.1002/pca.800
Derksen GC, Van Beek TA (2002) Rubia tinctorum L. Stud Nat Prod Chem 26:629–684. https://doi.org/10.1016/S1572-5995(02)80016-3
Derksen GC, van Beek TA, de Groot Æ, Capelle A (1998) High-performance liquid chromatographic method for the analysis of anthraquinone glycosides and aglycones in madder root (Rubia tinctorum L.). J Chromatogr A 816:277–281. https://doi.org/10.1016/S0021-9673(98)00492-0
Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defense gene expression in Arabidopsis suspension cultures. Biochem J 330:15–120. https://doi.org/10.1042/bj3300115
Dhingra V, Rao KV, Narasu ML (2000) Current status of artemisinin and its derivatives as antimalarial drugs. Life Sci 66:279–300. https://doi.org/10.1016/s0024-3205(99)00356-2
Dihazi A, Jaiti F, Zouine J, El Hassni M, El Hadrami I (2003) Effect of salicylic acid on phenolic compounds related to date palm resistance to Fusarium oxysporum f. sp. albedinis. Phytopath Medit 42:9–16. https://doi.org/10.14601/Phytopathol_Mediterr-1686
Ding CK, Wang CY, Gross KC (2002) Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta 21:895–901. https://doi.org/10.1007/s00425-001-0698-9
Dong J, Wan G, Liang Z (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and anti-oxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148:99–104. https://doi.org/10.1016/j.jbiotec.2010.05.009
Edahiro JI, Nakamura M, Seki M, Furusaki S (2005) Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of l-phenylalanine into the medium. J Biosci Bioeng 99:43–47. https://doi.org/10.1263/jbb.99.43
El Houssine Bouiamrine LB, Ibijbijen J, Nassiri L (2017) Fresh medicinal plants in middle atlas of Morocco: trade and threats to the sustainable harvesting. J Med Plants 5:123–128
El-Mawla AMAA (2012) Influence of certain abiotic elicitors on production of anthraquinones in cell cultures of Rubia tinctorum. Spatula DD 2:89–94. https://doi.org/10.5455/SPATULA.20120528021745
Giri A, Narasu ML (2000) Transgenic hairy roots. recent trends and applications. Biotechnol Adv 18:1–22. https://doi.org/10.1016/s0734-9750(99)00016-6
Govindaraju S, Arulselvi PI (2018) Effect of cytokinin combined elicitors (L-phenylalanine, salicylic acid and chitosan) on in vitro propagation, secondary metabolites and molecular characterization of medicinal herb–Coleus aromaticus Benth (L). J Saudi Soc Agric Sci 17:435–444. https://doi.org/10.1016/j.jssas.2016.11.001
Gaudeul M, Till-Bottraud I (2004) Reproductive ecology of the endangered Alpine species Eryngium alpinum L. (Apiaceae): phenology, gene dispersal and reproductive success. Ann Bot 93:711–721. https://doi.org/10.1093/aob/mch098
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Ann Rev Plant Biol 40:347–369. https://doi.org/10.1146/annurev.pp.40.060189.002023
Han YS, Van der Heijden R, Verpoorte R (2001) Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant Cell Tiss Org 67:201–220. https://doi.org/10.1016/s0031-9422(01)00296-5
Hao W, Guo H, Zhang J, Hu G, Yao Y, Dong J (2014) Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. The Science World J DOI. https://doi.org/10.1155/2014/843764
Jacob A, Malpathak N (2005) Manipulation of MS and B5 components for enhancement of growth and salsodine production in hairy root cultures of Solanum khasianum Clarke. Plant Cell Tiss Org 80:247–257. https://doi.org/10.1007/s11240-004-0740-2
Kalyoncu F, Cetin B, Saglam H (2006) Antimicrobial activity of common madder (Rubia tinctorum L.). Phytother Res 20:490–492. https://doi.org/10.1002/ptr.1884
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3:1222–1239. https://doi.org/10.5897/JMPR.9000026
Kawasaki Y, Goda Y, Yoshihira K (1992) The mutagenic constituents of Rubia tinctorum. Chem Pharm Bull 40:1504–1509. https://doi.org/10.1248/cpb.40.1504
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00462
Kinnosuko O, Takahito I, Keiko K (1991) Madders Dye in Plant Cell Culture in Japan (Atsuchi K (ed)), pp 138–142. CMC, Japan
Koca N, Karaman Ş (2015) The effects of plant growth regulators and l-phenylalanine on phenolic compounds of sweet basil. Food Chem 166:515–521. https://doi.org/10.1016/j.foodchem.2014.06.065
Krantev A, Yordanova R, Janda T, Szala G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931. https://doi.org/10.1016/j.jplph.2006.11.014
Kubota H, Sato K, Yamada T, Maitani T (1995) Phytochelatins (class lll metallothioneins) and their desglycyl peptides induced cadmium in normal root cultures of Rubia tinctorum. Plant Sci 110:157–166. https://doi.org/10.1016/0168-9452(95)04020-U
Lajkó E, Bányai P, Zámbó Z, Kursinszki L, Szőke E, Kőhidai L (2015) Targeted tumor therapy by Rubia tinctorum L.: Analytical characterization of hydroxyanthraquinones and investigation of their selective cytotoxic, adhesion and migration modulator effects on melanoma cell lines (A2058 and HT168-M1). Cancer Cell Int. https://doi.org/10.1186/s12935-015-0271-4
Largia MJV, Pothiraj G, Shilpha J, Ramesh M (2015) Methyl jasmonate and salicylic acid synergism enhances bacoside a content in shoot cultures of Bacopa monnieri L. Plant Cell Tiss Org 122:9–20. https://doi.org/10.1007/s11240-015-0745-z
Mahalakshmi R, Eganathan P, Parida AK (2013) Salicylic acid elicitation on production of secondary metabolite by cell cultures of Jatropha curcas L. J Pharm Pharmaceut Sci 5:655–659
Mathur L, Yadav RK (2011) Effect of salicylic acid on trigonelline production in Trigonella foenum-graecum L. cell suspension culture. Inter Referred Res J 1:137–138
Mobin M, Wu CH, Tewari RK, Paek KY (2015) Studies on the glyphosate induced amino acid starvation and addition of precursors on caffeic acid accumulation and profiles in adventitious roots of Echinacea purpurea (L.) Moench. Plant Cell Tiss Org 120:291–301. https://doi.org/10.1007/s11240-014-0606-1
Mora-Herrera ME, Peralta-Velázquez J, López-Delgado HA, García-Velasco R, González-Díaz JG (2011) Efecto del ácido ascórbico sobre crecimiento, pigmentos fotosintéticos y actividad peroxidasa en plantas de crisantemo. Rev Chapingo Ser Hortic 17:73–81
Mulabagal V, Tsay HS (2004) Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:472–497
Orban N, Boldizar I, Szücs Z, Bela D (2008) Influence of different elicitors on the synthesis of anthraquinone derivatives in Rubia tinctorum L. cell suspension cultures. Dyes Pigm 77:249–257. https://doi.org/10.1016/j.dyepig.2007.03.015
Palacio L, Cantero JJ, Cusidó R, Goleniowski M (2011) Phenolic compound production by Larrea divaricata Cav. plant cell cultures and effect of precursor feeding. Process Biochem 46:418–422. https://doi.org/10.1016/j.procbio.2010.08.029
Raskin I (1992) Role of salicylic acid in plants. Ann Rev Plant Biol 43:439–463. https://doi.org/10.1146/annurev.pp.43.060192.002255
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defense: Its role in plant growth and development. J Exp Bot 62:3321–3338. https://doi.org/10.1093/jxb/err031
Sánchez-Chávez E, Barrera-Tovar R, Muñoz-Márquez E, Ojeda-Barrios DL (2011) Efecto del ácido salicílico sobre biomasa, actividad fotosintética, contenido nutricional y productividad del chile jalapeño. Rev Chap Ser Hortic 17:63–68
Schulte U, El-Shagi H, Zenk MN (1984) Optimization of 19 Rubiaceae species in cell culture for the production of anthraquinones. Plant Cell Rep 3:51–54. https://doi.org/10.1007/BF00270970
Senaratna T, Touchell D, Bunn E, Dixon K (2000) Acetylsalicylic acid (aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plant. Plant Growth Regul 30:157–161. https://doi.org/10.1186/s40529-018-0222-1
Singleton VL, Rossi JR (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid. Am J Enol Vitic 16:144–158
Sudha G, Ravishankar GA (2003) Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiol Plant 25:249–256. https://doi.org/10.1007/s11738-003-0005-4
Thiruvengadam M, Rekha K, Rajakumar G, Lee TJ, Kim SH, Chung IM (2016) Enhanced production of Anthraquinones and Phenolic Compounds and biological activities in the cell suspension cultures of Polygonum multiflorum. Int J Mol Sci 17:1912. https://doi.org/10.3390/ijms17111912
Vázquez-Díaz DA, Salas-Pérez L, Preciado-Rangel P, Segura-Castruita MA, González-Fuentes JA, Valenzuela-García JR (2016) Efecto del ácido salicílico en la producción y calidad nutracéutica de frutos de tomate. Rev Mex Cienc Agríc 17:3405–3414
Westendorf J, Marquardt H, Poginsky B, Dominiak M, Schmidt J, Marquardt H (1990) Genotoxicity of naturally occurring hydroxyanthraquinones. Mutat Res 240:1–12. https://doi.org/10.1016/0165-1218(90)90002-j
Zhao J, Davis LC, Verpoorte R (2004) Elicitor signal transduction leading to production of plant secondary metabolites. Biotech Advanced 23:283–333. https://doi.org/10.1016/j.biotechadv.2005.01.003
Funding
The authors are thankful to TUBITAK (Scientific and Technological Research Council of Turkey) for the financial support for this research Project (TOVAG, Project No: 215O057).
Author information
Authors and Affiliations
Contributions
The authors have made the following declarations regarding their contributions: TD and NGB conceived the design of the experiments. TD and ÖAA monitored the research work. TD collected and analyses sample data. TD and NGB contributed to writing the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Ali R. Alan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demirci, T., Aras Ascı, Ö. & Göktürk Baydar, N. Influence of salicylic acid and L-phenylalanine on the accumulation of anthraquinone and phenolic compounds in adventitious root cultures of madder (Rubia tinctorum L.). Plant Cell Tiss Organ Cult 144, 313–324 (2021). https://doi.org/10.1007/s11240-020-01952-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01952-w