Abstract
The aim of this study was to determine the effects of 24-epibrassinolide (24-eBL) and l-phenylalanine (l-phy) on the root growth, total phenolics, total flavonoids, and caffeic acid derivatives’ (CADs) accumulation in Agrobacterium rhizogenes-mediated hairy roots of Echinacea purpurea. For this aim, different concentrations of 24-eBL (0.5, 1.0 and 2.0 mg L−1) and l-phy (100, 500, and 1000 µM) were applied to hairy roots and the roots were harvested five times at 10 day intervals. After harvest, hairy roots were evaluated for fresh root weight, dry root weight, growth index, and the contents of total phenolics, total flavonoids, and CADs. The highest fresh root weight, dry root weight, and growth index were obtained from the roots treated with 0.5 mg L−1 24-eBL and harvested at 50 days after treatment, while l-phy had no significant influence on root growth. Among the 24-eBL applications, 1.0 mg L−1 24-eBL was found as the optimum concentration, providing the highest total phenolics, total flavonoids, cichoric acid, caftaric acid, echinacoside, and p-coumaric acid contents. On the other hand, 500 μM l-phy was determined as the most suitable l-phy treatment for giving the maximum values in terms of all investigated parameters. In terms of harvesting period, 50th day was the most appropriate harvest time for secondary metabolite accumulation. Consequently, the results showed that 24-eBL and l-phy treatments can be successfully increased the production of secondary metabolites in transgenic hairy roots of E. purpurea and 24-eBL implementations could be a more effective strategy when compared to l-phy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Echinacea is a genus under Asteraceae family which constitutes perennial plants native to North America. Among the genus, Echinacea purpurea, Echinacea angustifolia, and Echinacea pallida are the most medicinally important plants used by Native Americans to treat a variety of conditions associated with inflammatory and allergic responses, including swollen gums, inflamed skin, sore throats, and gastrointestinal disorders (Kindscher 1989). E. purpurea is still a very popular medicinal plant used throughout the world in the form of capsulated root extracts or teas, especially to treat upper respiratory diseases. It was reported that E. purpurea extracts had positive effects on various viruses (Pleschka et al. 2009), respiratory tract infections (Schapowal et al. 2015), and inflammatory disorders, even including skin diseases like atopic eczema (Oláh et al. 2017). In addition to these activities, in vitro studies suggest that E. purpurea extracts have high antioxidant, antidiabetic, and antihypertensive properties (Chiou et al. 2017).
There are accumulating scientific evidence on the plant’s strong medicinal activities due to various bioactive compounds including caffeic acid derivatives (CADs), alkylamides, phenolics, and flavonoids (Kumar and Ramaiah 2011). E. purpurea is a plant with a medicinal activity that cannot be attributed to any single chemical. This eliminates the possibility of making synthetics that can provide the same medical effects. For this reason, production must necessarily be met from E. purpurea plants (Jones et al. 2009). In E. purpurea, although valuable metabolites are found in small amounts in the aerial parts of the plant, the main synthesis organs are their roots. Because of all these attractive properties of the bioactive compounds, E. purpurea herbal supplements have been in great demand in recent years. Therefore, wild E. purpurea populations are threatened due to extensive harvesting and traditional methods are unable to meet increasing demands (Mobin et al. 2015; Stiegler 2017). In addition, collecting plants from nature has also many limitations such as a mass infestation of plant materials by microorganisms, the variability of active ingredients, shortage of pure and standard plant materials, dependence on seasons, land, and labor (Dörnenburg and Knorr 1997). Therefore, in vitro techniques have become very important to produce secondary metabolites. In vitro secondary metabolite production techniques not only eliminate the limitations caused by collection from nature, but also make it possible to obtain valuable metabolites of high quantity and purity at any time of the year (Dörnenburg and Knorr 1997). And it is also possible and easier to increase metabolite production with different applications in in vitro conditions.
A. rhizogenes-mediated hairy root production is one of the most important approaches for the biosynthesis of valuable root-derived metabolites in an aseptic condition (Hu and Du 2006). A. rhizogenes-mediated hairy root cultures have a couple of advantages including their relatively fast growth rates (in plant growth regulator-free media), genetic and biochemical stability for long periods of time, and capacity for organogenesis-associated synthesis of metabolites (Shanks and Morgan 1999).
Another strategy of influencing secondary metabolite production is exogenous applications. Different treatments and physical applications were tested to increase the amount of secondary metabolites in E. purpurea such as light, gibberellic acid (GA3), triazoles, nitric oxide, ultrasound, and salinity (Abbasi et al. 2007; Wu et al. 2007; Jones et al. 2009; Liu et al. 2012; Sabra et al. 2012). It is of utmost importance that environmentally and human-friendly practices be implemented, while applications to increase the production of secondary metabolites in plants do not adversely affect plant viability. Brassinosteroids (BRs), which are expressed as the new generation of hormones, have the growth-promoting properties as auxins, gibberellins, and cytokinins, and possess the appropriate properties to be used as potential elicitors of secondary metabolism (Tanaka et al. 2003). BRs are known to take roles in many important physiological events such as plant growth, seed germination, flowering, rooting, cell expansion, and prolongation (Clouse and Sasse 1998). They were also found to be effective in alleviating the adverse effects of various stress factors (Farooq et al. 2009; Çoban and Göktürk Baydar 2016), and in stimulating the synthesis of plant secondary metabolites (Naeem et al. 2012; Ahammed et al. 2013; Çoban and Göktürk Baydar 2017).
Another approach used to increase the production of secondary metabolites in in vitro plant cultures is the addition of precursors into nutrient media (Contin et al. 1999). l-phenylalanine (2-amino-3-phenylpropanoic acid, C9H11NO2) (l-phy), an aromatic amino acid that is hydrophobic, is a compound that initiates the synthesis of many phenolic compounds by phenylpropanoid and flavonoid metabolic pathways in cells. Aromatic amino acids, such as phenylalanine, tyrosine, and tryptophan, are known to act as a precursor of a wide variety of secondary metabolites such as phenolic acids, flavonoids, lignins, coumarins, alkaloids, glucosinolates, and cyanogenic glycosides (Tzin and Galili 2010; Ng et al. 2016), especially cichoric acid and chlorogenic acid Mobin et al. 2015). l-phy is known to initiate the phenylpropanoid and flavonoid metabolic pathways as a precursor for the biosynthesis of phenolic compounds, enabling the synthesis of many phenolic compounds (Koca and Karaman 2015). In the step of producing caffeic acid derivatives from chorismate via the shikimic acid pathway, it is known that the phenylalanine ammonium lyase enzyme uses phenylalanine as the substrate (Fig. 1). Thus, phenylalanine is an important amino acid in the biosynthesis pathway of caffeic acid derivatives (Mobin et al. 2015).
Schematic representation of CADs putative biosynthesis pathways in Echinacea spp. Enzyme names are abbreviated as follows: DHAPS 3-deoxy-d-arabino-heptulosonate-7-phosphate-synthase, DHQS dehydroquinate synthase, DHD-SDH 3-dehydroquinate dehydratase/shikimate 5-dehydrogenase, SK shikimate kinase, EPSPS EPSP synthase, CS chorismate synthase, PAL phenylalanine ammonia lyase; C4H cinnamate 4-hydroxylase, 4CL 4-hydroxycinnamoyl CoA ligase, C3H, p-coumarate 3-hydrolase, CQT caffeoyl-CoA/quinic acid caffeoyl transferase, HIT hydroxycinnamoyl-CoA/tartaric acid hydroxycinnamoyl transferase (Murthy et al. 2014; Liu et al. 2016)
To the best of our knowledge, there is no study about the effects of exogenously applied BRs and L-phy on root growth and CADs production in Agrobacterium rhizogenes-mediated hairy roots of E. purpurea. Therefore, the aim of this study was to test the effects of l-phy and 24-eBL, exogenously applied to transgenic E. purpurea hairy roots, on several root growth parameters and production of the bioactive compounds.
Materials and methods
Plant materials
Seeds of E. purpurea were obtained from Isparta University of Applied Sciences, Department of Field Crops, Isparta/TURKEY. Surface sterilization of seeds was performed by the use of 70% ethanol for 30 s, 15% sodium hypochlorite with two drops of Tween 20 for 15 min. After rinsing 3 times with sterile distilled water, seeds were cultured on MS medium at 25 °C, according to Liu et al. (2006).
Hairy root cultures
Agrobacterium rhizogenes strain ATCC 43,057 used in the present study was purchased from ATCC, Manassas, VA. The strain was grown on the YMB medium (Liu et al. 2006). After 4 weeks of growth, axenic leaves from the germinated seedlings were cut into explants. The explants were inoculated by being dipped into an A. rhizogenes suspension (OD600) for 30 min under a vacuum pressure of 400 mmHg and then were washed three times with sterile distilled water to remove excess bacteria. After 3 days of cocultivation with bacteria in MS medium supplemented with 6 g L−1 agar and 30 g L−1 sucrose, the leaf explants were transferred to solid MS medium added 30 g L−1 L sucrose and 250 mg L−1 cefotaxime. Then, the cultures incubated at 25 °C in a growth chamber under the dark condition to induce hairy roots. Five weeks after inoculation, the hairy roots were excised from infected E. purpurea explants (Fig. 2a) and cultured on plant growth regulator-free MS medium supplemented with 30 g L−1 sucrose and 250 mg L−1 cefotaxime. The procedure was repeated three times to ensure that no bacterial cell colony survived. Afterward, sterile 2 g hairy root tips were transferred to 100 mL of liquid plant growth regulator-free MS medium supplemented with 50 g L−1 sucrose in a 250 mL Erlenmeyer flask. All roots were grown in the dark at 25 °C on a rotary shaker at about 105 rpm. The roots were sub-cultured every 21 days in the liquid media of the same composition (Fig. 2b, c).
Southern blot analysis
Isolation of genomic DNA was performed using the Qiagen DNeasy Plant Mini Kit. The Southern blot technique was performed on the basis of the protocol of Maniatis et al. (1982). Total root DNA was digested with HindIII restriction enzyme at 37 for 16 h. The genomic DNA fragments were electrophoresed in 1% agarose gel and 1% TBE running buffer. Blot was set using 10 × SSC solution and DNA samples were transferred to N + nylon membrane (Amersham, England) for 20 h. Transferred DNA fragments were immobilized on the membrane by UV exposure for 45 s followed by 1-h incubation at 80 °C. rolC gene was used as a probe and labeling was performed using PCR DIG Probe Synthesis Kit (Roche, USA). Hybridization was performed at 42 °C for 16 h and the detection was performed by the use of DIG-High Prime DNA Labelling and Detection Starter Kit II (Roche, USA) according to supplier’s instructions.
24-eBL and l-phy treatments
Hairy roots that were to be used in the applications continued to grow in dark conditions for 10 days, so that they could skip the lag phase and continue growing. At the end of this period, 24-eBL at 0.5, 1.0, and 2.0 mg L−1 and l-phy at 100, 500, and 1000 µM concentrations were added to the media by use of sterile automatic tipped pipettes at appropriate concentrations following filter sterilization, separately. The stock solution of 24-eBL was prepared by dissolving in ethanol and the final volume was maintained by distilled water, while the stock solution of l-phy (l-phenylalanine, Sigma-Aldrich) was prepared with distilled water. Then roots were cultured at a temperature of 20 °C, 3 h of light and 21 h of darkness, which was determined to be suitable for root development and secondary metabolite accumulation according to Wu et al. (2007). The samples for the analyses were taken five times at 10-day intervals (10th, 20th, 30th, 40th, and 50th days) starting from the 10th day, following the applications. Experiments were set up in a completely randomized design. Experiments were performed in triplicate and three flasks were used for each replication.
Determination of growth measurements
The hairy roots (2 g/100 ml) kept under different 24-eBL and l-phy applications were harvested and the fresh weights of plants were recorded. The plants were dried at room temperature to a constant weight, and then, the dry weights of plants were expressed as g.
The growth index of the hairy roots was calculated using the following equation:
Extraction and determination of total phenolics, total flavonoids, and CADs
The extraction of hairy roots was carried out using the method of Liu et al. (2006). Briefly washed roots were dried at 50 °C until constant weight and dried root samples were ground into a fine powder using a mortar and pestle. Samples were extracted twice with 70% methanol added 0.1% phosphoric acid in an ultrasonic bath for 30 min. The methanol fractions were pooled and filtered by using 0.45 µm Whatman microfilters.
The total phenolic content of the hairy roots was determined using the Folin–Ciocalteu colorimetric method (Singleton and Ross 1965). The absorbance of the extracts was measured at 765 nm using a spectrophotometer (T70 Plus Dual Beam/Arlington, USA). The total phenolic contents of the extracts were calculated using the calibration curve prepared gallic acid standards and expressed the results as mg gallic acid equivalents (mg GAE g−1 DW). Data presented are an average of three measurements.
Total flavonoid content was determined spectrophotometrically using the method of Wu et al. (2007). Briefly, 0.50 mL root extract was mixed with 2.5 mL of distilled water, followed by the addition of 150 µL of a 5% sodium nitrite solution. After 6 min, 0.30 mL of a 10% aluminum chloride solution was added and the mixture was allowed to stand for 5 min. Then, 1 mL of sodium hydroxide was added to the mixture and the absorbance at 510 nm was measured immediately. The total amount of flavonoids was calculated as catechin equivalent in mg/g using the calibration curve prepared from standard catechin solutions.
Chromatographic analyses were carried out on a Shimadzu model HPLC system (Shimadzu Corp., Kyoto, Japan). The separation of CADs was performed by the method of Liu et al. (2006). Reversed-phase (RP)-HPLC analysis was done using an SCL-10Avp system controller, an LC-10AD VP pump, a DGU-14a degasser, a CTO-10 A VP column heater, and a Diode Array Detector with wavelengths which set at 278 nm. The 250 × 4.6 mm i.d. 5 µm column used was filled with Agilent Eclipse XDB-C18 (Wellborn, Germany). The flow rate was 0.8 mL min−1, the injection volume was 20 µL, and the column temperature was set at 30 °C. For gradient elution, mobile phase A contained Ultrapure water containing 0.1% phosphoric acid (HPLC grade, %85 Sigma-Aldrich); solvent B contained acetonitrile (HPLC grade ≥ 99.9%, Sigma-Aldrich). The following gradient was used: 0–30 min, 10–20% B; 30–110 min, 20–80% B. The data were integrated and analyzed using the Shimadzu Class-VP Chromatography Laboratory Automated Software system. The root extracts, standard solutions, and mobile phases were filtered by a 0.45 µm pore size membrane filter (Millipore Co. Bedford, MA). The amounts of CADs in the root extracts were calculated as mg/g DW, using external calibration curves obtained for each standard. In the samples, cichoric acid, chlorogenic acid, caftaric acid, caffeic acid, echinacoside, and p-coumaric acid as CADs were determined. Data presented are an average of three measurements.
Statistical analysis
Data were subjected to analysis of variance with mean separation by Tukey’s multiple range tests. Differences were considered statistically significant at the p ≤ 0.01 and p ≤ 0.05 levels.
Results and discussion
In this study, a series of experiments were carried out to determine the effects of 24-eBL and l-phy on growth and secondary metabolite accumulation in E. purpurea hairy roots transformed by Agrobacterium rhizogenes and the results are presented below.
Verification of transgenic lines by southern blot analysis
The Southern blot analysis has been frequently used in hairy root transformation studies to verify transgenic lines and to determine the copy number of the transgenes as an efficient and reliable method (Wang and To 2004; Liu et al. 2006). In this study, Southern blot analysis confirmed the transgenic nature of different hairy root lines and revealed different transgene copy numbers. The hybridization signals observed on hairy root Line 1 indicated the presence of 2 copies of transgenes, whereas Line 2 and 3 carried 3 copies, Line 4 carried 5 copies, and Line 5, 6, and 7 carried 4 copies of rol family transgenes (Fig. 3). Positive control signal C ( +) was obtained by rolC probe hybridization of the pure rolC gene amplification product. Different hairy root lines were investigated for the parameters of fresh root weight, dry root weight, root growth index, total phenolic content and CADs’ contents, and Line 7, which exhibited robust growth and high capacity of secondary metabolite production was selected as the hairy root line to be used in all 24-eBL and l-phy applications, to be able to eliminate differences potentially arise from having different genetic backgrounds (data not shown).
Effects of 24-eBL and l-phy applications on hairy root growth traits
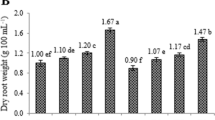
Growth traits including fresh weight, dry weight, and root growth index values were examined to understand the effects of 24-eBL and l-phy treatments on hairy root growth at different concentrations. All growth traits examined in this study were not significantly different among treatments and harvesting periods except 0.5 mg L−1 24-eBL treatment, which produced significant increases at the 50th day samples (Table 1). The root growth parameters increased as the harvest time extended and the highest values were reached at the 50th day samples. It is known that the growth indexes of hairy root cultures change depending on the growth of linear root, the formation of new growth spots, and the secondary thickening in the roots. The apical meristem provides longitudinal growth, but at the same time, it creates new growth spots that provide the formation of lateral roots. From these new growth points, lateral (capillary) root initiation occurs (Liu et al. 2006). The growth index increases with the length of hairy roots and the number of lateral roots formed. Accordingly, as the root growth index increases, the root fresh weight and root dry weight also rise (Giri and Narasu 2000; Kuzovkina and Schneider 2006) just like the results obtained from this study. The root fresh weights of the 50th day samples at which the highest values were obtained changed between 6.89 and 12.63 g 100 mL−1, and the root dry weights varied from 0.65 to 0.90 g 100 mL−1. In a previous study on the effects of KNO3, CaCl2, and MgSO4 ratios in the nutrient medium in E. purpurea transgenic hairy root cultures, the control group was found to have the fresh weight of 2.52 g 100 mL−1 and dry weight of 0.13 g 100 mL−1 in the roots after 4 weeks. In another study, the dry weight of E. purpurea hairy roots harvested at the 40th day was 1.22 g 100 mL−1 (Abdoli et al. 2013). These differences in hairy root growth parameters can vary depending on the culture conditions, nutrient content, harvest time, number of copies of T-DNA found in transgenic roots, position of integration in the plant genome, and different expression levels of genes (Abbasi et al. 2007; Jacob and Malpathak 2005; Liu et al. 2006, 2012).
Previous studies have shown that 24-eBL increases root growth and development not only stressed but also non-stressed plants when applied at the appropriate concentration and development period (Bao et al. 2004; Rady 2011; Sathiyamoorthy and Nakamura 1990). BRs are known to stimulate growth and development by promoting both cell division and cell enlargement (Clouse and Zurek, 1991; Oh and Clouse, 1998). It also induces plant growth by increasing the activities of the BRU1 and TCH4 genes, which encode xyloglucan endotransglycosylase (XET) proteins responsible for cell wall relaxation (Cosgrove 1997; Felner 2003).
In a study, the effects of l-phy treatment on the development of E. purpurea non-transgenic root cultures; l-phy administered at concentrations of 500, 1000, and 5000 μM was increased root dry weight and growth index to the control, with the highest values being obtained as 1.35 g 100 mL−1 and 12.5 with 500 μM l-phy treatments (Mobin et al. 2015). However, Edahiro et al. (2005) and Palacio et al. (2011) reported that high l-phy concentrations in strawberries and Larrea divaricata largely prevented cell growth. In the present study, l-phy had no significant effect on root development. These differences may arise depending on whether the roots are transgenic or not, depending on culture conditions, nutrient content, l-phy concentrations used, and the difference in harvest periods (Giri and Narasu 2000; Jacob and Malpathak 2005; Mobin et al. 2015).
Effects of 24-eBL and l-phy applications on total phenolics, total flavonoids, and CADs’ production in hairy roots
In this study, the effects of 24-eBL and l-phy treatments at different concentrations and harvest times on the accumulation of secondary metabolites of E. purpurea hairy root cultures were investigated. In this context, the first criteria examined were the total phenolic substances and total flavonoids. Both amounts increased linearly with increasing time to harvest and the highest amounts were obtained from hairy roots harvested at 50th day (Table 2). Both 24-eBL and l-phy treatments have been found to significantly increase the total amount of phenolic substances and total flavonoids compared to the control. It was also found that the positive effect of 24-eBL treatments on total phenolic substance and total flavonoid synthesis was higher than l-phy. The highest total amount of phenolic compounds and total flavonoids were obtained from the roots harvested at 50th day in treatments of 1.0 mg L−1 24-eBL which were 59.41 mg g−1 and 37.64 mg g−1, respectively.
Studies of exogenous treatments on the accumulation of secondary metabolites in E. purpurea plants are limited, and in these studies, it was examined the effects of nitric oxide (Wu et al. 2007), GA3 and triazoles (Jones et al. 2009), salt (Sabra et al. 2012), and ultrasonic treatments (Liu et al. 2012). There is no study on the effects of BRs on the total phenolic and total flavonoid contents in the E. purpurea, neither on the effects of external conditions nor in vitro conditions. However, it is known that BR treatments in different plants are highly effective in increasing the total phenolic substance and flavonoid content (Ahammed et al. 2013; Koca and Karaman 2015; Çoban and Göktürk Baydar 2016).
In the transgenic hairy root cultures of the E. purpurea, l-phy treatments have never been used before and that there is only one report concerning non-transgenic roots. Mobin et al. (2015) found that 500 μM l-phy applied to non-transgenic E. purpurea adventitious roots increased the total phenolic content by 29% and the total flavonoid content by 18% when compared to control. Similarly, it was determined that l-phy added to nutrient media, capsicum in Capsicum frutescens (Lindsey and Yeoman 1984), taxol in Taxus cuspidata (Fett-Neto et al. 1994), and rotenoid production in Cassia occidentalis (Vats and Kamal 2014) significantly increased.
In this present study, quantities of CADs, which are very important compounds in Echinacea species, have also been examined. It has been found that 24-eBL treatments, l-phy treatments, and harvest periods made significant differences in the accumulation of CADs including cichoric acid, chlorogenic acid, caftaric acid, echinacoside, and p-coumaric acid (Table 3), while caffeic acid was not found in the samples. According to the results, accumulation of cichoric acid, the primary compound used for quality control with caftaric acid and chlorogenic acid occurring at lower concentrations in E. purpurea roots, increased with extended culture period, and highest values were reached at the 50th day samples. Furthermore, the amount of cichoric acid significantly increased with 24-eBL and l-phy treatments when compared to control; but it was observed that 24-eBL treatments were more effective than l-phy treatments and promoted the accumulation of more cichoric acid. In particular, 24-eBL treatment at a concentration of 1.0 mg L−1 was chosen as the most appropriate treatment for the accumulation of cichoric acid and increased the amount of cichoric acid 3.22-fold compared to the control. l-phy treatment at 500 μM concentration increased the amount of cichoric acid 2.33 fold compared to the control, but 1000 μM l-phy treatment reduced its amount. Similarly, Mobin et al. (2015) reported that the amount of cichoric acid was increased by 23.62% with 500 μM l-phy treatments in non-transgenic E. purpurea plant roots, but significant reductions in the amount of cichoric acid were observed with increasing l-phy concentration.
Another CAD examined in this study was chlorogenic acid, which was obtained at the highest amounts on the 50th day and 24-eBL treatment was more effective than the l-phy treatment. With the treatment of 0.5 mg L−1 24-eBL in which the highest amount of chlorogenic acid was obtained, 4.08 times more chlorogenic acid was obtained compared to control treatment. Among the l-phy treatments, 500 μM l-phy, which were found to be the most effective concentration, resulted in a 2.11-fold increase. These results suggest that 24-eBL treatments can be used effectively in increasing chlorogenic acid production of cultures as well as cichoric acid. It was reported that 1000 μM l-phy applied to non-transgenic E. purpurea roots increased the amount of chlorogenic acid by 14.5% compared to control, while a significant decrease of 69.81% was obtained by increasing the concentration to 5000 μM (Mobin et al. 2015). It was also reported at the previous studies that the amount of chlorogenic acid, as well as cichoric acid and caftaric acid, can be increased significantly by using photoperiod change (Abbasi et al. 2007), GA3, and triazole treatments (Jones et al. 2009) and ultrasonic sound waves (Liu et al. 2012).
According to the results of this study, the accumulation of caftaric acid increased in direct proportion to the harvest time and reached the highest amount on the 50th day. 24-eBL and l-phy treatments were also found to increase caftaric acid production. 1 mg L−1 24-eBL, and 500 μM l-phy treatments were found to be more effective than the control group and other treatments. However, Mobin et al. (2015) reported that l-phy at concentrations of 500, 1000, and 5000 μM applied to non-transgenic E. purpurea roots produced no significant difference in the amount of caftaric acid compared to the control, which can be resulted depending on the different nature of transgenic roots and harvesting periods.
Echinacoside, another important bioactive compound, was found to be increased through the harvest time and reached its highest level on the 50th day. All 24-eBL and l-phy treatments also increased the amount of echinacoside significantly compared to the control. 500 μM l-phy treatment increased echinacoside production 3.36-fold compared to the control treatment. No studies have been found in the literature on the amount of echinacoside in transgenic hairy root cultures of the E. purpurea.
In this study, the changes of p-coumaric acid content were also examined in the roots applied 24-eBL and l-phy and harvested different culture times. Because p-coumaric acid is of great importance for the production of CADs. As a matter of fact, p-coumaric acid, which plays a key role in the biosynthesis step of CADs, has been transformed into cinnamic acid with the Phenylalanine ammonia lyase (PAL, EC 4.3.1.24) activity of l-phy and then into p-cumarol Coenzyme A, and combined with quinic acid and shikimic acid to provide biosynthesis of other CADs, especially chlorogenic acid (Boudet 2007; Tuan et al. 2014). The amounts of p-coumaric acid reached the highest values on the 40th day and did not change on the 50th day. l-phy treatments were found to increase the production of p-coumaric acid more than 24-eBL treatments. Naeem et al. (2012) stated that BRs increase metabolite accumulation by triggering the internal genetic potential responsible for secondary metabolite production. As a matter of fact, there are several reports of the increased different metabolites such as phenolics, essential oil levels in plants after BRs’ applications (Ahammed et al. 2013; Çoban and Göktürk Baydar 2017; Naeem et al. 2012). The external application of precursors also increases the accumulation of secondary metabolites. Indeed, it was reported that l-phy as a precursor increased flavonoids and phenolics in many plants by stimulating the necessary enzymatic pathways (Koca and Karaman 2015; Al-Gendy et al. 2015).
Conclusion
The results of this study revealed that 24-eBL and l-phy treatments significantly increased the levels of the investigated secondary metabolites in E. purpurea hairy roots. It has also been observed that 24-eBL applications generally produced superior results over l-phy applications, especially in terms of root growth parameters, total phenolics, total flavonoids, cichoric acid, chlorogenic acid, and caftaric acid. The most appropriate treatment was determined to be 1 mg L−1 24-eBL, which resulted in the highest total phenolic content, total flavonoid, cichoric acid, caftaric acid, echinacoside, and p-coumaric acid contents among all investigated 24-eBL applications. On the other hand, 500 μM l-phy treatments were found to be more successful than all other l-phy concentrations. l-phy at 100 and 500 µM concentrations also gave the highest values of echinacoside and p-coumaric acid among all treatments. As the harvesting period, the 50th day was determined as the most suitable harvesting period. Based on the results, the use of BR and precursors is effective in increasing the amounts of important bioactive secondary compounds in E. purpurea hairy roots which has become a popular herbal supplement in recent years with its immense medical benefits. In addition, the study confirmed the previous findings that hairy roots produced by Agrobacterium rhizogenes transferred rol genes, which presents an effective secondary metabolite production system in E. purpurea plant.
Author contribution statement
Design and implementation of the experiment: TD and NGB. Transformation and southern blot analyses: TD and UÇA. 24-eBL and l-phy treatments: TD and NGB. Determination of growth parameters and secondary metabolites: TD and NGB. Analysis and interpretation of data: TD and NGB. Writing the manuscript and preparing figures and the tables: TD. Final revision: NGB.
References
Abbasi BH, Tian CL, Murch SJ, Saxena PK, Liu CZ (2007) Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep 26:1367–1372
Abdoli M, Moieni A, Naghdi Badi H (2013) Influence of KNO3, CaCl2 and MgSO4 concentrations on growth and cichoric acid accumulation in hairy root culture of purple coneflower (Echinacea purpurea L.). J Med Plants 1(45):75–84
Ahammed GJ, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ (2013) Brassinosteroid regulates secondary metabolism in tomato towards enhanced tolerance to phenanthrene. Biol Plant 57(1):154–158
Al-Gendy AA, Bakr RO, El-Gindi OD (2015) Production of flavonoids and phenolic compounds by elicitation of Iphiona mucronata (Forssk.) Asch. Schweinf (Asteraceae) callus and suspension cultures. IJPPR 30(1):1293–1300
Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z (2004) Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 134(4):1624–1631
Boudet AM (2007) Evolution and current status of research in phenolic compounds. Phytochem 68(22):2722–2735
Chiou SY, Sung JM, Huang PW, Lin SD (2017) Antioxidant, antidiabetic and antihypertensive properties of Echinacea purpurea flower extract and caffeic acid derivatives using in vitro models. J Med Food 20(2):171–179
Clouse SD, Sasse JM (1998) Brassinosteroids: Essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49:427–451
Clouse SD, Zurek D (1991) Molecular analysis of brassinolide action in plant growth and development. In: Cutler HG, Yokota T, Adam G (eds) Brassinosteroids; chemistry, bioactivity and applications, ACS syrup series 474. American Chemical Society, Washington, DC, pp 122–140
Çoban Ö, Göktürk Baydar N (2016) Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Ind Crops Prod 86:251–258
Çoban Ö, Göktürk Baydar N (2017) Brassinosteroid modifies growth and essential oil production in peppermint (Mentha piperita L.). J Plant Growth Regul 36(1):43–49
Contin A, Van Der Heijden R, Verpoorte R (1999) Effects of alkaloid precursor feeding and elicitation on the accumulation of secologanin in a Catharanthus roseus cell suspension culture. PCTOC 56(2):111–119
Cosgrove DJ (1997) Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement. Plant Cell 9(7):1031–1041
Dörnenburg H, Knorr D (1997) Challenges and opportunities for metabolite production from plant cell and tissue cultures. Food Technol 51:47–53
Edahiro JI, Nakamura M, Seki M, Furusaki S (2005) Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of l-phenylalanine into the medium. J Biosci Bioeng 99(1):43–47
Farooq M, Wahid A, Basra SMA (2009) Improving water relations and gas exchange with brassinosteroids in rice under drought stress. J Agron Crop Sci 195(4):262–269
Felner M (2003) Recent progress in brassinosteroid research: hormone perception and signal transduction. In: Hayat S, Ahmad A (eds) Brassinosteroids: bioactivity and crop productivity. Kluwer Academic Publishers, Dordrecht
Fett-Neto AG, Zhang WY, Dicosmo F (1994) Kinetics of taxol production, growth, and nutrient uptake in cell suspensions of Taxus cuspidate. Biotechnol Bioeng 44:205–210
Giri A, Narasu M (2000) Transgenic hairy roots: recent trends and application. Biotechnol Adv 18:1–22
Hu ZB, Du M (2006) Hairy root and its application in plant genetic engineering. J Integr Plant Biol 48(2):121–127
Jacob A, Malpathak N (2005) Manipulation of MS and B5 components for enhancement of growth and salsodine production in hairy root cultures of Solanum khasianum Clarke. PCTOC 80:247–257
Jones AMP, Saxena PK, Murch SJ (2009) Elicitation of secondary metabolism in Echinacea purpurea L. by gibberellic acid and triazoles. Eng Life Sci 9(3):205–210
Kindscher K (1989) Ethnobotany of purple coneflower (Echinacea angustifolia Asteraceae) and other Echinacea species. Econ Bot 43(4):498–507
Koca N, Karaman Ş (2015) The effects of plant growth regulators and l-phenylalanine on phenolic compounds of sweet basil. Food Chem 166:515–521
Kumar KM, Ramaiah S (2011) Pharmacological importance of Echinacea purpurea. IJPBS 2(4):304–314
Kuzovkina IN, Schneider B (2006) Genetically transformed root cultures—generation properties and application in plant sciences. Prog Bot 67:275–324
Lindsey K, Yeoman MM (1984) The synthetic potential of immobilised cells of Capsicum frutescens Mill cv. Annuum. Planta 162(6):495–501
Liu C, Long J, Zhu K, Liu L, Yang W, Zhang H, Li L, Xu Q, Deng X (2016) Characterization of a citrus R2R3-MYB transcription factor that regulates the flavonol and hydroxycinnamic acid biosynthesis. Sci Rep 6:25352
Liu CZ, Abbasi BH, Gao M, Murch SJ, Saxena PK (2006) Caffeic acid derivatives production by hairy root cultures of Echinacea purpurea. J Agric Food Chem 54:8456–8460
Liu R, Li W, Sun LY, Liu CZ (2012) Improving Root growth and cichoric acid derivatives production in hairy root culture of Echinacea purpurea by ultrasound treatment. Biochem Eng J 60:62–66
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual, vol 545. Cold Spring Harbor Laboratory, Cold Spring Harbour
Mobin M, Wu CH, Tewari RK, Paek KY (2015) Studies on the glyphosate induced amino acid starvation and addition of precursors on caffeic acid accumulation and profiles in adventitious roots of Echinacea purpurea (L.) Moench. PCTOC 120(1):291–301
Murthy HN, Kim YS, Park SY, Paek KY (2014) Biotechnological production of caffeic acid derivatives from cell and organ cultures of Echinacea species. Appl Microbiol Biotechnol 98(18):7707–7717
Naeem M, Idrees M, Alam MM, Aftab T, Khan MMA (2012) Brassinosteroid-mediated enrichment in yield attributes, active constituents and essential oil production in Mentha arvensis L. Russ Agric Sci 38(2):106–113
Ng TLM, Karim R, Tan YS, Teh HF, Danial AD, Ho LS, Khalid N, Appleton DR, Harikrishna JA (2016) Amino acid and secondary metabolite production in embryogenic and non-embryogenic callus of Fingerroot ginger (Boesenbergia rotunda). PLoS ONE 11(6):e0156714
Oh MH, Clouse SD (1998) Brassinolide affects the rate of cell division in isolated leaf protoplasts of Petunia hybrid. Plant Cell Rep 17(12):921–924
Oláh A, Szabó-Papp J, Soeberdt M, Knie U, Dähnhardt-Pfeiffer S, Abels C, Bíró T (2017) Echinacea purpurea-derived alkylamides exhibit potent anti-inflammatory effects and alleviate clinical symptoms of atopic eczema. J Dermatol Sci 88(1):67–77
Palacio L, Cantero JJ, Cusidó R, Goleniowski M (2011) Phenolic compound production by Larrea divaricata Cav. plant cell cultures and effect of precursor feeding. Process Biochem 46(1):418–422
Pleschka S, Stein M, Schoop R, Hudson JB (2009) Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1 H7N7) and swine-origin H1N1 (S-OIV). Virol J 6(1):197
Rady MM (2011) Effect of 24-epibrassinolide on growth yield antioxidant system and cadmium content of bean (Phaseolus vulgaris L) plants under salinity and cadmium stress. Sci Hortic 129(2):232–237
Sabra A, Adam L, Daayf F, Renault S (2012) Salinity-induced changes in caffeic acid derivatives alkamides and ketones in three Echinacea species. Environ Exper Bot 77:234–241
Sathiyamoorthy P, Nakamura S (1990) In vitro root induction by 24-epibrassinolide on hypocotyl segments of soybean (Glycine max L.) Merr. Plant Growth Regul 9(1):73–76
Schapowal A, Klein P, Johnston SL (2015) Echinacea reduces the risk of recurrent respiratory tract infections and complications: a meta-analysis of randomized controlled trials. Adv Ther 32(3):187–200
Shanks JV, Morgan J (1999) Plant ‘hairy root’culture. Curr Opin Biotechnol 10(2):151–155
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. AJEV 16(3):144–158
Stiegler CD (2017) Echinacea: herbal medicine with a wild history. Ethnobiol Lett 8(1):56–57
Tanaka K, Nakamura Y, Asami T, Yoshida S, Matsuo T, Okamoto S (2003) Physiological roles of brassinosteroids in early growth of Arabidopsis: brassinosteroids have a synergistic relationship with gibberellin as well as auxin in light-grown hypocotyl elongation. J Plant Growth Regul 22(3):259–271
Tuan PA, Kwon DY, Lee S, Arasu MV, Al-Dhabi NA, Park NI, Park SU (2014) Enhancement of chlorogenic acid production in hairy roots of platycodon grandiflorum by over-expression of an Arabidopsis thaliana transcription factor AtPAP1. Int J Mol Sci 15(8):14743–14752
Tzin V, Galili G (2010) The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. In: The Arabidopsis book/American Society of Plant Biologists, vol 8
Vats S, Kamal R (2014) Cassia occidentalis L. (a new source of rotenoids): it’s in vitro regulation by feeding precursors and larvicidal efficacy. PCTOC 116(3):403–409
Wang HM, To KY (2004) Agrobacterium-mediated transformation in the high-value medicinal plant Echinacea purpurea. Plant Sci 166(4):1087–1096
Wu CH, Tewari RK, Hahn EJ, Paek KY (2007) Nitric oxide elicitation induces the accumulation of secondary metabolites and antioxidant defense in adventitious roots of Echinacea purpurea. J Plant Biol 50:636–643
Acknowledgements
The authors are thankful to TUBITAK (Scientific and Technological Research Council of Turkey) for the financial support for this research Project (TOVAG, 1001, project no: 213O257).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Araniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demirci, T., Çelikkol Akçay, U. & Göktürk Baydar, N. Effects of 24-epibrassinolide and l-phenylalanine on growth and caffeic acid derivative production in hairy root culture of Echinacea purpurea L. Moench.. Acta Physiol Plant 42, 66 (2020). https://doi.org/10.1007/s11738-020-03055-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03055-7