Abstract
Slash pine (Pinus elliottii Engelm.) has strong adaptability, early growth and high turpentine yield, and it is widely planted in southern China. Breeding of disease-resistant varieties is an effective way to prevent and control development of pine needle brown spot on slash pine in China. Plant regeneration by somatic embryogenesis has been achieved for slash pine, but large-scale production of somatic embryos remains restricted by several factors. We tested different embryogenesis conditions from time of initiation to maturation and studied genetic stability at simple sequence repeat (SSR) loci of regenerated plants in slash pine. Immature zygotic embryos of four open-pollinated mother trees were used for initiation of somatic embryogenesis. Seed sources (families) had a significant impact on initiation of embryonal suspensor mass (ESM, p < 0.05); however, the addition of abscisic acid ABA (1–2 mg/L) and phytosulfokine PSK (0.5 mg/L) could effectively improve initiation rate by up to 36%. Seed family 27 was the most favorable female parent for ESM initiation. The production (number of somatic embryos) was significantly increased by adding ABA (5 mg/L) during suspension culture before transferring to maturation media (p < 0.05). We tested the genetic stability of 14 regenerated plants at SSR loci, and 88.9% plants with normal phenotypes were found not to have genetic variation. We clarified the somatic embryogenesis conditions suitable for slash pine and established somatic embryo maturation technology.
Key message
We clarified the somatic embryogenesis conditions suitable for slash pine and established somatic embryo maturation technology by studying several related influencing factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Slash pine (Pinus elliottii Engelm.) is a fast-growing evergreen tree of the genus Pinus, which is native to the southeastern United States (Gholz et al. 1985). Slash pine was introduced into China in the late 1940s and began to be planted in southern China on a large scale in the late 1970s. Due to its rapid growth, wide adaptability, high resin content and other advantages, it has become one of the main wood and resin dual-use species in southern China (Wen et al. 2004). Pine needle brown spot has occurred on the introduced slash pine in southern China since 1978, severely restricting the development of slash pines (Li et al. 1987). Therefore, it is of great economic and ecological significance to carry out research on resistance breeding of slash pine. Selection of healthy trees from stands severely infested with pine needle brown spot was initiated from 1982 in Fujian, China (Ye et al. 1991). A resistant seed orchard was established using these resistant individuals in 1986 (Ye and Li 1996). However, traditional breeding methods struggle to meet the requirements of large-scale mass production in a short period of time.
Somatic embryogenesis has been suggested as the most promising tool for rapid clonal propagation of large numbers of conifer plants (Gupta et al. 1993), and it can help to capture the greatest benefits from traditional breeding programs by multiplying trees with desirable characteristics for plantation forestry (Pullman and Bucalo 2014). Since 1985, when somatic embryogenesis from immature zygotic embryos of Picea abies (Chalupa 1985; Hakman et al. 1985) and Larix deciduas (Nagmani and Bonga 1985) were reported, in vitro embryogenesis of conifers has made remarkable progress. Many different coniferous species have shown the ability to produce embryogenic tissue, including multiple pine species. At least 27 of the 115–120 known Pinus species, including Pinus banksiana, Pinus caribaea, Pinus desiflora, Pinus nigra, Pinus pinaster, Pinus radiata, Pinus sylvestris, Pinus taeda, and Pinus thunbergii, are reported to go through somatic embryogenesis (Pullman and Bucalo 2011). Initiation of embryogenic tissue and production of Stage-1 somatic embryos on solid media in slash pine was first reported by Jain et al. (1989), while successful regeneration of plantlets via somatic embryogenesis was first reported by Liao and Amerson (1995a, b); Newton et al. (2005) described a detail protocol for plant regeneration via somatic embryogenesis in slash pine; however, further protocol modifications are needed. More recently, Nunes et al. (2018) reported a somatic embryogenesis system with an initiation rate of 10.2% and maturation rate of 52% in hybrid P. elliottii × P. caribaea.
Although research on somatic embryogenesis of conifers in China started relatively late, great progress has been made. Somatic embryogenesis has been initiated from mature zygotic embryos of Pinus massoniana and Pinus yunnanesis (Huang et al. 1995a, b). The regeneration of complete embryos or plantlets via somatic embryogenesis from mature zygotic embryos has been reported for P. taeda and P. elliottii (Tang et al. 1997, 1998). Wu et al. (2013), Zhang et al. (2016) and Hu et al. (2019) also carried out systematic studies on the somatic embryogenesis of slash pine, and obtained regenerated plantlets. However, existing research results have shown that somatic embryogenesis of slash pine is still comparatively restricted, with low efficiency in the initiation of embryogenic callus and maturation of somatic embryos.
As stated above, somatic embryogenesis has been achieved successfully in many pine species; however, initiation and maturation frequency is often low. Several studies have focused on improving initiation or maturation by experimenting with culture medium formulations incorporating plant growth regulators, hormone inhibitors and polyamines (Pullman and Bucalo 2011, 2014; Pullman et al. 2005a, b). Percentages of somatic embryogenesis initiation in slash pine are improved through the use of paclobutrazol, a gibberellin biosynthesis inhibitor (Pullman et al. 2005a, b). Peptide hormones such as phytosulfokine (PSK) are known to contribute to the initial step of cellular differentiation, proliferation, and redifferentiation in conifers (Sarmast 2016). Abscisic acid (ABA) not only improves embryogenic tissue initiation (Pullman et al. 2003; Pullman and Skryabina 2007), but also improves early stage somatic embryo growth in maintenance medium (Pullman et al. 2005a, b).
Somatic embryogenesis has proved to be a favorable tool for large-scale propagation of superior forest trees. However, high concentrations of plant growth regulators supplemented in the medium and prolonged culture under artificial conditions can be potential inducers of somaclonal variation in vitro (DeVerno 1995). Somaclonal variation has been detected in embryogenic cultures of conifers including Picea glauca (DeVerno et al. 1999), L. deciduas (Von Aderkas et al. 2003), P. sylvestris (Burg et al. 2007), and P. pinaster (Marum et al. 2009). Genetic variations can be analyzed using different molecular markers, for example, random amplified polymorphic DNA (RAPD; Isabel et al. 1993; DeVerno et al. 1999), restriction fragment length polymorphism (RFLP; DeVerno et al. 1994), and simple sequence repeats (SSR; Burg et al. 2007; Marum et al. 2009). No information is currently available regarding somaclonal variation of embryogenic cultures in slash pine.
The objective of this study was to develop an efficient somatic embryogenesis protocol for slash pine. For this purpose, we examined immature zygotic embryos from four open-pollinated mother trees (family) of disease-resistant P. elliottii for the capacity to undergo somatic embryogenesis. The effects of the genotype of the parent trees, developmental stage of zygotic embryos, and the formulation of tissue culture medium, especially ABA and PSK, on embryonal suspensor mass (ESM) initiation were evaluated, along with the influence of ABA, maltose and inositol on somatic embryo maturation capacity. To validate our protocol, the genetic stability of regenerated plantlets was analyzed based on microsatellite variability at several SSR loci.
Materials and methods
Plant material
Green cones from four open-pollinated disease-resistant P. elliottii mother trees (family) (7#, 27#, 30#, and 32#), containing immature seeds, were collected from Xi’po State-owned Forest Farm, Huaan, Fujian (25° 06′ 34.58″ N, 117° 33′ 8.47″ E), from 28 June to 10 July 2019 (four collection dates). At least three cones were collected from each mother tree. These cones were surface-disinfected using alcohol cotton and stored in the dark at 4 °C for 1 week. Immature seeds were then removed from the cones, sterilized with 70% (v/v) ethanol for 1 min followed by 30% (w/w) H2O2 for 20 min and rinsed three times with sterile distilled water. Finally, whole megagametophytes were removed aseptically from immature seeds without damage and cultured horizontally on initiation medium (Zhang et al. 2016). These were cultured at 23 ± 2 °C in the dark.
Initiation and proliferation of embryogenic tissue
Microscopic observation of developing zygotic embryos
Zygotic embryos were removed from megagametophytes on each collection date and screened under a stereomicroscope (Leica MZ16, Wetzlar, Germany) to visualize and evaluate their developmental stages quickly. The embryo developmental stages were sorted according loblolly pine zygotic embryo development stage system developed by Pullman and Webb (1994), which is based on embryo morphology.
Effect of family on ESM initiation
To determine the effect of genotype on ESM initiation, about 20 megagametophytes (mostly at stages 2–5) without seed coat were picked from each of the 4 open-pollinated mother trees, respectively, and placed onto initiation medium. Two initiation media were tested, named YD1 medium and YD7 medium (Zhang et al. 2016). YD1 medium was composed of LP basic medium (Arnold and Eriksson 1977) containing basal salts and vitamins supplemented with 2 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 1 mg/L 6-benzyladenine (6-BA), 3% (w/v) maltose, 1 g/L myo-inositol, 500 mg/L casein acid hydrolysate, 250 mg/L 2-(4-morpholino) ethanesulfonic acid (MES) and 500 mg/L l-glutamine for ESM initiation. In the second medium, 2 mg/L 2,4-D and 1 mg/L 6-BA were replaced with 2 mg/L 1-naphthylacetic acid (NAA), 0.6 mg/L 6-BA and 0.6 mg/L kinetin (KT) (Table 1), which was the only difference between these 2 media. Before autoclaving, the pH of the medium was adjusted to pH 5.8 with KOH or HCl and then 6.5 g/L agar was added. Each treatment comprised 20 megagametophytes and was repeated at least 3 times.
Effect of ABA or PSK concentration on ESM initiation
Two families (seed families 7 and 27) collected on 2 July were tested, beginning with medium YD1, to determine the effect of 4 concentrations of ABA (0, 1, 2 and 4 mg/L) on ESM initiation. Two families (seed families 7 and 27) collected on 7 July were tested, beginning with medium YD1, to determine the effect of PSK (the PSK used in the study was synthesized by Nanjing Peptide Biotech Ltd.) concentration (0, 0.1 and 0.5 mg/L) on ESM initiation. Experiments comprised three replications of 20 megagametophytes per test medium per seed family. The rate of ESM initiation was recorded after 7–8 weeks in subculture.
Maintenance and proliferation of embryogenic tissue
After 7–8 weeks on initiation media in the dark, ESM were separated from the micropylar end of mega gametophytes and transferred to fresh maintenance medium for promotion of proliferation. The maintenance solid medium consisted of LP basic medium supplemented with 1 mg/L NAA, 0.3 mg/L 6-BA, 0.3 mg/L KT, 1.5% (w/v) maltose, 1 g/L myo-inositol, 500 mg/L casein acid hydrolysate, 250 mg/L MES and 500 mg/L l-glutamine. Embryogenic tissue (ET) was cultured in the dark at 23 ± 2 °C and subcultured onto fresh maintenance medium every 15 days.
Maturation of somatic embryos
Suspension of embryogenic cultures
Embryogenic line 1807-1 showing the most vigorous maturity was tested for maturation of somatic embryos. At 12–13 days after subculture, 1 g of fresh ESM was suspended in 30 mL of liquid proliferation medium for establishment of embryogenic cell suspension cultures. Liquid proliferation medium contained NAA (0.5 mg/L), 6-BA (0.15 mg/L) and KT (0.15 mg/L) to maintain proliferative growth. Erlenmeyer flasks were agitated on shaking tables at 90 rpm and cultured at 25 °C in the dark. Liquid cultures were subcultured every week. ESM was subcultured in fresh medium at a ratio of 1:2 (v/v) by transferring 10 mL cell suspension to a 100 mL flask containing 20 mL of fresh liquid medium; 5 mL of cell suspension was then spread on filter paper (Whatman No. 2, 55 mm). Lastly, filter papers with cells were placed on the surface of maturation medium (Zhang et al. 2016). The maturation medium consisted of LP basic medium supplemented with 2 mg/L ABA, 130 g/L PEG 8000, 1.5 mg/L GA3, 3% (w/v) maltose, 1 g/L myo-inositol, 500 mg/L casein acid hydrolysate, 250 mg/L MES and 500 mg/L l-glutamine. For each maturation treatment, at least 3 replicates (maturation medium with filter papers with cells) were incubated for 10 weeks in the dark, at 23 ± 2 °C.
Effect of ABA concentration on somatic embryo maturation
In order to test the effect of ABA concentration (0, 2, 5, 10 mg/L) on somatic embryo maturation in suspension culture, ABA was added to suspension liquid medium devoid of plant growth regulators (PGRs). Other additives were at the same concentrations as in the liquid medium. Flasks were also placed on a shaking table (90 rpm) in the dark at 23 ± 2 °C. After 1 week of incubation, the suspension ESM were inoculated on the same maturation medium (Zhang et al. 2016).
Effect of processing times with ABA on somatic embryo maturation
The above-mentioned ESM suspension proliferative cells were transferred into a liquid medium containing 5 mg ABA and cultured for 1 week. After 1 week of incubation with 5 mg/L ABA, ESM was subcultured in fresh liquid medium containing 5 mg/L ABA without PGRs at the same ratio of 1:2 (v/v). The subculture period was also 1 week. One week of cultivation means the first of the number of processing times (M5-1), 2 weeks of cultivation means the 2nd (M5-2) and so on for the 3rd (M5-3). At each subculture stage, 5 mL of the cell suspension was transferred onto filter paper placed on maturation medium to determine any effect of processing times (M5-1, M5-2, M5-3) with ABA on maturation ability.
Effect of maltose and inositol on SE maturation
To test the effects of maltose and inositol on somatic embryo maturation efficiency, different concentrations of maltose and inositol were added to the ESM suspension with liquid proliferation medium (Table 2). The number of mature somatic embryos was recorded after 10 weeks. Somatic embryo production from suspensions of different treatment was expressed as number of embryos per solid maturation medium for each maturation experiments.
Microscopic observation of suspension cells
Cell suspensions treated with different liquid media were settled for 5 min in Erlenmeyer flasks. Cells were transferred onto glass slides via a dropper and stained directly with 2% (w/v) acetocarmine for 1 min. The samples were then stained with 0.5% (w/v) Evan’s blue for 30 s and rinsed with distilled sterile water. Finally, stained cells were covered with a coverslip and placed under a Stereomicroscope with 40-fold magnification for observation (Montalbán et al. 2012). The staining of the cells in the suspension and the structure of the embryo were observed.
Germination and plant conversion
Approximately 100 somatic embryos from embryogenic cell line (ECL) 1807-1 were transferred onto LP basal medium without PGRs for germination. Culture plates were incubated for the first 3–5 days in the dark followed by transfer to light with an intensity of ~ 36 µmol/m2/s from cool white fluorescent illumination. After germination for 1 month, somatic embryos were transferred to rooting medium containing NAA and indole-3-butyric acid (IBA), and allowed to take root. After about 70 days of light culture, regenerated plants were obtained.
DNA isolation and quantification
Fourteen regenerated plants were tested, nine with normal phenotype and five had abnormal phenotype-poor rooting. DNA extraction was performed using a Bioteke DP3111 Plant Genomic DNA Extraction Kit (Beijing Bioteke Biotechnology Ltd.) following instructions provided by the manufacturer, using 100 mg regenerated plants for each sample. DNA quality was checked by agarose gel electrophoresis and ultraviolet absorption.
SSR amplification and fragment analysis
Seven pairs of SSR primers from slash pine and loblolly pine were selected for their high level of polymorphism, clear bands and good repeatability for genetic diversity analysis. PCR reactions for analysis of 1-5872, 3-5021, 4-3919, 11-3030 (Zhao 2016), 2094, 4062 and 4147 (Li et al. 2014) were performed in a final volume of 30 µl using Tsingke’s Gold Mix (green) (Cat. No. TSE101). Capillary electrophoresis was performed using an ABI3730 sequencer, and the GeneMapper 5 software was used to analyze the peak pattern at this site. When a mismatch of more than 2 bp was observed compared to the size of the original allele, the allele was considered to be mutated (Table 3).
Statistical analysis
Data were analyzed by a one-way analysis of variance (ANOVA) using GraphPad Prism Software, version 6.01. The data are expressed as the mean ± standard deviation. The data consists of the rate of ESM initiation and the number of somatic embryos.
Results
Initiation and proliferation of embryogenic tissue
According to Pullman’s criteria for the developmental stage of zygotic embryos of loblolly pine, the development of zygotic embryos of disease-resistant P. elliottii was divided into eight stages (Fig. 1). With the development of zygotic embryos, the embryo proper changed from indistinct and translucent to white and opaque, along with the radial increase of the suspensor. At the same time, the cotyledons gradually elongated, overtopping the shoot apical meristem, but the cotyledons did not completely close, and when viewed from above at a right angle, the shoot apical meristem was still visible. Using the embryo staging system of Pullman and Webb (1994), 88% of immature zygotes from open-pollinated seeds of mother trees collected on 28 June, 2019 were at 2–5 stage, and the proportion of immature zygotes collected on 2 July, 2019 stage decreased to 81%. As time to seed collection increased, the maturity of zygote embryo gradually increased, but mainly concentrated in stage 2–5.
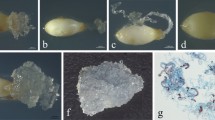
Photographs of Pinus elliottii zygotic embryos stripped from female megagametophyte. The embryo developmental stages of Pinus elliottii zygotic embryos were sorted according Pinus taeda zygotic embryo development stage system developed by Pullman and Webb (1994). Sequence of slash pine somatic embryo development showing similar developmental stages 1–8. Stage 1: pro-embryos had formed. The embryo proper is indistinct and translucent. Stage 2: stages showed early cleavage polyembryony. Multiple embryos are proliferating and developing. The suspensor has elongated but has not increased radially. Stages 3–6: the “bullet” stage which the dominant embryo is clearly developed. The embryo proper is beginning to become white and opaque. Cotyledon tissue gradually developed at the top of the zygotic embryo. Stages 7–8: the cotyledons have elongated but not closed, and when viewed at a right angle from above, the shoot apical meristem is still visible
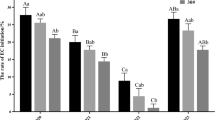
After placement of the female gametophyte on initiation medium for 2 weeks, the female gametophyte (FG) began to expand and white or translucent ESM were seen extruding from the micropylar end of the megagametophyte in several explants. On the surface of some megagametophytes, a yellow, compact callus formed in addition to ESM. Only proliferating ESM were considered for quantification of ECL initiation. For ECL establishment, we tested four different seed families and two different initiation mediums, consisting of LP basal formulations with different combinations of the PGRs 6-BA, 2,4-D, NAA and KT. There were some differences in the responses of immature FGs of P. elliottii from different seed families in different initiation media. There was no significant difference (p < 0.05) between mean initiation rates (12–15%) among the four seed families in YD1. Initiation of embryogenic tissue (ET) was significantly affected by seed family in YD7. The highest mean initiation percentages were obtained for seed family 27 (31.7%) inoculated in YD7 medium, which were significantly different (p < 0.05) from those of the other seed families. Mean initiation rates among the other three seed families in YD7 were lower than those in YD1. The lowest mean initiation percentages were observed for seed family 32 (3%), and differences were statistically significant (Fig. 2a).
ABA had a significant effect on the initiation of embryogenic callus. When ABA was not added to the medium, the callus initiation rate in seed families 7 and 27 was low. When the ABA concentration was 1 mg/L, the initiation rate in family 7 increased, but not significantly, while the initiation rate in family 27 increased significantly. Addition of 2 mg/L ABA resulted in a statistically significant increase in callus initiation over that without ABA (p < 0.05), with the initiation rate highest in family 27, reaching 35%. When the ABA concentration was increased to 4 mg/L, the callus initiation rates in families 7 and 27 were significantly reduced again (Fig. 2b). Therefore, addition of ABA to the medium increased average callus initiation, and addition of 1–2 mg/L ABA could effectively improve the initiation rate of embryogenic callus in disease-resistant slash pine.
Not all levels of PSK added to the medium were able to increase average callus initiation. Initiation percentages of about 13% were produced on YD1 medium with 0 or 0.1 mg/L PSK in seed family 7 after 5–6 weeks of incubation in the dark. The initiation rate of embryogenic callus in family 27 was significantly higher. The highest mean initiation percentages were obtained in families 7 (20%) and 27 (23%) on media with 0.5 mg/L PSK, which was significantly different (p < 0.05) from the media without PSK (Fig. 2c). Therefore, the appropriate addition of PSK could effectively increase the initiation rate of disease-resistant slash pine embryogenic callus.
Maturation of somatic embryos
Addition of ABA during suspension culture can effectively improve the ability of somatic embryos to mature. After ESM (1807-1) had been cultured for 1 week in liquid medium containing ABA but not other PGR, cell structure was observed under a microscope prior to transfer onto maturation medium.
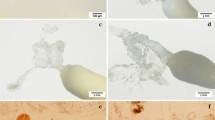
Proembryogenic masses (PEMs) are composed of two types of cell: small, densely cytoplasmic cells, which stain red with acetocarmine; and enlarged, highly vacuolated cells, which are more or less elongated and permeable to Evan’s blue (Filonova et al. 2000). The three stages of PEM (I–III) (Filonova et al. 2000) and early somatic embryo exist at the same time in embryogenic tissue. In control samples without ABA, most cells were in the PEM III stage, and cells cultured with 2 mg/L ABA were not much different from those cultured without ABA. The number of cells aggregated by the PEMs of PEM III was increasing, and the suspensor was continuously extended and formed. The embryo head was aggregated and embryo-suspensor was scattered around. When the concentration of ABA was 5 mg/L, embryonic cells gradually showed the stage of the early SE. The embryo-suspensor gathered at one end of the densely cytoplasmic cells, and the embryo head showed significant polarity development. The aggregated state of the embryo head was less obvious when the concentration was increased to 10 mg/L (Fig. 3a).
Effect of ABA concentration on somatic embryo maturation. a Morphology of embryogenic cells growing in liquid medium cultured at different ABA concentration. b Cells were treated with suspensions of different ABA concentrations and then transferred to solid maturation medium. Somatic embryos obtained after 70 days of solid culture. The concentrations of ABA from left to right are 0, 2, 5, and 10 mg/L. c Number of somatic embryos obtained from solid maturation medium after 70 days. Data represent mean ± SD of three replicates. Different lowercase letters indicate significant difference (p < 0.05) by Duncan’s test
After about 70 days of ESM culture, cotyledonary somatic embryos were isolated and quantified from each maturation medium. Production (number of somatic embryos) was significantly (p < 0.05) affected by the concentration of ABA; production peaked at 5 mg/L, followed by a significant decline at 10 mg/L (Fig. 3b, c). We continued to culture ESM in liquid medium containing ABA (5 mg/L), and changes after each treatment could be observed under the microscope. As the number of treatments increased, the number of early somatic embryos in the field of vision also gradually increased. A thick cytoplasmic embryonic mass aggregated at the apex, followed by a large number of long suspensor cell clusters (Fig. 4a). After 3 treatments (each 1 week), the number of somatic embryos increased significantly, reaching up to 51 per dish (Fig. 4b, c). The enlarged part of the photo shows the complete cotyledon embryo with high quantity and high quality. (Fig. 4b).
Effect of time with ABA on somatic embryo maturation. a Morphology of embryogenic cells growing in liquid medium cultured for different times. b Cells were treated with suspensions of different processing times and then transferred to solid maturation medium. Somatic embryos obtained after 70 days of solid culture. The processing times with ABA from left to right are M5-1, M5-2 and M5-3. M5-1, 1 week of cultivation with 5 mg/L ABA; M5-2, 2 weeks of cultivation with 5 mg/L ABA; M5-3, 3 weeks of cultivation with 5 mg/L ABA. c Number of somatic embryos obtained from solid maturation medium after 70 days. Data represent mean ± SD of three replicates. Different lowercase letters indicate significant difference (p < 0.05) by Duncan’s test
There was no significant change in cell structure when the concentration of maltose or inositol was reduced to 1/2. Early somatic embryos appeared, and relatively shaped early somatic embryos aggregated regularly. Without maltose or inositol, the cytoplasmic embryonic cell mass was relatively loose (Fig. 5a). Furthermore, the production of somatic embryos without maltose or inositol was significantly lower than that with maltose and inositol (15 g, 1 and 7.5 g, 0.5 g), whereas there was no significant difference in production between the 2 concentrations of maltose and inositol (Fig. 5b, c). The enlarged part of the photo shows the complete cotyledon embryo with quality (Fig. 5b).
Effect of maltose and inositol on somatic embryo maturation. a Morphology of embryogenic cells growing in liquid medium cultured at different maltose and inositol concentrations (Table 2). b Cells were treated with suspensions of different maltose and inositol concentrations and then transferred to solid maturation medium. Somatic embryos obtained after 70 days of solid culture. The concentrations of maltose and inositol from left to right are MI-1, MI-2 and MI-3. MI-1, 15 g maltose and 1 g inositol; MI-2, 7.5 g maltose and 0.5 g inositol; MI-3, 0 g maltose and 0 g inositol. c Number of somatic embryos obtained from solid maturation medium after 70 days. Data represent mean ± SD of three replicates. Different lowercase letters indicate significant difference (p < 0.05 by Duncan’s test)
Germination and plant conversion
Cotyledonary somatic embryos (1807-1) cultured for 5 days in the dark were transferred to light (Fig. 6a). After 1 month, the embryonic axis of 85 of 100 (85%) somatic embryos had extended and cotyledons gradually opened, but roots failed to develop (Fig. 6b). Somatic embryos with undeveloped roots were transferred to rooting medium to induce rooting (Fig. 6c). After 70 days, about 75% somatic embryos developed root ends with white root tips and eventually formed whole regenerated plants (Fig. 6d).
Genetic stability in regenerated plants
We analyzed the genetic stability of SSR loci of regenerated plants from the same ECL. 5 of 14 (35%) plants used for SSR analysis showed an abnormal phenotype, and 4 of these plants displayed amplification patterns different from those of most other plants with normal phenotype (Table 4). In addition, one plant with a normal phenotype showed allele size variation. The highest mutation rate (14.3%) was observed at the 1-5872 and 11-3030 loci (Table 5), where genetic variation was found in two individuals. No SSR allele size variation was detected at loci 3-5021 and 4-3919. Allele size variation was detected in five individual plants (Table 5); however, only one of these individuals had a normal phenotype.
Discussion
In this study, we established an effective procedure for plant regeneration by somatic embryogenesis of disease-resistant P. elliottii by investigating seed families from four mother trees obtained from the Xi’po State-owned Forest Farm (Huaan, Fujian) in 2019. Some studies of somatic embryogenesis in P. elliottii (Liao and Amerson 1995a, b; Newton 1995) have been described, but information on success rates is limited. With this protocol we described, it is possible to obtain a high ESM initiation frequency, and the somatic embryos maturation capacity also been improved.
The ESM initiation of slash pine is closely related to family, zygotic embryo development stage, basic medium, PGRs, PGR concentration and other factors, which are key for initiation (Pinto et al. 2008). In our experiments, different seed families showed significantly different abilities for ESM initiation, from up to 31.7% to only 3%. Similar significant differences in somatic embryogenesis response among pine families were also found in P. elliottii (Liao and Amerson 1995a, b), P. taeda (Tang et al. 2001; Pullman and Johnson 2002), P. pinaster (Miguel et al. 2004; Park et al. 2006), P. pinea (Carneros et al. 2009), P. radiata (Hargreaves et al. 2009), Pinus halepensis (Montalbán et al. 2013) and hybrid P. elliottii × P. caribaea (Nunes et al. 2018).
ABA was added to the original medium to produce a higher initiation rate for most seed families. Media containing 1–2 mg/L ABA increased the average ESM initiation rate. ABA plays an important role in maintaining the physiological and metabolic balance of cells as well as in embryo development because of its presence in early embryos and female gametophyte tissues. Handley (1997, 1999) also reported, in two US patents, increased somatic embryo initiation in loblolly pine using 5–120 mg/L ABA. Pullman (2003) found that all concentrations of ABA tested (0.25–5.0 mg/L) increased initiation, and the addition of 1 mg/L ABA produced consistent increases in somatic embryo initiation in loblolly pine. Ma et al. (2012) and other researchers have demonstrated through experiments and practice that the addition of ABA (1 ± 0.5 mg /L) can increase the somatic embryo initiation rate in pine.
Studies have shown that PSK-α plays an important regulatory role in plant growth, development, reproduction and response to the external environment, and is closely related to cytokinin and mitogen-mediated signal transduction pathways (Matsubayashi et al. 1999). Studies have shown that plant sulfopeptides can improve the somatic embryogenesis of plants, such as successfully promoting Japanese cedar (Cryptomeria japonica) (Igasaki et al. 2003) somatic embryogenesis. Some studies have found that in ESM initiation, a low concentration of PSK-α (0.1 mg/L) can effectively improve the response of explants and increase the initiation rate (Chen et al. 2013). The addition of PSK significantly improved the regeneration ability of somatic embryos and organs, thereby producing regenerated plants of both pea cultivars (Ochatt et al. 2018). The combination of PSK with auxins and cytokinins can promote somatic embryogenesis induction, and the formation of callus depends intensively on the combination of IAA, TDZ and PSK (Gałuszka et al. 2019).
Many factors affecting somatic embryo maturation, such as genotype, osmotic potential and PGRs have been reported (Silveira et al. 2004; Hazubska-Przybył et al. 2016). In our experiments, ABA was added to the liquid medium before the cells were placed on solid maturationmedium to adjust the state of ESM and the development of the original embryo. Higher somatic embryo production was associated with increased concentration and treatment times of ABA. Production was very low with culture in liquid medium without ABA, while production increased to 15 somatic embryos per dish when cultured in liquid medium with 5 mg/L ABA. In addition, the highest production (51 somatic embryos per dish) was obtained after 3 treatments with 5 mg/L ABA. Studies have been conducted to evaluate the effect of ABA in in vitro suspension cultures, but there are few reports describing the effect of ABA in suspension on maturation in conifer. ABA can affect the formation of preglobular embryonic structures while increasing production (Fernando et al. 2009). In a study by Zouine et al. (2005), embryogenic callus placed in a liquid medium containing about 2.64 mg/L ABA produced an average of 72 embryos/100 mL of medium within 2 months, significantly higher than the average of 16 embryos/100 mL of solid medium containing about 2.64 mg/L ABA. The effects of ABA at different concentrations (0–26.43 mg/L) on somatic embryos were studied in date palm (Al-Khayri and Al-Bahrany 2012). Addition of 0.26 mg/L ABA to the liquid medium appears to be essential for inhibiting the growth and development of somatic embryos, while high concentrations of ABA inhibit the elongation of somatic embryos at the small globular stage, leading to synchronization of embryoid size. The best synchronized development was found at 13.22–26.43 mg/L among the ABA concentrations tested (0-26.43 mg/L) in date palm (Alwael et al. 2017).
Cells in liquid media absorb nutrients quickly, while growth inhibitors released, such as phenolic resins, are quickly diluted to harmless levels because they spread faster through the liquid system. Negative effects on growth are therefore minimized. At the same time, because it saves labor, time and space, culture establishment in liquid medium can be widely promoted (Gupta and Timmis 2005). In addition to serving as a carbon source for ESM, sugar is also an important penetrant. Using appropriate concentrations of sugar at the somatic embryo maturation stage can not only promote the occurrence of somatic embryos but also effectively suppress the appearance of deformed embryos. Current studies have found that maltose has a greater effect on somatic embryo maturation than other types of sugar (Becwar et al. 1990). By reducing the concentration of maltose and inositol, we reduced the osmotic potential of the liquid culture medium and obtained more somatic embryos. However, the presence of a carbon source has an important effect on the development of somatic embryos and cannot be completely absent. Osmotic potential has been reported to be important for embryo development and maturity (Stasolla and Yeung 2003). In the work of Torres et al. (2001), ABA and osmotic preconditioning (mannitol) were shown to improve both the total number and the synchronization of sweet potato (Ipomoea batatas Lam.)somatic embryos, consistent with our results. The obtained somatic embryos were hard to take root in the germination medium, similar to results for other somatic embryos obtained without treatment with liquid medium (data not shown). Whether this is caused by the ABA needs to be further explored.
Regulation of somatic embryo germination and plant regeneration conditions is beneficial for increasing production and plant regeneration rate. The addition of IBA and NAA to the plant regeneration medium is beneficial for promoting root development and cotyledon elongation (Zhang et al. 2016). Nunes et al. (2018) reported that plantlet conversion rates were approximately 45 and 86%, respectively, and were related to genotype. In our study, higher regeneration rates were obtained, which may be related to genotype and selection of high-quality somatic embryos.
Concern over the genetic fidelity of plants produced by lengthy in vitro culture has been raised many times. Microsatellites have been proved to be highly sensitive markers for monitoring genetic variation during in vitro culture (Burg et al. 2007; Helmersson et al. 2008; Marum et al. 2009). When genetic stability was analyzed using four variable nuclear microsatellite loci in embryogenic cultures and zygotic embryos of Scots pine, significant differences in variation among families were found (Burg et al. 2007). No genetic variations were detected during early stages of somatic embryogenesis in two genotypes of Norway spruce using microsatellite markers (Helmersson et al. 2004), while mutations were observed in somatic plantlets derived from 6 out of 38 clones (Helmersson et al. 2008). Marum et al. detected genetic variations at 7 SSR loci in embryogenic cell lines of maritime pine after 6, 14 and 22 months under proliferation conditions, and 5 out of 52 somatic plantlets showed abnormal phenotypes (2009). However, no correlation was observed between genetic stability and abnormal germinants (Marum et al. 2009). In our study, most plants did not have mutations at the SSR loci tested, and mutations usually occurred only in plants with abnormal phenotypes. Although somaclonal variation risk appears limited based on results available today, it is still important to pursue research and analyses of the cause and results of such variability (Egertsdotter 2019).
In summary, this is a report describing the factors that play a crucial role in initiation and maturation in somatic embryogenesis of disease-resistant slash pine. We confirmed that zygotic embryo developmental stages ranging from 2 to 5 were suitable for ESM initiation, and we have identified the most favorable seed family for embryogenesis initiation. Besides, ABA treatment enhanced not only the initiation but also the maturation of somatic embryos. Although there is still a gap before application to large-scale production, we believe that the current protocol is helpful for labour savings in the future, and could potentially accelerate the breeding programme against pine needle brown spot disease.
Data availability
The data sets supporting the results of this article are included within the article and its additional files.
Code availability
Not applicable.
Consent for publication
The authors that the submission is original work and is not under review at any other publication.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- KT:
-
Kinetin
- BA:
-
6-Benzylaminopurine
- NAA:
-
1-Naphthylacetic acid
- ABA:
-
Abscisic acid
- IBA:
-
Indole-3-butyric acid
- ESM:
-
Embryonal suspensor mass
- ECL:
-
Embryogenic cell line
- PGR:
-
Plant growth regulator
- FG:
-
Female gametophyte
- PEM:
-
Proembryogenic masses
- PSK:
-
Phytosulfokine
- SSR:
-
Simple sequence repeat
- RAPD:
-
Random amplified polymorphic DNA
- RFLP:
-
Restriction fragment length polymorphism
References
Al-Khayri IM, Al-Bahrany AM (2012) Somatic embryo development in date palm (Phoenix dactylifera L.). Biotechnology 11(6):318–325. https://doi.org/10.3923/biotech.2012.318.325
Alwael HA, Naik PM, Al-Khayri JM (2017) Synchronization of somatic embryogenesis in date palm suspension culture using abscisic acid. In: Date palm biotechnology protocols, vol I. Humana Press, New York, pp 215–226. https://doi.org/10.1007/978-1-4939-7156-5_18
Arnold SV, Eriksson T (1977) A revised medium for growth of pea mesophyll protoplasts. Physiol Plant 39(4):257–260. https://doi.org/10.1111/j.1399-3054.1977.tb01879.x
Becwar MR, Nagmani R, Wann SR (1990) Initiation of embryogenic cultures and somatic embryo development in loblolly pine (Pinus taeda). Can J For Res 20(6):810–817. https://doi.org/10.1139/x90-107
Burg K, Helmersson A, Bozhkov P, Von Arnold S (2007) Developmental and genetic variation in nuclear microsatellite stability during somatic embryogenesis in pine. J Exp Bot 58(3):687–698
Carneros E, Celestino C, Klimaszewska K, Park Y-S, Toribio M, Bonga JM (2009) Plant regeneration in Stone pine (Pinus pinea L.) by somatic embryogenesis. Plant Cell Tissue Organ Cult 98(2):165–178. https://doi.org/10.1007/s11240-009-9549-3
Chalupa V (1985) Somatic embryogenesis and plantlet regeneration from cultured immature and mature embryos of Picea abies (L.) Karst. Commun Inst For Cech 14:57–63
Chen JH, Zhang YJ, Wu YY, Wang PK, Wang GP, Shi JS (2013) Effects of phytosulfokine on the somatic embryogenesis of Liriodendron hybrids (L. chinense × L. tulipifera). Sci Silvae Sin 49(02):33–38. https://doi.org/10.11707/j.1001-7488.20130206
DeVerno LL (1995) An evaluation of somaclonal variation during somatic embryogenesis. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. Kluwer, Dordrecht, pp 361–377
DeVerno LL, Charest PJ, Bonen L (1994) Mitochondrial DNA variation in somatic embryogenic cultures of Larix. Theor Appl Genet 88:727–732. https://doi.org/10.1007/BF01253977
DeVerno LL, Park Y, Bonga J, Barrett J, Simpson C (1999) Somaclonal variation in cryopreserved embryogenic clones of white spruce [Picea glauca (Moench) Voss.]. Plant Cell Rep 18(11):948–953
Egertsdotter U (2019) Plant physiological and genetical aspects of the somatic embryogenesis process in conifers. Scand J For Res 34(5):360–369. https://doi.org/10.1080/02827581.2018.1441433
Fernando SC, Weerakoon LK, Gunathilake TR (2009) Micropropagation of coconut through plumule culture. Cocos 16:1–10. https://doi.org/10.4038/cocos.v16i0.1003
Filonova LH, Bozhkov PV, Arnold S (2000) Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J Exp Bot 51(343):249–264. https://doi.org/10.1093/jexbot/51.343.249
Gałuszka A, Gustab M, Tuleja M (2019) In vitro morphogenetic responses from obligatory apomictic Taraxacum belorussicum Val. N. Tikhom seedlings explants. Plant Cell Tiss Organ Cult 139:505–522. https://doi.org/10.1007/s11240-019-01694-4
Gholz HL, Fisher RF, Prichett WL (1985) Nutrient dynamics in slash pine plantation ecosystems: ecological archives. Ecology 66(3):647–659. https://doi.org/10.2307/1940526
Gupta PK, Timmis R (2005) Mass propagation of conifer trees in liquid cultures—progress towards commercialization. Plant Cell Tissue Organ Cult 81(3):339–346. https://doi.org/10.1007/s11240-004-6654-1
Gupta PK, Pullman GS, Timmis R et al (1993) The biotechnology of somatic embryogenesis. Biotechnology 11(4):454–459. https://doi.org/10.1038/nbt0493-454
Hakman I, Fowke LC, von Arnold S, Eriksson T (1985) The development of somatic embryos in tissue cultures initiated from immature embryos of Picea abies (Norway Spruce). Plant Sci 38:53–59. https://doi.org/10.1016/0168-9452(85)90079-2
Handley LW III (1997) Method for regeneration of coniferous plants by somatic embryogenesis in culture media containing abscisic acid. US Patent 5,677,185
Handley LW III (1999) Method for regeneration of coniferous plants by somatic embryogenesis in culture media containing abscisic acid. US Patent 5,856,191
Hargreaves CL, Reeves CB, Find JI, Gough K, Josekutty P, Skudder DB, van der Maas SA et al (2009) Improving initiation, genotype capture, and family representation in somatic embryogenesis of Pinus radiata by a combination of zygotic embryo maturity, media, and explant preparation. Can J For Res 39(8):1566–1574. https://doi.org/10.1139/X09-082
Hazubska-Przybył T, Kalemba EM, Ratajczak E, Bojarczuk K (2016) Effects of abscisic acid and an osmoticum on the maturation, starch accumulation and germination of Picea spp. somatic embryos. Acta Physiol Plant 38(2):59. https://doi.org/10.1007/s11738-016-2078-x
Helmersson A, Jansson G, Bozhkov PV, von Arnold S (2008) Genetic variation in microsatellite stability of somatic embryo plants of Picea abies: a case study using six unrelated full-sib families. Scand J For Res 23(1):2–11. https://doi.org/10.1080/02827580701820043
Helmersson A, von Arnold S, Burg K, Bozhkov PV (2004) High stability of nuclear microsatellite loci during the early stages of somatic embryogenesis in Norway spruce. Tree Physiol 24:1181–1186. https://doi.org/10.1093/treephys/24.10.1181
Hu JW, Guo WB, Deng PL, Zhong SY, Wang WM, Zhao FC et al (2019) Somatic embryogenesis and plant regeneration of Pinus massoniana and its hybrids. J South China Agric Univ 40(1):113–121
Huang JQ, Wei ZM, Xu ZH (1995a) Somatic embryogenesis and plantlet regeneration from callus of mature zygotic embryos of masson pine. Chin Sci Bull 40(1):72–75
Huang JQ, Wei ZM, Xu ZH (1995b) Study on somatic embryogenesis of mature pine zygotic embryos in Pinus yunnanesis Franch. Acta Biol Exp Siniea 28(4):371–379
Igasaki T, Akashi N, Ujino-Ihara T, Matsubayashi Y, Sakagami Y, Shinohara K (2003) Phytosulfokine stimulates somatic embryogenesis in Cryptomeria japonica. Plant Cell Physiol 44(12):1412–1416. https://doi.org/10.1093/pcp/pcg161
Isabel N, Tremblay L, Michaud M, Tremblay FM, Bousquet J (1993) RAPDs as an aid to evaluate the genetic integrity of somatic embryogenesis-derived populations of Picea mariana (Mill.) B.S.P. Theor Appl Genet 86:81–87. https://doi.org/10.1007/BF00223811
Jain SM, Dong N, Newton RJ (1989) Somatic embryogenesis in slash pine (Pinus elliottii) from immature embryos cultured in vitro. Plant Sci 65(2):233–241. https://doi.org/10.1016/0168-9452(89)90070-8
Li CD, Ye JR, Han ZM (1987) Development of pine needle brown spot in young pine forest of Pinus elliottii. J Nanjing For Univ 11(1):1–7
Li YL, Zhao FC, Li XZ, Wu HS, Li FM, Zhong SY, Zhang YZ, Cai J, Guo WB (2014) Analysis of genetic diversity of Pinus elliottii and P. caribaea germplasm resources. For Environ Sci 30(6):9–14. https://doi.org/10.3969/j.issn.1006-4427.2014.06.002
Liao YK, Amerson HV (1995a) Slash pine (Pinus elliottii Engelm.) somatic embryogenesis. I. Initiation of embryogenic cultures from immature zygotic embryos. N For 10(2):145–163. https://doi.org/10.1007/BF00033404
Liao YK, Amerson HV (1995b) Slash pine (Pinus elliottii Engelm.) somatic embryogenesis. II. Maturation of somatic embryos and plant regeneration. N For 10(2):165–182. https://doi.org/10.1007/bf00033405
Ma X, Bucalo K, Determann RO, Cruse-Sanders JM, Pullman GS (2012) Somatic embryogenesis, plant regeneration, and cryopreservation for Torreya taxifolia, a highly endangered coniferous species. In Vitro Cell Dev Biol Plant 48(3):324–334. https://doi.org/10.1007/s11627-012-9433-4
Marum L, Rocheta M, Maroco J, Oliveira MM, Miguel C (2009) Analysis of genetic stability at SSR loci during somatic embryogenesis in maritime pine (Pinus pinaster). Plant Cell Rep 28(4):673–682. https://doi.org/10.1007/s00299-008-0668-9
Matsubayashi Y, Morita A, Matsunaga E, Furuya A, Hanai N, Sakagami Y (1999) Physiological relationships between auxin, cytokinin, and a peptide growth factor, phytosulfokine-α, in stimulation of asparagus cell proliferation. Planta 207(4):559–565. https://doi.org/10.1007/s004250050518
Miguel C, Gonçalves S, Tereso S, Marum L, Maroco J, Oliveira MM (2004) Somatic embryogenesis from 20 open-pollinated families of Portuguese plus trees of maritime pine. Plant Cell Tissue Organ Cult 76(2):121–130. https://doi.org/10.1023/B:TICU.0000007253.91771.e3
Montalbán IA, De Diego N, Moncaleán P (2012) Enhancing initiation and proliferation in radiata pine (Pinus radiata D. Don) somatic embryogenesis through seed family screening, zygotic embryo staging and media. Acta Physiol Plant 34(2):451–460. https://doi.org/10.1007/s11738-011-0841-6
Montalbán IA, Setién-Olarra A, Hargreaves CL, Moncaleán P (2013) Somatic embryogenesis in Pinus halepensis Mill.: an important ecological species from the Mediterranean forest. Trees 27(5):1339–1351. https://doi.org/10.1007/s00468-013-0882-0
Nagmani R, Bonga JM (1985) Embryogenesis in subcultured callus of Larix deciduas. Can J Res 15:1088–1091
Newton RJ, Tang W, Jain SM (2005) Slash pine (Pinus elliottii Engelm.). In: Jain S, Gupta P (eds) Protocol for somatic embryo genesis in woody plants. Springer, Dordrecht, pp 1–10
Newton RJ (1995) Somatic embryogenesis in woody plants. Dordrecht: Kluwer Acad 45:90–108
Nunes S, Marum L, Farinha N, Pereira VT, Almeida T, Sousa D et al (2018) Somatic embryogenesis of hybrid Pinus elliottii var. elliottii × P. caribaea var. hondurensis and ploidy assessment of somatic plants. Plant Cell Tissue Organ Cult 132(1):71–84. https://doi.org/10.1007/s11240-017-1311-7
Ochatt S, Conreux C, Moussa Mcolo R et al (2018) Phytosulfokine-alpha, an enhancer of in vitro regeneration competence in recalcitrant legumes. Plant Cell Tissue Organ Cult 135:189–201. https://doi.org/10.1007/s11240-018-1455-0
Park Y-S, Lelu-Walter MA, Harvengt L, Trontin JF, MacEacheron I, Klimaszewska K, Bonga JM (2006) Initiation of somatic embryogenesis in Pinus banksiana, P. strobus, P. pinaster and P. sylvestris at three laboratories in Canada and France. Plant Cell Tissue Organ Cult 86(1):87–101. https://doi.org/10.1007/s11240-006-9101-7
Pinto G, Silva S, Park Y, Neves L, Araújo C, Santos C (2008) Factors influencing somatic embryogenesis induction in Eucalyptus globulus Labill.: basal medium and anti-browning agents. Plant Cell Tissue Organ Cult 95(1):79–88. https://doi.org/10.1007/s11240-008-9418-5
Pullman GS, Bucalo K (2011) Pine somatic embryogenesis using zygotic embryos as explants. In: Thorpe T, Yeung E (eds) Plant embryo culture: methods and protocols. Humana Press, New York, pp 267–291. https://doi.org/10.1007/978-1-61737-988-8_19
Pullman GS, Bucalo K (2014) Pine somatic embryogenesis: analyses of seed tissue and medium to improve protocol development. N For 45:353–377
Pullman GS, Buchanan M (2003) Loblolly pine (Pinus taeda L.): stage-specific elemental analyses of zygotic embryo and female gametophyte tissue. Plant Sci 164(6):943–954. https://doi.org/10.1016/s0168-9452(03)00080-3
Pullman GS, Johnson S (2002) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation rates. Ann For Sci 59(5–6):663–668. https://doi.org/10.1051/forest:2002053
Pullman GS, Skryabina A (2007) Liquid medium and liquid overlays improve embryogenic tissue initiation in conifers. Plant Cell Rep 26:873–887
Pullman GS, Webb DT (1994) An embryo staging system for comparison of zygotic and somatic embryo development. In: TAPPI R&D Division biological sciences symposium, pp 31–34. http://hdl.handle.net/1853/1824
Pullman GS, Namjoshi K, Zhang Y (2003) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation with abscisic acid and silver nitrate. Plant Cell Rep 22(2):85–95. https://doi.org/10.1007/s00299-003-0673-y
Pullman GS, Johnson S, Van Tassel S, Zhang Y (2005a) Somatic embryogenesis in loblolly pine (Pinus taeda L.) and Douglas fir (Pseudotsuga menziesii): improving culture initiation with MES pH buffer, biotin, and folic acid. Plant Cell Tissue Organ Cult 80:91–103
Pullman GS, Mein J, Johnson S, Zhang Y (2005b) Gibberellin inhibitors improve embryogenic tissue initiation in conifers. Plant Cell Rep 23(9):596–605. https://doi.org/10.1007/s00299-004-0880-1
Sarmast MK (2016) Genetic transformation and somaclonal variation in conifers. Plant Biotechnol Rep 10:309–325. https://doi.org/10.1007/s11816-016-0416-5
Silveira V, Floh EIS, Handro W, Guerra MP (2004) Effect of plant growth regulators on the cellular growth and levels of intracellular protein, starch and polyamines in embryogenic suspension cultures of Pinus taeda. Plant Cell Tissue Organ Cult 76(1):53–60. https://doi.org/10.1023/A:1025847515435
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tissue Organ Cult 74(1):15–35. https://doi.org/10.1023/A:1023345803336
Tang W, Guo Z, Ouyang F (1997) Plantlet regeneration via somatic embryogenesis in slash pine. J Plant Resour Environ 6(2):8–11
Tang W, Guo Z, Ouyang F (1998) Direct somatic embryogenesis and plantlet regeneration from mature zygotic embryos of loblolly pine. Chin J Appl Environ Biol 4(2):103–106
Tang W, Guo Z, Ouyang F (2001) Plant regeneration from embryogenic cultures initiated from mature loblolly pine zygotic embryos. In Vitro Cell Dev Biol Plant 37(5):558–563. https://doi.org/10.1007/s11627-001-0097-8
Torres AC, Ze NM, Cantliffe DJ (2001) Abscisic acid and osmotic induction of synchronous somatic embryo development of sweet potato. In Vitro Cell Dev Biol Plant 37(2):262–267. https://doi.org/10.1007/s11627-001-0047-5
Von Aderkas PRP, Hristoforoglu K, Ma Y (2003) Embryogenesis and genetic stability in long term megagametophyte-derived cultures of larch. Plant Cell Tissue Organ Cult 75:27–34
Wen X, Kuang Y, Shi M, Li H, Luo Y, Deng R (2004) Biology of Hylobitelus xiaoi (Coleoptera: Curculionidae), a new pest of slash pine, Pinus elliottii. J Econ Entomol 97(6):1958–1964. https://doi.org/10.1093/jee/97.6.1958
Wu LJ, Weng QY, Chen D (2013) Factors affecting maturation of somatic embryos of slash pine. Fujian J Agric Sci 28(4):372–376
Ye JR, Li CD (1996) Research progress on resistance of Pinus elliottii to pine needle brown spot in China. For Res 9(2):189–195
Ye JR, Han ZM, Li CD, Zheng P, Zhou CG, Chen G, Hu CY, Xu GH, Zhou GH, Min SB, Gan SP (1991) Establishment technique of resistance clones seed orchard of slash pine to brown spot disease. J Nanjing For Univ 15(2):23–29
Zhang CY, Zhu LH, Tan JJ, Chen TT, Pan J, Liang F, Ye JR (2016) Somatic embryogenesis and plantlet regeneration of disease-resistant Slash Pine (Pinus elliottii Engelm.) to Brown Spot Needle Blight. J Northeast For Univ 44(06):17–22. https://doi.org/10.3969/j.issn.1000-5382.2016.06.006
Zhao H (2016) EST-SSR primer design and analysis the genetic relationship in Pinus elliottii. Jiangxi Agricultural University, Nanchang
Zouine J, El Bellaj M, Meddich A, Verdeil JL, El Hadrami I (2005) Proliferation and germination of somatic embryos from embryogenic suspension cultures in Phoenix dactylifera. Plant Cell Tissue Organ Cult 82(1):83–92. https://doi.org/10.1007/s11240-004-6914-0
Acknowledgements
This research was financially supported by the National Key Research and Development Program of China (No. 2017YFD0600104), the National Natural Science Foundation of China (No. 31971659) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Thanks are due to Xi’po State-owned Forest Farm for supplying pine cones. We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Author information
Authors and Affiliations
Contributions
FY and LHZ developed the idea of the study, participated in its design and coordination and helped to draft the manuscript. XRX and XK contributed to the acquisition and interpretation of data. LHZ and JRY revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Informed consent
The authors have seen and agree with the contents of the manuscript.
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, F., Xia, XR., Ke, X. et al. Somatic embryogenesis in slash pine (Pinus elliottii Engelm): improving initiation of embryogenic tissues and maturation of somatic embryos. Plant Cell Tiss Organ Cult 143, 159–171 (2020). https://doi.org/10.1007/s11240-020-01905-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01905-3