Abstract
Loblolly pine (Pinus taeda L.) culture initiation was improved by the addition of abscisic acid (ABA) (3.7 µM), silver nitrate (20 µM), and guanosine 3′,5′-cyclic monophosphate, 8-bromo-, sodium salt (10 µM) to the medium and by raising cytokinin levels in the presence of 50 mg/l activated carbon (AC). Basal medium contained modified 1/2-P6 salts, 50 mg/l AC, Cu and Zn added to compensate for adsorption by AC, 1.5% maltose, 2% myo-inositol, 500 mg/l casamino acids, 450 mg/l glutamine, 2 mg/l α-naphthaleneacetic acid (NAA), 0.55 mg/l 6-benzylaminopurine (BA), 0.53 mg/l kinetin, and 2 g/l Gelrite. Across 32 open-pollinated families initiation ranged from 0 to 53.4%, with an average of 17.9%. Further optimization of cytokinins to 0.63 mg/l BA and 0.61 mg/l kinetin along with the removal of ABA maintained initiation at 18.2% across 19 families. Survival of 2001 new initiations was tracked for 4–6 months. Survival averaged 28.8%. A test of 68 new initiations tracked closely for 4 months demonstrated that at least 80% of the cultures lost did not grow after transfer to the multiplication media, suggesting that many new initiations abort during the initiation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pine plantations in the Southern U.S. are forecast to rise by 67% from 32 million acres in 1999 to 54 million acres in 2040 (Wear and Greis 2001). Loblolly pine (LP, Pinus taeda L.) is the major species planted across the south with 1–1.5 billion trees planted annually (Schultz 1999). Clonal propagation technology is expected to play a major role in future reforestation of LP and other coniferous species if costs are acceptable and if a high-enough percentage of high-value genotypes can be successfully propagated and established in the plantation setting (Timmis 1998).
Somatic embryogenesis (SE) has been reported for many commercially important gymnosperms (Fowke et al. 1993; Park 2002; Sutton 2002; Tautorus et al. 1991). SE proceeds through a sequence of steps in vitro including initiation, multiplication, maturation, and germination. Cultures may be stored cryogenically, facilitating low-cost storage, field evaluation, selection, and recovery of the highest value clones. The first report of SE in LP (Pinus taeda L.) occurred in 1987 (Gupta and Durzan 1987). Since then several reports have focused on LP along with abundant patent activity (Becwar and Pullman 1995; Pullman and Webb 1994). Factors currently limiting commercialization of SE for LP include low initiation, poor culture survival, culture decline over time causing eventual loss of embryo production, and the inability of somatic embryos to fully mature, resulting in low germination and slow initial growth of somatic seedlings.
Low initiation at 1–5% is often reported for LP (Becwar et al. 1990; Becwar and Pullman 1995; Gupta and Durzan 1987; Li and Huang 1996; Li et al. 1998). Several patents contain methods for improved initiation frequencies for LP (Becwar et al. 1995; Handley 1997, 1999), while Pullman and Johnson (2002) recently reported 16% initiation across ten open-pollinated LP families. These low levels have provided a block for the scientific and commercial use of SE to multiply valuable LP genotypes. To capture the gains of long-term LP breeding programs and genetic engineering improvements, clonal propagation methods must work on a wide range of genotypes.
Kapik (1994) and Kapik et al. (1995) used an indirect ELISA method to estimate abscisic acid (ABA) levels in loblolly pine zygotic tissues. ABA levels were calculated either on a micromole basis assuming the endogenous ABA was uniformly distributed throughout the available water, or on a dry weight basis. When calculated on a micromole basis, peaks occurred in mid-development and at the end of embryo development (Fig. 1). ABA concentrations presented on a dry weight basis showed a different pattern, being highest in both female gametophyte and embryo tissues from early-stage embryos. The presence of ABA throughout embryo development suggested to us that ABA may improve culture initiation.
(+) ABA levels in 1993 zygotic whole ovules, embryos and female gametophytes on a micromole basis (seed source: WA 93). Stages 9A, 9B, etc. are equivalent to 9.1, 9.2, etc. (Kapik 1994)
Ethylene is known to have significant effects on many tissue culture systems including shoot culture, organogenesis, and embryogenesis. Although in some cases its influence seems negligible, in many types of tissue culture ethylene may act either as a promoter or inhibitor depending on the species (Biddington 1992). Effects on conifer SE seem to vary as well, ranging from an ethylene-mediated reduced embryogenic tissue growth (Kumar et al. 1989) or an inhibition of maturation (Kong and Yeung 1994) to no alteration in growth or somatic embryo maturation in Picea abies or P. sitchensis (Kvaalen 1994; Selby et al. 1996). Several reports have indicated improved embryogenesis when ethylene inhibitors, such as silver nitrate, were included in the medium (Auboiron et al. 1990; Roustan et al. 1989, 1990). We speculated that changes in ethylene through either stimulation or inhibition would improve LP initiation.
Although low levels of initiation may occur without auxin or cytokinin in other pines (Aitken-Christie and Parkes 1996; Smith 1996), auxin and cytokinin activity seem to be necessary for optimal LP initiation. As plant science begins to understand the mode of action of auxins and cytokinins, it is becoming clear that these plant hormones initiate a chain of cascading events that end in the resulting growth response (Hutchinson and Kieber 2002; Kepinski and Leyser 2002). Chemicals are becoming known that can stimulate or inhibit particular steps of the cascade. These chemicals may improve or substitute for the activity of hormones. Guanosine 3′:5′–cyclic monophosphate (cGMP) has been shown to be involved in phytochrome signal transduction and hormone signaling (Walden 1998) and can substitute for kinetin stimulation of stomatal guard cell opening (Gehring 1999). In the investigation reported here, we investigate the ability of cGMP to substitute for or supplement auxin and or cytokinin activity.
Activated carbon (AC) is known to adsorb cytokinins (Ebert et al. 1993; Pullman and Gupta 1991). Pullman and Gupta (1991) developed a working initiation medium for Douglas-fir by supplementing the initiation medium with 2.5 g/l AC and increasing the 6-benzyladenine (BA) and kinetin levels approximately 100 times to 0.2 μM each to compensate for cytokinin adsorption by AC. Since the initiation medium in the present study contains a small amount of activated carbon (50 mg/l), it may be beneficial to optimize the cytokinin content.
The objectives of the present research were: (1) to determine the effect of ABA on LP culture initiation, (2) to determine the effect of ethylene regulators on culture initiation, (3) to determine the effect on initiation of increased levels of cytokinin, and (4) to determine the effect of cGMP on initiation. The overall focus of the research in this report was the development of a SE initiation system that would work across a diversity of genetic material of Pinus taeda.
Materials and methods
Plant materials, seed sterilization, and dissection
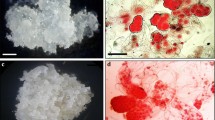
Loblolly pine cones were collected weekly in early to mid-July 1996–1999 from the same individual open-pollinated mother trees in clonal seed orchards, shipped on ice, and received within 24–48 h. Collections also occurred in mid-January by Westvaco/Rigesa, Celulose from breeding orchards near Canhinhas, Santa Catarina, Brazil. Cones were stored at 4–5°C for 1–9 weeks. Those containing seeds with embryos mostly at stages 2–4 (Pullman and Webb 1994) were used for initiation tests as described by Pullman and Johnson (2002).
Media, culture conditions, and replication
Medium 505 (Pullman and Johnson 2002, Table 1) was a starting point for this research. Medium pH was adjusted with KOH or HCl after the addition of all ingredients except the gelling agent or filter-sterilized materials. Media were autoclaved at 121°C for 20 min. Explants were cultured on 2 ml of medium contained in individual wells of Costar no. 3526 Well Culture Cluster Plates wrapped in two layers of Parafilm and incubated at 23–25°C in the dark. Unless otherwise indicated, experiments consisted of three replications of ten explants per test medium and cone collection.
Extrusion and initiation success and statistical analysis
Within 1–4 weeks extrusion occurs when one or more zygotic embryos push out of the megagametophyte micropylar end and become visible, protruding from the gametophyte or onto the medium. At 5–7 weeks, somatic embryos begin to form on the zygotic embryos. These somatic embryos then continue the proliferation or multiplication process to form a mass of embryogenic tissue. These phases can be evaluated as percentage (%) extrusion, percentage of explants forming three or more somatic embryos (visible through a dissecting microscope), and percentage of explants achieving a target mass or size. Percentage extrusion and initiation (explants with three or more somatic embryos visible) were routinely evaluated 9–10 weeks after placement of the megagametophytes on media. Treatments were arranged in a completely randomized design. Data were analyzed by analysis of variance, and significant differences between treatment means were determined by the Duncan Multiple Range Test at the 5% level of significance. Extrusion and initiation percentages for individual replicates were transformed by \(\arcsin \sqrt {(\% )} \) prior to ANOVA analysis. ANOVA tests analyzed seed source as one of the factors tested along with evaluation of interactions between factors. Seed sources frequently differed significantly in response (P=0.05). Unless indicated otherwise, interactions between factors were not statistically significant.
Initiation on medium containing abscisic acid
Experiment 1
During the initiation process cultures form embryogenic tissue with somatic embryos at stages 1–2 (Pullman and Webb 1994). Given the information that zygotic embryos at stages 1 and 2 contain approximately 1 μM ABA in the water associated with tissue fresh weight (Kapik 1994; Fig. 1), we hypothesized that a similar level of ABA added to the culture medium would improve culture initiation. Since initiation medium 505 contains 50 mg/l AC and AC is known to adsorb ABA (Pullman and Gupta 1991), an experiment was designed with a 2×2 factorial arrangement to test the effect of 0 mg/l or 1 mg/l ABA (3.7 µM) on initiation in the presence and absence of AC. Medium 505 (Pullman and Johnson 2002) was altered to reduce the level of Gelrite from 2.0 g/l to 1.0 g/l (= medium 534). In this experiment, medium 534 was modified and tested as follows: (534) no modification; (547) addition of 1 mg/l ABA; (544) removal of 50 mg/l AC; (545) removal of 50 mg/l AC and addition of 1 mg/l ABA. Cone collections from each of three different mother trees were tested for initiation with each medium.
Experiment 2
Having observed the benefit of 1 mg/l ABA in Experiment 1, we next tested medium 505 (Table 1) with ABA added at 0.25, 0.5, 1.0, 2.0, or 5.0 mg/l. Four seed sources were tested.
Experiment 3
A third experiment was carried out in a 2×4 factorial design using medium 505 (Table 1) supplemented with 0 mg/l or 1.0 mg/l ABA and four concentrations of glutamine (450, 650, 1,000, and 1,250 mg/l). Four seed sources were tested.
Initiation on medium containing silver nitrate
Experiment 4
Medium 505 was tested unmodified or supplemented with ethylene inhibitors or an ethylene-releasing compound. Cobalt chloride (10 μM and 50 μM) and nickel chloride (20 μM and 100 μM), two ethylene biosynthesis inhibitors, were tested along with silver nitrate (20 μM and 100 μM), which is an ethylene action inhibitor. Ethephon, an ethylene-releasing compound, was also tested at concentrations of 0.1, 1.0, and 10 mg/l. Cobalt chloride, nickel chloride, and silver nitrate were added prior to autoclaving, and ethephon was added subsequent to autoclaving as filter-sterilized. Four cone collections were tested.
Experiment 5
Medium 505 supplemented with 0 mg/l or1.0 mg/l ABA and 0, 10, 20, or 30 μM AgNO3 was next tested with three cone collections in a 2×4 factorial arrangement.
Initiation on medium containing 8-Br-cGMP
Experiments 6A and 6B
Two small experiments were conducted to determine if 8-Br-cGMP (Calbiochem, Catalog no. 203820), a cell permanent cGMP analogue, might be able to replace auxin, cytokinin, or both in the initiation medium. In the first experiment, medium 505 was tested (1) unmodified, (2) with the auxin (NAA) removed and replaced by 10 µM 8-Br-cGMP, (3) with the cytokinins (BA/kinetin) removed and replaced with 10 µM 8-Br-cGMP, (4) with both auxin and cytokinins removed and replaced with 10 µM 8-Br-cGMP, or (5) with medium 505 supplemented with 10 µM 8-Br-cGMP. Four seed sources were tested. A similar experiment was also carried out with the following changes. Basal salts of medium 505 were altered to reduce the levels of boron and calcium to 1/2- and 3/4-strength, respectively. Salt modifications were based on ongoing experiments (data not shown) to mimic metal concentrations present in female gametophyte tissue during seed development (Pullman and Buchanan 2003). In addition, the 8-Br-cGMP content was increased in these treatments to 25 µM.
Experiment 7
Modified medium 888 (Table 1) was tested in a 2×4 factorial arrangement with full-strength or 3/4-strength calcium combined with four levels of 8-Br-cGMP (0, 5, 10, 15 µM). Three Brazilian seed sources were tested.
Cytokinin optimization in the presence of AC
Experiment 8
Durzan and Gupta (1987) reported Douglas-fir initiation on medium containing 20 μM each of BAP and kinetin with no AC, while Pullman and Gupta (1991) reported initiation of Douglas-fir using medium containing 2.5 g/l AC and 200 μM each of BA and kinetin. This suggests that 2.5 g/l AC adsorbed 180 μM (200 μM−20 μM=180 M) of each cytokinin. We estimated that 50 mg/l (1/50 of 2.5 g) AC would adsorb about 3.6 μM (0.8 mg) of each cytokinin. In a preliminary experiment testing four cone collections, we compared initiation on medium 505 (0.45/0.43 mg/l BA/kinetin) and medium 505 with the levels of BA and kinetin each approximately doubled to 1.0 mg/l.
Experiment 9
The results from experiment 8 suggested testing an intermediate increase in cytokinins, therefore we decided to increase the level of cytokinins in medium 505 by approximately 25%. Medium 505 was tested in a 2×3 factorial design with two levels of cytokinins (0.45/0.43 or 0.55/0.53 mg/l BA/kinetin) and three treatments testing (1) no ABA or silver nitrate, (2) inclusion of 1.0 mg/l ABA and 20 μM silver nitrate, and (3) inclusion of ABA (1.0 mg/l), 20 μM silver nitrate, and 10 μM 8-Br-cGMP. Four cone collections from Brazil were tested.
Experiment 10
This experiment again used a 2×4 factorial design, beginning with medium 716 (Table 1), to test two levels of 8-Br-cGMP (0 μM and 10 μM) with four levels of cytokinins (0.45/0.43, 0.55/0.53, 0.63/0.61, 0.76/0.74 mg/l BA/kinetin). All media contained a background of medium 505 salts, 1.0 mg/l ABA, and 20 mM AgNO3.
Removal of ABA and initiation across several years and media with multiple families
Two years of initiation data confirmed the benefit of including ABA in the initiation media. Late in 1997, it became apparent that other laboratories had also experimented with the addition of ABA to initiation media (Aitken-Christie and Parkes 1996; Handley 1997, 1999). U.S. Patents 5,677,185 and 5,856,191 were granted for the use of ABA during somatic embryo initiation for a list of Pinus species (Handley 1997, 1999). New Zealand researchers also applied, a few days after Handley, for a world patent on a similar concept for initiation in conifers and other woody species (Aitken-Christie and Parks 1996). To avoid intellectual property issues, we decided to run initiation tests with multiple seed sources using media 945 with ABA removed (medium 1042).
Survival of new initiations over time
Li and Huang (1996) and Pullman and Johnson (2002) both reported that new initiations lose the ability to grow over time. Further, Pullman and Johnson (2002) divided the culture loss into three categories: (1) new initiations that failed to grow when transferred from the initiation medium; (2) cultures that grew poorly for several subcultures after initiation and then stopped; (3) cultures that grew well for a period, and then declined in growth rate over time with eventual death. To confirm earlier observations, new initiations from all initiation tests were pooled together during 1995 and 1996, grown on medium 16 with 2.5 g/l gellan gum (Pullman and Johnson 2002), transferred every 2–3 weeks, and survival by mother tree determined after 4–6 months. Also, to examine this problem further, a population of 68 new initiations were grown on the same medium, transferred every 3 weeks, and their rate of survival and contamination determined with every transfer for a period of 15 weeks.
Results and discussion
Initiation on medium containing ABA
Experiment 1
Media containing ABA averaged higher initiation for most seed sources (Table 2). While differences were not statistically significant between individual treatments, when analyzed with the 2×2 factorial design, ABA treatments resulted in higher initiation (statistically significant at P=0.10). The effect of ABA did not appear to be altered by the presence of 50 mg/l AC.
Experiment 2
All levels of ABA added to the medium showed increased average initiation (Table 3). Additions of 0.5 mg/l and 5.0 mg/l ABA resulted in statistically significant increases in initiation at P=0.05.
Experiment 3
Extrusion and initiation percentages are shown in Table 4. When analyzed using the factorial design Extrusion percentage increased as glutamine increased; differences between 450 mg/l and 1,250 mg/l glutamine were statistically significant (P=0.05). Differences in extrusion percentages were also statistically significant between media differing in ABA levels, increasing from 44% to 59%. Initiation percentages, however, did not increase with increasing glutamine, indicating that as extrusion increased in response to glutamine, somatic embryo formation on extruded tissues decreased. ABA again increased initiation as seen in prior experiments, and differences were again statistically significant. Average initiation on media without ABA was 4.1% versus 15.5% on media with 1.0 mg/l ABA.
All concentrations of ABA tested (0.25–5.0 mg/l) increased initiation, and the addition of 1 mg/l ABA produced consistent increases in initiation. Handley (1997, 1999) also reported, in two U.S. patents, increased initiation in loblolly pine using 5–120 mg/l ABA. Aitken-Christie and Parkes (1996) found that ABA increased initiation in Pinus radiata using dissected fertilized embryos at the pre-cotyledonary bullet stage and media without auxins or cytokinins present.
It is interesting to note that the range of ABA we found effective (0.25–5.0 mg/l=0.96–19.2 µM) covers the concentration range (1–4 µM) that Kapik (1994; Fig. 1) found in stage 1–2 zygotic embryos and female gametophyte when he assumed ABA is dissolved in embryo or megagametophyte water. Both Kong et al. (1997) and Carrier et al. (1999) measured ABA in developing seed tissues of white spruce. Kong et al. (1997) found ABA to be present in whole seed from 0–4 weeks after fertilization at levels of about 5–6 ng/seed. After 4 weeks, ABA increased, followed by a decrease at 6 weeks. Carrier et al. (1999) found ABA to be present at the highest concentrations in both the megagametophyte and seed coat 11 days after pollination (before fertilization). When calculated on a water content basis, ABA concentration in the whole seed was 11 µM 11 days after pollination and 7 µM 38 days after pollination (when pro-embryos were first present). Thus, ABA embryo content was the highest during early embryo development.
The presence of ABA in early-stage embryos and female gametophyte tissue suggests a role in embryo development. Kong et al. (1999) point out that the high levels of (+) ABA observed in their investigation and in LP (Kapik et al. 1995) occurred prior to the major increase in seed weight. Alternative hypotheses include ABA stimulation of cleavage polyembryony or enhanced tolerance of stress. High endogenous ABA levels in zygotic embryos are present when embryo multiplication through cleavage polyembryony is active (Becwar and Pullman 1995; MacKay et al. 2001). Krueger and Becwar (2001) reported that ABA added to the post-cryopreservation recovery medium improved growth and somatic embryo production from cryopreserved LP embryogenic tissue lines. We also found post-initiation culture growth to be improved on medium containing ABA (data not presented). Both of these stimulatory effects of ABA are on embryogenic tissue overcoming stressful treatments.
Initiation on medium containing silver nitrate
Experiment 4
Extrusion and initiation in medium 505 averaged 47.8% and 1.6%, respectively, across all cone collections (data not presented). Extrusion differences between unmodified medium 505 and other media were not statistically significant except for the medium containing 100 μM silver nitrate where extrusion was reduced to 21.7%. Differences in initiation between unmodified medium 505 and other media were not statistically significant except for the medium containing 20 μM silver nitrate where initiation increased to 7.6%. In medium 505 only one cone collection produced embryogenic tissue, whereas in medium 505+20 μM silver nitrate three of the four cone collections produced initiations. In these tests the ethylene promoter, ethephon, and ethylene biosynthesis inhibitors, CoCl2 and NiCl2, did not increase initiation. However, AgNO3, an ethylene action inhibitor, increased initiation when added to medium at 20 μM, suggesting further testing. The interaction between seed source and treatment was statistically significant (P=0.05).
Experiment 5
Media supplemented with ABA again showed significantly higher initiation. The combination of ABA and AgNO3 further increased initiation (Table 5). AgNO3 at concentrations of 20–30 μM appeared to be optimal. The addition of ABA to the medium resulted in a statistically significant increase in initiation averages from 3.9% to 8.9%. AgNO3 increased initiation averages from 3.9% (no AgNO3) to 8.3% (20 μM AgNO3), but the differences were not statistically significant. Additional experiments (data not shown) were carried out with medium 505—(1) with no or 1 mg/l ABA alone, (2) 10–30 μM AgNO3 alone, or (3) ABA and silver nitrate together. All experiments showed improved initiation with ABA alone. Silver nitrate alone was not always beneficial. The combination of silver nitrate and ABA often caused the highest initiation. The most consistently beneficial amount of silver nitrate combined with ABA was 20 μM.
Ethephon, an ethylene-releasing agent, and two ethylene biosynthesis inhibitors, cobalt and nickel chloride, did not alter initiation for LP. However; silver nitrate, when added to medium in the range of 20 μM to 30 μM, increased initiation in LP, especially when combined with ABA. Silver ions, in the form of nitrate or thiosulphate, are a strong inhibitor of ethylene action (Beyer 1976). Silver nitrate and other ethylene inhibitors may stimulate embryogenesis in species having high endogenous levels of ethylene and inhibit embryogenesis in species with a lower endogenous ethylene concentration (Biddington et al. 1988; Cho and Kasha 1989). When Wann et al. (1989) compared ethylene production in embryogenic and non-embryogenic tissue for five coniferous species, including LP, ethylene production in non-embryogenic tissue was always greater than that in embryogenic tissue, with the differences ranging from 2.4- to 345-fold. Loblolly pine showed the greatest difference between embryogenic and non-embryogenic tissue of all of the species studied, thereby supporting the hypothesis that low ethylene exposure is necessary for initiation. Wann et al. (1989) were able to predict embryogenic tissue production in advance of visual appearance for some cultures based on ethylene evolution. The two spruce species examined, Picea abies and P. glauca, showed ethylene differences of only 11.7- and 2.4-fold, respectively.
Media supplementation with silver nitrate improves embryogenesis in numerous angiosperms, including carrot (Roustan et al. 1990), coffee (Fuentes et al. 2000), and date palm (Al-Khayri and Al-Bahrany 2001). Ethylene has been shown to accumulate during embryogenesis in P. glauca and at higher levels inhibited embryogenesis (Kumar et al. 1989). However, in Picea sitchensis and P. abies, manipulations in ethylene did not alter embryogenic tissue growth or maturation (Kvaalen 1994; Selby et al. 1996). The presence of ethylene inhibitors such as silver nitrate has been shown to benefit white spruce maturation (Kong and Yeung 1994, 1995). In one of the few studies examining the effects of silver nitrate on initiation and embryogenic tissue growth in conifers, the presence of 10–100 μM AgNO3 in induction medium reduced embryogenesis and the recovery of blue spruce plantlets (Afele and Preveen 1995). Li and Huang (1996) tested the effect of silver nitrate on LP initiation and saw benefits in the same concentration range as our work. When their medium, a modification of that of Gupta and Pullman (1991), was supplemented with 29.4 µM AgNO3, initiation increased from 0% to 3% in the first year of the study and from 3% to 5.3% in the second year.
Initiation on medium containing 8-Br-cGMP
Experiments 6A and6B
For both experiments, 8-Br-cGMP had similar effects. In general, all of the treatments in which 8-Br-cGMP substituted for auxin or cytokinin showed reduced extrusion and initiation, suggesting that 8-Br-cGMP does not substitute for auxin or cytokinin activity (differences were not statistically significant). Both of the treatments in which the control medium was supplemented with 10 µM or 25 µM 8-Br-cGMP showed either initiation for some cone collection(s) not seen with other treatments or raised initiation averages (not statistically significant). The interaction in Experiment 6B between seed source and treatment was statistically significant (P=0.05).
Experiment 7
Extrusion percentage differences between treatments were not statistically significant (Table 6), while initiation percentage differences between treatments were statistically significant (Table 6). As seen in prior experiments, medium 505 produced less initiation than treatments containing ABA and silver nitrate. Initiation on media differing in calcium content or 8-Br-cGMP content did not differ significantly. However, when an analysis was performed using the factorial arrangement, medium supplemented with 10 μM 8-Br-cGMP resulted in the highest average initiation. Two of the three cone collections tested showed the highest initiation on medium 889, which contains ABA, AgNO3, and 10 μM 8-Br-cGMP. See also the results for experiment 9.
The addition of the cell-permeable analogue of cGMP, 8-Br-cGMP, often did not cause statistically significant increases in initiation. However, initiation averages were usually higher in the presence of 8-Br-cGMP, and some cone collections appeared to respond favorably to 8-Br-cGMP. With the potential benefit and no discernable disadvantages other than cost, we decided to leave this ingredient in the medium for now. When initiation levels improve further we will retest the value of this medium component.
Cytokinin optimization in the presence of AC
Experiment 8
Initiation on medium 505 averaged 5% versus that on media with increased cytokinin (4%); the differences were not statistically significant. This suggested to us that we may have raised the level of the cytokinins too much and should test lower concentrations of the supplements.
Experiment 9
Extrusion percentages did not differ significantly (P=0.05) between treatments (Table 7). However, initiation successively increased as silver nitrate, ABA, raised levels of cytokinins, and 8-Br-cGMP were each added (Table 7). The differences were statistically significant between media containing none versus all of these additives. When analyzed by the 2×3 factorial arrangement, raised cytokinin levels increased initiation from 21.3% to 26.7%; the differences were statistically significant at P=0.06. When initiation results from media 886–889 (Table 7) were analyzed by a 2×2 factorial arrangement with two levels of cytokinin and two levels of cGMP, an increase in the level of cytokinin increased initiation from 22.1% to 29.6% and the inclusion of 10 µM 8-Br-cGMP resulted in initiation increasing from 23.3% to 28.3%. The differences were statistically significant at P=0.10 for both changes.
Experiment 10
All of the increased cytokinin levels resulted in greater initiation, with the differences being statistically significant (Table 8). When analyzed in the factorial design, the four increased levels of cytokinins resulted in average initiation percentages of 14.7%, 22.5%, 24.0%, and 21.6%, respectively. 8-Br-cGMP did not change initiation, but as seen in earlier experiments, some cone collections respond well to 8-Br-cGMP (see data for UC 5-1036, Table 8). An optimal level of cytokinins appeared to be 0.63 mg/l BA and 0.61 mg/l kinetin. A statistically significant interaction occurred between cytokinin level and cGMP, suggesting that cGMP was not effective at lower cytokinin concentrations.
Optimization of the cytokinin levels resulted in statistically significant increases in initiation. Increasing both BA and kinetin from 0.45/0.43 mg/l to 0.63/0.61 mg/l, an increase of approximately 40%, raised initiation in the presence of 50 mg/l AC. Fifty milligrams per liter AC adsorbed approximately 0.36 mg/l of combined cytokinins or 7.2 mg adsorbed per gram AC. This was lower than our original estimate that 50 mg/l AC may be able to adsorb about 0.9 mg of each cytokinin. Van Winkle (2000), using a liquid medium based on 1/2-BLG and the same level of AC that we use, observed that 0.3 g/l AC-T1 adsorbed 63.4 mg/l to 64.5 mg/l BA after 6 h or 15 days, respectively. Ebert et al. (1993) reported that 2.5 g/l AC adsorbed 99.4% (11.12 mg/l) of the BA in a 5×10-5 M (11.25 mg/l) liquid solution within 5 days. Both Ebert and Taylor (1990) and Van Winkle et al. (2003) observed faster adsorption of materials by AC in liquid media.
Removal of ABA and initiation rates across several years and media with multiple families
Initiation results for 1996–1999 for seed from many open-pollinated trees on several media are shown in Table 9. Initiation on medium 505 averaged 8.5% and 7.4% during the 1996 and 1997 experiments. With the addition of ABA, AgNO3, 8-Br-cGMP, and increased cytokinin [0.55 mg/l BA and 0.53 mg/l kinetin (medium 889)], initiation almost tripled, averaging 17.9% across 32 families. When cytokinins were optimized at 0.63 mg/l BA and 0.61 mg/l kinetin and ABA was removed (medium 1042), initiation was similar, averaging 18.2% across 19 families.
Survival of new initiations over time
During 1995 and 1996, a combination of new initiations from medium 505 and modifications thereof were tracked for survival from 18 open-pollinated families. In 1995, new initiations from 436 explants were tracked over 6 months, resulting in 97 surviving cultures—a 22% survival rate. In 1996, an additional 765 new initiations were followed for 4–6 months, resulting in 249 surviving cultures—a 33% survival rate. Figure 2 demonstrates how the initial phase of culture loss occurred. Approximately 80% of the new initiations never grew after transfer onto the multiplication medium. A further 5% did not grow initially but slowly recovered and began growth later. This population was not tracked long enough to observe culture decline after 6 months. In this experiment, a significant number of cultures were lost due to contamination.
The inability of new initiations of LP to become stable multiplying cultures represents a major limitation for future commercial use of SE technology. Culture survival after initiation was found to be 22–33% after 4–6 months. Most of the loss occurred when embryogenic tissue grown on the initiation medium failed to continue growth when transferred to a multiplication medium. Since we use the visual presence of at least three somatic embryos (seen through a dissecting scope) as an indicator of initiation, it is possible that many cultures begin the initiation process and then abort. An alternative hypothesis is that new embryogenic tissue formed on the initiation medium cannot adjust to changes in medium ingredients as the tissue is transferred to multiplication medium.
Conclusions
When this research began, initiation for LP on medium 505 (Pullman and Johnson 2002) averaged 8% across 25 diverse cone collections over 2 years (Table 9). Medium 505 was developed through the use of megagametophyte osmotic profile research (Pullman 1997), modeling AC uptake of 2, 4-dichlorophenyloxyacetic acid (Toering 1995) and research aimed at understanding the effect of pH and AC on mineral availability (Van Winkle 2000; Van Winkle and Pullman 2003; Van Winkle et al. 2003). We continued with the approach of improving the tissue culture process by studying natural embryo development and the change in the availability of components in a medium over time by testing the effect on initiation of media supplementation with ABA concentrations similar to the endogenous ABA levels found in seed tissues as well as the addition of compounds involved in hormone signaling or the enhancement/repression of ethylene. The modification of medium 505 through the addition of 3.7 μM ABA, 20 μM AgNO3, 10 μM 8-Br-cGMP, and increased levels of cytokinins (2.44 μM BA and 2.46 μM kinetin) almost tripled initiation to 17.9% across 32 families. With cytokinins further optimized at 2.80 μM BA and 2.83 μM kinetin and the ABA removed, initiation across 19 families averaged 18.2%.
Abbreviations
- ABA :
-
Abscisic acid
- AC :
-
Activated carbon
- BA :
-
6-Benzylaminopurine
- 8-Br-cGMP :
-
Guanosine 3′,5′-cyclic monophosphate, 8-bromo-, sodium salt
- NAA :
-
α-Naphthaleneacetic acid
References
Afele JC, Preveen KS (1995) Somatic embryogenesis in blue spruce (Picea pungens Englemann). In: Jain S, Gupta P, Newton RE (eds) Somatic embryogenesis in woody plants, vol 3. Kluwer, Dordrecht, pp 99–109
Aitken-Christie J, Parkes BD (1996) Improved embryogenesis process for initiation and maturation. International application under the patent cooperation treaty (PCT). WO 96/37096, international publication date: 28 November 1996
Al-Khayri JM, Al-Bahrany AM (2001) Silver nitrate and 2-isopentyladenine promote somatic embryogenesis in date palm. Sci Hortic 89:291–298
Auboiron E, Darron MP, Michaux-Ferriere N (1990) Influence of atmospheric gases, particularly ethylene, on somatic embryogenesis of Hevea brasiliensis. Plant Cell Tissue Organ Cult 21:31–37
Becwar MR, Pullman GS (1995) Somatic embryogenesis in loblolly pine (Pinus taeda L.). In: Mohan Jain S, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 3. Gymnosperms. Kluwer, Dordrecht, pp 287–301
Becwar MR, Nagmani R, Wann SR (1990) Initiation of embryogenic cultures and somatic embryo development in loblolly pine (Pinus taeda). Can J For Res 20:810–817
Becwar M, Chesick E, Handley L III, Rutter M (1995) Method for regeneration of coniferous plants by somatic embryogenesis. U.S. Patent 5,413,930 Issued May 9, 1995
Beyer EM (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58:268–271
Biddington NL (1992) The influence of ethylene in plant tissue culture. Plant Growth Regul 11:173–187
Biddington NL, Sutherland RA, Robinson HT (1988) Silver nitrate increases embryo production in anther culture of Brussels sprouts. Ann Bot 62:181–185
Carrier DJ, Kendall EJ, Bock CA, Cunningham JE, Dunstan DI (1999) Water content, lipid deposition, and (+)-abscisic acid content in developing white spruce seeds. J Exp Bot 50:1359–1364
Cho U, Kasha KJ (1989) Ethylene production and embryogenesis from anther cultures of barley (Hordeum vulgare). Plant Cell Rep 8:415–417
Durzan DJ, Gupta PK (1987) Somatic embryogenesis and polyembryogenesis in Douglas-fir cell suspension culture. Plant Sci 52:229–235
Ebert A, Taylor F (1990) Assessment of the changes of 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult 20:165–172
Ebert A, Taylor F, Blake J (1993) Changes of 6-benzylaminopurine and 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult 33:157–162
Fowke LC, Attree SM, Binarova P, Galway ME, Wang H (1993) Conifer somatic embryogenesis for studies of plant cell biology. Cell Dev Biol 31:1–7
Fuentes SRL, Calheiros MBP, Nanetti-Filho J, Vieira LGE (2000) The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue Organ Cult 60:5–13
Gehring CA (1999) Natriuretic peptides—a new class of plant hormone? Ann Bot 83:329–334
Gupta PK, Durzan DJ (1987) Biotechnology of somatic polyembryogenesis and plantlet regeneration in loblolly pine. Biotechnology 5:147–151
Gupta PK, Pullman GS (1991) Method for reproducing coniferous plants by somatic embryogenesis using abscisic acid and osmotic potential variation. U.S. Patent No. 5036007. Issued July 30, 1991
Handley L III (1987) Method for regeneration of coniferous plants by somatic embryogenesis in culture media containing abscisic acid. U.S. Patent 5,677,185. October 14, 1997
Handley L III (1999) Method for regeneration of coniferous plants by somatic embryogenesis in culture media containing abscisic acid. U.S. Patent 5,856,191. January 5, 1999
Hutchinson CE, Kieber JJ (2002) Cytokinin signaling in Arabidopsis. Plant Cell [Suppl]:S47-S59
Kapik RH (1994) Changes in abscisic acid concentration during zygotic embryogenesis in Loblolly pine (Pinus taeda) as determined by indirect ELISA. PhD thesis, Institute of Paper Science and Technology, Atlanta, Ga.
Kapik RH, Dinus RJ, Dean JF (1995) Abscisic acid and zygotic embryogenesis in Pinus taeda. Tree Physiol 15:405–409
Kepinski S, Leyser O (2002) Ubiquination and auxin signaling: A degrading story. Plant Cell [Suppl]:S81-S95
Kong LS, Yeung EC (1994) Effects of ethylene and ethylene inhibitors on white spruce somatic embryo maturation. Plant Sci 104:71–80
Kong L, Yeung E (1995) Effects of silver nitrate and polyethylene glycol on white spruce (Picea glauca) somatic embryo development: enhancing cotyledonary embryo formation and endogenous ABA content. Physiol Plant 93:298–304
Kong L, Attree SM, Fowke LC (1997) Changes in endogenous hormone levels in developing seeds, zygotic embryos and megagametophytes in Picea glauca. Physiol Plant 101:23–30
Kong L, Attree SM, Evans DE, Binarova P, Yeung EC, Fowke LC (1999) Somatic embryogenesis in white spruce: studies of embryo development and cell biology. In: Mohan Jain S, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 4. Kluwer, Dordrecht, pp 1–28
Krueger SA, Becwar MR (2001) Recovering cryopreserved conifer embryogenic cultures. Canadian Intellectual Property Office, Patent application. Filed May 25, 2000
Kumar PP, Richard WJI, Thorpe TA (1989) Ethylene and carbon dioxide accumulation, and growth of cell suspension cultures of Picea glauca (white spruce). J Plant Physiol 135:592–596
Kvaalen H (1994) Ethylene synthesis and growth in embryogenic tissue of Norway spruce: effects of oxygen, 1-aminocyclopropane-1-carboxylic acid, benzyladenine and 2,4-dichlorophenoxyacetic acid. Physiol Plant 92:109–117
Li XY, Huang FH (1996) Induction of somatic embryogenesis in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 32:129–135
Li XY, Huang FH, Gbur EE Jr (1998) Effect of basal medium, growth regulators and phytagel concentration on initiation of embryogenic cultures from immature zygotic embryos of loblolly pine (Pinus taeda L.). Plant Cell Rep 17:298–301
MacKay J, Becwar MR, Park YS, Perfetti C, Cordero JC, Pullman G, Lockart L (2001) Genetics of somatic embryogenesis in loblolly pine. In: Dean J (ed) Proc South For Tree Improve Conf. School of Forest Resources, University of Georgia, pp 40–47. http://www.forestry.uga.edu/warnell/sftic/files/ExtendedAbstractBookv4.pdf
Park YS (2002) Implementation of somatic embryogenesis in clonal forestry: technical requirements and deployment strategies. Ann For Sci 59:651–656
Pullman GS (1997) Osmotic measurements of whole ovules during loblolly pine embryo development. In: Proc TAPPI Biol Sci Symp. TAPPI Press, Atlanta, Ga., pp 41–48
Pullman GS, Buchanan M (2003) Loblolly pine (Pinus taeda L.): Stage-specific elemental analyses of zygotic embryo and female gametophyte tissue. Plant Sci 164:943–954
Pullman GS, Gupta PK (1991) Method for reproducing coniferous plants by somatic embryogenesis using adsorbent materials in the development stage. U.S. Patent No. 5034326. Issued July 23, 1991
Pullman GS, Johnson S (2002) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation rates. Ann For Sci 59:663–668
Pullman GS, Webb DT (1994) An embryo staging system for comparison of zygotic and somatic embryo development. In: TAPPI R&D Div Biol Sci Symp. TAPPI Press, Atlanta, Ga., pp 31–34
Roustan JP, Latche A, Fallot J (1989) Stimulation of Daucus carota somatic embryogenesis by inhibitors of ethylene synthesis: cobalt and nickel. Plant Cell Rep 8:182–185
Roustan JP, Latche A, Fallot J (1990) Control of carrot somatic embryogenesis by AgNO3, an inhibitor of ethylene action: effect on arginine decarboxylase. Plant Sci 67:89–95
Schultz RP (1999) Loblolly—the pine for the twenty-first century. New For 17:71–88
Selby C, McRoberts WC, Hamilton JTG, Harvey BMR (1996) The influence of culture vessel head-space volatiles on somatic embryo maturation in Sitka spruce [Picea sitchensis (Bong.) Carr.] by butylated hydroxytoluene, a volatile antioxidant released by Parafilm. Plant Cell Rep 16:192–195
Smith D (1996) Growth medium. U.S. Patent No. 5,565,355. Issued October 15, 1996
Sutton B (2002) Commercial delivery of genetic improvement to conifer plantations using somatic embryogenesis. Ann For Sci 59:657–661
Tautorus TE, Fowke LC, Dunstan DI (1991) Somatic embryogenesis in conifers. Can J Bot 69:1873–1899
Timmis R (1998) Bioprocessing for tree production in the forest industry: conifer somatic embryogenesis. Biotechnology 14:156–166
Toering A (1995) Examining the relationship between 2,4-dichlorophenoxyacetic acid and activated charcoal in plant tissue culture media, MSc. thesis, Institute of Paper Science and Technology, Atlanta, Ga.
Van Winkle S (2000) The effect of activated carbon on the organic and elemental composition of plant tissue culture medium. PhD thesis, Institute of Paper Science and Technology, Atlanta, Ga.
Van Winkle S, Pullman GS (2003) The combined impact of pH and activated carbon on the elemental composition of plant tissue culture media. Plant Cell Rep 21:(in press)
Van Winkle S, Johnson S, Pullman GS (2003) The impact of Gelrite and activated carbon on the elemental composition of plant tissue culture media. Plant Cell Rep (in press)
Wann SR, Becwar MR, Nagmani R, Feirer RP, Johnson M (1989) Biochemical differences between embryogenic and nonembryogenic calli of conifers. Trees 3:173–178
Walden R (1998) The alphabet soup of plant intracellular signaling: enter cyclic nucleotides. Curr Opin Plant Biol 5:419–423
Wear DN, Greis JG (2001) Draft November 19, 2001: The southern forest resource assessment summary report. http://www.srs.fs.fed.us/sustain/
Acknowledgements
We thank the member companies of the Institute of Paper Science and Technology for financial support and Boise Cascade, Westvaco, Union Camp, and Georgia Pacific for cone collections. We also thank Dr. Gary Peter for discussions concerning potential auxin or cytokinin signaling compounds. We are grateful for the help of J. Ezzell, X. Feng, A. Holmgren, S. Johnson, H. Schindler, M. Snyder, and S. Sayre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.C. Phillips
Rights and permissions
About this article
Cite this article
Pullman, G.S., Namjoshi, K. & Zhang, Y. Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation with abscisic acid and silver nitrate. Plant Cell Rep 22, 85–95 (2003). https://doi.org/10.1007/s00299-003-0673-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0673-y