Abstract
Garden cress (Lepidium sativum L., Brassicaceae) is one of the most popular leafy vegetables which is widely used, and has also various medicinal properties and industrial usage. Small and very delicate leaves of this short period and fast growing species cause lots of crop losses along production to consumption; so it was supposed that increase in thickness and size of the leaves via induction of polyploidy possibly will improve post-harvest quality. Primary trial proved that seed treatments, via immersion of dry and wet seeds in different concentrations and durations of colchicine, were completely ineffective. Thereafter dropping method was conducted on apical bud of cotyledon and two true leaf stages with different concentrations of colchicine (0, 0.05, 0.1, 0.2, 0.5 and 0.75% w/v). Treatment on cotyledon stage was not fruitful because of sensitivity to colchicine and dying of small seedlings; but apical bud treatment in two true leaf stage resulted in inducing some polyploid plants. The best result was obtained by 0.5% colchicine concentration, inducing 9.33% tetraploid plants. Chromosome counting and flowcytometric analysis of morphologically putative plants confirmed chromosome doubling in garden cress from 2n = 2x = 16 to 2n = 4x = 32. Tetraploid plants comparing diploid ones specified by increasing in leaf size and thickness, stem diameter, stomata size, number of chloroplasts in stomata guard cells, seed weight and on the contrary, decreasing in stomata count and height of plants, percentage of seed germination and also germination rate.
Key message
In this research, we have tested various methods and different levels of colchicine for the polyploidy induction in garden cress, and the results of polyploidy induction have been studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Garden cress (Lepidium sativum L.) is a diploid species (2n = 2x = 16) from the family Brassicaceae. Easy growing, high nutrition value and light spiciness results in that it is one of the most popular and widely consumed fresh leafy vegetable. It is also very famous in folk medicine for hepato-protective, anti hypertensive, diuretics, fracture and respiratory disorder healing, anti-inflammation, laxative and many other therapeutic applications (Eddouks et al. 2005; Gilani et al. 2012). It has the potential for biofuel usage (Nehdi et al. 2012), because of the fast and short period growing, as well. The genus Lepidium has several species that are found in the most temperate and subtropical regions (Wadhwa et al. 2012). Ethiopia is known as the primary center of origin for L. sativum, but it is native to southwest Asia where its cultivation started many centuries ago and from where spread to Western Europe. Garden cress is a very old crop and is known in various languages with different names include the Persian word Turehtezuk, the Latin Nasturtium, the Greek Kardamon and Arabic Tuffa and Hurf (Hernández Bermejo and EstebanLeon 1994).
Garden cress is an annual plant the basal leaves have long petioles and leaf margin combination of smooth and slash (Wadhwa et al. 2012). The inflorescences are dense and the flowers have white or pink petals (Grubben and Denton 2004). It can grow in any type of climate and soil condition without extensive addition of fertilizers. Weeds also cannot develop in garden cress field because of its fast germination and growing.
Polyploidy has been identified as one of the important factors in the evolution of plants (Stebbins 1971). Polyploidy is unique field of plant breeding that has an important role in creating morphological and physiological changes in plants (Dhooghe et al. 2011). Ploidy manipulation is considered as a valuable tool for generating diversity, which increases the range of germplasm and provides the basis for plant breeding (Madon et al. 2005; Nakano et al. 2006; Stanys et al. 2006; Ye et al. 2010). Polyploidy probably can increase the size of leaves, flowers, the thickness of the roots and the stress tolerance and also can change the color of the leaves and cause a compact growth of the plant (Liu et al. 2007). Induction of polyploidy via changes in cell division activities resulting in the increase of nuclear DNA content (Sugiyama 2005), has been introduced since many years ago (Blakeslee and Avery 1937). Polyploidy is a breeding tool to increase the diversity of ornamental plants (Shao et al. 2003; Ye et al. 2010), resistance to diseases and environment stresses (Zhang et al. 2010) and also increasing and variation in content of secondary metabolites (Jesus-Gonzalez and Weathers 2003; Majdi et al. 2010; Moeini et al. 2013). Out of many applicable methods for inducing changes in chromosome number, the use of chemicals especially colchicine is a well-known method (Jaskani et al. 2005; Thao et al. 2003). Colchicine prevents the separation of chromosomes by connecting to tubulin dimmers and preventing the formation of microtubules (Dhawan and Lavania 1996; Petersen et al. 2003).
Small and very delicate leaves of garden cress cause lots of crop losses along production to consumption; so in this study induction of polyploidy was examined as a tool for improving its quality via increasing leaf size and thickness that morphological and cytological characteristics of the plants are analyzed.
Materials and methods
Polyploidy induction
Six separate experiments were designed to induce polyploidy. In the first and the second experiments dry and wet seeds implemented by immersion in different concentrations (0, 0.05, 0.1, 0.2 and 0.5% w/v) and durations (4, 8, 12 and 24 h) of colchicine in three replications using factorial experiment based on completely randomized design. For the third and the fourth experiments, at first seeds of garden cress were germinated in cultivation trays in greenhouse at 25 °C, then apical buds treated respectively in cotyledon and two true leaf stages with dropping 5 µl of different concentrations of colchicine (0, 0.05, 0.1, 0.2, 0.5 and 0.75% w/v) for three consecutive days. The experiments were designed completely randomized and each replication contained 72 seedlings. At last because of ineffectiveness of primary dry and wet seed treatments, they were repeated by higher concentrations of colchicine (0, 0.5, 0.75, 1 and 1.5% w/v) as fifth and sixth experiments. Seedlings were grown in greenhouse for 2 months until flowering and seed maturation.
Ploidy level assessment
Chromosome counting
Mature seeds of diploid and putative tetraploid plants were germinated on damp filter paper in petri dishes at room temperature. For somatic chromosome counting 0.5–1 cm long fresh root tips were collected from quickly growing seeds. Root tips were pre-treated with the 0.002 M 8-hydroxyquinoline for 5 h at 4 °C. The root tips were washed with distilled water three times (each 7 min) at room temperature. They were fixed in fresh Carnoy Fixative (ethanol:glacial acetic acid; 3:1) for at least 24 h at 4 °C. Fixed root tips were subsequently washed three times with distilled water and preserved in 70% (v/v) ethanol at 4 °C in refrigerator until use. For cytological preparations, root tips hydrolyzed in 1 M HCl for 10 min at 60 °C in water bath and 2% aceto-carmine for 1 h were used as the stain for squash technique. Images of the chromosomes were captured with a digital camera (Sony, Japan) interfaced to a CX21 Olympus microscope (Olympus Optical Co. Ltd., Tokyo, Japan).
Flow-cytometric analysis
After colchicine treatments, ploidy level of plants was investigated by the method described by Loureiro et al. (2007). To extract plant nuclei, 25 mg leaf sample of L. sativum with the same mass of Solanum lycopericum cv. Stupicke, as internal standard (2C = 1.96 pg DNA, Doležel et al. 1992) was used. Then 1 ml of Woody Plant Buffer (WPB) was added to a petri dish containing the plant tissues, which was chopped by a sharp razor blade in ice-cold nucleic extraction buffer of Partec CyStain PI and filtrated through a 30 µm nylon-mesh (Partec, Münster, Germany). The isolated nuclei by 50 µl of RNase were added to prevent staining of double-stranded RNA, followed by adding 50 µl staining solution propidium iodide (PI, Fluka). The nuclei suspension was analyzed by BD FACSCanto II flow cytometer (BD Biosciences, Bedford, MA, USA) in order to determine genomic 2C DNA content by using BD FACSDiva™ Software. To analyze data were transferred to Flowing Software version 2.5.0 to be editable in Partec FloMax ver. 2.4e (Partec, Münster, Germany). By using the method of Dolezel et al. (2003) and Dolezel and Bartos (2005) the absolute DNA amount of a sample was calculated based on the values of the G1 peak means as follows: Sample 2C DNA (pg) = (Sample G1 peak mean/Standard G1 peak mean) × Standard 2C DNA (pg).
Morphological and physiological measurements
Morphologic characters such as plant height, petiole diameter, leaf area, leaf thickness, plant wet and dry weight, time and period of flowering, 1000-seeds weight, percent and rate of seed germination were measured. Furthermore leaf chlorophyll content (CCI) was evaluated by SPAD-502 (Konica Minolta). Density and dimensions of stomata were measured on the axial leaf epiderm by the nail varnish method (Smith et al. 1989) using a Sony digital camera interfaced to the CX21 Olympus microscope (Olympus Optical Co. Ltd.) at × 20 and × 100 magnification respectively, for 30 leaves from three confirmed diploid and tetraploid plants. For chloroplast count in stomata guard cells Huamán (1995) method was used. Pollen grains were collected from mature flowers at anthesis and their diameters were measured at × 100 objective under the light microscope for 30 samples.
Statistical analysis
Survival rate and tetraploidy induction which came from influence of colchicine was evaluated by analysis of variance. Induction efficiency = % seedling survival × % tetraploidy induction (Bouvier et al. 1994). Tetraploidy induction efficiency was calculated. Induction efficiency for tetraploidy ranges from 0 to 100, 0 shows no tetraploidy induction and 100 indicating that all plants are tetraploid. The average (n = 30) between diploid and tetraploid plants for width and density of stomata, length, chlorophyll count in stomata retaining cells, pollen grains size, fresh and dry weight, seed weight, germination percentage, germination rate and CCI by Student’s t test was evaluated.
Results and discussion
Ploidy induction
As mentioned in materials and methods, dry and wet treatment of seeds by different concentrations (0, 0.05, 0.1, 0.2 and 0.5% w/v) and durations (4, 8, 12 and 24 h) of colchicine (first and second experiment), was completely ineffective. Seed treatment by higher concentrations of colchicine (0, 0.5, 0.75, 1 and 1.5% w/v) also didn’t have any desired result; however duration of colchicine treatment significantly affected the rate of surviving plants (P < 0.01). In other word short time were ineffective and long time treatments had dramatic destructive effect on germination. This adverse result was not proposed before since seed treatment is known as the most practical method for induction of polyploidy in many plants (Omidbaigi et al. 2010; Quan et al. 2004). Dhamayanthi and Gotmare (2010) emphasized seed treatment was more efficient and reliable method in cotton as compared to other seedling and cuttings treatments.
In dropping experiments, treatment on cotyledon stage was not fruitful. Based on mean comparisons, 0.05 and 0.1% colchicine treatment on apical bud in two true leaf stage had not also any significant effect for induction of tetraploids comparing to control, but 0.2 and 0.5% colchicine were effective (Fig. 1a). The best rate of doubling occurred in 0.5%; however < 40% of seedlings were survive in this concentration (Fig. 1b). Though the colchicine induction efficiency, a defined index for considering seedling survival and tetraploid rate induction concurrently, determines that 0.5% colchicine was the most efficient treatment (Fig. 1c). Other researchers have also used this index for tetraploid induction analysis (Lehrer et al. 2008; Majdi et al. 2010). The inverse correlation between colchicine concentrations and survival rate observed in this study, which was confirmed by reports from other plants (Chakraborti et al. 1998; Khosravi et al. 2008; Majdi et al. 2010; Moieni et al. 2013; Sikdar and Jolly 1994). Also in vitro-polyploidy induction on Trachyspermum ammi by applying 0.05% (w/v) colchicine for 24 h on germinating seeds showed successful duplication of chromosome number in tetraploid plants with 11.53% efficiency (Noori et al. 2017).
Morphological and physiological measurements
Colchicine treatment reduced stem growth in the first month after treatment, but after that, both treated and non-treated seedlings grow similarly. Reducing the growth rate of plants treated with colchicine in the first month after treatment may be due to colchicine-induced physiological changes, which reduces the amount of cell division. Initial backwardness in growth has also been reported in other studies (Majdi et al. 2010; Sikdar and Jolly 1994). The most of first leaves of the tetraploid had a distorted appearance, but the subsequent leaves seemed normal. Plant height, wet and dry weight of diploid and tetraploid plants was significantly (P < 0.01) different (Fig. 2; Table 1). Petiole diameter, leaf thickness and leaf area of tetraploid plants was also significantly (P < 0.01) different (Fig. 3; Table 1). The tetraploid plants had larger and thicker leaves (Fig. 4). In most plants, polyploidy induction creates larger reproductive and vegetative organs (Adaniya and Shira 2001). Derived plants by increased biomass and secondary metabolites are often more valuable for their improved fodder, ornamental or medicinal features (Gao et al. 1996). Length and width of stomata in tetraploid leaves were larger than those in diploid leaves (P < 0.01; Table 1); nevertheless stomata density was reduced to almost half in tetraploids (Table 1; Fig. 6). A reduction in the stomata density in tetraploids was observed in other plants as well (Chakraborti et al. 1998; Majdi et al. 2010; Omidbaigi et al. 2010; Pour Mohammadi et al. 2012). Chloroplast number in stomata guard cells increased by polyploidy. The number of chloroplasts in stomata guard cells of tetraploid plants was obviously more and typically doubled up diploids (Fig. 5; Table 1). Pollen grains were significantly larger and furthermore seed weight increased in tetraploids (P < 0.01; Table 1; Figs. 7, 8). The enlarged stomata, chloroplast number in guard cells, pollen grains, seed weight, seed size, petiole diameter, flowering time and period of induced tetraploid are also observed in other plants (Majdi et al. 2010; Tavan et al. 2015; Thao et al. 2003). It was reported increasing DNA content led to further cell growth and increasing cell size in tetraploid Arabidopsis, Mitracarpus hirtus and Artemisia annuain (Banyai et al. 2010; Breuer et al. 2007; Pansuksan et al. 2014).
There was no significant difference for chlorophyll content in diploid and tetraploid plants. Chlorophyll content has been explored to be higher in polyploid plants comparing with lower ploidy levels (Molin et al. 1982). However the effects of increased ploidy level cannot be anticipated all the time; for example the chlorophyll content was reported constant in different ploidy levels of Atriplex confertifolia (Warner and Edwards 1989) and Acacia mearnsii (Mathura et al. 2006) and even lower in tetraploid sugar beet genotypes compared with diploid ones (Beyaz et al. 2013). Tetraploidy induction caused decreasing in plant height, delay in flowering and expanding the flowering period compared to diploids (Table 1). Decreasing in height of induced polyploid plants has been perceived before in Rudbeckia species and hybrids (Oates et al. 2012), sugar beet (Beyaz et al. 2013) and Thymus persicus (Tavan et al. 2015).
Tetraploid plants unlike diploids produced a few seeds which had lower percentage and slower germination rate. The mean germination rate were 76% and 4/16 in induced tetraploid plants, while they were 98% and 5/20 in diploid controls (P < 0.01), respectively. It is determined that auto-tetraploid seeds typically germinate slower than diploid ones (Levin 2002). Polyploid watermelon seeds have poor germination and low seedling vigor obviously (Jaskani et al. 2006). Seeds by equal biomass of tetraploid plants germinated faster and to a higher percentage than those from diploid plants in Dactylis glomerata L. (Bretagnolle et al. 1995). Tetraploids in Vicia cracca L. had heavier seeds than diploids and greater germination rates (Eliášová and Münzbergová 2014).
Cytological studies
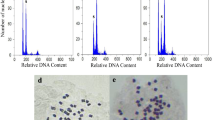
Although it was difficult to count very small-sized chromosomes in L. sativumin initial observations, repeated attempts were shown the natural samples had a chromosome number of 2n = 2x = 16 (Fig. 9a). Sharma and Sikka (1976) determined the chromosome number of L. sativum as 2n = 16. Johnston et al. (2005) in evolution of genome size in Brassicaceae reported L. sativum 3n = 24. Chromosome counts for root meristematic cells of colchicine-treated plants was significantly (P < 0.01) increased evidently doubled (2n = 4x = 32) in the induced tetraploid plants compared with the diploids (2n = 2x = 16). Chromosome counting is the most direct method for analysis of ploidy (Tang et al. 2010). Colchicine is an agent that inhibits the formation of spindle fibers and effectively arrests mitosis at the metaphase stage which is used in plant breeding programs (Majdi et al. 2010; Pourmohammadi et al. 2012).
DNA content estimated using flow-cytometric (FCM) analysis, confirmed the results of chromosome counting. It was clearly determined in FCM histograms, associating the standard (Solanum lycopericum cv. Stupicke) and garden cress nucleus peaks, chromosome duplication by colchicine treatment was achieved (Fig. 10). According to results the mean 2C DNA contents determined for diploid and tetraploid plants 1.39 and 2.69 pg, respectively. The mean CV for FCM measurements of both sample and reference standard plants were < 6%. Johnston et al. (2005) was stated that Brassicaceae plants have a relatively narrow range (0.16 pg < 1C < 1.95 pg) of DNA content. This is the first report for quantifying the DNA content of L. sativum.
Conclusion
The study demonstrated that viable tetraploid plants with superior characteristics, compared to diploid plants, could be produced in garden cress. The trials indicated that the time of colchicine treatment is very critical for effectiveness of chromosome doubling induction in this plant. Seed treatment by colchicine which is the most well-known manner in many species, but didn’t work in garden cress at all, because higher concentration colchicine had destructive effect and prevented germination entirely. It may be interpreted in relation to very fast germination of this species. Based on this hypothesis, it is difficult to find the proper condition for seed treatment in very fast germinating plants, though further studies are necessary. Apical buds treatment with 0.5% w/v colchicine in two true leaf stages with 5 µl of colchicine by dropping method for three consecutive days induced tetraploidization in L. sativum. The accurate morphological assessment showed obvious differences which might be a useful and confidential tool for determining ploidy level in of plants. However the traits like size of leaves or seeds are not certain enough but the number of chloroplasts in stomata guard cells seems an easy and very reliable characteristic for this purpose.
This is the first report of the successful induction of tetraploids in L. sativum using colchicine treatment. As mentioned before small and very delicate leaves of garden cress cause lots of crop losses along production to consumption. Tetraploidy improved its quality via increasing leaf size and thickness and hopefully it result in introduction of new lush cultivar of this valuable leafy vegetable with better market and consumption properties.
References
Adaniya S, Shira D (2001) In vitro induction of tetraploid ginger (Zingiber officinali Roscoe) and its pollen fertility and germinability. Sci Hortic-Amsterdam 88:277–287
Banyai W, Sangthong R, Karaket N, Inthima P, Mii M, Supaibulwatana K (2010) Over production of artemisinin in tetraploid Artemisia annua L. Plant Biotechnol 27:427–433
Beyaz R, Alizadeh B, Gürel S, Oscan SF, Yıldız M (2013) Sugar beet (Beta vulgaris L.) growth at different ploidy levels. Caryologia 66:90–95
Blakeslee AF, Avery AG (1937) Methods of inducing doubling of chromosomes in plants: by treatment with colchicine. J Hered 28:393–411
Bouvier L, Pillon FR, Lespinasse Y (1994) Oryzalin as an efficient agent for chromosome doubling of haploid apple shoots in vitro. Plant Breed 113:343–346
Bretagnolle F, Thompson JD, Lumaret R (1995) The influence of seed size variation on seed germination and seedling vigour in diploid and tetraploid Dactylis glomerata L. Ann Bot-London 76:607–615
Breuer C, Stacey NJ, West CE, Zhao Y, Chory J, Tsukaya H, Azumi Y, Maxwell A, Roberts K, Sugimoto-Shirasu K (2007) BIN4, a novel component of the plant DNA topoisomerase VI complex, is required for endo-reduplication in Arabidopsis. Plant Cell 19:3655–3668
Chakraborti SP, Vijayan K, Roy BN, Qadri SMH (1998) In vitro induction of tetraploidy in mulberry (Morus alba L.). Plant Cell Rep 17:799–803
Dhamayanthi KPM, Gotmare V (2010) Induction of polyploidy in two diploid wild cotton (G. armourianum and G. aridum) species by colchicine treatment. Electron J Plant Breed 1(4):966–972
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87:81–89
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult 104:359–373
Dolezel J, Bartos J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot-London 95:99–110
Dolezel J, Sgorbati S, Lucretti S (1992) Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol Plant 85:625–631
Dolezel J, Bartos J, Voglmayr H, Greilhuber J (2003) Nuclear DNA content and genome size of trout and human. Cytometry 51:127–129
Eddouks M, Maghrani M, Zeggwagh NA, Michel JB (2005) Study of the hypoglycaemic activity of Lepidium sativum L. aqueous extract in normal and diabetic rats. J Ethnopharmacol 97:391–395
Eliasova A, Munzbergova Z (2014) Higher seed size and germination rate may favour autotetraploids of Vicia cracca L. (Fabaceae). Biol J Linn Soc 113:57–73
Gao SL, Zhu DN, Cai ZH, Xu DR (1996) Autotetraploid plants from colchicine treated bud culture of Salvia miltiorrhiza. Plant Cell Tissue Organ Cult 47:73–77
Gilani AH, Rehman NU, Mehmood MH, AlKharfy KM (2012) Species differences in the antidiarrheal and antispasmodic activities of Lepidium sativum and insight into underlying mechanisms. Phytother Res 27(7):1086–1094
Grubben GJH, Denton OA (2004) Plant resources of Tropical Africa, 2. Vegetables. PROTA Foundation, Wageningen
Hernandez Bermejo J, EstebanLeon J (1994) Neglected crops: 1492 from a different perspective. Food and Agriculture Organization of the United Nations, Rome
Huaman Z (1995) Tecnicas citologicas para determinar el numero cromosomico y la fertilidad de las papas. Centro Internacional de la Papa, Lima, p 18
Jaskani MJ, Kwon SW, Kim DH (2005) Comparative study on vegetative, reproductive and qualitative traits of seven diploid and tetraploid watermelon lines. Euphytica 145:259–268
Jaskani MJ, Kwon SW, Kim DH, Abbas H (2006) Seed treatments and orientation affects germination and seedling emergence in tetraploid watermelon. Pak J Bot 38:89
Jesus-Gonzalea LD, Weathers PJ (2003) Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep 21:809–813
Johnston JS, Pepper AE, Hall AE, Chen ZJ, Hodnett G, Drabek J, Lopez R, Price HJ (2005) Evolution of genome size in Brassicaceae. Ann Bot-London 95:229–235
Khosravi P, Kermani MJ, Nematzadeh GA, Bihamta MR, Yokoya K (2008) Role of mitotic inhibitors and genotype on. chromosome doubling of Rosa. Euphytica 160:267–275
Lehrer JM, Mark HB, Lubell JD (2008) Induction of tetraploidy in meristematically active seeds of Japanese barberry (Berberis thunbergii var. Atropurpurea) through exposure to colchicine and oryzalin. Sci Hortic-Amsterdam 119:67–71
Levin DA (2002) The role of chromosomal change in plant evolution. Oxford University Press, Oxford
Liu G, Li Z, Bao M (2007) Colchicine-induced chromosome doubling in Platanus acerifolia effect on plant morphology. Euphytica 157:145–154
Loureiro J, Rodriguez E, Dolezel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot-London 100(4):875–888
Madon M, Clyde MM, Hashim H, Mohdyusuf Y, Mat H, Saratha S (2005) Polyploidy induction of oil palm through colchicine and oryzalin treatments. J Oil Palm Res 17:110–123
Majdi M, Karimzadeh G, Malboobi MA, Omidbaigi R, Mirzaghaderi G (2010) Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): morphological, physiological, cytological and phytochemical changes. Hortscience 45(1):1–6
Mathura S, Fossey A, Beck S (2006) Comparative study of chlorophyll content in diploid and tetraploid black Wattle (Acacia mearnsii). Forestry 79(4):381–388
Moeini A, Abdoli M, NaghdiBadi HA (2013) Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea. Acta Physiol Plant 35:2075–2083
Molin WT, Meyers SP, Baer GR, Schrader LE (1982) Ploidy effects of isogenic populations of alfalfa II. Photosynthesis, chloroplast number, ribulose-1,5-bisphosphate carboxylase, chlorophyll, and DNA in protoplasts. Plant Physiol 70:1710–1714
Nakano M, Nomizu T, Mizunashi K, Suzuki M, Mori S, Kuwayama S, Hayashi M, Umehara H, Oka E, Kobayashi H (2006) Somaclonal variation in Tricyrtis hirta plants regenerated from 1-year-old embryogenic callus cultures. Sci Hortic 110:366–371
Nehdi IA, Sbihi H, Tan CP, Al-Resayes SI (2012) Garden cress (Lepidium sativum L.) seed oil as a potential feedstock for biodiesel production. Bioresour Technol 126:193–197
Noori SA, Norouzi M, Karimzadeh G, Shirkool K, Niazian M (2017) Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tissue Organ Cult 130:543–551
Oates KM, Ranney TG, Touchell DH (2012) Influence of induced polyploidy on fertility and morphology of Rudbeckia species and hybrids. Hortscience 47:1217–1221
Omidbaigi R, Mirzaeea M, Hassani ME, Sedghi-Moghadam M (2010) Induction and identification of polyploidy in basil (Ocimum basilicum) medicinal plant by colchicine treatment. Int J Plant Prod 4(2):87–98
Pansuksan K, Sangthong R, Nakamura I, Mii M, Supaibulwatana K (2014) Tetraploid induction of Mitracar pushirtus by colchicine and its characterization including antibacterial activity. Plant Cell Tissue Organ Cult 117:381–391
Petersen KK, Hagberg P, Kristiansen K (2003) Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult 73:137–146
Pourmohammadi P, Moieni A, Ebrahimi A, Javidfar F (2012) Doubled haploid plants following colchicine treatment of microspore-derived embryos of oilseed rape (Brassica napus). Plant Cell Tissue Organ Cult 108(2):251–256
Quan K, Guolu L, Qigao G, Xiaolin L (2004) Polyploid induction of Arctium lappa by colchicine. Plant Physiol Commun 40:157–158
Shao J, Chen C, Deng X (2003) In vitro induction of tetraploid in pomegranate (Punica granatum). Plant Cell Tissue Organ Cult 75:241–246
Sharma AK, Sikka K (1976) Chromosome studies in Cruciferae. Res Bull Univ Calcutta Cytogenetics Lab 3:33–34
Sikdar AK, Jolly MS (1994) Induced polyploidy in mulberry (Morus spp.): induction of tetraploids. Sericologia 34:105–116
Smith S, Weyers JDB, Berry WG (1989) Variation in stomatal characteristics over the lower surface of Commelina communis leaves. Plant Cell Environ 12:653–659
Stanys V, Weckman A, Staniene G, Duchovskis P (2006) In vitro induction of polyploidy in Japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult 84:263–268
Stebbins GL (1971) Chromosomal evolution in higher plants. Edward Arnold, London
Sugiyama S (2005) Polyploidy and cellular mechanisms changing leaf size: comparison of diploid and autotetraploid populations in two species of Lolium. Ann Bot-London 96:931–938
Tang ZQ, Chen DL, Song ZJ, He YC, Cai DT (2010) In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tissue Organ Cult 102:213–220
Tavan M, Mirjalili MH, Karimzadeh G (2015) In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics ofThymuspersicus (Lamiaceae). Plant Cell Tissue Organ Cult 122:573–583
Thao NTP, Ureshino K, Miyajima I, Ozaki Y, Okubo H (2003) Induction of tetraploids in ornamental Alocasia through colchicine and oryzalin treatments. Plant cell Tissue Organ Cult 72:19–25
Wadhwa S, Panwar MS, Agrawal A, Saini N, Patidar N (2012) A review on pharmacognostical study of Lepidium sativum. Adv Res Pharm Biol 2(4):316–323
Warner DA, Edwards GE (1989) Effects of polyploidy on photosynthetic rates, photosynthetic enzymes, contents of DNA, chlorophyll, and sizes and numbers of photosynthetic cells in the C4 dicot Atriplex confertifolia. Plant Physiol 91:1143–1151
Ye YM, Tong J, Shi XP, Yuan W, Li GR (2010) Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci Hortic-Amsterdam 124:95–101
Zhang XY, Hu CG, Yao JL (2010) Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J Plant Physiol 167:88–94
Author information
Authors and Affiliations
Contributions
AA and ML conducted the experiments and wrote the manuscript, MN in the cytogenetic section helped and GK helped to improve the manuscript and Flow cytometric study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
The experiments were performed according to the current laws of Islamic Republic of Iran.
Additional information
Communicated by Alison M.R. Ferrie.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aqafarini, A., Lotfi, M., Norouzi, M. et al. Induction of tetraploidy in garden cress: morphological and cytological changes. Plant Cell Tiss Organ Cult 137, 627–635 (2019). https://doi.org/10.1007/s11240-019-01596-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01596-5