Abstract
Artemisia annua L. is presently the sole natural source of antimalarial drug artemisinin. We established dual cultures of A. annua callus or regenerated plantlets with endophytic Penicillium oxalicum B4 to explore endophyte-mediated effects on artemisinin biosynthesis. Although A. annua callus could not produce artemisinin with or without the endophyte, simultaneous growth stimulation of the endophyte and inhibition of A. annua callus were observed in dual cultures. In an in vitro dual culture of endophyte-regenerated plantlets, the endophyte enhanced growth and artemisinin content of host plant. The endophyte could simultaneously induce oxidative stress in regenerated plantlets through the generation of reactive oxygen species (ROS) including O •−2 and H2O2, which was then accompanied by the activation of antioxidant enzymes such as peroxidase, catalase and superoxide dismutase during the later stages. There was a significant increase in amorphadiene synthase (ADS) and amorpha-4,11-diene monooxygenase (CYP71AV1) transcripts in dual culture of endophyte-plantlets. The induced ROS could modulate the expression of those key genes for artemisinin biosynthesis and might be responsible for conversion of artemisinin acid into artemisinin production. Our results demonstrated that endophytic P. oxalicum B4 could be applied as a promising means to enhance artemisinin production in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
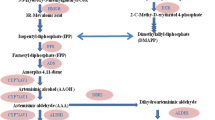
Artemisia annua L. (Asteraceae) is the important plant source of the potent antimalarial drug artemisinin. As to the industrial scale, artemisinin is isolated from leaves and flower buds of A. annua although the total synthesis of artemisin and semi-synthetic artemisinin through a fermentation process have been established in labs without the desired commercial feasibility till date (Corsello and Garg 2015). The artemisinin content in the plant materials, ranging from zero to 0.86 % (w/w, dry weight, DW), was disclosed to be dependent on the variety, cultivation and geographic condition (Singh et al. 1988; Woerdenbag et al. 1994).
It is widely believed that the synthesis of secondary metabolites in plants is part of the defense responses to elicitors and pathogenic attacks. In our previous reports (Wang et al. 2001; 2002), fungal elicitors have been tested for the elicitation of artemisinin production in hairy root cultures of A. annua. In recent years the defensive mutualism involving the production of host plants by microbial endophytes has received considerable attention (Hardoim et al. 2015). Endophytes, a special group of microorganisms living in the internal tissues of healthy plants without causing apparent pathogenic symptoms, may confer great benefits to plants such as growth enhancement, stimulated nutrient acquisition and stress tolerance (Rodriguez et al. 2009; Subramanian et al. 2015). Many endophytes protect host plants from natural enemies including animal herbivores and pathogenic microbe (Carroll 1988; Wang et al. 2015). Alkaloid toxins, fungitoxic sesquiterpene and other antibiotic metabolites from endophytes appeared to be the basis of insect resistance, mammalian toxicity and antaganism towards microbial pathogens (Tan and Zou 2001). Thus, endophytes are becoming a promising source of agriculturally and medicinally important metabolites. Lu et al. (2000) reported in vitro production of plant hormone indole-3-acetic acid (IAA) and new antifungal metabolites 3β,5α-dihydroxy-6β-acetoxy-ergosta-7,22-diene and 3β,5α-dihydroxy-6β-phenylacetyloxy-ergosta-7,22-diene by endophytic Colletotrichum sp. from A. annua. Out of 39 endophytes isolated from the internal stem tissue of A. annua, 21 were found to produce in vitro inhibitory substances to most of the tested phytopathogens (Liu et al. 2001). Daldinone C, D and new benzo[j]fluoranthene-based secondary metabolites produced by endophyte Hypoxylon truncatum IFB-18 from A. annua, were of substantial cytotoxic activity against the colon cancer SW1116 cells (Gu et al. 2007). In our previous studies (Wang et al. 2001; 2002), oligo- or polysaccharides derived from encophytic fungi of A. annual were used successfully to stimulate accumulation of artemisinin in A. annua hairy root cultures. With the elicitation of partially purified oligosaccharides, artemisinin production was increased by 51.6 % in the hairy root cultures (Wang et al. 2006). Recently the mycelia extracts of our most effective endophytic isolate (Penicillium sp.) were found to simulate both growth and artemisinin biosynthesis in A. annua seedling (Yuan et al. 2011). Taking into account that artemisinin was of allelopathic potential on weeds (Lydon et al. 1997), it is interesting to note that the accumulation of such an allelochemical can be stimulated by the endophte.
Production of callus and axenic plantlets under tissue culture conditions provided a simple and efficient system in understanding of plant–microbial interactions (Nowak et al. 1998). The dual culture of plant host calli and endophytes has been a simplified system to explore the interaction between grasses, crops and endophytic bactria or fungi (Sieber et al. 1990; Peters et al. 1998). Although there have been many reports on the complicated interactions between the host plants and endophytic fungi (Rodriguez et al. 2009), it is still unclear whether and how the endophyte is involved in the growth and artemisinin biosynthesis of the host A. annua. Better knowledge of their interactions may lead to understand artemisinin biosynthesis and develop new culture management for artemisinin production. In continuation of our characterization of biosynthetic regulation on artemisinin (Wang et al. 2009; Pan et al. 2014), we herewith wish to explore the interactions using dual culture of endophytic fungi with A. annua. As artemisinin production in A. annua was localized in glandular trichomes on the surface of leaves, shoots and flowers (Duke et al. 1994), it has been proved that artemisinin was produced in differentiated tissues such as shoots, leaves and flowers, but was undetected in callus or cell cultures (Ferreira and Janick 1996). In this study, undifferentiated callus-endophyte dual culture was tested to investigate growth interaction between the endophyte Penicillium oxalicum B4 and the callus of its host A. annua. The oxidative stress and its relationship with the activation of key genes for artemisinin biosynthesis, and artemisinin production were investigated in dual culture of endophyte-regenerated plantlets.
Materials and methods
Endophytic strain and culture condition
An endophytic isolate (B4) was isolated from fresh stems of healthy A. annua plants (collected in July 2008 in Zijin Mount of Nanjing, Jiangsu Province, China). The isolation of plant endophytes was carried out by the procedure as described previously by Schulz et al. (1993) with some modification. Briefly, random segments (ca. 1 cm-long) of stems were surface sterilized by immersing sequentially in 75 % (v/v) ethanol for 3 min, and in 50 % (v/v) solution of commercial available bleach (approximately 2.5 % (v/w) sodium hypochlorite) for 5 min, followed by rinsing thrice with sterilized distilled water. After dried with sterilized gauze, each surface-sterilized rod was cut aseptically into 0.5 cm long segments (both ends were discarded, so 25 segments per plant were prepared) and placed on WA (1.5 % water agar supplemented with 200 U/mL ampicillin and 150 μg/mL streptomycin sulphate to inhibit the bacterial growth) plates sealed with parafilm. All plates were kept in dark at 28 °C for up to 30 days depending on the growth rate of the endophytic fungi. The endophytic fungus isolates were successively transferred by tip-cut technique onto potato dextrose agar (PDA) plates till pure cultures were obtained (Strobel et al. 1996). Penicillium spp. was the most common endophytic species isolated from A. annua, making up 11 of the total 82 isolates. A strain of the Penicillium sp. (strain B4) was selected according to previous investigation (Yuan et al. 2011) and identified as P. oxalicum B4 through consulting documented data of its morphology as well as the 5.8S, 28S, ITS1 and ITS2 rDNA sequence (GenBank accession number FJ196840). The strain was cultured routinely and stored at 4 °C on PDA medium.
Callus induction and plantlet regeneration
The seeds of A. annua (cv. CQF39), obtained from Yunnan Academy of Agricultural Sciences in China, were sterilized with 0.1 % (w/v) mercuric chloride for 60 s and washed with sterile distilled water three times. Seeds were germinated on MS medium (Murashige and Skoog 1962) containing 2 % (w/v) sucrose at pH 5.8. Germination started within 7 days and plantlets were used for callus induction. Young leaves of 3-week old seedlings were cut into small pieces and callus was induced on MS medium containing 0.5 mg/L 6-benzylaminopurine (6-BA) and 1.0 mg/L 1-naphthaleneacetic acid (NAA) in continuous darkness at 25 ± 1 °C. Subcultures of the callus were carried out every 20 days and maintained at 25 ± 1 °C under 16 h photoperiod provided by coolwhite florescent lights at 100 μmol/m2 s.
Embryogenic callus (Fig. S1A) was induced on MS medium supplemented with 1.0 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D) (Choi et al. 2007). To produce regenerated plantlets, embryogenic calli were transferred to MS medium containing 5.0 mg/L 6-BA and 1.0 mg/L NAA and incubated at 25 ± 1 °C under 16 h photoperiod provided by coolwhite florescent lights at 100 μmol/m2 s. The regenerated plantlets were subcultured every month (Fig. S1B). Rooting plantlets of 30 days old on 1/2 MS medium containing 1.0 mg/L NAA were employed in the experiments of dual cultures.
Dual cultures
Dual culture of the endophyte with callus
Dual culture of endophyte-callus was established according to the previous report (Peters et al. 1998). 0.5 g fresh callus (2-week old) and a 5-mm agar plug from a 7-day-old fungal colony was inoculated simultaneously 2 cm from opposing sides of Petri dish (90 mm diam) containing 25 mL of callus multiplication medium, where callus and the endophyte were at a distance of approx. 4.5 cm from each other (Fig. S1C). Petri dishes were inoculated with callus or fungus alone as the respective control. Controls and dual cultures were grown at 25 ± 1 °C under 16 h photoperiod (light intensity 100 μmol/m2 s). The fungal colony diam in two directions: dk (diam in direction of the callus) and ds (perpendicular diam) every other day. The average radius (ra) was calculated as: ra = (dk + ds)/4. Callus growth was analyzed by determining the fresh weight (FW) of calli during culture. The dual culture was terminated when fungal growth reached the callus. Six replicates of dual culture and control were made.
Dual culture of the endophyte with plantlets
The regenerated plantlets were transferred to 1/2 MS medium containing 1.0 mg/L NAA for rooting induction. After 30-day culture each plantlet was inoculated with one piece of B4 mycelial disk (5 mm), which was placed upside down on the medium (0.5 cm away from the plantlet caudex) (Fig. S1D). An equal size of PDA was put as a control (Ren and Dai 2012). All treatments were conducted in a sterile environment and replicated at least three times to examine reproducibility. The dual culture was carried out under fluorescent lights at 100 μmol m2/s with a 16-h photoperiod at 25 ± 1 °C. To verify successful colonization by the endophyte, plantlet segments from the treatment were surface sterilized separately and incubated on PDA medium to reisolate the endophyte. Plantlet height, root length, and fresh weight (FW) were measured at 30 days post inoculation (dpi). The dry weight (DW) was obtained by drying the harvested plantlets at 50 °C in an oven until constant weight. Each treatment had 3 replicates, and each replicate consisted of 5 plantlets.
Measurement of reactive oxygen species (ROS) generation
During dual culture of endophyte-plantlets, O •−2 in whole plantlets was quantified by monitoring the nitrite formation from hydroxylamine in the presence of O •−2 as described by Elstner and Heupel (1976). Hydrogen peroxide (H2O2) content from the control and endophyte-treated plantlets was carried out by the method described by Velikova et al. (2000).
Treatment of diphenylene iodonium (DPI) and exogenous H2O2
For tests on the effect of ROS on endophyte-induced responses and artemisinin synthesis, NADPH oxidase inhibitor DPI (50 μM) were applied during the dual culture of endophyte-plantlets, while 25 mM H2O2 was added as ROS donors. Their dosages used in the experiments were chosen based on our previous study (Zheng et al. 2010). DPI and H2O2 were sterilized by filtering through 0.22 μm sterile filters (Millipore), and directly sprayed on plantlets and repeated every 5 days. The control was treated with the same amount of distilled water only.
Enzyme Assays
Plant tissue (0.5 g) was powdered in liquid nitrogen in a chilled pestle and mortar and homogenized in 4.0 ml chilled 50 mM potassium phosphate buffer (pH 7.0) containing 1.0 % (w/v) insoluble polyvinylpolypyrrolidone and 1.0 mM phenylmethylsulfonylfluoride, 1.0 mM EDTA, 1.0 mM dithiothreitol (DTT) and 0.2 % (v/v) triton X-100. The homogenate was centrifuged at 10,000g for 10 min at 4 °C. The supernatant was stored at 2 °C and used for enzyme assays within 4 h. Catalase (EC 1.11.1.6, CAT) activity was estimated in a reaction mixture containing 500 μM H2O2 in 10 ml 100 mM phosphate buffer (pH 7.0) and 1.0 ml suitably diluted tissue extract. H2O2 decomposed after 5 min reaction was assayed by reading absorbance 240 nm of both blanks and samples (Bisht et al. 1989). CAT activity is expressed as unit (μmol H2O2)/mg protein. Peroxidase (EC 1.11.1.7X, POD) activity was usually determined spectrophotometrically as described Dias and Costa (1983). POD activity is expressed as unit (μmol H2O2)/mg protein. Superoxide dismutase (EC 1.15.1.1, SOD) activity was measured by the photochemical method as described by Giannopolitis and Ries (1977). One unit of SOD activity was defined as the amount of enzyme required to cause a 50 % inhibition of the rate of nitroblue tetrazolium chloride (NBT) reduction at 560 nm. The total protein concentration was measured by the Bradford method (1976).
Determination of artemisinin and artemisininic acid content
The plantlets from each treatment were carefully collected and leave samples were dried at 50 °C in an oven until constant weight. Dry leaf material (100 mg) was used for the extraction of artemisinin and artemisininic acid quantified using a high-performance liquid chromatography (HPLC) method (Zhao and Zeng 1985; Zhang et al. 2005). HPLC analysis conditions: Agilent 1260 system equipped with 250 × 4.6 mm, 5 μm Agilent HC-C18 column. Calibration curves were made with artemisinin (Sigma, USA) and artemisininic acid standard (Aokebio, China).
RNA extraction and quantitative real-time PCR (RT-qPCR)
Leaves of A. annua plantlets were sampled randomly after different treatment, immediately frozen in liquid nitrogen and store at −80 °C. Total RNA was isolated from different treated and control leaves by using the RNAprep Pure Plant Kits (Tian Gen Biotech, China) according to the manufacturer’s instructions. The concentration and purity of RNA were determined by measuring A260 and A280. Reverse transcription and fluorescent quantitative PCR were performed with purchased First Strand cDNA Synthesis Kit (Fermentas, Canada) and FastStart Universal SYBR Green Master (Roche, Switzerland). Amplifications were performed in CFX96 Touch Real-Time PCR Detection System (Bio-Rad, USA), with initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 30 s, 56 °C for 30 s and 72 °C for 15 s, and a final extension at 72 °C for 10 min. The housekeeping gene β-actin was chosen as the internal reference. The primers designed for the validation of the target genes from A. annua were listed in Table S1.
Statistical analysis
Experiments were performed at least in triplicate, and the data are expressed as the mean ± standard deviation (SD). Statistical significance was determined via a one-way ANOVA followed by Duncan’s multiple range test with the SPSS 13.0 software (SPSS Inc., Chicago, IL). Differences in means were considered to be significant for P values <0.05. A Student’s t test was used for statistical comparisons of two means.
Results
Effect of the endophtye on growth and artemisinin content
To analyze growth interaction between endophytes and host plants, we established the dual culture model of endophytic P. oxalicum B4 and undifferentiated A. annua callus (Fig. S1). In comparison to the monoculture controls, the average radius of the colonies of the endophyte was greater in combination with callus of the host A. annua (Fig. 1a). In contrast to an areal growth of 19.6 cm2 (ra = 2.5 cm) of the fungal colony in monoculture, that in dual culture with the host was 36.3 cm2 (ra = 3.4 cm) after 10 days of incubation. On the other hand, growth of A. annua calli was inhibited significantly starting from day 5 of incubation, up to 42 % on the day 10 by the endophyte (Fig. 1b). Simultaneously, partial browning and necrotic lesions of calli were observed. Artemisinin was undetected in callus with and without the endophyte. However, in dual culture of endophyte-regenerated plantlets, the fresh weight and plant height in the endophtye-inoculated groups were greater than that of control (Table 1), indicating that endophytic fungi was beneficial to the growth of A. annua plantlets. We also compared the effect of the endophyte on the contents of artemisinin and its direct precursor artemisinic acid in leaves of plantlets. When 30-day-old rooting plantlets were exposed to the endophyte for 30 days, the content of artemisinin reached to 1.32 mg/g DW, a 43.5 % increase over the control. However, there was no marked enhancement for the precursor artemisinic acid concentration.
Comparison of growth of endophytic P. oxalicum B4 in dual culture with controls (monocultures). a The radius of the fungal colony; b the fresh weight of calli. 0.5 g fresh callus (2-week old) and a 5-mm agar plug from a 7-day-old fungal colony was inoculated on MS medium containing 0.5 mg/L 6-BA and 1.0 mg/L NAA. The results are represented by their mean ± standard deviation (SD) of six replicates
Effect of the endophtye on ROS production
Figure 2 shows the time courses of ROS production after the endophyte inoculation in the dual culture of endophyte-plantlets. A higher O •−2 production over that of the control was detected after 3 days of inoculation. The endophyte-induced O •−2 production exhibited a biphasic time course, reaching the first higher plateau around day 9, and starting the second phase from day 18 and reaching the second plateau around day 24, after the inoculation of the endophyte. The O •−2 production in dual culture was 2.4-fold higher than that of the control in the first phase (day 9), and 1.7-fold higher in the second phase (day 24) (Fig. 2a). On the other hand, the endophyte induced a later production of H2O2 in A. annua plantlets, reaching a broad peak around day 24 post inoculation (Fig. 2b).
Time course of O •−2 (a) and H2O2 (b) production in A. annua plantlets in dual culture with endophytic P. oxalicum B4. Each 30-day-old rooting plantlets was inoculated with one piece of mycelial disk (5 mm) placed upside down on the medium (0.5 cm away from the plantlet caudex). An equal size of potato dextrose agar was put as a control. The results are represented by their mean ± standard deviation (SD) of triplicate samples (5 plantlets for each replicate)
Effects of the endophtye on the activities of antioxidant enzymes
The activities of antioxidant enzymes POD and SOD assessed in dual cultures showed some similar patterns (Fig. 3a, b). During the early stage (day 1–9) of the dual cultures, there was no significant differences in activities of POD and SOD between the dual cultures and monocultures of plantlets while both activity were stimulated in the dual culture after day 10. The CAT activity was also enhanced in dual cultures after day 12 (Fig. 3c). The activities of those antioxidant enzymes reached their peak levels around day 20 after endophyte inoculation, indicating that the redox change and the induced defensive responses might be involved in the dual cultures.
The activities of peroxidase (POD), superoxide dismutase (SOD) and catalase (CAT) during the dual cultures of A. annua plantlets with endophytic P. oxalicum B4. Each 30-day-old rooting plantlets was inoculated with one piece of mycelial disk (5 mm) placed upside down on the medium (0.5 cm away from the plantlet caudex). An equal size of potato dextrose agar was put as a control. The results are represented by their mean ± standard deviation (SD) of triplicate samples (5 plantlets for each replicate)
Effects of ROS on artemisinin accumulation in the dual culture
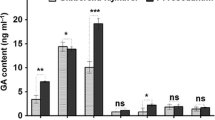
To investigate whether ROS are involved in the induction of the endophyte inoculation on artemisinin biosynthesis, A. annua plantlets were treated with exogenous H2O2 or DPI (an inhibitor of NADPH oxidase) treatments. As in Fig. 4, treatment with DPI significantly reduced endophyte-induced artemisinin increase (B4 + DPI vs B4), which was significantly stimulated by the treatment with exogenous H2O2 (B4 + H2O2 vs B4). On the other hand, we found H2O2 alone could reduce the content of artemisinic acid of A. annua plantlets in monocultures. Although the endophyte inoculation did not cause any changes in artemisinic acid content (B4 vs control), the combination treatment with DPI in dual cultures (B4 + DPI) increased its content significantly while H2O2 still depressed the accumulation of artemisinic acid in plantlets (B4 + H2O2 vs B4). These results suggested that ROS could be involved in the induced artemisinin production.
The effects of DPI and exogenous H2O2 on contents of artemisinic acid and artemisinin in A. annua plantlets during the dual cultures with endophytic P. oxalicum B4. Each 30-day-old rooting plantlets was inoculated with one piece of mycelial disk (5 mm) placed upside down on the medium (0.5 cm away from the plantlet caudex). An equal size of potato dextrose agar was put as a control. DPI (50 μM) and H2O2 (25 mM) were sprayed directly on plantlets and repeated every 5 days. The results are represented by their mean ± standard deviation (SD) of triplicate samples (5 plantlets for each replicate). Letters show statistical significance (P < 0.05)
Effects of the endophyte on transcription changes of artemisinin biosynthetic genes
To observe the transcriptional chnages of artemisinin biosynthetic genes during the dual cultures, the relative expression level of genes encoding six vital enzymes was measured by qPCR. As shown in Fig. 5a, mRNA levels of amorphadiene synthase (ADS) and amorpha-4,11-diene monooxygenase (CYP71AV1) were observed to be stimulated significantly. However, the enhanced expression of both genes was inhibited significantly in presence of 50 μM DPI (B4 + DPI vs B4 in Fig. 5b, c). The expression of ADS was stimulated by H2O2 alone or with the inoculation of the endophyte (Fig. 5b), while H2O2 didn’t cause any change in CYP71AV1 expression (Fig. 5c). All these results reveal the possible complicated relationship among ROS in the activation of artemisinin biosynthetic genes in dual culture of endophyte-plantltes.
Q-PCR analysis of artemisinin biosynthetic gene transcripts in plantlets of A. annua in dual culture with endophytic P. oxalicum B4 (a), and responses of the expression of ADS (b) and CYP71AV1 (c) gene to NADPH oxidase inhibitor DPI (50 μM) and H2O2 (25 mM), respectively. The results are represented by their mean ± standard deviation (SD) of triplicate samples (5 plantlets for each replicate). Letters show statistical significance (P < 0.05)
Discussion
As less investigated microorganisms colonizing inner host plant tissues, endophytes are obviously a rich and reliable source for secondary metabolites exhibiting a variety of biological activity such as growth promotion of the host plant and improvement of the hosts’ ecological adaptability (Tan and Zou 2001). Liu et al. (2001) reported that most of endophytes isolated from the internal stem tissue of A. annua could produce in vitro inhibitory substances against phytopathogens. Colletotrichum sp. ascertained as en endophytic fungus in A. annua, was reported to produce new antimicrobial compounds and plant hormone IAA (Lu et al. 2000), suggesting that the endophyte could relate to growth and metabolism of the host plant. Li et al. (2012) found although the inoculation of an endophytic actinomycete YIM63111 (Pseudonocardia sp.) inhibited the growth of A. annua plantlets, it stimulated accumulation of artemisinin. In our previous studies (Wang et al. 2001; 2002), oligo- or polysaccharides derived from endophytic Colletotrichum sp. were used successfully as elicitors to stimulate artemisinin production in A. annua hairy root cultures. Recently the mycelia extracts of an effective endophytic Penicillium isolate were found in our lab to promote both growth and artemisinin biosynthesis in A. annua seedling (Yuan et al. 2011). In this study, although growth of A. annua calli was inhibited in dual culture with the endophyte (Fig. 1), the plantlet growth and artemisinin content was enhanced markedly (Table 1). Artemisinin was proved not only to be an antimalarial drug for human health, but also known to be very effective against a wide spectrum of microorganisms including protozoa, bacteria, fungi and viruses as well as serve as a selective insecticide and phytoalexin (Jessing et al. 2014). The synthesis of such metabolite in plants is believed to be part of the defense responses to pathogenic attacks and stress environment. In the present study, it is interesting to note that synthesis of such an allelochemical can be enhanced (at least in dual cultures) by the action of P. oxalicum B4, an indigenous endophyte originally derived from native A. annua plants. To our knowledge, this is the first report on the induction of artemisinin biosynthesis by fungal endophyte.
Despite the progress in general understanding of plant–microbe interactions, the relationships between fungal endophytes and their respective plant hosts remain highly complex (Wu et al. 2009). Production of axenic plantlets under tissue culture conditions provides a good simplified system to explore the complex interaction. Interactions between plant calli and their endophytes have been studied by many groups (Sieber et al. 1990; Hendry et al. 1993; Peters et al.1998). Sieber et al. (1990) used the dual cultures to investigate the interactions between the endophyte Cryptodiaporthe hystrix and the callus of its host Acer macrophyllum. A significant growth stimulation of the fungus and simultaneous inhibition of callus growth observed therein were revealed in our present study in dual cultures of A. annua callus with the endophyte (Fig. 1). Also in agreement with our results are those of Lu and Clay (1994) on dual culture of endophyte Aktinsonella with their host calli. The authors suggested that growth of fungi on medium with host calli correlated positively with compatibility of the host calli, and secreted metabolites from endophytic fungi could have been responsible for the growth inhibition of plant calli. However, in our results from dual cultures with plantlets, the endophyte exhibited positive effects on the growth of A. annua plantlets at least on MS medium (Table 1). In contrast to the uninoculated controls, A. annua plantlets had significant enhancement in plant height and biomass. It has mostly been reported that endophytic fungal species enhanced plant growth due to their regulation on plant physiology and host protection under biotic and abiotic stresses (Schulz and Boyle 2005). Plant growth-promoting endophytic fungi could increase plant nutrition efficiently (Malinowski and Belesky 1999) and secreted phytohormones such as gibberellins and IAA (Lu et al. 2000; Khan et al. 2012). An endophytic Colletotrichum sp. from A. annua was proved to produce IAA and some new secondary metabolites in vitro (Lu et al. 2000). While the precise mechanism on growth enhancement by endophytic P. oxalicum B4 needs to be further elucidated, our results suggest that growth response of host plant to endophytes may be different during undifferentiated callus and seedling growth stage. Additionally, in order to better understand endophyte–host interactions, host defense reactions must be studied.
Increasing evidence proved the endophyte protection (defensive mutualism) of host plants was based on the enhanced antioxidant capacity induced by endophytes (White and Torres 2010). As the antioxidants are the result of ROS production by endophytes, ROS is part of a mechanism whereby endophytic fungi alter host cell membranes to facilitate nutrient acquisition and enhance stress tolerance (Tanaka et al. 2006). Consistent with this, we found that the endophyte inoculation induced oxidative stress through the generation of ROS such as O •−2 and H2O2 in dual culture of endophyte-plantlets (Fig. 2), which was then accompanied by the activation of POD, CAT and SOD (Fig. 3). Although oligosaccharide elicitor from endophytic Colletotrichum could also trigger higher activities of POD in A. annua hairy root cultures (Wang et al. 2002), but the characteristic of the induction was divergent from the living endophyte. The responses of hairy roots to the elicitor was quick while maximum activity of POD reached within 1 day and then strikingly decreased to control level within 3 days. The intense physiological reaction (cellular apoptosis) occurred during the elicitation (Wang et al. 2002). Our present results showed that the endophyte-induced ROS production experienced a longer process of increment and remained a higher level at the end of dual cultures (30 days). Unlike most fungal elicitors, which can lead to a biomass decrease (Wang and Wu 2013), the endophyte in present study could stimulate both growth and artemisinin accumulation concomitantly of A. annua. It suggested that ROS-producing endophytes with growth-promoting capacity could be better elicitors to improve the quality of the medicinal herb.
Beside the ROS burst and several increases of antioxidant enzyme activities in dual culture of endophyte-plantlets, the increase of artemisinin contents as a possible defensive response were recorded in our study (Table 1). This observation, along with the fungal elicitors to induce artemisinin biosynthesis by our previous report (Wang et al. 2009; Wang and Wu 2013), highlighted that artemisinin is an inducible secondary metabolite involved in plant defensive responses to fungi. Our present study has also shown that the induction of artemisinin biosynthesis by the endophyte was strongly dependent on the induced ROS production (Fig. 4). H2O2 generated through methylene blue sensitized photo-oxygenation was reported to convert artemisinic acid to another artemisinin precursor artemisinin B (EI-Feraly et al. 1986). The oxidation reaction of the ∆4,5 double bond in both artemisinic acid and dihydroartemisinic acid in A. annua plants was found to be involved in the biotransformation to artemisinin (Brown and Sy 2004). Our study clarified the points that O •−2 from the endophyt-induced oxidative burst may play a role in stimulating the conversion from artemisinic acid to artemisinin whereas exogenous H2O2 stimulated endophyt-induced artemisinin accumulation, although H2O2 alone suppressed the content of artemisinic acid (Fig. 4). In our previous studies (Wang et al. 2001; 2002), both oligosaccharide and polysaccharide elicitor induced oxidative damage to hairy roots of A. annua and the oxidative stress in turn altered artemisinin concentration and yield. These studies thus support our observations that ROS including O •−2 and H2O2 were generated during dual cultures with the endophyte and might be responsible for conversion of the immediate precursors into artemisinin production. On the other hand, there also was a significant increase in ADS and CYP71AV1 transcripts in endophyte-inoculated plantlets in dual cultures (Fig. 5), indicating a high capacity to produce artemisinin precursors. Endophytic fungi have also been shown to stimulate rate-limiting enzymes in the terpenoid pathway, namely 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) and 1-deoxy-d-xylulose5-phosphate synthase (DXR) (Gao et al. 2011). CYP71AV1 and cytochrome P450 oxidoreductase (CPR) expression were up-regulated in A. annua upon inoculation with actinobacteria strain YIM 63111 (Li et al. 2012). The qPCR data presented here clearly demonstrated that NADPH oxidase inhibitor DPI treatment resulted in significant decrease in induced transcription level, but exogenous H2O2 had opposite effects on ADS gene expression (Fig. 5b, c). It can be suggested that the expression of those key genes could be modulated by ROS production induced by the endophyte in dual cultures.
In conclusion, this study suggests that fungal endophytes may interact with host plant A. annua to stimulate plant growth and artemisinin production. Since the interactions between the endophytes and the plant hosts have not been well characterized and their potential benefits on secondary metabolites not been fully explored, further efforts aiming at such interactions are desired to understand the mutualistic mechanism, to utilize the interaction for improved production of key metabolites, finding of novel messenger molecular and bioactive metabolites. Furthermore, it also suggests strategies of reintroduction of endophytes to improve the quality of medicinal herbs.
References
Bisht SS, Sharma A, Chaturvedi K (1989) Certain metabolic lesions of chromium toxicity in radish. Indian J Agric Biochem 2:109–115
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein–dye binding. Anal Biochem 72:248–254
Brown GD, Sy LK (2004) In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron 60:1139–1159
Carroll G (1988) Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology 69:2–9
Choi PS, Min SR, Ko SM, Liu JR (2007) Somatic embryogenesis and plant regeneration in tissue cultures of Artemisia annua L. J Plant Biotechnol 34:197–200
Corsello MA, Garg NK (2015) Synthetic chemistry fuels interdisciplinary approaches to the production of artemisinin. Nat Prod Rep 32:359–366
Dias MA, Costa MM (1983) Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leave. J Exp Bot 34:537–543
Duke MV, Paul RN, Elsohly HN, Sturtz G, Duke SO (1994) Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int J Plant Sci 155:365–372
EI-Feraly FS, AI-Meshal IA, Alyahya MA, Hifnawy MS (1986) On the possible role of qinghao acid in the biosynthesis of artemisinin. Phytochemistry 25:2777–2778
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium-chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Ferreira JFS, Janick J (1996) Roots as an enhancing factor for the production of artemisinin in shoot cultures of Artemisia annua. Plant Cell Tiss Org Cult 44:211–217
Gao FK, Yong YH, Dai CC (2011) Effects of endophytic fungal elicitor on two kinds of terpenoids production and physiological indexes in Euphorbia pekinensis suspension cells. J Med Plants Res 5:4418–4425
Giannopolitis CN, Ries SK (1977) Superoxide dismutase: I. Occurrence in higher plants. Plant Physiol 59:309–314
Gu W, Ge HM, Song YC, Ding H, Zhu HL, Zhao XA, Tan RX (2007) Cytotoxic benzo[j]fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua. J Nat Prod 70:114–117
Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compante S, Campisano A, Döring M, Sessitsch A (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320
Hendry SJ, Boddy L, Lonsdale D (1993) Interactions between callus cultures of European beech, indigenous ascomycetes and derived fungal extracts. New Phytol 123:421–428
Jessing KK, Duke SO, Cedergreeen N (2014) Potential ecological roles of artemisinin produced by Artemisia annua L. J Chem Ecol 40:100–117
Khan AL, Hamayun M, Kang S-M, Kim Y-H, Jung H-Y, Lee J-H, Lee I-J (2012) Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol 12:3
Li J, Zhao G-Z, Varma A, Qin S, Xiong Z et al (2012) An endophytic pseudonocardia species induces the production of artemisinin in Artemisia annua. PLoS One 7(12):e51410
Liu CH, Zou WX, Lu H, Tan RX (2001) Antifungal activity of Artemisia annua endophyte cultures against phytopathogenic fungi. J Biotechnol 88:277–282
Lu M, Clay K (1994) Differential growth of Aktinsonella species on host grass calli. Mycologia 86:667–673
Lu H, Zou WX, Meng JC, Hu J, Tan RX (2000) New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci 151:67–73
Lydon J, Teasdale JR, Chen PK (1997) Allelopathic activity of annual wormwood (Artemisia annua) and the role of artemisinin. Weed Sci 45:807–811
Malinowski D, Belesky DP (1999) Neotyphodium coenophialum-infection affects the ability of tall fescue to use sparingly available phosphorous. J Plant Nutrition 22:835–853
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plantarum 15:473–497
Nowak J, Asiedu SK, Bensalim S, Richards J, Stewart A, Smith C, Stevens D, Sturz AV (1998) From laboratory to applications: challenges and progress with in vitro dual cultures of potato and beneficial bacteria. Plant Cell Tiss Org Cult 52:97–103
Pan WS, Zheng LP, Tian H, Li WY, Wang JW (2014) Transcriptome responses involved in artemisinin production in Artemisia annua L. under UV-B radiation. J Photochem Photobiol B Biol 140:292–300
Peters S, Draeger S, Aust H-J, Schulz B (1998) Interactions in dual cultures of endophytic fungi with host and nonhost plant calli. Mycologia 90:360–367
Ren CG, Dai CC (2012) Jasmonic acid is involved in the signaling pathway for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. BMC Plant Biol 12:128
Rodriguez RJ, White JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330
Schulz B, Boyle C (2005) The endophytic continuum. Mycolog Res 109:661–686
Schulz B, Wangke U, Draeger S, Aust H-J (1993) Endophtyes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res 97:1447–1450
Sieber TN, Sieber-Canavesi F, Dorworth CE (1990) Simultaneous stimulation of endophytic Cryptodiaporthe hystrix and inhibition of Acer Macrophyllum callus in dual culture. Mycologia 82:569–575
Singh A, Vishwakarma RA, Husian A (1988) Evaluation of Artemisia annua strain for higher artemisinin production. Planta Med 54:275–277
Strobel G, Yang XS, Sears J, Kramer R, Sidhu RS, Hess WM (1996) Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 142:435–440
Subramanian P, Kim K, Ramasamy K, Sundaram S, Sa T (2015) Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobium japonicum MN110. Plant Growth Regul 76:327–332
Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18:448–459
Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B (2006) Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. Plant Cell 18:1052–1066
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wang JW, Wu JY (2013) Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. Adv Biochem Eng Biotechnol 134:55–89
Wang JW, Zhang Z, Tan RX (2001) Stimulation of artemisinin production in Artemisia annua hairy roots by the elicitor from the endophytic Colletotrichum sp. Biotechnol Lett 23:857–860
Wang JW, Xia ZH, Tan RX (2002) Elicitation on artemisinin biosynthesis in Artemisia annua hairy roots by the oligosaccharide extract from the endophytic Colletotrichum sp. B501. Acta Bot Sin 44:1233–1238
Wang JW, Zheng LP, Tan RX (2006) The preparation of an elicitor from a fungal endophyte to enhance artemisinin production in hairy root cultures of Artemisia annua L. Chinese J Biotechnol 22:829–834 (in Chinese)
Wang JW, Zheng LP, Zhang B, Zou T (2009) Stimulation of artemisinin synthesis by combined cerebroside and nitric oxide elicitation in Artemisia annua hairy roots. Appl Microbiol Biotechnol 85:285–292
Wang HL, Zheng JR, Ren XY, Yu T, Varma A, Lou BG, Zheng XD (2015) Effects of Piriformospora indica on the growth, fruit quality and interaction with tomato yellow leaf curl virus in tomato cultivars susceptible and resistant to TYCLV. Plant Growth Regul 76:303–313
White JF, Torres MS (2010) Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol Plantarum 138:440–446
Woerdenbag HJ, Pras N, Nguyen GC, Bui TB, Rein B, van Uden W, Nguyen VB, Sieb B, Lugt CB (1994) Artemisinin, related sesquiterpenes, and essential oil in Artemisia annua during a vegetation period in Vietnam. Planta Med 60:272–275
Wu CH, Bernard SM, Andersen GL, Chen W (2009) Developing microbe–plant interactions for applications in plant-growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microb Biotechnol 2:428–440
Yuan YF, Dong T, Wang JW (2011) Effect of endophytic Penicillium sp. Y2 on growth and artemisin biosynthesis of plantlets in tissue cultures of Artemisia annua L. Amino Acids Biot Res 33:1–4 (in Chinese)
Zhang YS, Ye HC, Liu BY, Wang H, Li GF (2005) Exogenous GA3 and flowering induce the conversion of artemisinic acid to artemisinin in Artemisia annua plants. Russ J Plant Physiol 52:58–62
Zhao SS, Zeng MY (1985) Spektrometrische hochdruck-flüssigkeits-chromatographische (HPLC) Untersuchungen zur Analytik von Qinghaosu. Planta Med 51:233–237
Zheng LP, Zhang B, Zou T, Chen ZH, Wang JW (2010) Nitric oxide interacts with reactive oxygen species to regulate oligosaccharide-induced artemisinin biosynthesis in Artemisia annua hairy roots. J Med Plants Res 4:758–765
Acknowledgments
The authors are grateful to the Graduate Program of Higher Education in Jiangsu Province (No. CXLX13-841), Suzhou Scholar Program (No. 14317363) and the projects sponsored by the NNSF (No. 81273487), PAPD and SRF for ROCS (No. K513201011) for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, L.P., Tian, H., Yuan, Y.F. et al. The influence of endophytic Penicillium oxalicum B4 on growth and artemisinin biosynthesis of in vitro propagated plantlets of Artemisia annua L.. Plant Growth Regul 80, 93–102 (2016). https://doi.org/10.1007/s10725-016-0162-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0162-2