Abstract
Flavonoids are widely distributed secondary metabolic products with many biological functions in plants. Further elucidation of the accumulation and localization patterns of its biosynthesis enzymes will broaden our understanding of flavonoids biosynthesis and regulation. Chalcone isomerase (CHI, EC 5.5.1.6) is an early-step enzyme in the flavonoids biosynthesis pathway. In this study, using an antibody specifically developed against grapevine CHI enzyme, we found that the accumulation of CHI protein exhibited temporal and spatial specificity. In grape berries, CHI was investigated mainly in the outer hypodermis cells of exocarp tissues, in the vascular bundles of mesocarp; and in the integument and the cells around the raphe of seeds. At the subcellular level, CHI was visualized in the cytoplasm, nucleus, and plastids (chloroplasts) of the exocarp cells, while only in the cytoplasm of mesocarp vascular bundle cells. In grapevine vegetable organs, the leaf mesophyll and phloem of leaf veins, the pith ray and primary phloem of stems, the primary phloem and endoderm of roots, and the young leaves, leaf primordium, and the growth point of leaf buds were CHI signal-positive. In these tissue cells, CHI was primarily observed in the cytoplasm, cell wall, and nucleus. The distinct localization patterns of CHI suggested the complexity of flavonoids biosynthesis in grapevine.
Key Message
The distribution of CHI protein exhibited temporal and spatial specificity. Different subcellular localization patterns were observed in grape berries and grapevine vegetable tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavonoids represent one of the most abundant classes of plant secondary metabolites, playing important functions in response to various biotic and abiotic stresses (Buer 2010). For the quality of fruits and their products like grapes and wine, flavonoids play a critical role by contributing to the color, the bitterness and astringency (Flamini et al. 2013). Moreover, many potential positive effects on human health have also been ascribed to these compounds (Georgiev et al. 2014). Due to the multitude functions, the synthesis and regulation of flavonoids have been studied intensely in recent years.
Because of containing abundant soluble flavonoids and the availability of genome sequence, grapevine (Vitis vinifera L.) has become one of the best studied fruit crops on flavonoids biosynthesis and regulation. Most of the enzymes and genes in flavonoid biosynthesis pathway have been well characterized (Supplementary Fig. 1, Petrussa et al. 2013). The expression patterns of flavonoid biosynthesis related genes have been elucidated clearly too (Jeong et al. 2008; Bogs et al. 2007). Additionally, a set of transcription factors including R2R3 MYBs (VvMYBA1/2/5a/5b), WD40 (VvWDR1 and VvWDR2), and bHLH (VvMYC1, VvMYCA1) have also been isolated and identified (Petroni and Tonelli 2011; Fang et al. 2018). These results give our better understanding of the regulation of flavonoids synthesis in grapevine. However, the researchers are mostly focused on its synthesis and regulation at transcriptional level, which does not fully reflect the translational and protein levels.
It is believed that flavonoids accumulation in grapevine is tissue-specific. Flavonoid compounds accumulate to high concentrations within grape berries, especially in the peripheral layers of berry skin and in some layers of the seed coat, whereas little flavonoids are detected in the berry mesocarp (Braidot et al. 2008). While in the grapevine seedlings, flavonoids have been investigated in most of the vegetable tissues including leaves, petioles, stems, leaf buds, stem tips, and roots (Wang et al. 2016). Within the cell, the flavonoid products are mostly stored in the vacuoles and the cell wall (Fang et al. 2018; Grotewold 2004). Some flavonoid endproducts have also been found in the nucleus of Arabidopsis root cells (Saslowsky et al. 2005). However, it is still confusing that at these sites flavonoids are synthesized in situ or delivered from other sites. The researches on the distribution and exact localization patterns of flavonoids biosynthesizing enzymes is of significance for clarifying flavonoids biosynthesis, accumulation and compartmentalization mechanism in grapevine.
Our previous studies have investigated the expression, tissue and subcellular distribution of Chalcone synthase (CHS) and Anthocyanidin synthase (ANS) in different grapevine tissues (Wang et al. 2016, 2011). Additional localization information from more other key enzymes will help to further clarify the mechanisms of flavonoids metabolism and accumulation in grapevine. Chalcone isomerase (CHI, EC 5.5.1.6), which is one of the early pivitol enzymes in flavonoid biosynthetic pathway, converts chalcone to naringenin. The cDNA of CHI has been obtained from a series of plants (Cheng et al. 2011; Zamora et al. 2013; Wang et al. 2012; Wu et al. 2018; Ma et al. 2019). And the CHI expression at gene level has been well studied (Yin et al. 2018; Azuma et al. 2012). But the available information on its expression, regulation and localization at protein level in grapevine is limited. In the oat primary leaves, CHI has been found to be localized almost entirely in the mesophyll and not in the epidermis where flavonoids predominantly accumulated (Knogge and Weissenböck 1986). In Arabidopsis, CHI has been detected in cotyledon epidermal cells and cortex cells of the root. At subcellular level, CHI has been found primarily localized not only in the endoplasmic reticulum but also in the nucleus and vacuole membrane (Saslowsky et al. 2005; Saslowsky and Winkel-Shirley 2001). But in soybean, GmCHIs from subfamilies 1, 2 and 4 have been found in the nucleus and cytoplasm, while GmCHI3 isoforms have been detected mainly in the chloroplast (Dastmalchi and Dhaubhadel 2015). Overall, the diverse distribution patterns of CHI were investigated in different plant species.
On this basis, in the present work, the accumulation of CHI, its tissue and subcellular localizations were investigated in different grapevine tissues via western blot, immune-histochemical localization, immunogold labeling, and confocal laser scanning microscope techniques. The results obtained in this study will enhance our knowledge of flavonoids biosynthesis and regulation in grapevine.
Materials and methods
Materials
Grape berries (V. vinifera L. cv. Cabernet Sauvignon) were sampled during the 2009 and 2010 growing seasons at 20, 30, 40, 50, 60, 70, 80, 90, 100 and 120 days after full bloom (DAFB) from a commercial vineyard in the suburbs of Beijing according to the previous described method (Chen et al. 2006). The harvested berries were immediately transported to the laboratory. The berry skin and flesh were separated manually. The vegetable tissues were prepared from 1-year-old Cabernet Sauvignon grapevine seedlings (V. vinifera L. cv.). The plants were raised in a greenhouse at 25 °C with 16 h light/8 h dark photoperiod and 65% relative humidity. Plants with uniform vegetative growth were selected for the study. Afterwards, some samples were embedded for further immune-localization experiments; the remainder were quickly frozen in liquid nitrogen and stored at − 80 °C until used.
The polyclonal antibody specifically developed against grape CHI (Wang et al. 2012) was used in the following immune tests. Anti-Plant Actin Mouse Monoclonal Antibody was purchased from Abbkine. Unless otherwise stated, all chemicals were purchased from Sigma.
Extraction and determination of total flavonoids and Naringenin content
For flavonoids extraction, the collected samples were ground in liquid nitrogen using a mortar and pestle. A 1 g subsample of the tissue was added to 3 ml of methanol containing 1% HCl. The solution was carefully blended, sonicated 3 s for 30 times, and set aside overnight at − 4 °C in darkness. Then the extract was centrifuged at 12,000×g for 20 min. The supernatant was retained and the residue was repeatedly extracted with the same solvent until it was colourless. Total flavonoids were measured according to methods described in Wolfe et al. (2003).
The naringenin in different grapevine tissues was extracted according to the methods described by Sun et al. (2006) with slight modification. In brief, 4 g of grapevine tissue was firstly ground to fine powder under liquid nitrogen and then was extracted by 100 ml methanol with agitation at room temperature under darkness for 48 h. Then, samples were processed with evaporation and purification. The final residue was recovered by 2 ml of 50% ethanol in water and stored at − 20 °C for further reverse phase HPLC analysis. HPLC analysis was performed according to the previous reported method (Wang et al. 2010a).
RT-PCR analysis
Total RNA extraction from all collected samples was performed according to the described procedure by Wen et al. (2005). Total RNA samples were DNase-treated with an RNase-free DNase (TaKaRa, Japan) prior to RT-PCR, to remove any contaminating genomic DNA. First-strand cDNA was synthesized in a 20 µl reaction mixture with the Reverse Transcription System A3500 (Promega, USA) according to the protocol provided by the manufacturer, using 1 µg total RNA. CHI transcript levels were measured by quantitative reverse transcription polymerase chain reaction (RT-PCR) following previous method of Wang et al. (2012). RNA was extracted from each sample twice. RT-PCR was conducted three times. The sequences of PCR products were analyzed at the Beijing Sunbiotech Company (Beijing, China) to confirm the identity.
Protein extraction and western blotting analysis
The extraction of total protein in the samples was based on the method described by Famiani et al. (2000). The protein concentration was detected using Bradford method (Bardford 1976). For each sample, 10 µg total protein were loaded per lane and separated by 12% polyacrylamide gel electrophoresis, which were then transferred to 0.45 mm nitrocellulose (Amersham Life Science) through an electro-transfer apparatus (Bio-Rad) by the method of Isla et al. (1998). Immunoblot analysis was performed with rabbit antibodies against recombinant V. vinifera CHI (1/800, v/v) at 25 °C. Thereafter, alkaline phosphatase conjugated anti-rabbit IgG (1/500, v/v) was applied as secondary antibody. The membrane staining was processed with the reported method (Wang et al. 2010b). The western blotting analysis for the Actin1 was used as protein control.
After immunoblotting, the band signal intensity, which was used to indicate CHI protein mounts, was determined by scanning the nitrocellulose via a densitometer using the ImageQuant software. For different samples, three independent experiments were performed. The significant tests for the investigated data of all the measurements were carried out using SPSS 13.0 software.
Immunohistochemical detection of CHI localization
The immunohistochemical localization of CHI was carried out by the published method (Hou and Huang 2005) with slight changes. In brief, the prepared sections were processed with deparaffinization and hydration, followed by a wash with 10 mM phosphate buffered saline (PBS, pH 7.0). Thereafter the sections were incubated with a blocking solution [10 mM PBS, 1.5% (w/v) glycine, 0.1% (v/v) Tween-20, and 5% (w/v) bovine serum albumin (BSA)] for 45 min at 25 °C, and then rinsed with regular salt rinse solution [RSR, 10 mM PBS, 0.88% (w/v) NaCl, 0.8% (w/v) BSA, 0.1% (v/v) Tween-20] for 5 min and 10 mM PBS supplemented with 0.8% (w/v) BSA for 1 min. Then each slide was added with 100 µl grape CHI antibodies (1:100, v/v) and incubated at 4 °C for 12 h in humidity chamber. After twice vigorous washes in high salt rinse solution [10 mM PBS, 2.9% (w/v) NaCl, 0.1% (w/v) BSA, 0.1% (v/v) Tween-20] and a 10-min rinse with RSR, slides were incubated with the alkaline phosphatase conjugated anti-rabbit IgG from goat (1 mg/l, Promega) (1:100, v/v). Finally, the slides were reacting with 200 µl Western Blue stabilized substrate for alkaline phosphatase for approximately 15 min after twice rinse with RSR and once in water. When the blue/green color was observed, the reaction was terminated by washing the sections with water. After dehydration, the sections were mounted with a cover glass for final photographing.
In addition, three types of negative control were detected to test specificity of the immunohistochemical procedure. In the first one, unspecific labeling of the alkaline phosphatase conjugated anti-rabbit IgG was tested by omitting the primary CHI antibody. In the second one, the specificity of CHI antibody was detected by using rabbit preimmune serum instead of the rabbit antibody. In the last one, the slides were applied with antibody absorbed by purified recombinant CHI protein.
Subcellular immunogold labeling of CHI
For immunogold localization, the ultrathin sections preparation and immunogold labeling were performed according to the published method with some modifications (Chen et al. 2006). Before the immunostaining, the ultrathin sections were reacted with purified CHI antibody in TBSTG after incubation with TBSTG buffer for 30 min. After twice washes of TBSTG buffer, the goat anti-rabbit IgG conjugated to 10-nm gold particles (1:100, v/v) was applied and incubated for 1 h, followed by three times washes with TBSTG and double-distilled water respectively. Finally, the sections were stained with 4% lead citrate and 2% uranyl acetate to improve electron visibility of immunogold particles, which were detected using a JEM-100S electron microscope (Kyoto, Japan).
The specificity of the immunogold labeling procedure was tested. Some samples were processed without primary antibody, with the rabbit preimmune serum substituted for the CHI antibody, or with the antibody absorbed with purified recombinant CHI, respectively.
Transient transformation of CHI-GFP in grape berry protoplasts and onion epidermis
The procedure of plasmid construction and transient transformation of CHI-GFP in grape berry protoplasts was carried out essentially according to the previous described methods (Wang et al. 2015). The nucleus was stained with 4′,6′-diamidino-2-phenylindole (DAPI) by the method of Wang et al. (2010b). The transient transformation of CHI-GFP in onion epidermis was conducted according to Scott et al. (1999). Using a Bio-Rad PDS-1000/He particle delivery system, the strips of onion (Allium cepa) bulb epidermis were bombarded with gold particles coated with plasmids. After incubated at 23 °C for 16 h, fluorescence of CHI-GFP was investigated by a confocal laser scanning microscope (Bio-Rad MRC 1024).
Results and discussion
Flavonoids are important secondary metabolites in plants that have diverse physiological and pharmacological functions (Buer 2010; Gouot et al. 2018; Georgiev et al. 2014). It is well known that the flavonoids biosynthesis is regulated temporally and spatially. To further understand mechanism of the spatial regulation, the distributions of CHI at cellular and subcellular levels in different grapevine tissues were investigated at present work.
Total flavonoids and naringenin content in different grapevine tissues
In an attempt to obtain more insight into the CHI distribution in different grapevine tissues, the accumulation of total flavonoids content and naringenin was evaluated in the collected materials. As shown in Fig. 1, the accumulation of flavonoids naringenin in grapevine was developmental stage dependent and organ-specific. In developing grape berry skins, abundant total flavonoids content (12.70 mg/g FW) was detected at young grape stage (20 DAFB), and then the content was decreased until 40 DAFB. From 50 DAFB to 80 DAFB, a rapid increase was observed in and total flavonoids content reached 26.84 mg/g FW. A slight increase was investigated at 90 DAFB. Thereafter, the content value increased gruadually toward 120 DAFB reaching the maximum level (29.92 mg/g FW). While in the developing berry flesh, a small quantity of total flavonoids was investigated. In different grapevine vegetable tissues, The maximum concentration of total flavonoids was found in leaf buds (39.29 mg/g FW), followed by stem tips (31.23 mg/g FW), stem phloem (stem P, 25.96 mg/g FW), leaves (27.85 mg/g FW), root (17.22) and petioles (15.53 mg/g FW); the minimum content was detected in the stem xylem (stem X, 5.23 mg/g FW). The accumulation pattern of total flavonoids content was consistent with our previous investigation (Wang et al. 2016).
Changes of Total flavonoids and naringenin content in different grapevine tissues. The above figure showes total flavonoids and naringenin content in the skin and flesh grape berries (Vitis vinifera L. cv. Carbernet Sauvignon) sampled at different developmental stages. The figure below showes total flavonoids and naringenin content in different grapevine vegetative tissues. Stem P, stem phloem; Stem X, stem xylem. Different letters indicate a statistical difference at P ≤ 0.05 among samples according to Duncan’s multiple range tests
As to naringenin, its content was detected lower than HPLC detection limit at early developmental stage (20 DAFB and 30 DAFB), then there was a slow increase of naringenin content in grape berry skins at 40 DAFB. Two accumulation peaks of naringenin content (0.56 and 0.72 mg/kg FW, respectively) were investigated at 50 DAFB and 90 DAFB (Fig. 1). The content of naringenin was also analyzed in developing berry fleshes, but the accumulation of naringenin was only detected at 60 DAFB and 100 DAFB (0.03 and 0.17 mg/kg FW. respectively, data not shown). The content of naringenin in different vegetable tissues of grape plants ranged from 1.36 mg/kg FW to 363.4 mg/kg FW, and there was a significant difference among the tissues. The maximum content of naringenin was presented in the root (363.4 mg/kg FW), followed by leaf buds (212.11 mg/kg FW), stem phloems (146.24 mg/kg FW) and stem xylems (83.42 mg/kg FW). Lower content of naringenin was detected in stem tips (4.21 mg/kg FW), petioles (4.68 mg/kg FW) and leaves (1.36 mg/kg FW).
CHI accumulation in different grapevine tissues
The changes of CHI accumulation in developing grape berry skins and fleshes were investigated by Western blotting (Fig. 2a, b). As shown in Fig. 2, a single polypeptide (approximately 25 kDa) was detected from the extracted total protein specifically. It is noted that the greatest accumulation of the CHI protein in berry skins happened at early developmental stage (20 DAFB), and then a gradual decrease was observed during 30 to 60 DAFB. Thereafter, a rapid increase of the CHI protein was detected at the time of veraison (approximately 70 DAFB), followed by a reduction during the later part of ripening stage (Fig. 2a), which was corresponding well to the CHI transcript accumulation patterns (Supplementary Fig. 2A). A significant difference on CHI protein accumulation patterns was investigated in grape berry flesh samples (Fig. 2b). Although maximal accumulation of CHI protein and gene transcript were also observed at 20 DAFB (Supplementary Fig. 2B), a decrease was detected during the successive development stage.
The accumulation of CHI in different grapevine tissues. a CHI accumulation in the skin of developing grape berries. b Western blotting analysis of CHI in the flesh of developing grape berries. Actin was used as the control protein. The data of signal intensity are means from three analyses of one of two independent replicates. Bars are standard errors (n = 3). Different letters indicate a statistical difference at P ≤ 0.05 among samples according to Duncan’s multiple range tests
Previous studies have pointed out flavonoids biosynthesis related genes including CHI gene expression is developmentally regulated (Bogs et al. 2007; Jeong et al. 2008). Here we also found the accumulation of CHI transcript and protein was developmental stage-dependent in both developing grape berry skin and flesh, and CHI was subjected to both transcriptional and translational regulation (Fig. 2). In developing grape berry skins, the peaks of CHI accumulation occurred at both early development stage and verasion, which was consistent with synthesis of flavonoid compounds (Fig. 1). Previous studies have shown that substantial quantities of proanthocyanidins (Pas) are accumulated in berry skins at early stage (Wang et al. 2011; Bogs et al. 2007). It is suggested that the accumulation of CHI in early samples may mainly responds to Pas accumulation, while the high-level expression of CHI at veraison parallels the onset of anthocyanin synthesis with other flavonoids biosynthesis enzymes in grape skin (Bogs et al. 2007).
Additionally, CHI transcript has been previously detected in almost all the grapevine vegetative tissues including leaves, tendrils, green canes, stems, and roots (Jeong et al. 2008). Here we also found that CHI protein and transcript was also accumulated in all the sampled vegetable organs. Substantial accumulation of CHI was detected in the leaf buds, stem X, leaves and stem tips. A Relatively small amount of CHI was investigated in petioles, roots and stems P (Fig. 3). The accumulation of CHI protein was basically consistent with the levels of mRNA in most organs. Meanwhile CHI protein accumulation in various grape tissues was similar to the patterns of total flavonoids accumulation (Fig. 1). Abundant accumulation of CHI was detected in colorless grapevine tissues such as leaves and root, which may be mainly involved in Pas, flavonols and flavan-3-ols biosynthesis (Wang et al. 2011; Jeong et al. 2008). While, in stem tip and petiole, large amount of CHI may respond to both anthocyanins and Pas synthesis. However in the stem phloem, high levels of flavonoids were detected in spite of the relatively low levels of CHI protein, while in the stem xylem, the higher level of CHI protein was accumulated and the total flavonoids content was low. Previous studies have pointed out the existence of transportation of flavonoids through the plant from one organ to another (Buer et al. 2008). The flavonoids synthesized in stem xylem might be transported to other tissues. The accumulation of flavonoids in different grapevine tissues may be the result of in situ biosynthesis and transportation from the other synthesis tissues. However, the transport mechanism of flavonoid between different grapevine tissues requires further investigation.
The accumulation of CHI gene transcript (upper artwork) and protein (bottom artwork) in the vegetative tissues. Stem P, stem phloem; Stem X, stem xylem. The data of signal intensity are means from three analyses of one of two independent replicates. Bars are standard errors (n = 3). Different letters indicate a statistical difference at P ≤ 0.05 among samples according to Duncan’s multiple range tests
In order to obtain more insights into the CHI accumulation in different grapevine tissues, the presumed CHI direct product naringenin content was evaluated in the collected materials (Fig. 1). Interestingly, the change patterns of naringenin content were contradictory comparing to total flavonoids (Fig. 1) and CHI protein accumulation (Figs. 2, 3). Although bioinformatics analysis indicated that CHI studied in present work belongs to plant type I chalcone isomerase, which use naringenin chalcone as a substrate and convert it into naringenin (Park et al. 2018). And its protein sequence has high similarity with Arabidopsis CHI (Supplementary Fig. 3), which has high activity in naringenin synthesis (Kaltenbach et al. 2018). It cannot exclude that naringenin, as an intermediate product, might be quickly used for the synthesis of different downstream flavonoid compounds or transported to other tissues (Buer et al. 2008). Furthermore, there are two copies of CHIs in grapevine. VvCHI2 may be also involved in the synthesis of naringenin in different grapevine tissues. And previous studies have investigated high expression level of VvCHI2 in grapevine (Jeong et al. 2008). But the functions and importance of CHIs in naringenin biosynthesis need to be made clear further by enzyme assay or transgenetic techniques.
Immunohistochemical localization of CHI in different grapevine tissues
In an attempt to observe the possible sites of flavonoids biosynthesis in grapevine, six tissues were sampled including grape berries (Fig. 4a), grape seeds (Fig. 4b), leaves (Fig. 4c), stems (Fig. 4d), roots (Fig. 4e), and leaf buds (Fig. 4f) to detect the tissue localization of CHI. In grape berries, CHI signals (blue–green color) were mainly localized in the exocarp outer hypodermis cells and mesocarp vascular bundles, little signals were detected in mesocarp parenchyma cells (Fig. 4a), which was consistent with previous research (Wang et al. 2012). Furthermore our previous researches have revealed that grapevine chalcone synthase (CHS) and Anthocyanidin synthase (ANS) were localized in the same type pericarp cells too (Wang et al. 2010b, 2016). And substantial flavonoids have been detected in hypodermal layers of grape berry skins (Falginella et al. 2012; Braidot et al. 2008; Gouot et al. 2018). It is suggested that flavonoid biosynthesis in grape skin may mostly happen in the exocarp outer hypodermis cells.
Immunohistochemical localization of CHI in different grapevine organs. a The distribution of CHI in grape berries; b tissue localization of CHI in grape seeds; c the distribution of CHI in grape leaves; d tissue localization of CHI in grapevine stems; e immunohistochemical localization of CHI in grapevine roots; f tissue localization of CHI in grapevine leaf buds. EP exocarp, MP mesocarp, II inner integument, OI outer integument, Em endosperm, R raphe, UE upper epidermis, LE lower epidermis, PT palisade tissue, ST spongy tissue, Epi epidermis, PPh primary phloem, PX protoxylem, PiR pith ray, CoP cortex parenchyma cells, Co cortex, En endoderm, Vc vascular cylinder, GP growth point, LP leaf primordium, YL young leaf. Bars = 50 µm
Flavonoids were found in most plant seeds, which play important functions in seed development (Lepiniec et al. 2006). Grape seeds are good source of flavonoids (Shi et al. 2003). Here we found CHI signals were primarily localized in inner integument, outer integument, and the cells around the raphe, while little CHI signals were investigated in other type cells of grape seeds (Fig. 2b). Together with the investigation of flavonoids in the inner integument, the inner cells of the soft seed coat, and cell walls of the outer integument (Cadot et al. 2006), it is indicated that flavonoids accumulated in the diverse structures of grape seed are synthesized in situ there. Furthermore the late-step enzyme of flavonoids biosynthesis ANS have also been detected in these tissues (Wang et al. 2011). However, in Arabidopsis seed, PAs are synthesized in the endothelium, the innermost cell layer of the inner integument (Lepiniec et al. 2006). It might be that localization of flavonids biosynthesis varies according to plant species.
In different grapevine vegetative tissues, CHI showed tissue-specific localization patterns (Fig. 4c–f). In the grape leaves (Fig. 4c), strong CHI signals were detected in both mesophyll and vein, but not in the epidermis. In leaf veins (Fig. 4c), CHI were mostly localized in the vascular bundles. In grapevine plant stems, intensive CHI signals were observed in the pith ray and primary phloem (Fig. 4d). While, in grapevine roots, the CHI was primarily localized in the endoderm and primary phloem (Fig. 4e). In leaf buds (Fig. 4f), substantial CHI protein were found in the leaf primordium, growth point and young leaves. Additionally, little CHI signal was found in the controls (Supplemetary Fig. 4), indicating that the antibody was specific and the unspecific signal was negligible.
Substantial evidences have shown the accumulation of flavonoid compunds in the epidermal tissues, which play important roles in protecting the underlying tissues from UV light damage, phytopathogens and so on (Agati et al. 2013; Buer 2010). And the distribution of CHS and CHI have been detected mainly in the epidermal cells in Arabidopsis (Saslowsky et al. 2005). Here, slight CHI signals were also investigated in the epidermal cells of grapevine leaves, but strong signals were mainly found in mesophyll (Fig. 4c), which consistent with the result observed in the oat primary leaves (Knogge and Weissenböck 1986). In the other grapevine vegetative tissues, CHI was mostly localized in the underlying tissues but not in the epidermal cells (Fig. 4c–f), which was in accordance with the distribution patterns of CHS and ANS detected in these tissues (Wang et al. 2016, 2011). Previous reports have pointed out the sites of biosynthesis are not necessarily the sites of accumulation and that the transport of flavonoid end products between cells may be involved (Braidot et al. 2008; Petrussa et al. 2013). Some putative transporters within and between cells such as ATP-binding cassette (ABC), multidrug and toxic compound extrusion (antho-MATE) and glutathione S-transferase (GST) have also been identified (Gouot et al. 2018; Biała and Jasiński 2018). Flavonoids biosynthesized in these tissues may be transported to the epidermal cells for a further glycosylation and accumulation or accumulated in situ to play important roles like affecting auxin transport and so on (Peer and Murphy 2007; Buer et al. 2008).
Furthermore, substantial CHI was investigated in vascular bundles not only in grape berries but also in vegetative tissues. This observation is consistent with the association of CHS and ANS with phloem tissue in different grapevine tissues (Wang et al. 2016, 2011). Several studies have confirmed the distribution of flavonoids in transport tissues (Gholami 2004; Wang et al. 2016). Here a large amount of naringenin was also detected in stem phloem and xylem (Fig. 1). These results suggested the existence of in situ flavonoids biosynthesis in vascular tissues. But the function of flavonoids play there does not know. Recent studies have investigated the flavonoid occurrence in vascular bundles, where they moved towards the root tip (Buer et al. 2008). Considering the role of vascular bundle in long distance transportation (Bondada et al. 2005), flavonoids biosynthesized in vascular tissues may be totally or partially transported from the vascular bundle to the other different tissues (Petrussa et al. 2013; Buer et al. 2008). The synthesis of flavonoids in vascular bundles is likely to be convenient for transportation. Furthermore, some flavonoid carriers in vascular bundles have been identified (Petrussa et al. 2013).
Subcellular localization of CHI
We investigated the subcellular localization of CHI in reproductive tissues (grape berry exocarp and mesocarp vascular tissue cells) and vegetable tissues (grapevine leave mesophyll, epidermis cells, stem and root cells) using immunogold electron-microscope technique respectively. Different subcellular localization sites of CHI were observed in grape berry tissues and grapevine vegetable tissues (Figs. 5, 6, 7, 8).
Immunogold electron microscope localization of CHI in the exocarp cells. a Ultrastructure of grape exocarp cells; b–d CHI visualized by gold particles resides in the cytoplasm (b), plastids (chloroplast, c); nucleus (b, d). Cyt cytoplasm, Chl chloroplast, CW cell wall, V vacuole, N nucleus. Bars = 10 µm in a, 0.5 µm in b–d
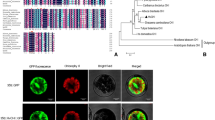
Subcellular localization of CHI-GFP fusion protein in the onion epidermis cells and the protoplast derived from grape berry suspension cells. a–c Transient expression of CHI-GFP fusion protein in the epidermis cells of onion. a Image of CHI-GFP green fluorescence; b bright-field images; c merged images. Bar = 100.0 µm. d–g Transient expression of CHI-GFP fusion protein in the protoplast derived from grape berry suspension cells. The laser-scanning confocal microscopy images are the CHI-GFP-fluorescence (d), DAPI-staining images (e), bright field (f), and merged images (g). Bars = 5.0 µm
Immunogold electron microscope localization of CHI in mesocarp vascular bundle cells. a Ultrastructure of mesocarp vascular tissue cells; b, c subcellular localization of CHI in sieve element. c, d Immunogold localization of CHI in companion cell. SE sieve element, CC companion cell, Cyt cytoplasm, CW cell wall, V vacuole. Bars = 0.5 µm
In grape berry exocarp cells, gold particles representing CHI were primarily distributed in the cytoplasm (Fig. 5b, c) and nucleus (Fig. 5b, d). Transient expression system provides an excellent platform to study protein subcellular localization in vivo (Zhao et al. 2016). Here, using transient expression system, the subcellular localization results of CHI in vivo from both homologous system (grape berry suspension cultured cells) and heterologous system (onion epidermis cells) were investigated. CHI mostly distributed in the cytoplasmand nucleus (Fig. 6), which was consistent with the results of immunogold microscopy. Meanwhile, gold particles were also detected in the plastids (chloroplasts) (Fig. 5c), no particles was labeled in the vacuole and the cell wall. Flavonoids have been investigated in many different locations in plant cell, including the plastids (Zhao and Dixon 2010). Moreover our previous results have suggested that abundant grape berry Cinnamate 4-hydroxylase (C4H), CHS and ANS were localized in the plastids of the exocarp cells (Chen et al. 2006; Wang et al. 2010b, 2016). It appears that plastid may be a novel subcellular flavonoids synthesis site in grapevine and the pathway enzymes of flavonoids biosynthesis including CHI are organized together to synthesize different flavonoid compounds more efficiently in situ there. Agati et al. (2013) have proposed that flavonoids located in chloroplasts play roles in removing singlet oxygen and H2O2 produced under excess stresses to prevent programmed cell death.
As shown in Fig. 7, the subcellular distribution of CHI in the mesocarp vascular bundle cells was investigated. The gold particles were mostly distributed in the cytoplasm in both the sieve element (Fig. 7b, c) and the companion cells (Fig. 7c, d). No particle was found in other organelles. Flavonoids biosynthesized in the cytoplasm may be convenient for symplastic translocation in the phloem (Ayre et al. 2003).
As to vegetable tissues, evidences from other plant have shown the cytoplasm and nucleus localization patterns of flavonoids biosynthesis enzymes (Saslowsky and Winkel-Shirley 2001; Saslowsky et al. 2005). Here, in grapevine, CHI was found distributed in cytoplasm of all the sampled vegetable tissue cells too (Fig. 8), and in nucleus of plant leaf mesophyll (Fig. 8c) and root cells (Fig. 8e). Additionally, a quantity of CHI was also investigated in the cell wall of the vegetable tissue cells (Fig. 8). Grapevine Phenylalanine ammonia-lyase (PAL) and CHS have been found in the cell wall (Chen et al. 2006; Wang et al. 2016). It is suggested that cell wall might be another subcellular flavonoids biosynthesis site in grapevine vegetable tissues. Braidot et al. (2008) have detected the accumulation of flavonols and tannins in the cell wall in grapevine, Which play important roles in protecting underlying organelles from solar radiation and so on (Agati et al. 2013). While, no gold particle was detected in the control samples (Supplementary Fig. 5).
Subcellular localization of CHI in different grapevine vegetable tissues. a–c Immunogold electron microscope localization of CHI in the mesophyll. d–f Immunogold labeling of CHI in the root cells. g–i Subcellular localization of CHI in grape stem cells. CW cell wall, Cyt cytoplasm, V vacuole, P plastid, Chl chloroplast, M mitochondrion, N nucleus. Bars = 2 µm in a and g, 10 µm in d, 0.5 µm in b, c, e, f and h, i
Intrestingly, the nuclear distributed pattern of CHI was detected in grape berry and some vegetable tissue cells. It was not surprising. Several other studies have reported the nuclear localization of some flavonoid bisosynthesis enzymes, which responds to in situ synthesis of flavonoids there (Saslowsky et al. 2005; Yu et al. 2008; Winkel 2017). The biosynthesis of flavonoids in the nuclear may serves to protect DNA from UV and oxidative damage (Khan et al. 2017) and so on.
In conclusion, diverse distributions of CHI were investigated in different grapevine tissues, which further suggested that grapevine flavonoids biosynthesis is complicated. And different subcellular localization patterns of CHI were detected between productive and vegetable tissues. In grape berries, a large amount of CHI was observed in cytoplasm, plastids, and nucleus of the exocarp cells. While in grapevine vegetable tissues, besides the cytoplasm and nucleus, the cell wall localization of CHI were investigated too. Together with our previous observation on the distribution of other flavonoids biosynthesis pathway enzymes including CHS and ANS, it is suggested that multiple flavonoids biosynthesis sites exist in grapevine, which need more complex regulation system. Further studies on regulatory mechanism of the different localization patterns will deepen our understanding of the flavonoids metabolism in grapevine.
Abbreviations
- PAL:
-
Phenylalanine ammonia-lyase
- C4H:
-
Cinnamate 4-hydroxylase
- CHI:
-
Chalcone isomerase
- CHS:
-
Chalcone synthase
- ANS:
-
Anthocyanidin synthase
- IgG:
-
Immunoglobulin fractions
- BSA:
-
Bovine serum albumin
- PBS:
-
Phosphate-buffered saline
- BS:
-
Blocking solution
- RSR:
-
Regular salt rinse solution
- DFAB:
-
Days after full bloom
References
Agati G, Brunetti C, Ferdinando MD, Ferrini F, Pollastri S, Tattini M (2013) Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol Biochem 72:35–45. https://doi.org/10.1016/j.plaphy.2013.03.014
Ayre BG, Keller F, Turgeon R (2003) Symplastic continuity between companion cells and the translocation stream: long-distance transport is controlled by retention and retrieval mechanisms in the phloem. Plant Physiol 131:1518–1528. https://doi.org/10.1104/pp.012054
Azuma A, Yakushiji H, Koshita Y, Kobayashi S (2012) Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 236:1067–1080. https://doi.org/10.1007/s00425-012-1650-x
Bardford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Biała W, Jasiński M (2018) The phenylpropanoid case—it is transport that matters. Front Plant Sci 9:1610. https://doi.org/10.3389/fpls.2018.01610
Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson SP (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol 143:1347–1361. https://doi.org/10.1104/pp.106.093203
Bondada BR, Matthews MA, Shackel KA (2005) Functional xylem in the post-veraison grape berry. J Exp Bot 56:2949–2957. https://doi.org/10.1093/jxb/eri291
Braidot E, Zancani M, Petrussa E, Peresson C, Bertolini A, Patui S, Macrì F, Vianello A (2008) Transport and accumulation of flavonoids in grapevine (Vitis vinifera L.). Plant Signal Behav 3(9):626–632. https://doi.org/10.4161/psb.3.9.6686
Buer CS (2010) Flavonoids: New roles for old molecules. J Integr Plant Biol 52(1):98–111. https://doi.org/10.1111/j.1744-7909.2010.00905.x
Buer CS, Muday GK, Djordjevic MA (2008) Implications of long-distance flavonoid movement in Arabidopsis thaliana. Plant Signal Behav 3(6):415–417. https://doi.org/10.4161/psb.3.6.5440
Cadot Y, Minana-castelloa MT, Chevalier M (2006) Anatomical, histological, and histochemical changes in grape seeds from Vitis vinifera L. cv Cabernet Franc during fruit development. J Agric Food Chem 54:9206–9215. DOI: 10.1021/jf061326f
Chen JY, Wen PF, Kong WF, Pan QH, Wan SB, Huang WD (2006) Changes and subcellular localizations of the enzymes that involved in phenylpropanoid metabolism during grape berry development. J Plant Physiol 163:115–127. https://doi.org/10.1016/j.jplph.2005.07.006
Cheng H, Li L, Cheng S, Cao F, Wang Y, Yuan H (2011) Molecular cloning and function assay of a chalcone isomerase gene (GbCHI) from Ginkgo biloba. Plant Cell Rep 30(1):49–62. https://doi.org/10.1007/s00299-010-0943-4
Dastmalchi M, Dhaubhadel S (2015) Soybean chalcone isomerase: evolution of the fold, and the differential expression and localization of the gene family. Planta 241(2):507–523. https://doi.org/10.1007/s00425-014-2200-5
Falginella L, Gaspero GD, Castellarin SD (2012) Expression of flavonoid genes in the red grape berry of ‘Alicante Bouschet’ varies with the histological distribution of anthocyanins and their chemical composition. Planta 236:1037–1051. https://doi.org/10.1007/s00425-012-1658-2
Famiani F, Walker RP, Tecsi L, Chen ZH, Proietti P, Leegood RC (2000) An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis Vinifera L.) berries. J Exp Bot 51:675–683. https://doi.org/10.1093/jxb/51.345.675
Fang J, Jogaiah S, Guan L, Sun X, Abdelrahman M (2018) Coloring biology in grape skin: a prospective strategy for molecular farming. Physiola plantarum 164(4):429–441. https://doi.org/10.1111/ppl.12822
Flamini R, Mattivi F, Rosso MD, Arapitsas P, Bavaresco L (2013) Advanced knowledge of three important classes of grape phenolics: anthocyanins, stilbenes and flavonols. Int J Mol Sci 14:19651–19669. https://doi.org/10.3390/ijms141019651
Georgiev V, Ananga A, Tsolova V (2014) Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 6:391–415. https://doi.org/10.3390/nu6010391
Gholami M (2004) Biosynthesis of anthocyanins in Shiraz grape berries. Acta Hortic (ISHS) 640:353–359. https://doi.org/10.17660/ActaHortic.2004.640.42
Gouot JC, Smith JP, Holzapfel BP, Walker AR, Barril C (2018) Grape berry flavonoids: a review of their biochemical responses to high and extreme high temperatures. J Exp Bot. https://doi.org/10.1093/jxb/ery392
Grotewold E (2004) The challenges of moving chemicals within and out of cells: insights into the transport of plant natural products. Planta 219:906–909. https://doi.org/10.1007/s00425-004-1336-0
Hou ZX, Huang WD (2005) Immunohistochemical localization of IAA and ABP1 in strawberry shoot apexes during floral induction. Planta 222:678–687. https://doi.org/10.1007/s00425-005-0014-1
Isla MI, Vattuone MA, Sampietro AR (1998) Essential group at the active site of Frapaeolum invertase. Phytochemistry 47:1189–1193. https://doi.org/10.1016/S0031-9422(97)00757-7
Jeong ST, Goto-Yamamoto N, Hashizume K, Esaka M (2008) Expression of multi-copy flavonoid pathway genes coincides with anthocyanin, flavonol and flavan-3-ol accumulation of grapevine. Vitis 47(3):135–140
Kaltenbach M, Burke JR, Dindo M, Pabis A, Munsberg FS, Rabin A, Kamerlin SCL, Noel JP, Tawfik DS (2018) Evolution of chalcone isomerase from a noncatalytic ancestor. Nat Chem Biol 14(6):548. https://doi.org/10.1038/s41589-018-0042-3
Khan NM, Ahmad I, Ansari MY, Haqqi TM (2017) Wogonin, a natural flavonoid, intercalates with genomic DNA and exhibits protective effects in IL-1β stimulated osteoarthritis chondrocytes. Chem Biol Interact 274:13–23. https://doi.org/10.1016/j.cbi.2017.06.025
Knogge W, Weissenböck G (1986) Tissue-distribution of secondary phenolic biosynthesis in developing primary leaves of Avena sativa L. Planta 167(2):196–205. https://doi.org/10.1007/BF00391415
Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57:405–430. https://doi.org/10.1146/annurev.arplant.57.032905.105252
Ma J, Fu X, Zhang T, Qian H, Zhao J (2019) Cloning and analyzing of chalcone isomerase gene (AaCHI) from Artemisia annua. Plant Cell Tissue Organ Cult (in press)
Park SH, Lee CW, Cho SM, Lee H, Park H, Lee J, Lee JH (2018) Crystal structure and enzymatic properties of chalcone isomerase from the Antarctic vascular plant Deschampsia antarctica Desv. PLoS ONE 13(2):e0192415. https://doi.org/10.1371/journal.pone.0192415
Peer WA, Murphy AS (2007) Flavonoids and auxin transport: modulators or regulators ? Trends Plant Sci 12(12):556–563. https://doi.org/10.1016/j.tplants.2007.10.003
Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci 181:219–229. https://doi.org/10.1016/j.plantsci.2011.05.009
Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A (2013) Plant flavonoids—biosynthesis, transport and involvement in stress responses. Int J Mol Sci 14:14950–14973. https://doi.org/10.3390/ijms140714950
Saslowsky D, Winkel-Shirley B (2001) Localization of flavonoid enzymes in Arabidopsis roots. Plant J 27:37–48. https://doi.org/10.1046/j.1365-313x.2001.01073.x
Saslowsky DE, Warek U, Winkel-Shirley B (2005) Nuclear localization of flavonoid enzymes in Arabidopsis. J Biol Chem 280:23735–23740. https://doi.org/10.1074/jbc.M413506200
Scott AC, Wyatt S, Tsou P, Robertson D, Allen NS (1999) Model system for plant cell biology: GFP imaging in living onion epidermal cells. Biotechniques 26:1125–1132. https://doi.org/10.2144/99266st04
Shi J, Yu J, Pohorly JE, Kakuda Y (2003) Polyphenolics in grape seeds—biochemistry and functionality. J Med Food 6:291–299. https://doi.org/10.1089/109662003772519831
Sun BS, Ribes AM, Leandro MC, Belchior AP, Spranger MI (2006) Stilbenes: quantitative extraction from grape skins, contribution of grape solids to wine and variation during wine maturation. Anal Chim Acta 563:382–390. https://doi.org/10.1016/j.aca.2005.12.002
Wang W, Tang K, Yang H-R, Wen P-F, Zhang P, Wang H-L, Huang W-D (2010a) Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiol Biochem 48(2–3):142–152. https://doi.org/10.1016/j.plaphy.2009.12.002
Wang HL, Wang W, Zhang P, Pan QH, Zhan JC, Huang WD (2010b) Gene transcript accumulation, tissue and subcellular localization of anthocyanidin synthase (ANS) in developing grape berries. Plant Sci 179:103–113. https://doi.org/10.1016/j.plantsci.2010.04.002
Wang HL, Wang W, Li H, Zhan JC, Huang WD (2011) Expression, tissue and sub-cellular localization of anthocyanidin synthase (ANS) in grapevine. Protoplasma 248:267–279. https://doi.org/10.1007/s00709-010-0160-6
Wang W, Wang HL, Wan SB, Zhang JH, Zhang P, Zhan JC, Huang WD (2012) Chalcone isomerase in grapevine: gene expression and localization in the developing fruit. Biol Plantarum 56(3):545–550. https://doi.org/10.1007/s10535-011-0216-2
Wang HL, Wang W, Zhan JC, Huang WD, Xu HY (2015) An efficient PEG-mediated transient gene expression system in grape protoplasts and its application in subcellular localization studies of flavonoids biosynthesis enzymes. Sci Horticult 191:82–89. https://doi.org/10.1016/j.scienta.2015.04.039
Wang HL, Wang W, Zhan JC, Yan AL, Sun L, Zhang GJ, Wang XY, Ren JC, Xu HY (2016) The accumulation and localization of chalcone synthase in grapevine (Vitis vinifera L.). Plant Physiol Biochem 106:165–176. https://doi.org/10.1016/j.plaphy.2016.04.042
Wen PF, Chen JY, Kong WF, Pan QH, Wan SB, Huang WD (2005) Salicylic acid induced the expression of phenylalanine ammonialyase gene in grape berry. Plant Sci 169:928–934. https://doi.org/10.1016/j.plantsci.2005.06.011
Winkel BS (2017) When an enzyme isn’t just an enzyme anymore. J Exp Bot 68(7):1387–1389
Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51:609–614. https://doi.org/10.1021/jf020782a
Wu YQ, Zhu MY, Jiang Y, Zhao DQ, Jun TAO (2018) Molecular characterization of chalcone isomerase (CHI) regulating flower color in herbaceous peony (Paeonia lactiflora Pall.). J Integr Agric 17(1):122–129. https://doi.org/10.1016/S2095-3119(16)61628-3
Yin YC, Zhang XD, Gao ZQ, Hu T, Liu Y (2018) The research progress of chalcone isomerase (CHI) in plants. Mol Biotechnol. https://doi.org/10.1007/s12033-018-0130-3
Yu XH, Chen MH, Liu CJ (2008) Nucleocytoplasmic-localized acyltransferases catalyze the malonylation of 7-O-glycosidic (iso) flavones in Medicago truncatula. Plant J 55:382–396. https://doi.org/10.1111/j.0960-7412.2008.03509.x
Zamora P, Pardo A, Fierro A, Prieto H, Zúñiga GE (2013) Molecular characterization of the chalcone isomerase gene family in Deschampsia antarctica. Polar biol 36(9): 1269–1280. https://doi.org/10.1007/s00300-013-1346-0
Zhao J, Dixon RA (2010) The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci 15(2):72–80. https://doi.org/10.1016/j.tplants.2009.11.006
Zhao FL, Li YJ, Hu Y, Gao YR, Zang XW, Ding Q, Wang YJ, Wen YQ (2016) A highly efficient grapevine mesophyll protoplast system for transient gene expression and the study of disease resistance proteins. Plant Cell Tissue Organ Cult 125:43–57. https://doi.org/10.1007/s11240-015-0928-7
Acknowledgements
This study was funded by the special fund of China Agriculture Research System (CARS-29), the National Natural Science Foundation of China (No. 31601712), Beijing Municipal Natural Science Foundation (No. 6182007), and the Natural Science Foundation of Beijing Academy of Agriculture and Forestry Science (No. QNJJ201604).
Author information
Authors and Affiliations
Contributions
Weidong Huang and Haiying Xu designed the experiments and revised the manuscript; Huiling Wang, Wei Wang, Jicheng Zhan performed the experiments; Huiling Wang and Wei Wang processed statistical analysis; Huiling Wang prepared the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Yu-Jin Hao.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Wang, W., Zhan, J. et al. Tissue-specific accumulation and subcellular localization of chalcone isomerase (CHI) in grapevine. Plant Cell Tiss Organ Cult 137, 125–137 (2019). https://doi.org/10.1007/s11240-019-01557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01557-y