Abstract
Linum usitatissimum: L. is well-known for production of pharmacologically important secondary metabolites. Due to their tremendous beneficial effects on human health, these compounds are receiving greater attention throughout the World, especially in the treatment of various types of cancers. In present study, we have developed an efficient protocol for production of lignans like secoisolariciresinol diglucoside (SDG) and lariciresinol diglucoside (LDG) and neo-lignans like dehydrodiconiferyl alcohol glucoside (DCG) and guaiacylglycerol-β-coniferyl alcohol ether glucoside (GGCG) by exploiting in vitro callus cultures of Flax. These cultures were established from stem and leaf explants, inoculated on Murashige and Skoog (MS) media supplemented with various concentrations of α-naphthalene acetic acid (NAA), thidiazuron (TDZ) and 6-benzyl adenine (BA). Results revealed that the leaf-derived calli (1.0 mg/l NAA) accumulated highest levels of biomass (DW; 15.7 g/l) and antioxidant activity, while highest production of total phenolics (111.09 mg/l) and flavonoids (45.02 mg/l) were observed in stem-derived calli (1.0 mg/l NAA). The high-performance liquid chromatography (HPLC) analysis revealed that the stem-derived calli (1.0 mg/l NAA) accumulated optimum concentrations of SDG (2.7 ± 0.021 mg/g DW), LDG (9.8 ± 0.062 mg/g DW) and DCG (13.8 ± 0.076 mg/g DW), while leaf-derived calli (1.0 mg/l NAA) showed optimum accumulation of GGCG (3.8 ± 0.022 mg/g DW) as compared to all other treatments. These results provided definite evidence that the NAA differentially influence the production of lignans and neo-lignans in callus culture of Flax. This study opens new dimensions to devise strategies to enhance the production of these valuable metabolites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Linum usitatissimum L. (Flax), is one of the oldest cultivated annual crop which has become a focus of great deal in both basic and applied research in the field of plant cell and biotechnology in recent years (Siegień et al. 2013). The translation of the Latin word usitatissimum is “the most useful one”, reflecting the numerous uses of this plant species. Flax is the only member of Linaceae that is of economic importance and its average worldwide production is 1,602,047 metric tonnes (Mejía 2007). Flax seed contains 38–40% of a drying oil (linseed oil) which have a wide range of industrial and nutritional applications (Millam et al. 2005). Linseed oil is also an excellent source of the omega-3 fatty acid (linolenic acid), Increasing its demand as a source of edible oil with significant percentages of omega-3 fatty acids (55%), which results in consumption of flax seeds as a functional food (Shim et al. 2014). Flax is also mixed with animal feed to improve digestion and reproductive performance of animals (Heimbach 2009; Turner et al. 2014). Besides having industrial and nutritional importance, Flax also has long history of medicinal uses due to the presence of feasible concentrations of antioxidant phenolic acids, proteins and flavonoids, etc (Kosińska et al. 2011; Liu et al. 2006).
Pharmacological importance of Flax is mainly due to presence of biologically active components like lignan, which is an important class of plant secondary metabolites (Adlercreutz 2007). Secoisolariciresinol diglucoside (SDG), matairesinol (MAT), secoisolariciresinol (SECO) and lariciresinol diglucoside (LDG) are the main lignans which represents over 95% of the total lignans in this plant, having numerous pharmacological activities (Touré and Xueming 2010). By the action of intestinal bacteria, these lignans are converted into mammalian lignans (enterodiols and enterolactones), which can potentially reduce the risk of certain types of cancers especially the breast, prostate and colon cancers (Gabr et al. 2016; Szewczyk et al. 2014; Wang et al. 2000). Besides lignans, Flax is also a good source of neo-lignans (dehydrodiconiferyl alcohol glucoside, DCG; and guaiacylglycerol-β-coniferyl alcohol ether glucoside, GGCG), which are used as principal agents in antifungal medicines (Beejmohun et al. 2007). Flax lignans and neo-lignans also have been reported for treatment of cardiovascular disorders (Rickard-Bon and Thompson 2003), hypercholesterolemia (Prasad 1997), diabetics (Hano et al. 2013), and diarrhea (Palla et al. 2015). Because of their important pharmacological and physiological properties, lignans and neo-lignans are currently well recognized, and many studies have been carried out to get a better knowledge of the biological processes involved in the biosynthesis, regulation and the accumulation of these valuable secondary metabolites in plants (Attoumbré et al. 2006; Beejmohun et al. 2007; Ford et al. 2001).
Plant in vitro cultures offer an alternative platform for production of pharmacologically important secondary metabolites, which are difficult to extract from field grown plants (Sasheva et al. 2013). Flax has been exploited for enhanced production of fiber and linseed oil but a few in vitro reports are available on the production of medicinally important lignans and neo-lignans (Beejmohun et al. 2007; Gabr et al. 2016; Hano et al. 2013). Therefore in vitro cultures techniques should be exploited in this species to enhance the production of these valuable metabolites. In vitro callus culture of Flax also have been reported for enhanced biosynthesis of antimicrobial silver nanoparticles due to accumulation of high levels of antioxidant secondary metabolites, which act as reducing as well as stabilizing agents (Anjum and Abbasi 2016). The principle objective of this study was to develop an efficient in vitro culture of Flax which accumulates high levels of lignans and neo-lignans by employing different types of explants and plant growth regulators (PGRs) either alone or in combination. In order to investigate the accumulation of these metabolites in Flax, callus cultures were established, characterized for total phenolic and flavonoid production and further evaluated for their lignan and neo-lignan contents by high performance liquid chromatography (HPLC). The antioxidant activity of callus culture was performed by using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and 2, 2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assays due to ease of their use, simplicity, inexpensiveness and sensitivity. In particular, the accumulation of SDG, LDG, DCG and GGCG (Fig. 1) was evaluated in relation to PGRs balance of the culture media and the type of explant used. To the best of our knowledge, this is the first comprehensive report for feasible production of LDG, DCG and GGCG in callus culture of flax having potential for scaling up to industrial level for enhanced production of these pharmacologically important secondary metabolites.
Materials and methods
Explant collection and establishment of callus cultures
Seeds of Linum usitatissimum L. (brown variety) were collected in 2014 from natural habitat of District Swat, Pakistan. Seeds were surface sterilized and inoculated on Murashige and Skoog basal medium (MS0) (Murashige and Skoog 1962) according to the method of Abbasi et al. (2011). For establishment of callus cultures, leaf (~0.5 cm2) and stem (~1.0 cm) explants were collected from 25-days old in vitro plantlets of Flax and cultured on MS media containing sucrose (30 g l− 1), agar (8 g l− 1) and various concentration (0.5–5.0 mg/l) of α-naphthalene acetic acid (NAA), thidiazuron (TDZ), 6-benzyl adenine (BA), either alone or in combination with 1.0 mg/l NAA. MS0 medium without any PGRs was used as control. These cultures were maintained at 25 ± 2 °C for 16 h/8 h (light/dark) photoperiod in growth room. For each concentration of PGRs used, experiment was performed in triplicate culture flasks and whole of the experiment was repeated twice. Data on callus induction frequencies was recorded on weekly basis and respective calli were subcultured after every 3 weeks on fresh MS medium supplemented with same PGRs. Calli were harvested from the culture media after 5 weeks of 3rd subculturing cycle and investigated for their fresh weight (FW) and dry weight (DW) determination.
Analytical scheme
For evaluation of total phenolic content (TPC), total flavonoid content (TFC) and free radical scavenging activity of calli, the samples were subjected to extraction according to the protocol described by Ali and Abbasi (2013) with minor modifications. Briefly, 200 mg of each dried sample of callus was mixed with 10 ml of methanol (99.9%) and kept these mixtures on rotatory shaker (50 rpm) for 24 h at room temperature. After that, these mixtures were sonicated for 25 min (Toshiba, Japan) and then vortexed for 5 min. Sonication and vortexing processes were repeated twice after every 30 min and finally centrifuged (6000 rpm) for 15 min. The supernatants were collected, syringe filtered and stored at 4 °C for further analysis.
For antioxidant activity determination, DPPH and ABTS free radical scavenging assays (FRSA) were used. DPPH assay was performed according to the protocol reported by Anjum and Abbasi (2016). Briefly, 20 µl of the extracted sample was mixed with 180 µl of DPPH (3.2 mg/100 ml methanol) and incubated the reaction mixture for 1h at room temperature (25 ± 2 °C). Absorbance of the reaction mixture was measured at 517 nm by using UV–Visible spectrophotometer (Shimadzu-1650; Japan). Methanolic extract of DPPH solution (0.5 ml) was used as standard. Finally the radical scavenging activity was calculated as %age of DPPH discoloration using the following equation;
where AC is absorbance of the solution when a callus extract was added at a particular concentration and AS is the absorbance of the DPPH solution (standard).
ABTS assay was performed according to the method reported by Tagliazucchi et al. (2010) with some modifications. Briefly, ABTS solution (7 mM) was reacted with potassium persulphate solution (2.4 mM) at a ratio of 1:1 (v/v). After incubation for 12–16 h at room temperature in the dark, 1.0 ml of the resulting ABTS+ solution was diluted with 60 ml of methanol resulting in an absorbance of 0.706 ± 0.001 at 734 nm. 20 µl of diluted samples of callus extracts were added to 1.96 ml of the resulting blue–green ABTS+ solution and incubated in the dark for 10 min at 37 °C. Blank was run in parallel in an identical manner without test samples. The percentage ABTS radical scavenging activity was calculated by using the following equation;
where AC is absorbance of the solution when a callus extract was added at a particular concentration and AS is the absorbance of the standard solution without test samples.
TPC was determined by using Folin–Ciocalteu (FC) reagent according to the method reported by Velioglu et al. (1998). Briefly, 20 µl of the extracted sample was mixed with 90 µl of the FC reagent (10× diluted with distilled water) and incubated for 5 min at room temperature (25 ± 2 °C). After incubation, 90 µl of sodium carbonate (6%, w/v) was added to above mixture and absorbance was measured at 725 nm by using UV–Visible spectrophotometer (Shimadzu-1650; Japan). Gallic acid (0–50 µg/ml) was used as standard for plotting calibration curve (R2 = 0.967) and the TPC was expressed as gallic acid equivalents (GAE)/g of DW. Total phenolic production was calculated by using the following formula and expressed in mg/l.
TFC was determined by using aluminum chloride (AlCl3) colorimetric method reported by Chang et al. (2002). Briefly, 20 µl of the extracted sample was mixed with 10 µl of potassium acetate (1 M) and 10 µl of AlCl3 (10%, w/v). This mixture was incubated for 30 min at room temperature (25 ± 2 °C) after addition of 160 µl of distilled water. Absorbance of the reaction mixture was measured at 415 nm by using UV–Visible spectrophotometer (Shimadzu-1650; Japan). Quercetin (0–50 µg/ml) was used as standard for plotting calibration curve (R2 = 0.967) and the TFC was expressed as quercetin equivalents (QE)/g of DW. Total flavonoid production was calculated by using the following formula and expressed in mg /l.
The quantification of two lignans (SDG and LDG) and two neo-lignans (DCG and GGCG) compounds was carried out on a Varian liquid chromatographic system as described by Fliniaux et al. (2014). Ultrasonic extraction for HPLC analysis was carried out by using protocol described by Corbin et al. (2015). Prior to HPLC injection, the extracted samples were centrifuged at 3000 rpm for 15 min and then filtered by using nylon syringe membrane (0.45 μm). Purospher (Merck) reverse phase (RP-18) column was used for separation and detection was carried out at 280 nm wavelength by using UV–Vis spectrophotometer. The compounds were quantified by using HPLC (composed of Varian Prostar 410 autosampler, Metachem Degasit, and Varian Prostar 335 Photodiode Array Detector) and identified by comparing their retention times and UV spectra to those of authentic reference standards. 0.2% acetic acid aqua solution (solvent A) and methanol (solvent B) was used as mobile phase. For mobile phase variation, a nonlinear gradient was applied with a flow rate of 0.8 ml/min as follows: from 0 to 40 min of A–B: 90:10 (v/v) to 30:70 (v/v), from 41 to 50 min of A–B: 30:70 (v/v) to 0:100 (v/v), and A–B: 0:100 (v/v) from 51 to 60 min (Corbin et al. 2015).
Statistical analysis
All experiments were conducted in a synchronized manner and each treatment was consisted of three replicates. Mean values of various treatments were subjected to analysis of variance by using Graph Pad Prism 5.01. Duncan’s multiple range test (Windows version 7.5.1, SPSS Inc., Chicago) was used to determine the significance at P < 0.05 (Duncan 1955). Origin software (8.5) was used for generation of all figures.
Results and discussion
Trends in callogenesis
Callogenesis from leaf and stem explants was screened for optimal production of biomass in response to different PGRs either alone or in combinations (Table 1). Callus was initiated from both explants in response to all applied treatments of PGRs except kinetin and dicamba, whose various concentrations (0.5–5 mg/l) resulted in direct shooting (data not shown). However, Callus induction frequency varied in response to different treatments of the PGRs and explants. Callogenesis started at the cut end of leaf and stem explants. In case of leaf explant, 100% callogenic frequency was found at (0.5, 1.0 and 2.0 mg/l) TDZ, 0.5 mg/l NAA and 1.0 mg/l BA. It was observed that the NAA and BA alone showed higher callus induction frequency as compared to their combination with 1.0 mg/l TDZ (Fig. 2a). The lowest callogenic frequency was observed in all treatments of NAA + TDZ combination. These findings are in harmony with the observations of Janowicz et al. (2012) for their study on Linum spp. They noticed higher callogenic frequencies in media supplemented with NAA or BA alone rather than in their combinations. Among the different tested treatments of PGRs, in the case of stem explant, 4.0 mg/l TDZ, (1.0, 2.0 mg/l) NAA, (1.0, 2.0 mg/l) BA and 1.0 mg/l BA + 1.0 mg/l TDZ concentration proved optimum (100%) callogenic response (Fig. 2b). A general trend was observed in both cases of explants, that higher concentrations of each tested PGRs inhibited callus induction frequency, which was possibly due to suppression of endogenous hormones. The difference between stem and leaf explants response to different PGRs concentrations could be a reflection of probable variances in different levels of endogenous hormones in the explant sources or different tissue sensitivities to these PGRs (Lisowska and Wysokinska 2000; Mathur and Shekhawat 2013).
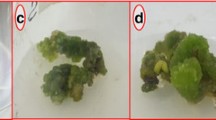
No callus formation from leaf or stem explants was observed on basal (MS0) medium. These findings are in agreement with previously available reports (Mathur and Shekhawat 2013; Ray et al. 2011). In the current study, morphological variations in texture and color of calli have also been observed under different PGRs treatments, which indicated the differences in biochemistry and physiology of explants. It was observed that in all treatments of PGRs, calli produced from stem were watery and friable (Fig. 3) unlike the leaf calli those presented compact and hard texture (Fig. 4). Similar results were reported by Cheng et al. (2006), in which calli induced from leaf and stem explants with morphological variations. Our results showed that exogenous hormones were essential for callus formation in Linum usitatissimum, and all the tested concentrations of TDZ and NAA showed optimum callogenesis. Among the explants used, stem was more responsive for callus induction than leaf explants.
Trends in biomass accumulation
In case of leaf explant, the highest value of callus biomass as fresh weight (FW; 355.07 g/l) and dry weight (DW; 15.7 g/l) was attributed to MS medium supplemented with 1.0 mg/l NAA compared with all other PGRs treatments used either alone or in combinations. While the minimum callus FW (32 g/l) and DW (3.7 g/l) was recorded in response to 5.0 mg/l NAA + 1.0 mg/l TDZ (Fig. 5a, b). However, among all the concentrations and combination of PGRs used, all treatments of NAA (0.5–5 mg/l) resulted in maximum accumulation of callus fresh as well as dry biomass. It suggests that NAA alone has enhancing effect on callus biomass accumulation as compared to all other PGRs tested, which is also evident from previous studies describing the callus formation frequency and biomass accumulation in Flax (da Cunha and Ferreira 1996; Siegień et al. 2013). Similar reports are also available for many other medicinally important plant species (Abbasi et al. 2011; Lisowska and Wysokinska 2000; Mathur and Shekhawat 2013; Rasool et al. 2012). Among all the tested concentrations (0.5–5.0 mg/l) of TDZ, BA, NAA + 1.0 mg/l TDZ and BA + 1.0 mg/l TDZ on leaf explant, maximum FW with the values 300, 239, 145, 96 g/l and DW with the values of 13.7, 11.08, 10.78, 7.2 g/l was recorded in response to 1.0 mg/l BA + 1.0 mg/l TDZ, 1.0 mg/l BA, 3.0 mg/l NAA + 1.0 mg/l TDZ, respectively (Fig. 5a, b).
a Fresh callus biomass and b dry callus biomass from leaf explant. c Fresh callus biomass and d dry callus biomass from stem explant on MS medium supplemented with TDZ, BA, NAA (0.5–5.0 mg/l), BA (0.5–5.0 mg/l) + TDZ 1.0 mg/l, NAA (0.5–5.0 mg/l) + TDZ 1.0 mg/l. Values are means of triplicates with the standard deviation. Means with different letters are statistically different at P ≥ 0.05
In case of stem explant, the highest value of callus FW (318.07 g/l) and DW (15.25 g/l) was recorded in response to 2.0 mg/l NAA while the minimum callus FW (30.01 g/l) and DW (3.5 g/l) was attributed with MS medium supplemented with 5.0 mg/l NAA + 1.0 mg/l TDZ (Fig. 5c, d). Application of 3.0 mg /l TDZ treatment also resulted in higher value of callus FW (316.03 g/l) parallel to 2.0 mg/l NAA treatment but low value of callus DW (12.31 g/l) which showed that callus formed in response to 3.0 mg /l TDZ has high moisture content as compared to 2.0 mg/l NAA-induced callus. Our results showed that the calli produced in response to all applied concentrations (0.5–5.0 mg/l) of TDZ have high values of FW in comparison with their respective DW, suggesting their higher moisture contents. These results are in coordination with the reports of Rasool et al. (2012), Fazal et al. (2014) and Danya et al. (2012). In the current study, irrespective of the explant used, maximum biomass accumulation was recorded in response to lower concentrations of each PGRs tested (0.5, 1.0, 2.0, 3.0 mg/l) either alone or in combinations, but further increase in PGRs concentration (4.0, 5.0 mg/l) significantly inhibited the callus biomass. Similar results were reported previously by Ali and Abbasi (2014) for callus culture of Artemisia absinthium. Our data suggested that the NAA or TDZ alone are the best stimulators among all the tested PGRs, for maximum biomass accumulation in callus cultures of Flax, which is endorsed by the previous reports about callus formation frequency and biomass accumulation in in vitro cultures of Flax (da Cunha and Ferreira 1996; Siegień et al. 2013).
Trends in total phenolic production
Significant variances in TPC profiles were shown by callus cultures of Flax. TPC were found to be dependent upon PGRs treatments and type of explant used. In case of leaf explant, maximum levels of TPC (6.5, 6.25, 5.8 mg/g DW) were found in response to 1.0, 2.0, 3.0 mg/l concentrations of NAA, respectively. After NAA, the calli induced in response to 4.0 mg/l concentrations of BA showed highest levels (5.38 mg/g DW) of TPC (Fig. 6a). These observations were supported by the findings of El-Baz et al. (2010), they found that the addition of higher level of BA as cytokinin and lower level of NAA as auxin yielded the highest TPC in the leaf-derived calli of Citrullus colocynthiss. The minimum values of TPC were recorded in all tested concentrations of NAA (0.5–5.0 mg/l) + 1.0 mg/l TDZ. Jain and Rashid (2001) also reported the minimum levels of TPC in shoot culture of Flax in response to TDZ in combination with NAA, but better levels of TPC were seen when TDZ was used alone. Maximum levels of phenolics production (correlated with DW) 103.04, 90.02, 72.51, 63.04 mg/l were observed in response to 1.0, 2.0, 3.0, 4.0 mg/l concentrations of NAA, respectively (Fig. 6c). Our results showed that a positive correlation existed between total phenolics production and biomass accumulation. Similar observations were reported by Ali and Abbasi (2014), they found that the total phenolics production is dependent on biomass accumulation.
a Total phenolic content (mg/g DW) from leaf explant callus culture and b from stem explant callus culture. c Total phenolic production (mg /l) from leaf explant callus culture and d from stem explant callus culture on MS medium supplemented with TDZ, BA, NAA (0.5–5.0 mg/l), BA (0.5–5.0 mg/l) + TDZ 1.0 mg/l, NAA (0.5–5.0 mg/l) + TDZ 1.0 mg/l. Values are means of triplicates with the standard deviation. Means with different letters are statistically different at P ≥ 0.05
In stem-derived calli, maximum levels of TPC (6.87, 6.40 mg/g DW) were found in response to MS medium supplemented with 1.0, 2.0 mg/l of NAA followed by 2.0 mg/l BA treatment which showed 5.8 mg/g DW of TPC. Low values of TPC were observed in all combinations of NAA (0.5–4.0 mg/l) + 1.0 mg/l TDZ except 1.0 mg/l NAA + 1.0 mg/l TDZ (Fig. 6b). Our results suggested that low concentrations of NAA showed maximum levels of TPC used either alone or in combinations. Similarly, highest levels of total phenolics production (111.09, 86.67, 69.06 mg/l) were shown by 1.0–3.0 mg/l of NAA, respectively (Fig. 6d). In agreement with our results, the highest TPC and phenolics production in response to lower concentrations of NAA and BA were reported by Kartnig et al. (1993), Maharik et al. (2009) and Chaâbani et al. (2015). In present study, we found that among different treatments of PGRs tested either alone or in combination, the lower levels of NAA showed maximum accumulation of TPC in callus culture of Flax. Among the explants used, stem-derived calli produced higher levels of TPC and total phenolics production as compared with leaf-derived calli. The variation in the production of these secondary metabolites in response to different treatments of PGRs are dependent on explant type and may involve the biosynthesis of key enzymes (PAL and/or TAL), which are strategic for the production of phenolic compounds (Maharik et al. 2009).
Trends in total flavonoid production
In comparison to TPC, the accumulation of TFC was also found to be dependent on PGRs treatments and explant used. However, the Production of flavonoids and TFC did not show any linear relationship with biomass accumulation like TPC. In case of leaf explant, maximum level of TFC (3.2 mg/g DW) was observed in response to 1.0 mg/l of NAA followed by 3.0 mg/l BA + TDZ 1.0 mg/l treatment which produced 3.1 mg/g DW of TFC (Fig. 7a). However, Low levels of TFC were observed in response to all treatments of BA (0.5–5.0 mg/l). Production of flavonoids was found to be highest (50.18, 43.94 mg/l) in calli produced from 1.0 to 2.0 mg/l treatments of NAA, respectively (Fig. 7c). As discussed earlier, a decline was observed in TPC production when the concentration of NAA was increased in the culture media. Similar results were recorded in case of TFC and endorsed by the observations of Janowicz et al. (2012) and El-Baz et al. (2010). Previously, no specific reports were available on the production of TPC and TFC in callus cultures of Flax. However, many researchers reported similar TPC and TFC profiling in in vitro cultures of other medicinal plants species (Bahri-Sahloul et al. 2014; Jain and Rashid 2001).
a Total flavonoid content (mg/g DW) from leaf explant callus culture and b from stem explant callus culture. c Total flavonoid production (mg GAE/l) from leaf explant callus culture and d from stem explant callus culture on MS medium supplemented with TDZ, BA, NAA (0.5–5.0 mg/l), BA (0.5–5.0 mg/l) + TDZ 1.0 mg/l, NAA (0.5–5.0 mg/l) + TDZ 1.0 mg/l. Values are means of triplicates with the standard deviation. Means with different letters are statistically different at P ≥ 0.05
In stem-derived calli, maximum level of TFC (3.22 mg/g DW) was found in response to MS medium supplemented with 3.0 mg/l BA + 1.0 mg/l TDZ followed by 1.0 mg/l NAA + TDZ treatment which showed 3.11 mg/g DW of TFC. Low values of TFC were observed in all treatments (0.5–5.0 mg/l) of BA (Fig. 7b). Maximum levels of flavonoids production (45.02, 35.08 mg/l) were resulted in response to low concentrations of NAA (1.0, 2.0 mg/l) (Fig. 7d). In agreement with our results, the highest TFC and flavonoids production in response to lower concentrations of NAA was reported by Fazal et al. (2014) for root cultures of Prunella vulgaris. Our results showed that the production of flavonoids was not restricted to biomass accumulation but vary with type and concentration of PGRs. Al Abdallat et al. (2011) and Bahorun et al. (2003) reported that the synthesis and production of antioxidant secondary metabolites are affected by different treatments of PGRs and elicitors.
Trends in antioxidant activities
In this study, we used two different methods (DPPH and ABTS) based on different mechanisms to evaluate the antioxidant capacity of callus cultures of Flax. According to Huang et al. (2005), no single method is adequate for the evaluation of the antioxidant activity of in vitro cultures of plants and foods. Results of DPPH and ABTS assays showed almost similar results of FRSA in all callus cultures of Flax. The minor differences in the FRSA evaluated by DPPH and ABTS may be attributed to the enhanced efficacy of DPPH assay in polar solvents (methanol) than non-polar solvents (Floegel et al. 2011; Schaich et al. 2015). In leaf-derived calli, maximum FRSA by DPPH (91.51%) and ABTS (86.21%) was observed in MS medium containing 2.0 mg/l TDZ (Fig. 8a, c). Other PGRs, either alone or in combination were found less effective in enhancing FRSA except a combination of 2.0 mg/l NAA + 1.0 mg/l TDZ, which greatly influenced FRSA (DPPH; 90.03% and ABTS; 87.05%). Lowest levels of FRSA were observed in combinations of (3.0–5.0 mg/l) BA + 1.0 mg/l TDZ. TPC generally correlates with antioxidant capacities, and various scientists showed a linear relationship between these two parameters (Djeridane et al. 2006; Kim et al. 2003). Biomass dependent and TPC independent antioxidant potential was observed in all treatments of TDZ (0.5–5.0 mg/l). Contrarily, TPC dependent and biomass independent antioxidant activity was observed in calli obtained in response to all treatments of (0.5–5.0 mg/l) NAA and 2.0 mg/l NAA + 1.0 mg/l TDZ. The current data is in agreement with the results of Grzegorczyk et al. (2007), they observed a high FRSA in Salvia officinalis shoot cultures grown on low levels of NAA. It showed that PGRs enhanced the production of important secondary metabolites which are independent of biomass accumulation.
a DPPH radical scavenging activity (%) from leaf explant and b from stem explant callus culture. c ABTS radical scavenging activity (%) from leaf explant and d from stem explant callus culture on MS medium supplemented with TDZ, BA, NAA (0.5–5.0 mg/l), BA (0.5–5.0 mg/l) + TDZ 1.0 mg/l, NAA (0.5–5.0 mg/l) + TDZ 1.0 mg/l. Values are means of triplicates with the standard deviation. Means with different letters are statistically different at P ≥ 0.05
In stem-derived calli, NAA added at 0.5, 1.0, 2.0 mg/l concentrations as the sole PGR in the medium significantly enhanced the FRSA to levels of 90.01, 93.8, 88.06% by DPPH and 88.12, 90.16, 87.06% by ABTS, respectively (Fig. 8b, d). These results suggested that the FRSA of stem-derived calli are dependent on TPC and callus biomass as these treatments of NAA also showed the highest TPC (Fig. 6b) and DW accumulation (Fig. 5d). In agreement with our results, the highest TPC and FRSA in response to lower concentrations of NAA was reported by Fazal et al. (2014). After NAA, the combinations of high levels (3.0, 4.0 mg/l) of NAA with 1.0 mg/l TDZ showed positive effects on FRSA (DPPH; 87.9, 87.06% and ABTS; 86.61, 84.13%). Salient feature of this study was that all TDZ (0.5-5.0 mg/l) treatments in stem-derived calli showed negative correlation of antioxidant activities with callus biomass, indicating that the effects of PGRs on secondary metabolism are usually biomass-independent and it is difficult to optimize growth conditions (media composition, PH and light) for highest production of biomass and secondary metabolites simultaneously (Ramawat and Mathur 2007).
Trends in accumulation of lignans and neo-lignans
The exogenous application of PGRs not only control the growth and developmental processes in plants but also regulate the production of secondary metabolites in in vitro cultures of plant species (Ali and Abbasi 2013; Coste et al. 2011). To enhance the accumulation of lignans and neo-lignans in callus cultures of Flax, we used different concentrations of PGRs along with different types of explants. All treatments of NAA, BA and NAA + 1.0 mg/l TDZ showed the biosynthesis of both lignans (SDG and LDG) and neo-lignans (DCG and GGCG), whereas none of the treatment of TDZ and BA + 1.0 mg/l TDZ showed accumulation of these metabolites in callus cultures of Flax (Table 2). Stem-derived callus obtained in response to 1.0 mg/l NAA showed the highest accumulation of SDG (2.7 ± 0.021 mg/g DW), LDG (9.8 ± 0.062 mg/g DW) and DCG (13.8 ± 0.076 mg/g DW), whereas the leaf-derived callus obtained in response to 2.0 mg/l NAA + 1.0 mg/l TDZ showed lowest accumulation of SDG (0.7 ± 0.0045 mg/g DW) and DCG (0.5 ± 0.0029 mg/g DW). Leaf-derived callus produced on 1.0 mg/l NAA showed highest accumulation of GGCG (3.8 ± 0.022 mg/g DW) as compared with other treatments (Table 2). In general, calli obtained in response to all treatments of NAA showed enhanced production of lignans and neo-lignans as compared to all other treatments. Many reports are available in the literature on different plant species in which the presence of auxin in the media showed enhanced production of secondary metabolites as compared to cytokinins or their combinations (Al Abdallat et al. 2011; Danya et al. 2012; Fazal et al. 2014). Our results are in agreement with the findings of Attoumbré et al. (2006), in which 1.0 mg/l of NAA stimulated the production of DCG in cell suspension of Flax. Similarly, Beejmohun et al. (2007) reported the biosynthesis of LDG, DCG and GGCG in coniferin-fed cell suspensions of flax but they could not detect SDG. Hano et al. (2006) treated the cell suspensions of Flax with different fungal elicitors and found that cell cultures accumulated SECO and DCG, whereas, SDG was not detected either in control or elicited cells. Similarly, Schmidt et al. (2010) also found that the roots of Flax accumulated trace amounts of SDG. Recently, Gabr et al. (2016) reported the synthesis of lignans (SECO, MAT and SDG) in callus and hairy root cultures of Flax but they could not detect the neo-lignans. No report is available on the biosynthesis of neo-lignans from callus culture of Flax yet. In the current study we made efforts to promote the accumulation of lignans and neo-lignans in callus cultures of Flax and reported the biosynthesis of DCG and GGCG first time in callus culture of Flax.
Conclusions
In this study, we have established the callus culture of Flax for feasible production of pharmacologically important lignans and neo-lignans (SDG, LDG, DGC and GGCG). Among all the tested PGRs, calli obtained in response to 1.0 mg/l of NAA showed optimum concentrations of SDG, LDG, DGC and GGCG, irrespective of the type of explant used. This showed that the NAA differentially increases the production of lignans and neo-lignans in callus culture of Flax Moreover, the accumulation of SDG, LDG, and DCG was found to be dependent on TPC and TFC, whereas the accumulation of GGCG was found to be dependent on biomass in callus cultures of Flax. Limited information is available regarding lignan and neo-lignan biosynthesis, accumulation and regulation in in vitro cultures of plants. The system presented here, may provide a new and efficient way to produce these secondary metabolites on large scale. Further studies are required to investigate its potential for enhanced production of lignans and neo-lignans by establishing cell cultures of Flax.
Abbreviations
- ABTS:
-
2, 2′-Azino-bis-3-ethylbenzothiazoline-6-sulphonic acid
- BA:
-
6-Benzyl adenine
- DCG:
-
Dehydrodiconiferyl alcohol glucoside
- DPPH:
-
2, 2-Diphenyl-1-picrylhydrazyl
- FRSA:
-
Free radical scavenging activity
- GGCG:
-
Guaiacylglycerol-β-coniferyl alcohol ether glucoside
- LDG:
-
Lariciresinol diglucoside
- MAT:
-
Matairesinol
- NAA:
-
α-naphthalene acetic acid
- SDG:
-
Secoisolariciresinol diglucoside
- SECO:
-
Secoisolariciresinol
- TDZ:
-
Thidiazuron
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
References
Abbasi BH, Khan M, Guo B, Bokhari SA, Khan MA (2011) Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tissue Organ Culture (PCTOC 105:337–344
Adlercreutz H (2007) Lignans and human health. Crit Rev Clin Lab Sci 44:483–525
Al Abdallat A, Sawwan J, Al Zoubi B (2011) Agrobacterium tumefaciens-mediated transformation of callus cells of Crataegus aronia. Plant Cell Tissue Organ Culture (PCTOC 104:31–39
Ali M, Abbasi BH (2013) Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind Crops Prod 49:400–406
Ali M, Abbasi BH (2014) Thidiazuron-induced changes in biomass parameters, total phenolic content, and antioxidant activity in callus cultures of Artemisia absinthium L. Appl Biochem Biotechnol 172:2363–2376
Anjum S, Abbasi BH (2016) Thidiazuron-enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L. Int J Nanomed 11:715
Attoumbré J et al (2006) High accumulation of dehydrodiconiferyl alcohol-4-β-d-glucoside in free and immobilized Linum usitatissimum cell cultures. Plant Cell Rep 25:859–864
Bahorun T, Aumjaud E, Ramphul H, Rycha M, Luximon-Ramma A, Trotin F, Aruoma OI (2003) Phenolic constituents and antioxidant capacities of Crataegus monogyna (Hawthorn) callus extracts. Food/Nahrung 47:191–198
Bahri-Sahloul R et al. (2014) Phenolic composition and antioxidant and antimicrobial activities of extracts obtained from Crataegus azarolus L. var. aronia (Willd.) Batt. ovaries calli. J Bot
Beejmohun V et al (2007) Coniferin dimerisation in lignan biosynthesis in flax cells. Phytochemistry 68:2744–2752
Chaâbani G et al. (2015) Effects of 2, 4-dichlorophenoxyacetic acid combined to 6-Benzylaminopurine on callus induction, total phenolic and ascorbic acid production, and antioxidant activities in leaf tissue cultures of Crataegus azarolus L. var. aronia. Acta Physiol Plant 37:1–9
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10
Cheng H, Yu L-J, Hu Q-Y, Chen S-C, Sun Y-P (2006) Establishment of callus and cell suspension cultures of Corydalis saxicola Bunting, a rare medicinal plant. Zeitschrift für Naturforschung C 61:251–256
Corbin C et al (2015) Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason Sonochem 26:176–185
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Culture (PCTOC 106:279–288
da Cunha ACG, Ferreira MF (1996) Somatic embryogenesis, organogenesis and callus growth kinetics of flax. Plant Cell Tissue Organ Culture (PCTOC) 47:1–8
Danya U, Udhayasankar M, Punitha D, Arumugasamy K, Suresh S (2012) In vitro regeneration of Tecomella undulata (Sm.) Seem-an endangered medicinal plant. Int J Plant Anim Environ Sci 2:44–49
Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem 97:654–660
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
El-Baz FK, Mohamed AA, Ali SI (2010) Callus formation, phenolics content and related antioxidant activities in tissue culture of a medicinal plant colocynth (Citrullus colocynthis). Nova Biotechnol 10:79–94
Fazal H, Abbasi BH, Ahmad N (2014) Optimization of adventitious root culture for production of biomass and secondary metabolites in Prunella vulgaris L. Appl Biochem Biotechnol 174:2086–2095
Fliniaux O et al (2014) Microwave-assisted extraction of herbacetin diglucoside from flax (Linum usitatissimum L.) seed cakes and its quantification using an RP-HPLC-UV system. Molecules 19:3025–3037
Floegel A, Kim D-O, Chung S-J, Koo SI, Chun OK (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of food composition analysis 24:1043–1048
Ford JD, Huang K-S, Wang H-B, Davin LB, Lewis NG (2001) Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside-hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linum u sitatissimum) Seed. J Nat Prod 64:1388–1397
Gabr AM, Mabrok HB, Ghanem KZ, Blaut M, Smetanska I (2016) Lignan accumulation in callus and Agrobacterium rhizogenes-mediated hairy root cultures of flax (Linum usitatissimum). Plant Cell Tissue Organ Culture (PCTOC) 1–13
Grzegorczyk I, Matkowski A, Wysokińska H (2007) Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chem 104:536–541
Hano C et al (2006) Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223:975–989
Hano C et al (2013) Flaxseed (Linum usitatissimum L.) extract as well as (+)-secoisolariciresinol diglucoside and its mammalian derivatives are potent inhibitors of α-amylase activity. Bioorganic medicinal chemistry letters 23:3007–3012
Heimbach J (2009) Determination of the generally recognized as safe status of the addition of whole and milled flaxseed to conventional foods and meat and poultry products. Flax Canada 2015:1
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Jain P, Rashid A (2001) Stimulation of shoot regeneration on Linum hypocotyl segments by thidiazuron and its response to light and calcium. Biol Plant 44:611–613
Janowicz J, Niemann J, Wojciechowski A (2012) The effect of growth regulators on the regeneration ability of flax (Linum usitatissimum L.) hypocotyl explants in in vitro culture. BioTechnol J Biotechnol Comput Biol Bionanotechnol 93
Kartnig T, Kögl G, Heydel B (1993) Production of flavonoids in cell cultures of Crataegus monogyna. Plant Med 59:537–538
Kim D-O, Chun OK, Kim YJ, Moon H-Y, Lee CY (2003) Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem 51:6509–6515
Kosińska A, Penkacik K, Wiczkowski W, Amarowicz R (2011) Presence of caffeic acid in flaxseed lignan macromolecule. Plant foods for human nutrition 66:270–274
Lisowska K, Wysokinska H (2000) In vitro propagation of Catalpa ovata G. Don. Plant Cell Tissue Organ Culture (PCTOC) 60:171–176
Liu Z, Saarinen NM, Thompson LU (2006) Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J Nutr 136:906–912
Maharik N, Elgengaihi S, Taha H (2009) Anthocyanin production in callus cultures of Crataegus sinaica boiss. Int J Acad Res 1:30–34
Mathur S, Shekhawat GS (2013) Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: an in vitro approach for production of stevioside. Acta Physiol Plant 35:931–939
Mejía D (2007) FAO (Food and Agriculture Organization of the United Nations)
Millam S, Obert B, Pret’ová A (2005) Plant cell and biotechnology studies in Linum usitatissimum—a review. Plant Cell Tissue Organ Culture (PCTOC) 82:93–103
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Palla AH, Khan NA, Bashir S, Iqbal J, Gilani A-H (2015) Pharmacological basis for the medicinal use of Linum usitatissimum (Flaxseed) in infectious and non-infectious diarrhea. J Ethnopharmacol 160:61–68
Prasad K (1997) Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis 132:69–76
Ramawat K, Mathur M (2007) Factors affecting production of secondary metabolites. Biotechnology secondary metabolites Science. Enfield 59–101
Rasool R, Ganai BA, Kamili AN, Akbar S (2012) Antioxidant potential in callus culture of Artemisia amygdalina Decne. Nat Prod Res 26:2103–2106
Ray B, Hassan L, Nasiruddin K (2011) In vitro regeneration of brinjal (Solanum melongena L.). Bangladesh J Agric Res 36:397–406
Rickard-Bon SE, Thompson LU (2003) The role of flaxseed lignans in hormone-dependent and independent cancer Flax. Genus Linum 181–203
Sasheva P, Letkarska G, Ionkova I (2013) Biotechnological production of podophyllotoxin and podophyllotoxin-related lignans in cultures of Linum thracicum Degen. Comptes Rendus de l’Academie bulgare des Sciences 66:1445–1450
Schaich K, Tian X, Xie J (2015) Hurdles and pitfalls in measuring antioxidant efficacy: a critical evaluation of ABTS, DPPH, and ORAC assays. J Funct Foods 14:111–125
Schmidt TJ et al (2010) Lignans in flowering aerial parts of Linum species–chemodiversity in the light of systematics and phylogeny. Phytochemistry 71:1714–1728
Shim YY, Gui B, Arnison PG, Wang Y, Reaney MJ (2014) Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: a review. Trends Food Sci Technol 38:5–20
Siegień I, Adamczuk A, Wróblewska K (2013) Light affects in vitro organogenesis of Linum usitatissimum L. and its cyanogenic potential. Acta Physiol Plant 35:781–789
Szewczyk M, Abarzua S, Schlichting A, Nebe B, Piechulla B, Briese V, Richter D-U (2014) Effects of extracts from Linum usitatissimum on cell vitality, proliferation and cytotoxicity in human breast cancer cell lines. J Med Plants Res 8:237–245
Tagliazucchi D, Verzelloni E, Bertolini D, Conte A (2010) In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem 120:599–606
Touré A, Xueming X (2010) Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Comprehen Rev Food Sci Food Safe 9:261–269
Turner T, Mapiye C, Aalhus J, Beaulieu A, Patience J, Zijlstra R, Dugan M (2014) Flaxseed fed pork: n–3 fatty acid enrichment and contribution to dietary recommendations. Meat Sci 96:541–547
Velioglu Y, Mazza G, Gao L, Oomah B (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46:4113–4117
Wang L-Q, Meselhy MR, Li Y, QIN G-W, Hattori M (2000) Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem Pharmaceut Bull 48:1606–1610
Author contributions
SA did the research work, data analyses and manuscript write-up. BHA conceived the idea, supervised the research work and critically reviewed the manuscript. CH performed the HPLC analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Anjum, S., Abbasi, B.H. & Hano, C. Trends in accumulation of pharmacologically important antioxidant-secondary metabolites in callus cultures of Linum usitatissimum L.. Plant Cell Tiss Organ Cult 129, 73–87 (2017). https://doi.org/10.1007/s11240-016-1158-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1158-3