Abstract
Adventitious root cultures of Prunella vulgaris L. were established in shaking flask system for the production of biomass and secondary metabolites. Adventitious root cultures were induced from callus cultures obtained from leaf explants on solid Murashige and Skoog (MS) medium containing combination of 6-benzyladenine (BA; 1.0 mg l−1) and naphthalene acetic acid (NAA; 1.5 mg l−1). Thereafter, 0.49 g inoculum was transferred to liquid MS medium supplemented with different concentrations of NAA (0.5–2.0 mg l−1). Growth kinetics of adventitious roots was recorded with an interval of 7 days for 49 days period. Highest biomass accumulation (2.13 g/l) was observed in liquid medium containing 1.0 mg l−1 NAA after 21 days of inoculation. However, other concentrations of NAA also showed similar accumulation pattern but the biomass gradually decreases after 49 days of inoculation. Adventitious roots were collected and dried for investigation of total phenolics (TP), total flavonoids (TF), and antioxidant activities. Higher TPC (0.995 GAE mg/g-DRB) and TFC (6.615 RE mg/g-DRB) were observed in 0.5 mg l−1 NAA treated cultures. In contrast, higher antioxidant activity (83.53 %) was observed 1.5 mg l−1 NAA treated cultures. These results are helpful in up scaling of root cultures into bioreactor for secondary metabolites production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Majority of the commercially important secondary metabolites have been widely isolated by solvent extraction from different parts of naturally growing plants. But there are some metabolites which are either produced in limited quantities in parental plants or difficult to synthesize under laboratory conditions [1, 2]. That is why, plant cell, tissue, and organ culture technology have received greater attention for producing metabolites of interest such as pharmaceuticals, neutraceutical, fine chemicals, pigments, and cosmetics [3]. These in vitro systems are specifically exploited for production due to their similarities to microbial cultures under similar or modified growth conditions [4]. Furthermore, to produce large amount of value added compounds, these culture system seems to be more promising and reliable than field cultivation which need high initial stock, laborious, and in fact, long-lasting process with greater possibility of fluctuation in quality and quantity of the resultant phytochemicals [5].
Among different classes of phytochemicals, polyphenols are the largest group which has proven to exhibit higher antioxidant activity as compared to carotenoids and vitamins [6–8]. Caffeic acid derivatives, lignans, gallotannins, ellegitannins, xanthones, and stilbenes are some of the examples of polyphenols [9, 10]. These compounds are effective in quenching toxic free radicals, stimulation of immune system, regulation of gene expression, apoptosis induction, enzyme activation, and interaction with cell-cycle arrest [11, 12]. Generally, there exist strong relationships between phenolics and flavonoids with antioxidant activity [13]. An interest in plant-based antioxidants has increased due to lower side effects as compared to synthetic antioxidant which sometimes induce carcinogenic effects [14]. Among different methods used for determination of antioxidant activities, DPPH is one of the simplest, inexpensive, and rapid methods used for the determination of antioxidant capacity of plant cells and derived products [1, 15]. DPPH activity is not restricted to specific antioxidant component, solid, or liquid samples, but is used for the overall antioxidant capacity of the samples.
Among different culture techniques, adventitious root culture is the most attractive system for the production of biomass and commercially important metabolites [16]. Easily up-scaling of adventitious root culture to bioreactor is another advantage of this method [17]. As compared to other culture techniques (cell, embryo, and intact plant), root culture (hairy and adventitious) is widely exploited for production of bioactive compounds due to high efficiency and similarities with those from mother plants [18]. Naturally, biotic and abiotic stress conditions alter the synthesis of bioactive compounds in plants. Therefore, the synthesis of bioactive compounds in adventitious root culture can be altered by exposure to different conditions. Furthermore, it is relatively easy to get control over physical and chemical environmental conditions of adventitious root cultures [19].
Prunella vulgaris L. (P. vulgaris) species belongs to family Labiatae and commonly known as “self-heal” [20, 21]. Chang and Yeung were the first scientists who observed the inhibitory activity of aqueous extracts of P. vulgaris against human immunodeficiency virus (HIV) [22]. Later on in 1989, Tabba and his team isolated, purified, and characterized “Prunellin” as an anti-HIV component from aqueous extracts of P. vulgaris [23]. After these reports, no one further worked on this species either to isolate Prunellin or to enhance its production. Majority of the available reports mainly focus on its biological activities. These activities include anti-inflammatory, anti-tumor, anti-viral, immune system stimulant, and production of T lymphocytes and cytokines [21, 24–28]. Till now, no reports are on hand on the development of adventitious root culture of P. vulgaris. Our lab team is trying to optimize and develop different in vitro culture protocols for this important species. Therefore, the main objective of the present study was to optimize adventitious root culture for the production of biomass and secondary metabolites. This simple protocol has the potential for commercial applications and the current study need HPLC fingerprinting of Prunellin.
Materials and Methods
Seed Germination and Explant Collection
Whole plants of P. vulgaris were collected from natural habitat of District Swat, Pakistan. These plants were authenticated through taxonomic markers in the Medicinal Botanic Centre (MBC), PCSIR Laboratories Complex, Peshawar. After plant authentication, seeds were collected in June 2012.These seeds were surface sterilized by using recent method of Ahmad et al. [2]. Seeds were washed several times with sterile distilled water after treatment with mercuric chloride (HgCl2; 0.2 %) and ethanol (70 %). Sterilized seeds were then inoculated to plant growth regulators free Murashige and Skoog (MS) [29] medium containing 30 g l−1 sucrose, solidified with 7–8 g l−1 agar; the pH was adjusted to 5.3 and autoclaved at 121 °C for 25 min. Inoculated flasks were placed in growth chambers at 25 °C ± 2 under 16/8 h photoperiod with light intensity of 40-mol m−2 s−1. After 30 days of inoculation, leaf explants were collected from in vitro grown plantlets for callus induction.
Callus Induction from Leaf Explants
Leaf explants (~3-4 mm2 pieces) of in vitro grown plantlets were placed on MS-medium containing combination of 6-benzyladenine (BA; 1.0 mg l−1) and naphthalene acetic acid (NAA; 1.5 mg l−1). After 30 days of explant inoculation, friable calli was collected for development of adventitious root culture.
Adventitious Root Culture and Growth Kinetics
For optimization of adventitious root culture, 0.49 g of 30 days old calli was transferred to 100 ml Erlenmeyer flasks containing 40 ml MS-basal media supplemented with different concentrations of NAA (0.5–2.0 mg l−1). These cultured flasks were placed in orbital shaker (120 rpm; Gallenkamp, England) at 25 °C for seven weeks period. Data regarding growth kinetics was collected with 7 days interval for a period of 49 days. Growth curve was established for the biomass accumulation of the rapidly growing adventitious root culture, obtained in response to different concentrations of NAA.
Adventitious Root Biomass Determination
For fresh biomass (FB), adventitious roots were collected from liquid media. These roots were carefully washed with sterile distilled water and pressed gently on filter paper (Whatman Ltd., England) to remove excess water and finally weighted (Sortorious digital balance; Germany). Similarly, for dry biomass (DB) determination, roots were dried in an oven (Thermo Scientific; Germany) at 50 °C and finally weighted. Fresh and dry biomass of adventious roots were expressed in gram/flask.
Analytical Methods
The dried biomass of adventitious root cultures was grinded in mortar and pestle for extract preparation. In test tube, 10 mg of the powdered material of each sample was mixed with 10 ml of ethanol. The solution was vertixed daily for 1 week. After 1 week, these solutions were centrifuged at 14,000 rpm for 15 min. The supernatent was used for determination of different activities. The total phenolic content in each sample was determined by using the recent methods of Ahmad et al. [2]. Briefly, 0.1 ml (2N) Folin-Ciocalteus reagent was mixed with 0.03 ml extract and 2.55 ml sterile distilled water. Before incubation for 6 min, the mixture was centrifuged (10,000 rpm; 14 min) and then filtered through 45 μm membrane in UV–visible spectrophotometer (Shimadzu-1650; Japan) cuvette. The absorbance of the resulted mixture was measured at 760 nm. Gallic acid (Sigma; 1.0–10 mg/ml; R 2 = 0.9878) was used for plotting standard calibration curve. Results as Gallic acid equivalent (GAE) mg/g of dry root biomass (DRB) were obtained from % TPC by using the following equation:
Where AS is the absorbance of the sample and AB is absorbance of blank, CF is the conversion factor from standard curve, and DF is the dilution factor.
The TFC in DRB was determined by using the method of Ahmad et al. [2] with little modification. Methanolic extract (0.25 ml) of treated samples was mixed with sterile distilled water (1.25 ml) and 0.075 ml 5 % (w/v) AlCl3. Before incubation (5 min) and centrifugation (10,000 rpm; 14 min), the solution was mixed with 0.5 ml of NaOH (1 M). The absorbance was checked at 510 nm with UV–visible spectrophotometer (Shimadzu-1650PC, Japan). Rutin (Sigma; 1.0–10 mg/ml; R 2 = 0.9866) was used for plotting standard calibration curve. The total flavonoid content was expressed as rutin equivalent (RE) mg/g-DRB of extracts.
DPPH radical scavenging activity (DRSA) in different samples was determined according to the method of Ahmad et al. [12] with little modifications. Briefly, ethanolic extract (1.0 ml; 5 mg/20 ml) of each sample was mixed with 2.0 ml of DPPH free radical solution (0.25 mg/20 ml × 4). The mixture was incubated in the dark for approximately 30 min. The absorbance of resulted mixture was measured at 517 nm at room temperature by using UV–visible spectrophotometer model (Shimadzu-1650PC, Japan). Finally, the radical scavenging activity was calculated as percentage of DPPH discoloration using the following equation:
Where AP represents absorbance of plantlets extract at 517 nm and AD is the absorbance of the DPPH solution without tissue extract.
Statistical Analysis
Analysis of replicated mean values, standard errors (±), and least significant difference (LSD) was carried out by using Statistix software (8.1 versions), and Origin Lab (8.5) software was used for graphical presentation.
Results and Discussions
The Effect of NAA Concentrations on Adventitious Roots Biomass and Growth Kinetics
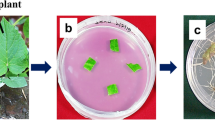
In the present study, adventitious root culture of P. vulgaris has been established (Fig. 1). Adventitious root biomass accumulation and growth kinetics were investigated for 49 days period. Initially, 0.498 g of callus inoculum was inoculated in liquid medium containing different concentrations of NAA (0.5, 1.0, 1.5, and 2.0 mg l−1). Maximum adventitious root biomass accumulation (2.13 g/l) was observed after 21 days of callus immersion in liquid medium containing 1.0 mg l−1 of NAA. The addition of 1.5 mg l−1 NAA also induced higher accumulation of biomass (1.95 g/l) but lower than 1.0 mg l−1 of NAA. Other NAA treated cultures also produced optimum biomass after 21 days. It shows that the production of adventitious root biomass in liquid culture is PGRs dependent. The growth kinetics showed that biomass accumulation increases up to 21 days; but later on, a gradual decline in biomass was observed from 28 to 49 days (Fig. 2). After 21 days, 0.5 mg l−1 NAA treated culture showed threefold increment in biomass, 1.0 mg l−1 NAA showed more than fourfold, 1.5 mg l−1 NAA showed fourfold, and 2.0 mg l−1 NAA showed more than threefold increment in biomass (Fig. 2). The root morphology and color remains the same throughout the experiment. Data regarding adventitious root culture in P. vulgaris is absent in the cited literatures; however, many studies revealed that the addition of NAA to solid/liquid medium stimulate adventitious root induction in high-valued medicinal plants. Lee et al. [30] reported that lower concentration of NAA accelerate adventitious root production in Aloe vera. Taylor et al. [31] observed that the addition of NAA to the medium induced adventitious roots in Lycopersicon esculentum. Hussain et al. [32] found that application of NAA is the best option to produce adventitious rooting in Eurycoma longifolia. NAA has also been reported to be superior to IBA for production of adventitious roots in Cornus mas and Ulmus parvifolia [33, 34]. The possible reason is that under in vitro conditions, plant cells and tissues more rapidly absorb and utilize NAA than IBA. Peeters et al. [35] recorded that the uptake of NAA was six times faster than IAA in tobacco explants. Similarly, Van Der Krieken et al. [36] observed that the uptake of IBA was 4 times faster than IAA. Contrarily to previous reports, in this investigation, different concentrations of NAA induced optimum adventitious rooting response in P. vulgaris liquid culture. Therefore, it is concluded that the addition of 1.0 or 1.5 mg l−1 NAA ensure the best medium for adventitious root production in P. vulgaris.
Optimization of adventitious root culture in P. vulgaris. a Mother plant of P. vulgaris used for explants collection. b Callus formation from leaf explants on solid medium containing 1.0 mg l−1 of BA and 1.5 mg l−1 of NAA. c Inoculation of calli to 100 ml Erlenmeyer flasks containing liquid MS medium and different NAA concentration and sequent shaking on orbital shaker at 120 rpm. d Development of adventitious roots. e Roots formation on 0.5 mg l−1 of NAA, f 1.0 mg l−1 NAA, g 1.5 mg l−1 NAA, and h 2.0 mg l−1 NAA
Fresh adventitious roots biomass accumulation in liquid culture of P. vulgaris after treatment with different concentrations of NAA (0.5, 1.0, 1.5, and 2.0 mg l−1). The growth kinetics of adventitious roots was studied for 49 days of culture period. Mean data were collected from experiments which were arranged in triplicates. Mean data and standard errors (±S.E) were calculated by using Statix software (8.1). For graphical presentation of data, OriginLab software (8.1) was used. Mean data are significantly different at P < 0.05
Biomass Independent Total Phenolics Content Production
In the present study, TPC was investigated in DRB of P. vulgaris. It has been observed that TPC production was completely dependent on PGR concentrations rather than biomass accumulation (Fig. 3). Maximum TPC (0.995 GAE mg/g-DRB) was observed in roots treated with 0.5 mg l−1 NAA in comparison with Gallic acid curve (R 2 = 0.9878). The production of dry biomass (33 mg/l) on the same medium is lower than other concentrations of NAA tested. It means that TPC production in liquid culture of P. vulgaris is independent of biomass accumulation. Higher DRB (86 mg/l) was observed in roots treated with 1.5 mg l−1 of NAA. But the production of TPC (0.263 GAE mg/g-DRB) is lower than 0.5 mg l−1 of NAA augmented medium. Significantly similar TPC (0.234 GAE mg/g-DRB) was observed in roots treated with 1.0 mg l−1 NAA. Biomass independent production of TPC was recently reported by Ali et al. [37] in callus cultures of Artemisia absinthium. It has been previously reported that the accumulation and biosynthesis of commercially important secondary metabolites in medicinal plant species is possible by varying the culture conditions, including the type of growth regulator and concentration [38–41]. In the present investigation, lowest TPC was observed in dry biomass of adventitious roots obtained in response to 2.0 mg l−1 NAA. However, lower NAA concentration enhanced the production of TPC (Fig. 3). Dornenburg and Knorr [42] and Chan et al. [43] reported that high auxin levels in culture medium often inhibit the production of secondary metabolites. The current results suggest that 0.5 mg l−1 NAA is suitable growth regulator for TPC production in adventitious root culture of P. vulgaris.
Biomass independent and PGRs dependent correlation of total phenolics content in adventitious root culture of P. vulgaris. Mean data were collected from experiments which were arranged in triplicates. Mean data and standard errors (±S.E) were calculated by using Statix software (8.1). For graphical presentation of data, OriginLab software (8.1) was used. Mean data are significantly different at P < 0.05
Biomass Independent Total Flavonoids Content Production
To investigate the production of flavonoids content in P. vulgaris, adventitious root culture initially tested more than 20 culture media using different auxin treatments with the aim of obtaining higher adventitious roots biomass. As compared to phenolics content, the production of TFC was also found to be PGR dependent. The production of flavonoids content did not show linear correlation with biomass accumulation. However, the production rate of TFC in adventitious root culture of P. vulgaris was higher than TPC. Maximum TFC (6.62 RE mg/g DRB) was observed in roots treated with 0.5 mg l−1 of NAA in comparison with Rutin calibration curve (R 2 = 0.9866, Fig. 4). As discussed earlier that when the concentration of NAA was increased in the culture media, a decline was observed in TPC production. But we did not observe similar response during TFC production because significantly similar production of TFC (5.51 RE mg/g DRB) was observed in roots treated with 1.5 mg l−1 NAA (Fig. 4). Furthermore, the dry biomass of roots was also higher at 1.5 mg l−1 of NAA. In contrast, the production of TPC and TFC in control roots was lower than NAA treated roots. The current results are in agreement with the reports of Ali et al. [37]. During current experiment, it was observed that the production of important metabolites was not restricted to biomass accumulation but vary with type and concentration of PGRs. Literature is scarce on production of secondary metabolites through adventitious root culture in Prunella, however, many workers reported that indole butyric acid (IBA) is more potent than NAA in production of biomass and secondary metabolites [44–46]. However, we observed positive effect of NAA and the differences in data may be due plant species and growth conditions.
Biomass independent and PGRs dependent correlation of total flavonoids content in adventitious root culture of P. vulgaris. Mean data were collected from experiments which were arranged in triplicates. Mean data and standard errors (±S.E) were calculated by using Statix software (8.1). For graphical presentation of data, OriginLab software (8.1) was used. Mean data are significantly different at P < 0.05
Biomass Dependent DPPH Radical Scavenging Activity (DRSA)
Free radicals are constantly generated in body that is highly reactive due to the presence of unpaired electrons. These unpaired electrons produce more toxic species by donating or taking electron from other molecules such as oxygen free radicals and some non-radical derivatives of oxygen [47, 48]. This discrepancy cause aging and lethal diseases including diabetes mellitus, arthritis, cancer, arteriosclerosis, and cardiovascular diseases [49]. The presence of different antioxidants in intact plants or in vitro culture neutralize the harmful effects of ROS by using endogenous antioxidative enzyme defense system such as ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD).
TPC and TFC showed biomass independent and PGRs dependent mode of production, but the DRSA in root cultures treated with different NAA concentrations showed linear correlation with biomass production. Higher DRSA (83.5 %) and biomass (86 mg/l) were observed in culture treated with 1.5 mg l−1 of NAA (Fig. 5). Significantly similar, DRSA (81.17 %) was also observed in roots grown on medium containing 1.0 mg l−1 of NAA as compared to control (24.1 %). Recently, Ali et al. [1, 37] and Tariq et al. [50] reported biomass dependent and PGRs independent, and biomass independent and PGRs dependent DPPH based antioxidant activity in callus and suspension cultures of Artemisia absinthium. Moreover, Lo et al. [51] reported higher DRSA in shoot culture containing roots in Dendrobium tosaense as compared to Dendrobium moniliforme and Dendrobium linawianum. Gulluce et al. [52] observed maximum DPPH activity in callus cultures of Satureja hortensis than synthetic butylated hydroxytoluene antioxidant agent. Similarly, Grzegorczyk et al. [53] reported DPPH activity in hairy and regenerated roots of Salvia officinalis.
Biomass dependent and PGRs independent correlation of total phenolics content in adventitious root culture of P. vulgaris. Mean data were collected from experiments which were arranged in triplicates. Mean data and standard errors (±S.E) were calculated by using Statix software (8.1). For graphical presentation of data, OriginLab software (8.1) was used. Mean data are significantly different at P < 0.05
Plant cell, tissue, and organ cultures are widely exploited for the production of high-valued secondary metabolites [54]. During in vitro cultures, different factors affect the production of important metabolites [55]. Auxin and cytokinin alone or their synergistic combinations significantly alter the production of metabolites depending on plant species. Kinetin enhances the production of anthocyanins in Haplopappus gracilus but inhibit it in populous cell cultures [56]. But we observed PGRs independent activity in adventitious root culture of P. vulgaris. However, as the concentration of NAA increases from 0.5 to 1.5 mg l−1, the DRSA also increases but the activity decreases with further increment in NAA (2.0 mg l−1) stock solution.
In conclusion, this is the first report on optimization of adventitious root culture in shaking flask system for production of biomass and metabolites. P. vulgaris root culture was investigated by treating leaf-derived callus cultures with different NAA concentrations to induce rooting. Among different NAA concentrations, 1.0 mg l−1 was found effective in fresh biomass accumulation after 21 days of inoculation. However, 0.5 mg l−1 NAA was effective for TPC and TFC production. Furthermore, these in vitro roots showed optimum antioxidant potential than control. Therefore, this study further need up gradation to bioreactor system for higher production of biomass and bioactive compounds for commercial applications.
References
Ali, M., Abbasi, B. H., & Haq, I. U. (2013). Industrial Crops and Products, 49, 400–406.
Ahmad, N., Abbasi, B. H., Fazal, H., Khan, M. A., & Afridi, M. S. (2014). Comptes Rendus Biologies, 337, 19–28.
Kolewe, M. E., Gaurav, V., & Roberts, S. C. (2008). Molecular Pharmacy, 5, 243–256.
Baque, M. A., Elgirban, A., Lee, E. J., & Paek, K. Y. (2012). Acta Physiology Plantarum, 34, 405–415.
Murthy, H. N., Hahn, E. J., & Paek, K. Y. (2008). Chinese Journal of Biotechnology, 24, 711–716.
Ciésla, Ł., Kowalska, I., Oleszek, W., & Stochmal, A. (2012). Phytochemical Analysis. doi:10.1002/pca.2379.
Ahmad, N., Fazal, H., Abbasi, B. H., & Ali, M. (2013). Forest Systems, 22, 559–563.
Ahmad, N., Abbasi, B. H., Fazal, H., & Rahman, U. R. (2013). Applied Biochemistry and Biotechnology, 169, 2004–2015.
Harborne, J. B. (2001). Natural Product Reports, 18, 361–379.
Matkowski, A. (2006). NATO science series: Life and behavioral sciences (pp. 129–148). Amsterdam: IOS Press.
Joo, S. S., Kim, Y., & Lee, D. I. (2010). Plant Pathology Journal, 26, 57–62.
Ahmad, N., Abbasi, B. H., & Fazal, H. (2013). Industrial Crops and Products, 49, 164–168.
Amid, A., Johan, N. N., Jamal, P., & Zain, W. N. W. M. (2011). African Journal of Biotechnology, 10, 18653–18656.
Matkowski, A. (2008). Biotechnology Advances, 26, 548–560.
Khan, M. A., Abbasi, B. H., Ahmed, N., & Ali, H. (2013). Industrial Crops and Products, 46, 105–110.
Wang, J., Man, S., Gao, W., Zhang, L., & Huang, L. (2013). Industrial Crops and Products, 41, 57–63.
Baque, M. A., Shiragi, M. H. K., Moh, S. H., Lee, E. J., & Paek, K. Y. (2013). In Vitro Cellular and Developmental Biology-Plant, 49, 737–749.
Cui, X.-H., Chakrabarty, D., Lee, E. J., & Paek, K. Y. (2010). Bioresource Technology, 101, 4708–4716.
Sivanandhan, G., Arun, M., Mayavan, S., Rajesh, M., Mariashibu, T. S., Manickavasagam, M., Selvaraj, N., & Ganapathi, A. (2012). Industrial Crops and Products, 37, 124–129.
Chen, Y., Yu, M., Zhu, Z., Zhang, L., & Guo, Q. (2013). PLoS ONE, 8, 1–7.
Rasool, R., Kamili, A. N., Ganai, B. A., & Akbar, S. (2009). Journal of Natural Sciences and Mathematics, Qassim University, 3, 21–26.
Chang, R. S., & Yeung, H. W. (1988). Antiviral Research, 9, 163–176.
Tabba, H. D., Chang, R. S., & Smith, K. M. (1989). Antiviral Research, 11, 263–273.
Chen, C. Y., Wu, G., & Zhang, M. Z. (2009). Chinese-German Journal of Clinical Oncology, 8, 426–429.
Chlopcíková, S., Psotová, J., Miketová, P., Sousek, J., Lichnovský, & Miketová, V. (2005). Fitoterapia, 76, 556–561.
Huang, R., Zhao, M., Yang, X., Huang, J., Yang, Y., Chen, B., Tan, J., Huang, J., Li, Z., Lv, Y., & Ji, G. (2013). PLoS ONE, 8, e77355.
Liu, G. M., Jia, X. B., Wang, H. B., Feng, L., & Chen, Y. (2009). Journal of Chinese Medicine Materials, 3, 1920–1926.
Zdarilova, A., Svobodova, A., Simanek, V., & Ulrichova, J. (2009). Toxicology In Vitro, 23, 386–392.
Murashige, T., & Skoog, F. (1962). Physiology Plantarum, 15, 473–497.
Lee, Y. S., Yang, T. J., Park, S. U., Baek, J. H., Wu, S. Q., & Lim, K. B. (2011). Plant Omics Journal, 4, 190–194.
Taylor, J. L. S., & van-Staden, J. (1998). Plant Growth Regulation, 26, 77–83.
Hussein, S., Ling, A. P. K., Ng, T. H., Ibrahim, R., & Paek, K. Y. (2012). Romanian Biotechnological Letters, 17, 7026–7035.
Durkovic, J., & Bukovska, J. (2009). Biologia Plantarum, 53, 715–718.
Thakur, R. C., & Karnosky, D. F. (2007). Plant Cell Reports, 26, 1171–1177.
Peeters, A. J. M., Gerads, W., Barendse, G. W. M., & Wullems, G. J. (1991). Plant Physiology, 97, 402–408.
Van Der Krieken, W. M., Breteler, H., Visser, M. H. M., & Mavridou, D. (1993). Plant Cell Reports, 12, 203–206.
Ali, M., & Abbasi, B. H. (2013). Applied Biochemistry and Biotechnology. doi:10.1007/s12010-013-0663-7.
Palacioa, L., Canterob, J. J., Cusidoc, R. M., & Goleniowski, M. E. (2012). Plant Science, 193–194, 1–7.
Rao, S. R., & Ravishankar, G. A. (2002). Biotechnology Advances, 20, 101–153.
Verpoorte, R., Contin, A., & Memelink, J. (2002). Phytochemistry Reviews, 1, 13–25.
Ribeiro Affonso, V., Ribeiro Bizzo, H., Salgueiro Lage, C. L., & Sato, A. (2009). Journal of Agricultural and Food Chemistry, 57, 6392–6395.
Dornenburg, H., & Knorr, D. (1995). Enzyme Microbiology and Technology, 17, 674–684.
Chan, L. K., Dewi, P. R., & Boey, P. L. (2005). Journal of Plant Biology, 48, 142–145.
Kim, Y. S., Hahn, E. J., Yeung, E. C., & Paek, K. Y. (2003). In Vitro Cellular and Developmental Biology–Plant, 39, 245–249.
Wu, C. H., Dewir, Y. H., Hahn, E. J., & Paek, K. Y. (2006). Journal of Plant Biology, 49, 193–199.
Lee, E. J. (2009). Ph.D. Thesis, Chungbuk National University, Cheong-Ju
Aliyu, A. B., Ibrahim, M. A., Ibrahim, H., Musa, A. M., Lawal, A. Y., Oshanimi, J. A., Usman, M., Abdulkadir, I. E., Oyewale, A. O., & Amupitan, J. O. (2012). Romanian Biotechnological Letters, 17, 7458–7465.
Shoji, H., Yamashiro, Y., & Koletzko, B. (2008). In H. S. Packer (Ed.), (p. 72) Boca Raton: CRC Press Taylor & Francis Group.
Gupta, V. K., & Sharma, S. K. (2010). International Journal of Biology and Chemistry, 4, 134–140.
Tariq, U., Ali, M., & Abbasi, B. H. (2014). Journal of Photochemistry and Photobiology B: Biology, 5, 264–271.
Lo, S.-F., Nalawade, S. M., Mulabagal, V., Matthew, S., Chen, C.-L., Kuo, C.-L., & Tsay, H.-S. (2004). Biological and Pharmaceutical Bulletin, 27, 731–735.
Güllüce, M., Sökmen, M., Daferera, D., Ağar, G., Özkan, H., Kartal, N., Polissiou, M., Sökmen, A., & Şahin, F. (2003). Journal of Agriculture and Food Chemistry, 51, 3958–3965.
Grzegorczyk, I., Matkowski, A., & Wysokińska, H. (2007). Food Chemistry, 104, 536–541.
Abouzid, S. F., El-Bassuon, A. A., Nasib, A., Khan, S., Qureshi, J., & Choudhary, M. I. (2010). International Journal of Applied Research of Natural Products, 3, 23–27.
Duangporn, P., & Siripong, P. (2009). American-Eurasian Journal of Agriculture and Environmental Sciences, 5, 258–263.
Seitz, H. U., & Hinderer, W. (1998). 5, 49–76 San Diego: Academic.
Acknowledgments
We acknowledge the support of Higher Education Commission of Pakistan (HEC) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fazal, H., Abbasi, B.H. & Ahmad, N. Optimization of Adventitious Root Culture for Production of Biomass and Secondary Metabolites in Prunella vulgaris L.. Appl Biochem Biotechnol 174, 2086–2095 (2014). https://doi.org/10.1007/s12010-014-1190-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1190-x