Abstract
The influence of fungus elicitor Aspergillus flavus on alkaloid yield was investigated in Catharanthus roseus. The study reveals increased yield of vinblastine and vincristine in cultivated tissues. Different concentrations of extract applied to solid MS medium were: 0.05 % (T1), 0.15 % (T2), 0.25 % (T3), and 0.35 % (T4) along with control (T0). The callus biomass, embryo formation and plant regeneration were studied in response to elicitor treatments. The embryogenic callus was induced from hypocotyls of in vitro germinated seeds and various tissues were exposed to fungal elicitation. The use of A. flavus fungal elicitation improved callus biomass growth, which later differentiated into embryos, maximum somatic embryo induction being in T2 (106.53/callus mass). Biochemical analysis revealed more accumulation of sugar, protein and proline in growing tissues especially amended with elicitor. The somatic embryos germinated into plantlets on 2.24 µM BA added MS medium. The percent germination, shoot-, root length of germinated somatic embryos were high in low doses of elicitation (T1/T2). The quantitative analysis of vinblastine and vincristine yield was conducted in different elicitor treated tissues by the use of HPTLC. Vinblastine yield was maximum in germinating embryos (0.837 µg gm−1 dry weight), A. flavus elicitation at T2 improved vinblastine yield further (0.903 µg gm−1 dry weight). Compared to vinblastine, the yield of vincristine was low and on A. flavus addition, maximum vincristine yield was noted (0.216 µg gm−1 dry weight). The highest 7.88 and 15.50 % increased yield of vinblastine and vincristine respectively was noted on A. flavus elicitated tissues. In order to understand the role of elicitor on plant defense responses various antioxidant enzymes activity were investigated as the addition of elicitor induced cellular stress on tissues. Maturated and germinating somatic embryos had high SOD activity and on elicitation the activity of enzymes was further increased, indicating extra cellular stress on tissues, which yielded enriched level of vinblastine and vincristine at T2/T1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catharanthus roseus commonly named Madagaskar periwinkle, is a tropical perennial plant belonging to the family Apocynaceae. It is a source of several important indole alkaloids of medicinal importance such as vinblastine, vincristine, ajmalicine, vindoline, catharanthine and serpentine (El-Sayed and Verpoorte 2007). Due to immense pharmaceutical importance and the low (approx. 0.0005 %) content of vinblastine and vincristine, the C. roseus has been regarded as an important model plant for plant secondary metabolism studies. During the last few decades, a comprehensive multidisciplinary attempt has been made in order to enrich alkaloids yield in C. roseus (Moreno 1995; Mukherjee et al. 2001; Mujib et al. 2003, 2012). The common strategies used are optimization of media and cultural conditions, use of superior high producing cell lines, addition of precursors, overexpression of key enzymes participating in metabolic engineering processes and other biotechnological techniques (Cheng et al. 2008; Rhee et al. 2010; Pawar et al. 2011).
In recent years, the use of biotic or abiotic elicitors in improving biomass with enriched product synthesis has become a crucial process strategy in plant biotechnological research (Murthy et al. 2014). These techniques also reduce processing time in obtaining active compounds in volume (Coste et al. 2011). These elicitors are a large target group of compounds, have been amended to medium at various stages of cultural growth for improving secondary compounds. Traditionally, the ‘elicitor’ may be defined as a substance, once introduced in small concentrations improves the biosynthesis of specific compounds by triggering cellular defense response (Zahid and Mujib 2012). Elicitor for a plant refers to compounds of various sources, stimulates physiological and morphological responses and induces compounds like phytoalexin of defensive nature (Mustafa et al. 2009). It is well understood that the treatment of elicitors or an attack of pathogen causes an array of defensive secondary metabolism to intact plants/cell cultures (Valluri 2009). Singh et al. (1998) suggested elicitors of diverse groups: (a) biotic elicitors such as bacterial and fungal cell walls or glycoproteins, (b) abiotic elicitors like UV irradiation, salt and various non-constitutive compounds, and (c) endogenous elicitors, which are signalling compounds of plant cells’ origin. A large number of biotic compounds have been recognised to be very efficient in enriching secondary metabolites and are exploited in a variety of cell cultures including C. roseus (Xu and Dong 2005). Yeast extract is used as a biotic elicitor in culture which induces synthesis of a variety of phytocompounds in several plant-microbe interaction investigations (Huttner et al. 2010; Cai et al. 2012). Induction of Arbuscular mycorrhizal fungi (a group of beneficial microorganisms) improved ajmalicine and serpentine levels in C. roseus roots suggesting that the mycorrhization (Zubek et al. 2012) has a major influence on alkaloid accumulation and this enhancement was considered to be due to changed expression pattern of genes related to plant defense system (Andrade et al. 2013). In Centella asiatica, fungal elicitation of Trichoderma harzianum, Colletotrichum lindemuthianum and Fusarium oxysporum improved biomass and asiaticoside accumulation in shoot culture (Prasad et al. 2013).
Beside biotic elicitors, a number of abiotic elicitors have widely been incorporated in culture to augment product synthesis in cultured tissues. These include harsh temperature, salinity, osmotic stress, ultra-violet rays (UV), heavy metals, other elements etc. (Lovkova et al. 2005; Elmaghrabi et al. 2013). In C. roseus itself, various abiotic compounds like NaCl, CaCl2, cerium (CeO2 and CeCl3), yttrium (Y2 O3) and neodymium (NdCl3), osmotic stress are used successfully for enhancing alkaloid yield (Zhao et al. 2001). These used elictors induce cellular stresses and enhance secondary compounds synthesis in several investigated genera (Ramani and Jayabaskaran 2008; Binder et al. 2009; Zahid et al. 2011). In cultivated tissues, elicitor induced cellular stress is assayed by monitoring antioxidant enzymes, which scavenge stress levels in tissues (Cai et al. 2012). Various enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) activities are often tested to ascertain the level of stress in plant tissues and was investigated in different plant genera (Samar et al. 2015). Although the enhancement of alkaloids is treatment—and cell line specific, the exploitation of biotic and abiotic elicitor in medium is a good economic approach for enriching important alkaloids of medicinal importance.
In this present study, Aspergillus flavus fungal elicitor was used as biotic elicitor and the yield of vinblastine and vincristine was measured in cultures. This is perhaps the first ever fungal (biotic) elicitation study on alkaloid yield, mediated through embryogenesis. The callus biomass growth and the biochemical alterations/associations during the course of growth and morphogenesis were also monitored.
Materials and methods
In vitro seed germination and culture condition
Seed germination and the process of cultural establishment of C. roseus L. (G). Don were done following Junaid et al. (2006) method. In a nutshell, the fruits/seeds were procured from herbal garden of Jamia Hamdard (Hamdard University). The material was earlier identified and voucher specimen (JH-002-98) was maintained. Twenty to twenty-five surface disinfected seeds were placed in 250 ml conical flask (Borosil, India) containing 50 ml of MS solid medium (Murashige and Skoog 1962) without any plant growth regulator (PGR). The germinated seedlings were cultured in in vitro conditions until the plantlets attained a height of 2–4 cm length. Various parts (nodal stem, leaf, and hypocotyl) were used and inoculated in test tubes (Borosil, India) as explants. For embryogenic callus induction, the MS medium was amended with 4.52 µM 2,4-Dichlorophenoxyacetic acid (2,4-D). For fast proliferation of embryo, the medium was fortified with 6.72 µM N6-Benzyladenine (BA) and 5.37 µM Naphthalene acetic acid (NAA). The medium was solidified with 8 g l−1 of agar, was boiled and poured into clean, dry culture tubes (6′ × 1″ Borosil); each tube contained 20 ml of medium. The pH of the medium was adjusted to 5.7 before autoclaving at 121 °C temperature. All the cultures were incubated at 25 ± 2 °C under 16-h photo period with cool white fluorescent tubes (100 µmol m−2 s−1).
Procurement, culture of fungi and preparation of elicitor
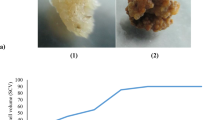
Aspergillus flavus (Fig. 1a) was obtained from the Department of Pathology, Indian Agricultural Research Institute (IARI), Pusa, New Delhi, India. The above fungus was grown in 100 ml conical flasks containing Potato dextrose agar. After 7d, the conical flasks of fungal growth were sterilized, and were filtrated by using Whatman no. 1 filter paper. The mycelium was washed several times with sterilized distilled water, stored at 4 °C after suspended in 100 ml water and was designated as culture media filtrate. The fungal mat was washed several times with sterilized distilled water and an aqueous extract was prepared (Staniszewska et al. 2003) by homogenising in a mortar and pestle. This extract was filtered through centrifugation at 5000 rpm and the supernatant was taken. It was later sterilized (designated as mat extract) and was kept at 4 °C for future investigations. Fungal elicitor of four different treatments i.e. 0.05 % (T1), 0.15 % (T2), 0.25 % (T3) and 0.35 % (T4) were prepared and were added to the culture medium. A control (T0) i.e. without fungal filtrate was also used for comparative evaluations of elicitor’s influence. Morphogenetic and biochemical studies were made at periodic intervals.
a Aspergillus flavus culture grown on Potato dextrose medium (bar 0.5 cm. b Embryogenic callus grown in MS, medium contained 4.52 μM 2,4-D and fungal elicitor T2 (bar 2 mm). c Embryo on maturation medium added with 2.60 μM GA3 and elicitor (bar 2 mm). d Germinated embryos at early stage with root (bar 0.5 cm)
Callus induction in fungus treated and non-treated conditions
Hypocotyls of 5–6 d old seedling were placed on MS, added with optimized 2, 4-D concentrations (4.52 µM). The A. flavus fungal elicitor with four different treatments were added in order to assess the effect of elicitors on callus induction and growth. A control i.e. without fungal filtrate was also used for comparison.
Proliferation, maturation and germination of embryo under the influence of biotic elicitors
The embryogenic callus (40–50 mg) was cultured on MS, supplemented with optimized concentration of BAP (6.62 µM) and NAA (5.36 µM) for embryo proliferation. The medium was additionally amended with above mentioned fungus with earlier indicated treatments. The somatic embryos were induced in masses and were counted, this stage was called as proliferation stage. The vincristine and vinblastine alkaloid were extracted from proliferated stages of embryo and some of the proliferated embryos were cultured in medium for embryo maturation. Somatic embryos on 2.89 µM GA3 added MS became coiled and green, a good sign of maturing embryos. The green matured embryos were later placed on same MS, amended with 2.22 µM BAP for germination. The above two stages (maturation and germination) of embryo development media were additionally amended with A. flavus extract with above indicated treatments. The somatic embryo started to germinate within a week or so and the germination percentage, shoot and root length were measured and compared to assess the impact of elicitor on embryos. Matured and germinating embryos were harvested and were oven-dried for extraction of vincristine and vinblastine alkaloids.
Fresh weight, dry weight and absolute dry mass % as measure of growth index
For growth index analysis, callus biomass i.e. the fresh-dry weight of calli of various growth stages were taken and investigated. For determination of fresh weight, the calli (with or without elicitor treated) were weighed immediately after isolation at regular interval (15, 30 and 45 d). For dry weight, the calli were dried at 60 °C for 18 h and was measured and finally the absolute dry mass was calculated by using Winkelmann et al. (2004) method and formula:
Estimation of total sugar, proline and protein
Total sugar estimation was made according to Dey method (1990). Different stages of tissues (0.5 g) were extracted twice with 90 % ethanol, and the extracts were pooled. The final volume of the pooled extract was made up to 25 ml with double distilled water. To an aliquot of 1.0, 1.0 ml 5.0 % phenol and 5.0 ml of concentrated analytical-grade sulphuric acid were added, and cooled in air. The optical density was measured at 485 nm. A solution containing 1.5 ml of 55 % glycerol, 0.5 ml ninhydrin and 4.0 ml double distilled water was used as a calibration standard.
For measurement of proline, 0.2 g of specific stages of tissues were homogenized in 5.0 ml 3 % aqueous sulfosalicylic acid and filtered through Whatman filter paper (No. 1). To 1.0 ml extract, 1.0 ml acid ninhydrin and 1.0 ml of glacial acetic acid were added and the reaction mixture was incubated at 100 °C for 1 h. The reaction mixture was placed on ice and extracted with 2.0 ml toluene. The proline content in the extract was subject to the spectrophotometric assay of Bates et al. (1973).
Protein was estimated by Bradford method (1976), 0.5 g tissue was ground in a pre-cooler mortar and pestle with 1.5 ml (0.1 M) phosphate buffer (pH 7.0), placed on ice and centrifuged at 5,000 rpm for 10 min. With 0.5 ml Trichloroacetic acid (TCA), the sample was again centrifuged at 5,000 rpm for 10 min. The supernatant was discarded, the pellet was washed with chilled acetone and dissolved in 1.0 ml of 0.1 N Sodium hydroxide (NaOH). Later, 0.5 ml aliquot was added with 5.0 ml of Bradford reagent, the optical density was measured at 595 nm.
Assay of antioxidant enzyme activity
Catalase (CAT)
The activity of CAT was measured following Aebi method (1984). It was measured by observing the decay in H2O2, a decrease measure at 240 nm absorbance in reaction mixture containing 1.0 ml of 0.5 M phosphate buffer (Na-phosphates, pH 7.5), 0.1 ml EDTA, 0.2 ml enzyme extract and 0.1 ml H2O2. The chemical reaction was continued for 3 min. The enzyme activity was represented as EU mg−1 protein min−1. Single unit of enzyme represents the amount used to decompose 1.0 µmol of H2O2/min. The activity was registered by using co-efficient of absorbance at 0.036 mM−1 cm−1.
Superoxide dismutase (SOD)
The activity of SOD was measured following Dhindsa et al. (1981) method. Different stages of tissues/embryos (0.1 g) were homogenised in 2.0 ml of extraction solution [0.5 M sodium phosphate buffer, pH 7.3 + 3.0 mM EDTA + 1.0 % (w/v) Polyvinylpyrollidone (PVP) + 1.0 % (v/v) Triton X100] and the mixture was centrifuged (10,000 rpm) at 4 °C. The enzyme activity was measured by the ability in inhibiting photo-chemical reduction. The assay mixture contained 1.5 ml reaction buffer, 0.2 ml methionine, 0.1 ml enzyme extract, equal amount of 1.0 M NaCO3 and 2.25 mM Nitro Blue Tetrazolium (NBT) solution, 3.0 mM EDTA, riboflavin, 1.0 ml of Millipore H2O. The whole mixture was kept in test tubes and incubated at 25 °C for 10 min under light. A 50 % loss in colour is considered to be 1.0 unit and the enzyme content was expressed as EU mg−1 protein min−1.
Ascorbate peroxidase (APX)
The Nakano and Asada (1981) method was used for determining APX activity. The assay mixture contained 1.0 ml 0.1 M sodium buffer, pH 7.2 + 0.1 ml EDTA + 0.1 ml enzyme extract. The ascorbate was added to the solution and the reaction mixture was run for 3 min at 25 °C. The APX activity was measured by observing the reduction of absorbance by ascorbate mediated breakdown of APX. Enzyme activity was measured by using co-efficient of absorbance 2.81 mM−1 cm−1. Similar to other enzymes, the activity was expressed in EU mg−1 protein min−1 i.e. one unit of enzyme determines the amount necessary in decomposing 1.0 µm of ascorbate/min.
Vinblastine and vincristine quantification through HPTLC
Method of extraction
The vinblastine and vincristine were extracted following earlier described methods (Miura et al. 1988; Junaid et al. 2010) and the content was measured in different in vitro grown tissues and was compared with standard vinblastine and vincristine, obtained from Sigma-Aldrich (St. Luis, MO, USA). The select tissues/embryos were collected from optimized media with their best growth. One gm (dry weight) of tissues/embryos was refluxed in 30 ml of methanol for 5 h; later the supernatant was warmed at 60 °C, finally the volume was reduced to 1–2 ml.
Standard stock solution preparation and calibration curve
One mg of vinblastine and vincristine each was dissolved in 1.0 ml methanol for making a stock solution concentration 1.0 mg ml−1. Various concentrations were prepared from stock solutions in obtaining 200, 400, 600, 800 and 1000 µg per band of standard and was run separately in HPTLC. Standard curve was plotted between peak area (y-axis) and concentration (x-axis), which showed good linearity.
HPTLC instrumentation and quantification of alkaloids in in tissues
For stationary phase, the coated Thin Layer Chromatography (TLC) aluminium sheets of size 20 × 10 cm with Silica gel (60 F 254, Merck) were used. The freshly prepared mobile solution (phase) contained toluene, carbinol, acetone and ammonia in the ratio of 40:20:80:2. The samples were applied by 100 µl micro syringe using Linomat 3 (CAMAG) applicator. The silica plates were air-dried for 10–15 min and kept in a chamber (Twin Through Chamber CAMAG, 20 × 10 cm) filled with mobile solution. The solvent system was allowed to move up to about 85 mm. The plates were later removed from the chamber and again air dried for about 10–20 min. The Silica gel plates were documented by using CAMAG Reprostar under UV-light without any spray of chemical on it. The vinblastine and vincristine containing stationary phase was scanned by the use of a CAMAG Scanner 3. The vinblastine and vincristine were scanned at 280 and 300 nm respectively. The peak of vinblastine and vincristine was fixed and the identification of alkaloids in tissue samples was made by comparing the peaks of standard alkaloids. Finally, the alkaloid yields were measured in µg gm−1 dry weight.
Statistical analysis
The data on the effect of A. flavus elicitor on callus growth, embryogenesis, differences in biochemical attributes, the antioxidant enzyme activity, the alkaloid yield and other parameters were analysed by one-way analysis of variance (ANOVAs). The data or the values are means of three replicates from two experiments and the presented mean values were separated using Duncan’s Multiple Range Test (DMRT) at p ≤ 0.05.
Results
Callus induction and biomass growth in response to elicitation
On 4.52 µM 2, 4-D added MS medium, the hypocotyls of in vitro grown seedlings produced profuse callus. The calli were friable, light yellow and fast growing; and later turned into embryogenic (Fig. 1b). This induced hypocotyl-calli were subject to various level of A. flavus elicitation and routinely sub cultured at regular intervals. The growth index is an indicator of cell division with fast proliferation of callus; therefore callus biomass growth was measured in response to various biotic elicitor treatments. We observed that with A. flavus elicitation, the growth of embryogenic callus was faster compared to control (Fig. 2a). The biomass of the calli increased up to T2, and in this treatment a maximum fresh-, dry- and absolute dry mass % were observed (1.55, 0.183 g and 11.803 % respectively). On elicitation, the calli appeared to be more friable and white especially in treatment with T1 and T2. Higher concentration of elicitors i.e. T3 and T4 were less responsive, callus turned light brown compact, and showed poor growth.
Growth index of embryogenic callus induction (a) and proliferating embryo stage (b) of tissue (30 days old) in different A. flavus elicitated treatments [Control (T0), 0.05 % (T1), 0.15 % (T2),0.25 % (T3) and 0.35 % (T4)]. Values are means ± standard errors of 3 replicates. Within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
Elicitor treatments, embryo numbers and growth index of proliferating embryos
Induced embryogenic calli were cultured on optimized 5.37 µM NAA and 6.72 µM BA added MS, and different concentrations of A. flavus extract was amended in order to observe elicitor’s influence on embryo number and growth (Table 1). The maximum fresh-, dry- and absolute dry weight were observed in T2 (2.066, 0.237 g and 11.442 % respectively) compared to other treatments and control, T0 (Fig. 2b). In all tested conditions, the embryogenic calli differentiated into embryos and in T2 of A. flavus elicitation, maximum number of embryos were formed (114.56/culture); next important treatment is T1 (106.53/culture), which also induced good numbers of embryos, the embryo numbers declined gradually at higher elicitor levels.
Aspergillus flavus elicitation and biochemical attributes
Sugar, proline and protein content
The sugar content was noted to be high at early induction stage, which increased marginally during embryo proliferation stage. On addition of increasing concentration of elicitors, sugar level increased further in both growing stages of tissues. Maximum amount of sugar was observed in A. flavus elicitated tissues in T2 (30.30 mg G−1). The proline level was also high at induction stage of tissues (9.24 mg G−1) but the accumulation declined with the growth and maturity of embryos. Total soluble protein on the other, was found to be less at induction stage compared to proliferating embryogenic stage; the comparative details are presented in (Fig. 3a, b).
Sugar, Proline and Protein content at embryogenic callus induction (a) and Proliferating embryo stage (b) of tissue (30 days old) in different A. flavus elicitated treatments [Control (T0), 0.05 % (T1), 0.15 % (T2),0.25 % (T3) and 0.35 % (T4)]. Values are means ± standard errors of 3 replicates. Within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
Maturation and plant regeneration of somatic embryos in response to A. flavus elicitation
The cotyledonary embryos were cultured on MS, amended with 2.60 µM GA3 for maturation; the medium was additionally added with fungal elicitors (Fig. 1c). In T1 and T2, the embryos turned green, elongated, coiled and which later germinated into plantlets (Fig. 1d). In T3 and T4, however, the embryo development was poor; a few remained in cotyledonary stage while other embryos turned brown; the embryos reaching to maturity were thin and had a poor growth. The embryos germinated into plantlets on 2.24 µM BA added MS medium. The percent germination, shoot-, root length of germinated somatic embryos were high in A. flavus elicitated conditions compared to control (Table 2).
Vinblastine and vincristine yield
The yield of vinblastine and vincristine was quantified in different in vitro cultivated tissues. The mobile phase showed a sharp standard vinblastine and vincristine peak. The regression analysis also showed a good linearity with r = 0.999 and 0.993 for vinblastine and vincristine respectively. It is evident from the Table 3 that vinblastine was maximum in maturation (0.787 µg gm−1 dry weight; Fig. 4a, b) and germinating stages of embryos (0.837 µg gm−1 dry weight; Fig. 5a, b) compared to other two i.e. induction and proliferating embryo tissues. With A. flavus elicitation at T2, the vinblastine yield was improved further (0.903 µg gm−1 dry weight), the T1 treatment was also equally efficient in promoting yield. Compared to vinblastine, the yield of vincristine was low and the content was maximum in germinating embryos compared to other stages. On A. flavus addition, improved vincristine yield was noted in cultured tissues (Table 4), maximum being in T2 (0.216 µg gm−1 dry weight), followed by T1 treatment (0.202 µg gm−1 dry weight). The maximum 7.88 and 15.50 % increased yield of vinblastine and vincristine respectively was noted on A. flavus elicitated treatment in T2 over control tissues.
SOD, CAT and APX activities
The germinating and maturated somatic embryos produced enhanced level of alkaloids especially on A. flavus elicitor treated culture. The addition of elicitor might also cause stress on tissues. To better understand the role of elicitor treatments on plant defense and later on secondary metabolism, the antioxidant activity of various enzymes were investigated as stress markers. Maturated and germinating somatic embryos had high antioxidant enzyme activities than the early embryogenic tissues. The anti-oxidant enzyme activities were even more on addition of A. flavus treatments, which indicated extra cellular stress on cultivated tissues. It is evident from Fig. 6a, b that the SOD activity was high in maturing (4.16 EU min−1 mg−1 proteins) and germinating (3.78 EU min−1 mg−1 proteins) stages of embryos compared to control (3.85 and 3.52 EU min−1 mg−1 proteins respectively), which yielded highest level of vinblastine and vincristine especially in T1 and T2. Compared to SOD, the CAT and APX activity was however, low in these two embryogenic stages (maturating and germinating embryos).
SOD, CAT and APX activity at maturation (a) and germinating embryo stage (b) of tissue (30 days old) in different A. flavus elicitated treatments [Control (T0), 0.05 % (T1), 0.15 % (T2), 0.25 % (T3) and 0.35 % (T4)]. Values are means ± standard errors of 3 replicates. Within each column, means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
Discussion
In this present study, the yield of vinblastine and vincristine was quantified following A. flavus elicitation in embryogenic cultures of C. roseus. The callus was first induced from hypocotyls on 2, 4-D added MS in which high frequency somatic embryos were formed on same PGR containing medium; other used auxins induced embryos at a lower rate. Here, the embryo differentiation was on embryogenic callus i.e. indirect, but in other observed cases the embryos were also formed directly on explants without intervening callus (Mujib and Samaj 2006). In both developmental pathways, the use of exogenous auxins/auxin analogues like 2, 4-D efficiently trigger embryogenesis. These synthetic auxin-analogs play a central signalling role in acquisition of embryogenic competence from somatic state (Song 2013; Feher 2015). In our study, A. flavus fungus extract was used at varying concentrations, of which T2 (0.15 %) was observed to be more efficient in promoting biomass growth compared to T1, T3 and T4. We also observed that the callus biomass and embryo numbers increased significantly in T2 with A. flavus elicitation. The induced embryos were more distinct and showed fast growth and development in elicitated condition. The present study indicated that high concentrations (T3, T4) of elicitation declined callus biomass growth by inhibiting cell division; and this reduction may be due to fungus extract toxicity or may be due to excessive availability of stress ion (Saiman et al. 2014). The ion induced osmotic imbalance with reduced growth was reported in several other investigated plants (Shibli et al. 2007; Elmaghrabi et al. 2013). In this present study, low level of A. flavus elicitated condition improved somatic embryo numbers in culture. Similar responses i.e. stress induced embryogenesis were earlier described in a number of previous observations (Benkirane et al. 2000; Kawana and Sasamoto 2008). Once embryo is induced, the presence of 2, 4-D in medium inhibits embryo development; therefore other PGRs combinations were tested and was suggested to be necessary (Pasternak et al. 2002; Feher 2015). The involvement of cytokinins alone or with low doses of weak auxin like NAA successfully influence in vitro embryogenesis and plant morphogenesis also (Mujib and Samaj 2006).
The influence of A. flavus biotic elicitor on biochemical attributes was investigated at various stages of embryogenesis. In this present study, extra sugar, protein and proline accumulation were noted at early stages of embryogenesis, which however, declined with increased level of elicitation. Similar increase of protein, phenolics, hydrogen peroxides and carbohydrate level in response to stress was noted in several investigated plant genera and this enhancement is considered to be a good adaptation mechanism in tolerant genotypes (D’Souza and Devaraj 2010; Samar et al. 2015). The protein level also increases gradually with the progress of tissues and the change of protein with growing developmental stage was reported earlier in other investigated plant materials (Roja Rani et al. 2005). In chickpea, enriched proline accumulation was noted at early embryo development stage and this proline level perhaps acts as an osmotic balancing agent, a reservoir of nitrogen or a source of energy to growing tissues (Kiran Ghanti et al. 2009). Here, in A. flavus elicitated tissues, increased accumulation of proline may be due to up regulation and over expression of proline synthesis gene (P5C6), which produces c-glutamyl kinase and glutamate-5-semialdehyde dehydrogenase enzymes participating in proline synthesis (Chen et al. 2009). Transcriptome data of damages of different plant species revealed that the biotic stress globally down-regulates photosynthesis light reaction, carbon reduction cycle and pigment synthesis genes and this low regulation has been suggested to be a part of defence manifestation (Bilgin et al. 2010). Kundu et al. (2013) similarly analysed proteomic changes and noted early accumulation of stress protein during ‘host-stress’ interaction in reactive oxygen species (ROS) metabolism, and this synthesis of stress and signal transduction proteins has been considered to be the central factor in cellular defence responses.
The cultivation of simple plant cell and tissue or complex organized structure are practised in vitro as an efficient renewable source for producing a variety of phytochemicals and the importance of these methods are reviewed in recent years (Mulabagal and Tsay 2004; Martin et al. 2008; Karuppusamy 2009; Siahsar et al. 2011). The callus and suspension are cultivated more frequently because of ease and possibility of scale-up in bioreactors. Beside bioreactor, a number of other important strategies such as liquid culture, use of mist, liquid-overlaying improve biomass/embryogenesis, to be used as raw materials for alkaloid synthesis (Fei and Weathers 2014). Liquid overlaying is a technique where a thin film of liquid nutrient is added on solid medium, which facilitate fast growth of callus and suspension (Zahid and Mujib 2012). The yield of active compounds is often high in complex differentiated structures like shoots, roots and leaves (Kornfeld et al. 2007; Vinterhalter et al. 2008). The extraction method is however, rigorous especially with metabolites, synthesised and accumulated in specialized cells or tissues (Facchini and De Luca 2008). Different techniques have recently been adopted for collection of alkaloids from specialized tissues (Verma et al. 2012). In this present study, we noted that the compact embryo structures like maturated- and germinating embryo synthesized higher vinblastine and vincristine compared to early stages of embryos. On A. flavus elicitated treatment 7.88 % vinblastine and 15.50 % vincristine increased yield were noted; this enhancement is little higher (0.08 %) for vinblastine and about 2.0 % more in vincristine than our previous reported observations. The same low level (T1/T2) of elicitation was earlier noted to be very efficient for improving callus biomass. This rapid growth of embryogenic callus may be due to fast mitosis of cells, triggered by cell cycle gene Waste Water Evry 1 (WWE1), which up regulated strongly in fast dividing cells (Sorrell et al. 2002; De Schutter et al. 2007). As the yield of alkaloids was high in advanced staged embryos, we tried to investigate the level of stress by measuring antioxidant enzyme activities in these cultivated tissues. The SOD activity was high in both these two tissues and on addition of elicitor the activity was further up. Increased SOD activity under various stresses was observed in several investigated plant genera (Samar et al. 2011, 2015). The CAT and APX activity also showed similar pattern with added levels of elicitors, although tissue—and dose specific variation was also not uncommon (Elkahoui et al. 2005). Beside the increase of stress marker enzyme activity and alteration of physiological reserves, molecular analysis indicates that over expression of Salt Overly Sensitive 1(SOS1) gene is a crucial adaptation event in response to stress, caused by biotic and abiotic compounds (Misic et al. 2012).
It is very evident from present study that the biotic elicitor promoted cultural growth in C. roseus and later stimulates enriched level of alkaloids, the underlying mechanism is still not fully understood. It was earlier reported that the biotic elicitors contain compounds like oligosaccharides and glycopeptides, evoking elicitation effect (Van der Heijden et al. 2004; Zizhen et al. 2015). Although the active principle is yet to be illustrated, the elicitation mechanism may be due to the concept based on ‘elicitor-receptor’ interaction (Radman et al. 2003), which activate signal transduction mechanisms by stimulating transcriptional control of cascade of defense genes, participating in alkaloid synthesis (Memelink et al. 2001; Mujib et al. 2012). Thus, the experimentations on elicitation are important and valuable as these promise to promote embryogenic biomass and regulate alkaloid biosynthesis in cultivated tissues.
Conclusion
The A. flavus fungus elicitor was used as biotic elicitor and the callus biomass growth, the embryogeny, plant regeneration and the alkaloid yield (vinblastine and vincristine) were investigated. Low doses of fungal elicitation were very efficient in improving callus biomass and embryo numbers. The percent germination, shoot-, root length of embryos were high in low elicitation level (T1/T2). Maturated and germinating somatic embryos had high level of alkaloids, which was further improved by elicitation. Addition of elicitation also caused cellular stress as was evidenced by high antioxidant enzyme activities. We therefore suggest that the synthesis of alkaloids may be enhanced by low elicitor doses and stress in advanced organised embryo structure.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Andrade SAL, Malik S, Sawaya ACHF, Bottcher A, Mazzafera P (2013) Association with arbuscular mycorrhizal fungi influences alkaloid synthesis and accumulation in Catharanthus roseus and Nicotiana tabacum plants. Acta Physiol Plant 35:867–880
Bates L, Waldren PP, Teare JD (1973) Rapid determination of free proline of water stress studies. Plant Soil 39:205–207
Benkirane H, Sabounji K, Chlyah A, Chlyah H (2000) Somatic embryogenesis and plant regeneration from fragments of immature inflorescences and coleoptiles of durum wheat. Plant Cell Tissue Org Cult 61:107–113
Bilgin DD, Zavala JA, Zhu JIN, Clough SJ, Ort DR, DeLucia E (2010) Biotic stress globally downregulates photosynthesis genes. Plant, Cell Environ 33(10):1597–1613
Binder BY, Peebles CA, Shanks JV, San KY (2009) The effects of UV-B stress on the production of terpenoidindole alkaloids in Catharanthus roseus hairy roots. Biotechnol Prog 25:861–865
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein, utilizing the principle of protein dye binding. Anal Biochem 72:248–541
Cai Z, Kastell A, Mewis I, Knorr D, Smetanska I (2012) Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Org Cult 108:401–409
Chen J-B, Wang S-M, Jing R-L, Mao X-G (2009) Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J Plant Physiol 166:12–19
Cheng DM, Yousef GG, Grace MH, Rogers RB, Gorelick-Feldman J, Raskin I, Lila MA (2008) In vitro production of metabolism enhancing phytoecdysteroids from Ajuga turkestanica. Plant Cell Tissue Org Cult 93(1):73–83
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Org Cult 106(2):279–288
D’Souza MR, Devaraj VR (2010) Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol Plant 32:341–353
De Schutter K, Joubes J, Cools T, Verkest A, Corellou F, Babiychuk E, Der Schueren E-V, Beeckman T, Kushnir S, Inze D, De Veylder L (2007) Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 19:211–225
Dey PM (1990) Methods in plant biochemistry. Carbohydarates, vol 2. Academic Press, London
Dhindsa RH, Plumb-Dhindsa R, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
Elkahoui S, Hernandez JA, Abdelly C, Ghrir R, Limam F (2005) Effects of salt on lipid peroxidation and antioxidant enzyme activities of Catharanthus roseus suspension cells. Plant Sci 168:607–613
Elmaghrabi AM, Ochatt S, Rogers HJ, Francis D (2013) Enhanced tolerance to salinity following cellular acclimation to increasing NaCl levels in Medicago truncatula. Plant Cell Tissue Org Cult 114:61–70
El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6:277–305
Facchini PJ, De Luca VD (2008) Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants. Plant J 54:763–784
Feher A (2015) Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849(4):385–402
Fei L, Weathers PJ (2014) From cells to embryos to rooted plantlets in a mist bioreactor. Plant Cell Tissue Org Cult 116:37–46
Huttner C, Beuerle T, Scharnhop H, Ernst L, Beerhues L (2010) Differential effect of elicitors on biphenyl and dibenzofuran formation in Sorbus aucuparia cell cultures. J Agric Food Chem 58(22):11977–11984
Junaid A, Mujib A, Bhat MA, Sharma MP (2006) Somatic embryo proliferation, maturation and germination in Catharanthus roseus. Plant Cell Tissue Org Cult 84:325–332
Junaid A, Mujib A, Sharma MP (2010) Variations in vinblastine production at different stages of somatic embryogenesis, embryo and field grown plantlets of Catharanthus roseus L. (G) Don, as revealed by HPLC. In Vitro Cell Dev Biol-Plant 46:348–353
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plant Res 3:1222–1239
Kawana Y, Sasamoto H (2008) Stimulation effects of salts on growth in suspension culture of a mangrove plant, Sonneratia alba, compared with another mangrove, Bruguiera sexangula and nonmangrove tobacco BY-2 cells. Plant Biotechnol 25:151–155
Kiran Ghanti S, Sujata KG, Rao S, Udayakumar M, KaviKishor PB (2009) Role of enzymes and identification of stage-specific proteins in developing somatic embryos of chickpea (Cicer arietinum L.) In Vitro Cell. Dev Biol-Plant 45:667–672
Kornfeld A, Kaufman PB, Lu CR, Gibson DM, Bolling SF, Warber SL, Chang SC, Kirakosyan A (2007) The production of hypericins in two selected Hypericum perforatum shoot cultures is related to differences in black gland culture. Plant Physiol Biochem 45:24–32
Kundu S, Chakraborty D, Kundu A, Pal A (2013) Proteomics approach combined with biochemical attributes to elucidate compatible and incompatible plant-virus interactions between Vigna mungo and Mungbean Yellow Mosaic India Virus. Proteome Sci 11:15. doi:10.1186/1477-5956-11-15
Lovkova MY, Buzuk GN, Sokolova SM, Buzuk LN (2005) Role of elements and physiologically active compounds in the regulation of synthesis and accumulation of indole alkaloids in Catharanthus roseus L. Appl Biochem Microbiol 41:299–305
Martin EK, Vishal G, Susan CR (2008) Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol Pharm 5:243–256
Memelink J, Verpoorte R, Kijne JW (2001) Orcanization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci 6:212–219
Misic D, Siler B, Zivkovic NJ, Simonovic A, Maksimovic V, Budimir S, Janosevic D, Durickovic M, Nikolic M (2012) Contribution of inorganic cations and organic compounds to osmotic adjustment in root cultures of two Centaurium species differing in tolerance to salt stress. Plant Cell Tissue Org Cult 108:389–400
Miura Y, Hirata K, Miyamoto K, Uchida K (1988) Formation of vinblastine from multiple shoot culture of Catharanthus roseus. Planta Med 54:8–20
Moreno PRH (1995) Influence of stress factors on the secondary metabolism in suspension cultured Catharanthus roseus cells. Ph.D. thesis, Leiden University, Leiden
Mujib A, Samaj J (2006) Somatic embryogenesis. Springer, Berlin
Mujib A, Ilah A, Gandotra N, Abdin MZ (2003) In vitro application to improve alkaloid yield in Catharanthus roseus. In: Govil JN, Kumar PA, Singh VK (eds) Biotechnology and genetic engineering. Recent progress in medicinal plants, vol IV. Sci. Tech., Houston, pp 415–440
Mujib A, Ilah A, Aslam J, Fatima S, Siddiqui ZH, Maqsood M (2012) Catharanthus roseus alkaloids: application of biotechnology for improving yield. Plant Growth Reg 68:111–127
Mukherjee AK, Basu S, Sarkar N, Ghosh AC (2001) Advances in cancer therapy with plant based natural products. Curr Med Chem 8:1467–1486
Mulabagal V, Tsay HS (2004) Plant cell cultures- an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Org Cult 118:1–16
Mustafa NR, Kim HK, Choi YH, Erkelens C, Lefeber AW, Spijksma G, van der Heijden R, Verpoorte R (2009) Biosynthesis of salicylic acid in fungus elicited Catharanthus roseus cells. Phytochemistry 70:532–539
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pasternak TP, Prinsen E, Ayaydin F, Miskolezi P, Potters G, Asard H, Van Onckelen A, Dudits D, Feher A (2002) The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast derived cells of alfalfa. Plant Physiol 129:1807–1819
Pawar KD, Yadav AV, Shouche YS, Thengane SR (2011) Influence of endophytic fungal elicitation on production of inophyllum in suspension cultures of Calophyllum inophyllum L. Plant Cell Tissue Org Cult 106(2):345–352
Prasad A, Mathur A, Kalra A, Gupta MM, Lal RK, Mathur AK (2013) Fungal elicitor-mediated enhancement in growth and asiaticoside content of Centella asiatica L. shoot cultures. Plant Growth Reg 69:265–273
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plants and microbial cell systems. Biotechnol Appl Biochem 37:91–102
Ramani S, Jayabaskaran C (2008) Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J Mol Signal 25(3):9
Rhee HS, Cho HY, Son SY, Yoon SYH, Park JM (2010) Enhanced accumulation of decursin and decursinol angelate in root cultures and intact roots of Angelica gigas Nakai following elicitation. Plant Cell Tissue Org Cult 101(3):295–302
Roja Rani A, Reddy VD, PrakashBabu P, Padmaja G (2005) Changes in protein profiles associated with somatic embryogenesis in peanut. Biol Plant 49:347–354
Saiman MZ, Mustafa NR, Pomahocova B, Verberne M, Verpoorte R, Choi YH, Schulte AE (2014) Analysis of metabolites in the terpenoid pathway of Catharanthus roseus cell suspensions. Plant Cell Tissue Org Cult 117:225–239
Samar F, Mujib A, Samaj J (2011) Anti-oxidant enzyme responses during in vitro embryogenesis in Catharanthus roseus. J Hort Sci Biotechnol 86(6):569–574
Samar F, Mujib A, Dipti T (2015) NaCl amendment improves vinblastine and vincristine synthesis in Catharanthus roseus: a case of stress signalling as evidenced by antioxidant enzymes activities. Plant Cell Tissue Org. Cult. doi:10.1007/s11240-015-0715-5
Shibli RA, Kushad M, Yousef GG, Lila MA (2007) Physiological and biochemical responses of tomato microshoots to induced salinity stress with associated ethylene accumulation. Plant Growth Reg 51:159–169
Siahsar B, Rahimi M, Tavasssoli A, Raissi AS (2011) Application of biotechnology in production of medicinal plants. Am-Euras J Agric Environ Sci 11:439–444
Singh G, Gavrieli J, Oakey JS, Curtis WR (1998) Interaction of methyl jasmonate, wounding and fungal elicitation during sesquiterpene induction in Hyoscyamus muticus in root cultures. Plant Cell Rep 17:391–395
Song Y (2013) Insight into the mode of action of 2,4-Dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol 56:106–113
Sorrell DA, Marchbank A, McMahon K, Dickinson JR, Rogers HJ, Francis D (2002) A WEE1 homologue from Arabidopsis thaliana. Planta 215:518–522
Staniszewska I, Krolicka A, Malinski E, Jojkowska E, Szafranek JZ (2003) Elicitation of secondary metabolites is in vitro cultures of Ammi majus L. Enzy Microbial Technol 33:565–568
Valluri JV (2009) Bioreactor production of secondary metabolites from cell cultures of periwinkle and sandalwood. Methods Mol Biol 547:325–335
Van der Heijden R, Jacobs DT, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus alkaloids: pharamacognosy and biochemistry. Curr Med Chem 11:607–628
Verma P, Mathur AK, Srivastava A, Mathur A (2012) Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloid pathway in Catharanthus roseus. Protoplasma 249:255–268
Vinterhalter B, Jankovic T, Sovikin L, Nikolic R, Vinterhalter D (2008) Propagation and xanthone content of Gentianella austriaca shoot cultures. Plant Cell Tissue Org Cult 94:329–335
Winkelmann T, Muβmann V, Serek M (2004) Cryopreservation of embryogenic cell suspension cultures of Cyclamen persicum Mill. Plant Cell Rep 23:1–8
Xu M, Dong J (2005) Elicitor-induced nitric oxide burst is essential for triggering catharanthine synthesis in Catharanthus roseus suspension cells. Appl Microbiol Biotechnol 67:40–44
Zahid SH, Mujib A (2012) Accumulation of vincristine in calcium chloride elicitated Catharanthus roseus cultures. Nat Prod J 2(9):307–315
Zahid SH, Mujib A, Maqsood M (2011) Liquid overlaying improves somatic embryogenesis in Catharanthus roseus. Plant Cell Tissue Org Cult 104:247–256
Zhao J, Hu Q, Guo YQ, Zhu WH (2001) Effects of stress factors, bioregulators, and synthetic precursors on indole alkaloid production in compact callus clusters cultures of Catharanthus roseus. Appl Microbiol Biotechnol 55:693–698
Zizhen L, Tiantian Z, Xiaoqian Z, Jia Z, Changqi Z (2015) An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules 20:1424–1433
Zubek S, Mielcarek S, Turnau K (2012) Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 22:149–156
Acknowledgments
The authors are highly thankful to University Grant Commission (UGC) and Department of Botany, Hamdard University (Jamia Hamdard) for receiving financial assistance and other research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Tonk, D., Mujib, A., Maqsood, M. et al. Aspergillus flavus fungus elicitation improves vincristine and vinblastine yield by augmenting callus biomass growth in Catharanthus roseus . Plant Cell Tiss Organ Cult 126, 291–303 (2016). https://doi.org/10.1007/s11240-016-0998-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-0998-1