Abstract
Plant secondary metabolites production is increased in response to both biotic and abiotic elicitors. This study investigates the impact of sodium nitroprusside (SNP) and Trichoderma harzianum on the molecular and biochemical characteristics of Catharanthus roseus cell suspensions. A leaf cell suspension cultured on a medium supplemented with 8 µM 2,4-D and 2 µM BAP was exposed to Trichoderma harzianum (1% v/v) and SNP (150 µM), and subsequently harvested at 12, 24, 48, and 72 h intervals. The highest catalase, ascorbate peroxidase, β (1–3) glucanase, and chitinase activities were recorded 48-hours after elicitation, and coincided with the highest expression levels of G10H (2.5-fold), T16H (1.5-fold), D4H (1.1-fold), DAT (1.9-fold), STR (5-fold), and CrPRX (2-fold) genes. A positive correlation was established between enzyme activities, Terpenoid Indole Alkaloid (TIAs) biosynthesis pathway genes, and the accumulation of vinblastine and vincristine. HPLC analyses showed that the amount of vinblastine and vincristine increased 1.84 and 1.93-fold, respectively, confirming that fungal extracts and SNP elicitors for 48 h significantly increased the vinblastine and vincristine accumulation and related biosynthesis gene in C. roseus plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkaloids constitute a class of more than 12,000 different low molecular weight nitrogen-containing compounds prevalent in various plant species (Facchini 2001). Owing to their biological activities, many alkaloids have found extensive applications in pharmacology (Zhou et al. 2010). Catharanthus roseus, a member of the Apocynaceae family, holds significance as both a medicinal and an ornamental plant. It serves as the exclusive source of the anticancer drugs vincristine and vinblastine (Alhaithloul et al. 2019; Birat et al. 2022). Vinblastine and vincristine bind to tubulin inducing polymerization, disrupting microtubule assembly, and ultimately arresting metaphase (Zandi 2021). However, the limited accumulation of these valuable alkaloids falls short of global demand (Zhang et al. 2018). Recent studies have made strides in elucidating the genes involved in Terpenoid Indole Alkaloid (TIAs) biosynthesis, the regulation of the TIAs biosynthesis pathway, and the transport of pathway intermediates (Liu et al. 2014). Despite this progress, comprehensive investigations into pathway genes, regulators, and TIA transporters are still needed to better understand the production of components vital for anticancer drug development in C. roseus (Pan et al. 2018).
Plants respond to various stresses, elicitors, or signal molecules by accumulating secondary metabolites (Thakur et al. 2019; Siddiqui et al. 2023). Elicitors, derived from biotic, abiotic, physical and chemical sources, play a pivotal role in enhancing secondary metabolite biosynthesis and accumulation by inducing defensive responses and physiological changes in plants (Baldi et al. 2009; Cai et al. 2012; Ramezani et al. 2018). The application of biotic or abiotic elicitors has proven to be one of the most effective ways to boost secondary metabolite production by in vitro plant cultures, reducing processing time for obtaining active compounds on a larger scale (Sahu et al. 2013; Coste et al. 2011).
Fungal elicitors, among various biotic elicitors, have gained prominence for stimulating secondary metabolite production in “in vitro” cultures (Singh et al. 2018). Understanding the role of elicitors in plant defense responses involves considering the activities of various antioxidant enzymes, as the addition of elicitors induces cellular stress in tissues (Tonk et al. 2016). Previous studies have highlighted the tissue and cell-specific control of TIAs biosynthesis pathway gene expression in response to biotic and abiotic stimuli in C. roseus (Kellner et al. 2015).
While the enhancement of alkaloids like vincristine and vinblastine is treatment and cell line-specific, the use of biotic and abiotic elicitors in the medium remains an economical approach to enriching valuable alkaloids for medicinal purposes (Tonk et al. 2016). Biotic elicitors have been employed to increase the production of secondary metabolites such as shikonin by cell cultures of Lithospermum erythrorhison, rosmarinic acid by Coleus blumei Benth. (Lamiaceae), and berberine by cell cultures of Coptis japonica Makino (Ionkova 2007). Fungal elicitation has demonstrated positive effects on the enrichment of various secondary metabolites, including ginsenoside (Tonk et al. 2016), and vincristine and vinblastine (Tonk et al. 2016). The accumulation of ajmalicine was significantly improved in C. roseus cell suspension, with optimum results supplementing the medium with 5% v/v concentrations of Aspergillus niger, Fusarium moniliforme, and Trichoderma viride as biotic elicitors (Namdeo et al. 2002). Fungal elicitation by Trichoderma harzianum, Colletotrichum lindemuthianum, and Fusarium oxysporum also increased biomass and asiaticoside accumulation in Centella asiatica (Prasad et al. 2013). Ming et al. (2013) reported that Trichoderma atroviride and polysaccharide fragments led to an enhancement of tanshinone in Salvia officinalis hairy roots. In a recent study, Ramezani et al. (2018) reported a 1.2-fold increase in Deacetoxyvindoline 4-hydroxylase (D4H) and a 0.7-fold increase in Deaccetylvindoline by vindoline 4-O-acetyltransferase (DAT) gene expression after 48 h of treatment, leading to enhanced vinblastine and vincristine production in C. roseus cell suspension inoculated with Trichoderma tomentosum elicitor, which proved to be more effective than Piriformospora indica.
Sodium nitroprusside (SNP) serves as a nitric oxide (NO) donor and NO play a fundamental role in plant growth (Khurana et al. 2011), signaling, regulating plant defense or stress responses (Amooaghaie and Korrani 2018), increasing alkaloid accumulation (Xu and Dong 2005), and antioxidant genes (Khan et al. 2017). Mahendran et al. (2021) reported a significant increase in deacylgymnemic acid, gymnemagenin, and gymnemic acid XVII in Gymnema sylvestre cell suspensions exposed to 20 µM SNP treatment. Elicitors, causing cellular stress, can be gauged by antioxidant enzyme activity, with enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) serving as crucial indicators (Fatima et al. 2015). The addition of Aspergillus flavus as a fungal elicitor to Catharanthus roseus callus cultures induced extracellular stress, resulting in improved vincristine and vinblastine yields and increased antioxidant enzyme activities (Tonk et al. 2016).
This study comprises two main steps. In the first step, in vitro callus from selected C. roseus explants were induced in different hormonal media, and cell suspensions were obtained and subjected to elicitor application. In the second step, the activity of selected enzymes and the expression of genes involved in the biosynthesis of vinblastine and vincristine, including Geraniol 10-hydroxylase (G10H), Strictosidine synthase (STR), and Catharanthus roseus peroxidase (CrPrx), were investigated using real-time quantitative PCR (qRT-PCR). Simultaneously, alkaloids production under the influence of Trichoderma fungi and SNP as elicitors in periwinkle cell suspension was assessed. The integrated results from enzyme activities, qRT-PCR, and HPLC data provide comprehensive insights into the effect of elicitors on the content of terpenoid alkaloids in C. roseus cell suspension.
Materials and methods
Plant material and in vitro culture conditions
Catharanthus roseus (C. roseus) seeds were procured from Syngenta Company, Basel, Switzerland. Surface sterilization involved a modified protocol (Tonk et al. 2016), utilizing 70% ethanol for 5 min, 1.5% sodium hypochlorite for 12 min, and subsequent rinsing with sterile distilled water at intervals of 10, 8, 6, 4, 2, and 1 min, respectively. Seeds were placed on a basal medium (Murashige and Skoog 1962) without plant growth regulators. After 2 weeks seeds germinated and various explants, including hypocotyl, cotyledon, node, leaf, and petiole (before reach to the blooming) were excised and cultured in the MS medium without PGRs as control and MS medium supplemented with 2, 4-D (2, 4, and 8 µM) and benzyl amino purine (BAP) at 0.5, 1, and 2 µM. The media were solidified with 7 g/l of agar, and the pH was adjusted to 5.7 ± 0.1 before autoclaving at 121 °C for 15 min. All cultures were incubated at 25 ± 2 °C under a 16-hour photoperiod regime with a cool white fluorescent light source (100 µM− 1 m− 2 s− 1 PFD).
Callus induction and cell suspension
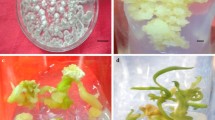
A factorial experiment in a completely randomized design was conducted in triplicate to evaluate the effect of PGRs and explant types on callus formation. After 4 weeks 2 types of calluses, soft-friable and hard, were found. Soft callus contained mostly round and elongated cells with thin cell walls and large vacuoles (Fig. 1, a). Callus derived from different explants was dried and an extract obtained by ultrasonic method, first 200 mg of the powdered dried callus was mixed with 2 ml 80% methanol and placed in an ultrasonic bath (40 khz, AVA-UB 5–20, AVA TEKS, Iran) at 40◦C for 45 min. Then mixture was filtered through Whatman filter paper (25 mm diameter, 0.45 mm pore size) and 20 µl of extract was injected into HPLC. Based on HPLC analyzed results 1 g of soft-friable callus derived from the explant with the highest vinblastine and vincristine percentage was selected and transferred to 250 ml liquid MS medium with the optimal hormonal treatment for callus growth, and shaken (120 rpm at 25 °C, under light) for cell suspension proliferation. The growth curve of cell suspension was measured by considering the volume of stationary cells and counting the number of cells during 13 days every day. The maximum volume of stationary cells of samples was observed on the 7th and 8th days, while the cell growth was stable after 9th day (Fig. 1, b). Cell suspension were subcultured every 7 days until an optimal cell growth rate was achieved. Cell growth curves were constructed using settled cell volume and cell count (Farjaminezhad et al. 2013; Junaid et al. 2006). Elicitor treatments were applied when the cells reached the exponential growth phase.
Procurement, culture of fungi and elicitor preparation
The Trichoderma harzianum strain was obtained from the Department of Plant Pathology, Bu-Ali Sina University, Hamedan, Iran. T. harzianum was cultured in PDA solid medium for 10 days at 28 °C, then transferred to PDA liquid medium and placed in a shaker incubator (120 rpm at 28 °C, under light) for two weeks. After sufficient growth of fungi, a Buchner funnel was used to filter mycelium and fungal spores. After drying fungal cells at 65 °C for 24 h, 10 g powdered cells were dissolved in 1 l distilled water, autoclaved at 120 °C for 20 min, then filtered through Whitman filter paper (25 mm diameter, 0.45 mm pore size), and the filtered extracts were used as an elicitor (Namdeo et al. 2002). Finally, 1% (v/v) concentration of fungal extract was applied to the cell suspension (Tashackori et al. 2016).
The abiotic elicitor used was 150 µM SNP, and the medium without elicitor served as a control. The cell suspension was harvested at 12, 24, 48, and 72 h after exposure to the elicitor. The harvested cell suspension was filtered through a Whitman filter paper (25 mm diameter, 0.45 mm pore size), excess water was removed at room temperature (25 °C), and then samples were stored at -80 °C for subsequent analysis of enzyme activities and RNA extraction.
Enzyme activities assessment
To measure the activities of catalase and ascorbate peroxidase, sodium phosphate buffer at 50 mM (pH 7) containing 2 mM EDTA was prepared. In order to prepare enzyme extract, cells grown in each treatment suspension were used. The initial cooling was done by liquid nitrogen and then samples were kept at -80 °C; 1 g cells were completely crushed with liquid nitrogen in a mortar and 1 ml of extraction buffer was added to 0.1 g of the powder, centrifuged for 15 min at 10,000 rpm at 4 °C, then floating solution was removed.
Catalase enzyme activity was measured using a modified Aebi (1984) method. Thus, 3 ml sodium phosphate buffer was poured into both control and sample cuvettes, 4.51 µl of 30% hydrogen peroxide was added, two cuvettes were placed into the spectrophotometer as calibration. 50 µl of plant extract was added to the sample cuvette and the changes in absorbance at 240 nm for 2 min were recorded. Finally, the enzyme activity was calculated using the absorption coefficient of oxygenated water at a 240 nm (0.036 mM− 1 cm− 1) and was expressed as units per mg of fresh weight. A unit of catalase enzyme activity was considered as the amount of enzyme that decomposes 1 µM of H2O2 per minute.
For ascorbate peroxidase enzyme activity, Aebi (1984) modified method was used. After calibrating as above, 50 µl of plant extract was added to the sample cuvette and the absorbance changes at 290 nm after 2 min were recorded. The amount of enzyme activity was calculated using the absorption coefficient of ascorbate at 290 nm (2.8 mM− 1 cm− 1) and expressed as units per mg of fresh weight. A unit of ascorbate peroxidase enzyme was considered as the amount of enzyme that oxidizes 1µM of ascorbate per minute.
The β (1–3) glucanase enzyme activity was measured by the Abeles and Forrence (1970) method with modifications. To extract glucanase, 0.75 g of cells were crushed in a mortar on ice with 1.5 ml cold sodium acetate buffer at 0.5 M. Then, the homogenized extract was transferred to 1.5 ml vials and centrifuged 20 min at 4 °C at 1400 rpm, the upper part was transferred to similar vials and kept at -20 °C. The reaction mixture was prepared from 30 µl of 4% Laminarin (Sigma) base solution prepared in 0.5 M sodium acetate buffer with pH5 and 30 µl of plant extract. The mixture was kept at 40 °C in a water bath for 30 min, then the reaction was stopped by adding 187 µl of dinitrosalicylic acid reagent and placed in boiling water at 100 °C for 5 min. The final volume was adjusted to 2 ml. Enzyme activity was determined as mg of glucose released per minute per 1 ml of plant extract. The absorption was at 500 nm and different concentrations of glucose were used to draw a standard curve.
Chitinase activity was assayed with a modified Fan et al. (2008) method, by homogenizing 1 g cells with 3 ml of 0.1 M sodium acetate buffer at pH 5, and centrifuging for 15 min at 13,000 rpm at 4 °C. The upper part contained chitinase enzyme, which was kept at -20 °C. Then, 1 ml of plant extract, 0.3 ml of 0.1 M sodium acetate buffer at pH 4.7 and 0.2 ml of colloidal chitin were mixed. They were kept for 12 h at 4 °C, and centrifuged at 12,000 rpm for 5 min at 4 °C. Then, 0.75 ml of the supernatant plus 0.25 ml of 1% dinitrosalicylic acid solution, 0.7 M NaOH and 0.1 ml of 10 M NaOH were placed for 5 min in boiling water (100 °C). The absorption at 582 nm was determined and different concentrations of N-acetyl-D-glucosamine were used to draw a standard curve.
Extraction and assay of vinblastine and vincristine
Following the protocol by Pan et al. (2010), samples were harvested, dried at 25 °C for a week, and pulverized. The assay involved dissolving 0.1 g of the sample’s powder in 1 ml of 85% methanol (HPLC grade), ultra sonication at 30 °C for 1 h, centrifugation at 14,000 rpm for 15 min, and collection of the supernatant for analysis. The vinblastine (code V03000 Sigma Aldrich) and vincristine (code V7988 Sigma) standards were purchased. 0.5 mg of vincristine and 0.5 mg of vinblastine were solved in 0.5 ml of HPLC methanol and 0.5 ml of acetonitrile respectively. In order to plot the standard curves, different concentrations of vincristine: 0.2, 0.4, 0.6, 0.8, 1, 2, 4, 6, 8 and 10 mg/l (ppm) and vinblastine: 100, 150, 200, 300, 400 and 500 mg/l were prepared and 20 µl injected into HPLC machine. Optical absorptions were recorded at 210 nm and the standard curve plotted. Chromatography was carried out using an HPLC manufactured by Knauer Scientific Instruments, Berlin, Germany. For the mobile phase, HPLC water and acetonitrile with a 50:50 ratios were used. Flow rate was set to 1 ml per minute and light absorption measured at 210 nm. Reverse phase-HPLC was utilized for the evaluation of indole alkaloids, with detection and quantification performed using a Smart line pump 1050, a Smart line UV Detector 2600, and DGU-14 A degasser. All the solutions were filtered through a 0.45 nm filter. Aliquots (80 µl) were injected into a C18 reversed-phase column (250 mm × 4.6 mm length, 5 μm particle size). Vinblastine and vincristine compounds were identified by comparing their retention times with standards, and their content was determined using a calibration curve.
Total RNA extraction and gene expression analyses
Total RNA extraction from control and elicited samples was carried out using the RNX-Plus Kit (Sinaclon, Iran), following the manufacturer’s instructions. Quality and quantity of RNA were assessed using agarose gel electrophoresis (1 g/l) and a Nanodrop spectrophotometer (Thermo Scientific, Germany), respectively. The first-strand cDNA was synthesized from RNA using the Sinaclon First-Strand cDNA synthesis kit (Tables 1 and 2). Primers for geraniol 10-hydroxylase (G10H), strictosidine synthase (STR), tabersonine 16-hydroxylase (T16H), desacetoxyvindoline 4-hydroxylase (D4H), deacetylvindoline-4-O-acetyltransferase (DAT), and Catharanthus roseus Apoplastic Peroxidase (CrPrx) genes were designed using OligoArchitect online software and synthesized by Pishgam Company, Iran (Table 3). Real-time PCR reactions were performed with Syber Green dye, following the Sinaclon protocol. The Ribosomal Protein S9 (RPS9) gene served as a housekeeping gene for data normalization. For each sample, Ct values of the reference genes were used as an internal control to normalize target gene expression. The results were calculated as relative changes (Fold changes) compared to the control sample using the 2 −ΔΔCt method (Livak and Schmittgen 2001).
Statistical analysis
Data were analyzed and correlation between phytochemical, molecular and metabolic evaluations was done by using one-way analysis of variance (ANOVA) in SAS software (version 9.4 Cary, NC, USA). The normality was checked by the Shapiro-Wilk test before we used data for the statistical analysis. Duncan’s multiple range test was used to compare the means. Differences were considered statistically significant when P ≤ 0.01. Each treatment included 3 replications for all experiments.
Results
Callus induction and cell suspension establishment and culture
Callus formation percentage and callus fresh weight were measured after four weeks. The effect of explants, hormonal treatment, and their interaction on callus induction percentage and callus fresh weight (Fig. 2, a) of C. roseus were significant (P ≤ 0.01). Comparisons between explants and hormonal treatments on callus formation also showed that the callus formation percentage of different explants was strongly influenced by hormonal content in the culture medium. Some explants, including hypocotyl, nodule, and leaf, showed a high callus formation percentage with most hormonal treatments, such as 4 µM 2,4-D and 2 µM BAP, while 8 µM 2,4-D and 2 µM BAP resulted in 100% callus formation. Conversely, the lowest percentage of callus formation from leaf explants was observed in the medium with 2 µM 2,4-D and 2 µM BAP.
(a) Interaction effect of C. roseus explants and hormonal treatments on callus formation percentage. In each column, the averages with the same letters have no significant difference at the 5% probability level based on Duncan’s multiple range test. (b) Interaction effect of C. roseus explants and hormonal treatments on callus fresh weight
Interaction effects of explant and hormonal treatment showed variable callus fresh weight for various explants in culture media with different hormonal treatments, as illustrated in Fig. 4. For hypocotyl explants, the highest callus fresh weight was observed on 2 µM 2,4-D and 0.5 µM BAP, followed by a combination of 2 µM 2,4-D and 1 µM BAP, while the lowest callus fresh weight was observed in the medium supplemented with 2 µM 2,4-D and 2 µM BAP (Fig. 2, b).
HPLC results showed that the simple effect of explants on vinblastine and vincristine in C. roseus was significant (Fig. 5). The highest amounts of vinblastine and vincristine were obtained from leaf explants 1.348 and 0.657 µg/g dry weight respectively, followed by hypocotyl 0.317 and 0.183 µg/g dry weight respectively. There was no significant difference between petiole, node, and cotyledon explants in the aforementioned alkaloids (Fig. 3). Hence, leaf explants were for the next stage, while 8 µM 2,4-D plus 2 µM BAP was the most desirable hormonal combination for callus growth, and was used for liquid culture and the preparation of cell suspensions.
The amount of vincristine and vinblastine in leaf-derived callus was 3.6 (0.7 µg/g dry weight) and 4.2-fold (1.3 µg/g dry weight) higher than in callus from hypocotyl explants, respectively (Fig. 5).
Enzymes activities
Application of Trichoderma and SNP significantly affected the activities of catalase, ascorbate peroxidase, β (1–3) glucanase, and chitinase in the treated C. roseus cell suspension. The effects of elicitors and the timing of their application on catalase activity were noteworthy. Specifically, T. viride + SNP and T. harzianum + SNP treatments led to a 2-fold and 1.9-fold increase in catalase activity compared to controls, respectively (Fig. 4, a). The interaction effect of elicitors and application timing on ascorbate peroxidase activity did not show a significant difference. However, the highest observed enzyme activity was in the T. harzianum + SNP (2.59 mg–1 protein min–1) and SNP (2.55 mg–1 protein min–1) treatments, respectively (Fig. 4, b). Additionally, a significant increase in ascorbate peroxidase activity was observed after 48 h of elicitor application (Fig. 4, c).
(a) The interaction effect of T. harzianum, T. viride and sodium nitroprusside elicitor treatment and elicitor application time on catalase enzyme activity in C. roseus cell suspension. (b) The interaction effect of T. harzianum, T. viride and sodium nitroprusside elicitor treatment on ascorbate peroxidase enzyme activity in C. roseus cell suspension. (c) The interaction effect on elicitor application time on ascorbate peroxidase enzyme activity in C. roseus cell suspension
As shown in Table 3, elicitors and the timing of their application influenced β (1–3) glucanase. It was found that β (1–3) glucanase activity increased by 2.4-fold in the T. harzianum and T. harzianum + SNP treatments, 48 h after elicitor application, compared to the control (Fig. 5, a). The data from Table 4 also revealed that elicitors and their time of application affected chitinase activity. Specifically, the T. harzianum and T. harzianum + SNP treatments resulted in a 3.7-fold increase in chitinase activity 48 h after elicitor application compared to controls (Fig. 5, b). Comparing the treatments, the activity of catalase and ascorbate peroxidase enzymes was higher in the SNP treatment than in the fungal treatments. The peak activity of these antioxidant enzymes occurred 48 h after the treatments. The most effective elicitor and application timing for increasing catalase and ascorbate peroxidase activity were for the T. harzianum and SNP treatment 48 h after application. After 72 h, due to the death of most cells in the suspension culture, enzyme activity decreased, reaching levels lower than those observed 12 h after treatment.
Furthermore, the activity of chitinase and β-1,3-glucanase enzymes in samples treated with T. harzianum was higher than that in T. viride, although no significant difference was observed between them. When comparing the impact of biological treatments and SNP elicitors on the activity of chitinase and β-1,3-glucanase enzymes, fungal treatments were found to increase the activity of these enzymes compared to SNP treatment. The highest activity of these enzymes was observed 24 h after treatment. Overall, the highest activity of chitinase and β-1,3-glucanase enzymes was obtained from the T. harzianum and SNP treatment after 24 h. However, after 48 h of treatment, the activity of these enzymes decreased, reaching levels lower than those observed 12 h after treatment with biotic elicitors and SNP.
The results showed that after 48 h of treatment with biotic elicitor and SNP, the activity of β-1,3-glucanase, chitinase, polyphenol oxidase, peroxidase, and phenylalanine ammonia-lyase increased and was recorded as 3.34, 2.11, 3.34, 2.17, and 2-fold higher than controls. In this study, there were non-significant differences between T. harzianum and T. viride elicitors.
Expression of TIAs pathway genes
The relative expressions of the G10H, T16H, D4H, DAT, STR, and CrPRX genes in the TIAs biosynthetic pathway of C. roseus cell suspension were significantly up regulated with elicitors and their time of application. The gene expression began to increase after 12 h, reaching its maximum level at 48 h, and subsequently decreased to levels lower than the initial values after 72 h. The T. harzianum + SNP treatment, applied after 48 h of elicitor application, resulted in the maximum expression of all considered genes. The impact of elicitors and application time on G10H gene expression revealed the highest relative expression in the T. harzianum + SNP treatment 48 h after elicitor application (2.53-fold compared to control) followed by 24 h after elicitor application (2.32-fold compared to control) (Fig. 4, c). For the T16H gene, a 1.5-fold and 1.37-fold increase in relative expression was observed in the T. harzianum + SNP and T. harzianum treatments 48 h after elicitor application, respectively (Fig. 5, a). The relative expression of the D4H gene was higher in the suspension treated with T. harzianum + SNP 48 h (1.18-fold compared to control) and 24 h after elicitor application (1.15-fold compared to control) (Fig. 5, b). A 1.98-fold and 1.68-fold increase in DAT gene relative expression was observed in the T. harzianum + SNP treatment after 48- and 24-hours of elicitor application, respectively (Fig. 6). The maximum relative expression of the STR gene was obtained from the T. harzianum + SNP treatment after 48 h (5.08-fold compared to control) and 24 h of elicitor application (4.46-fold compared to control) (Fig. 6). For the CrPRX gene, the T. harzianum + SNP treatment after 48 and 24 h of elicitor application caused a 2.07-fold and 1.79-fold increase, respectively (Fig. 6).
(a) The interaction effect of T. harzianum, T. viride and sodium nitroprusside elicitor treatment and elicitor application time on β-1 and 3-glucanase enzyme activity in C. roseus cell suspension. (b) The interaction effect of T. harzianum, T. viride and sodium nitroprusside elicitor treatment and elicitor application time on chitinase enzyme activity in C. roseus cell suspension
The lowest expression value for all genes was observed in the control samples. In summary, the utilized elicitors stimulated the expression of the six key genes in the vinblastine and vincristine biosynthesis pathway within the TIAs biosynthetic pathway, leading to the accumulation of TIAs.
Vinblastine and vincristine alkaloid yield
The influence of Trichoderma fungi and SNP, the timing of elicitor application, and the interactive effects of Trichoderma fungi and SNP with elicitor application time on the amounts of vinblastine and vincristine alkaloids in C. roseus cell suspension were found to be statistically significant at a 1% probability level (Fig. 7, a and b). The interaction effect of Trichoderma fungi and SNP, along with the elicitor application time, revealed that the maximum levels of vinblastine (Fig. 7, a) and vincristine (Fig. 7, b) alkaloids occurred in the treatment involving T. harzianum + SNP 48 h after elicitor application, exhibiting a 1.84-fold and 1.93-fold increase compared to the control, respectively. Additionally, the combination of T. harzianum + SNP after 24 h of elicitor application resulted in a 1.69-fold and 1.87-fold increase in vinblastine and vincristine alkaloids compared to controls, respectively. The controls, with varying elicitor application times, displayed the lowest amounts of vinblastine and vincristine alkaloids, and these values were not significantly different from each other. This study provides clear evidence that the biotic elicitor stimulates an enriched level of alkaloids in C. roseus cell suspension.
(a) The interaction effect of Trichoderma harzianum elicitor and sodium nitroprusside elicitor treatment and application elicitor time on vinblastine alkaloid amount in C. roseus cell suspension. (b) The interaction effect of Trichoderma harzianum and sodium nitroprusside elicitors and application time on vincristine alkaloid amount in C. roseus cell suspension
Correlation between phytochemical, molecular and metabolic evaluations
The interplay between enzymatic activity, molecular, and metabolic parameters in C. roseus cell suspension under the influence of Trichoderma fungi and SNP elicitors revealed a positive and significant correlation. This correlation was observed between catalase, ascorbate peroxidase, β-1 and 3-glucanase, and chitinase enzyme activities with the relative expression of G10H, T16H, D4H, DAT, STR, CrPRX STR, and CrPRX genes involved in the biosynthesis of vinblastine and vincristine alkaloids.
Based on these results, it can be inferred that T. harzianum, SNP elicitor, and the timing of elicitor application induced a defense response, leading to an increase in enzymatic activity. Consequently, this increase in enzymatic activity stimulated the relative expression of genes in the biosynthetic pathway of C. roseus cell suspension. The confirmation of this inference lies in the enhanced production of vinblastine and vincristine alkaloids. The heightened synthesis of these alkaloids may be attributed to the increased activity of antioxidant enzymes (catalase and ascorbate peroxidase) and defense enzymes (β-1 and 3-glucanase and chitinase), or an upregulation in the expression of genes integral to the biosynthetic pathway of these alkaloids (refer to Table 4).
Discussion
The initial phase of the experiments aimed to screen for the optimal explants and medium, focusing on achieving high callus formation percentages, substantial callus fresh weight, and elevated levels of vinblastine and vincristine. In the subsequent section, the study delved into investigating the impact of T. harzianum, SNP elicitors, and the timing of elicitor application on enzymatic activity and the expression of the TIAs biosynthetic pathway genes in C. roseus cell suspension.
Farhadi et al. (2021) investigated the effects of BAP, 2,4-D, and NAA on the percentage and weight of calluses from the roots and hypocotyl of C. roseus. They found that these were highest from hypocotyl explants cultured with 1 µM BAP and 1 µM 2,4-D. C. roseus hypocotyl explant in MS medium supplemented with 4.5 µM 2,4-D with 2 µM BAP had the highest callus formation. Fragile, light-colored, and fast-growing calluses were produced that could produce somatic embryos. In the present study, the combination treatment of 4 µM 2,4-D and 1 µM BAP and 4 µM 2,4-D and 2 µM BAP induced callus formation in most of the explants. The positive effects of 2,4-D and BAP on callus formation percentage and callus weight have been reported in many medicinal plants, such as Stevia rebaudiana (Keshvari et al. 2018), Taxus baccata, Calotropis procera (Amirkavei Najafabadi et al. 2020) and Mentha piperita (Ahmad et al. 2021), and are consistent with the results of this study. The balance between organic and inorganic nutrients, carbon sources, plant growth regulators, stresses, and plant growth stages can affect the biosynthetic pathway of alkaloids.
Aslam et al. (2010) investigated the vincristine alkaloid content in non-embryonic versus embryogenic callus from leaf, root, and node explants. Their results showed the highest amount of vincristine alkaloid in the embryonic germination stage (10.04 micrograms per gram of dry weight). Also, plants regenerated from somatic embryos had 2.2 µg per gram of dry weight of vinblastine more than plants grown in the field. Their results showed that vinblastine alkaloid was not detected in the roots, and the highest vinblastine alkaloid was observed in the leaf explant. On the other hand, vinblastine alkaloid in the in vitro condition was 12.3 µg/g of dry weight, and in field condition, it was 9.4 µg/g of dry weight.
Liu et al. (2021) emphasized that cell suspension and hairy roots represent optimal methods for studying the biosynthesis pathway of indole alkaloids in periwinkle. However, earlier research had used seedlings (Mortensen et al. 2019), leaves (Sharma et al. 2018), and petals (Schweizer et al. 2018) to investigate gene expression related to the synthesis of valuable alkaloids in this plant. For this study, C. roseus cell suspensions derived from leaf callus were employed.
The genetic control of secondary metabolite production is well-established, yet its accumulation in the plant is triggered by biotic stresses and SNP elicitors (Verma et al. 2017). Fungal elicitors, in particular, enhance the production of secondary metabolites, especially those integral to plant defense mechanisms. Fungi and plants share cell walls, but the composition of fungal cell walls differs from that of plants. Elicitors target cell wall compounds, activating the plant’s defense system in response to the elicitor’s message (Orbán et al. 2008). Over time, a decrease in cell growth affected by fungal elicitors may be attributed to reduced primary metabolism and the initiation of secondary metabolism. Interestingly, there are reports of increased growth influenced by fungal elicitors (Wang et al. 2001).
SNP serves as a nitric oxide source, acting as a free radical with wide-ranging physiological consequences in plant cells (Hayat et al. 2010). The signaling role of nitric oxide in regulating essential growth processes, development, and defense responses has been well-documented (Hong et al. 2008). Nitric oxide activity in plant tissues and cells typically occurs in response to abiotic stresses, pathogen attacks, and challenges from fungal elicitors. Its prominent role lies in signaling and regulating defense responses to stresses, leading to an increased production of secondary metabolites (Senthil 2020), as well as the activation of enzymes and the expression of genes related to their biosynthetic pathways (Ma et al. 2021). Tonk et al. (2016) conducted a study investigating the effect of Aspergillus flavus fungal elicitor on vinblastine and vincristine content in periwinkle in vitro culture. Their results showed that adding 0.15% of fungal elicitor to the culture medium, in comparison with other concentrations, led to the highest activity of superoxide dismutase, catalase, and ascorbate peroxidase.
SNP stimulates enzymes and genes involved in the scavenging of free radicals, induces stress, and triggers the stress response mechanism by stimulating enzymes and genes related to defense. The reduction of fusarium wilt was investigated in tomatoes by treating with biotic and SNP elicitors (Chakraborty et al. 2021).
Given the positive effects observed with fungal elicitors and SNP in this study, their influence on the relative expression of genes involved in the biosynthetic pathway of vincristine alkaloid was explored. It is worth noting that the relative expression of G10H, T16H, D4H, DAT, STR, and CrPRX genes involved in the biosynthetic pathway of vinblastine and vincristine alkaloids in C. roseus cell suspension under treatment of T. harzianum and SNP elicitors had not been examined at various elicitor application times.
The present findings align with previous publications that highlight the positive impact of biotic elicitors on the biosynthesis pathway of TIAs. Pandey et al. (2016) explored the influence of endophytic fungi (Choanephora infundibulifera and Curvularia spp.) on enhancing the expression of vindoline genes in periwinkle. Following inoculation with Curvularia spp., the elicitor led to the expression of various genes, including STR (0.7-fold increase), SGD (0.2-fold decrease), T16H (0.3-fold decrease), 16OMT (1-fold increase), D4H (1.7-fold increase), DAT (5.3-fold increase), and PRX1 (2-fold increase) compared to the control. Choanephora infundibulifera elicitor also induced changes in the relative expression of key genes in the biosynthetic pathway of alkaloids. Ramezani et al. (2018) reported that the treatment of Piriformospora indica and Trichoderma tomentosum at 1% v/v in periwinkle cell suspension proved more effective than T. tomentosum alone. Gene expression initiated after 24 h of treatment, with the expression of D4H and DAT genes peaking at 48 h, showing a highest gene expression of 1.2-fold and 0.7-fold, respectively. After 72 h, gene expression levels receded to control levels.
Liang et al. (2018) studied the effect of the biotic elicitor Aspergillus flavus on the expression of TIAs production genes in C. roseus meristem-derived cell suspension. The results demonstrated that the combined treatment with 25 mg/l elicitor after 48 h yielded the most desirable outcomes. The relative expression of D4H, G10H, GES, IRS, LAMT, SGD, STR, TDC, and ORCA3 genes increased by 49.4, 1.75, 1.71, 1.42, 3.12, 2.33, 2.87, 2.51, and 5.97-fold compared to controls. These findings indicated a correlation between the increased expression of specific genes and the enhancement of vindoline, catharanthine, and ajmaline alkaloids in periwinkle cells.
The application of SNP elicitor was shown to increase the production of secondary metabolites and stimulate the expression of genes in the biosynthetic pathway of valuable medicinal compounds in plants (Zhou et al. 2010). Mahendran et al. (2021) reported that SNP at different concentrations (5, 10, 20, and 40 µM) in the cell suspension of Gymnema sylvestre increased valuable secondary metabolites and their biosynthetic genes. A concentration of 20 µM, applied up to day 40 of treatment, resulted in the highest fresh and dry cell weight. The maximum accumulation of gymnemic diacyl acid, gymnemagnine, and gymnemic acid was observed after 40 days in the treatment of 20 µM SNP, showing a substantial increase compared to the control. They attributed this increase to a change in the expression pattern of genes involved in the biosynthesis of these compounds and recommended the 20 µM SNP treatment as a viable strategy for large-scale production of these secondary metabolites at an industrial level.
Liang et al. (2012) demonstrated that the application of the SNP elicitor (100 µM) in Salvia hairy root culture significantly enhanced tanshinone production. This treatment led to a substantial upregulation in the expression of two key genes, HMGR and DXR, within the biosynthesis pathway of tanshinone alkaloids, showing increases of 16.7-fold and 14.1-fold, respectively.
In a study by Wang et al. (2009), the application of cerebroside (30 µg/ml) and SNP (10 µM) in the hairy roots of Artemisia reported that SNP alone did not induce changes in the expression of genes involved in the biosynthesis pathway of artemisinin sesquiterpene alkaloids. However, the results of qRT-PCR revealed that the combined treatment of these two elicitors resulted in the highest expression levels of HMGR (9.3-fold) and DXR (6.6-fold) genes compared to the controls.
In a study evaluating the role of SNP as a nitric oxide donor in secondary metabolite production, Xu and Dong (2005) found that the treatment of 10 mmol L-1 SNP in the cell suspension of periwinkle increased the production of ajmalicine and catharanthine by 1.6 and 2.9-fold, respectively. This increase was accompanied by a significant upregulation of genes associated with the production of ajmalicine and catharanthine alkaloids.
Zhou et al. (2010) investigated the transcriptional response of the catharanthine alkaloid biosynthesis pathway in hairy root culture of periwinkle using methyl jasmonate (50 mg/l) and SNP (10 mg/l) elicitors. Their findings suggested that SNP elicitor alone and in combination with methyl jasmonate caused overexpression of ORCA3, resulting in decreased catharanthine levels. Additionally, there was a significant enhancement of CPR and ZCT transcription. Methyl jasmonate increased the expression of several genes, including TDC, STR, MECS, SLS, SGD, and G10H, and this effect was partially inhibited by SNP, suggesting an antagonistic role of methyl jasmonate and SNP in the biosynthesis of catharanthine alkaloid.
These studies collectively emphasize the intricate interactions and diverse effects of SNP elicitor on secondary metabolite production and gene expression in different plant systems.
The results revealed that SNP elicitor, both alone and in combination with methyl jasmonate, induced overexpression of ORCA3, leading to a subsequent decrease in catharanthine levels. Additionally, there was a significant enhancement in CPR and ZCT transcription. Methyl jasmonate, when applied individually, increased the expression of TDC, STR, MECS, SLS, SGD, and G10H genes. However, this upregulation was inhibited in the presence of SNP. This complex response involving transcriptional regulators and pathogen-related genes suggests a potential antagonistic role of methyl jasmonate and SNP in the biosynthesis of catharanthine alkaloid.
In a study conducted by Li et al. (2011), various concentrations of SNP (0, 0.1, 1, and 10 mM) were utilized to investigate their effects on growth and alkaloid production in the hairy root culture of periwinkle. The results indicated that 10 mM of SNP acted as a complete inhibitor of hairy root growth, and both 1- and 10-mM SNP were found to be toxic, leading to a reduction in the production of TIAs. In contrast, treatment with 0.1 mM SNP up to day 9 resulted in an increase in the alkaloids of ajmalicine and taberzonin, accompanied by a decrease in catharanthine and serpentine alkaloids. The maximum levels were reached on day 14 but declined by day 30. Notably, the treatment with 0.1 mM SNP concentration up to day 12 led to a reduction in the expression of STR, ORCA3, and Crgbf1 genes. However, by day 21, the relative expression of ZCT1 gene had doubled compared to the control, and until the 28th day, the relative expression of G10H gene exhibited an upward trend.
Previous studies have demonstrated the ability of elicitors to exert control over a large number of transcription factor regulators involved in the biosynthesis pathway of TIAs, stimulating the expression of these genes at both the biochemical and molecular levels (Khataee et al. 2019). However, the findings of our research indicate that the simultaneous application of a biotic elicitor and SNP has a more pronounced effect in stimulating the production of secondary metabolites. These results align with earlier studies, underscoring the enhanced efficacy of the combined treatment involving the Trichoderma elicitor (T. harzianum) and the abiotic elicitor (SNP). This combined approach proves more effective in influencing enzyme activity, regulating the relative expression of the identified genes, and determining the quantities of vinblastine and vincristine alkaloids in the C. roseus cell suspension culture.
Conclusion
Based on the results, we recommend employing leaf explants in conjunction with 8 µM 2,4-D and 2 µM BAP for liquid culture and the preparation of cell suspension. Furthermore, the utilization of the T. harzianum elicitor, combined with the SNP elicitor applied 48 h after, is advised for enhancing enzyme activities and promoting the expression of genes involved in the biosynthesis pathway of vinblastine and vincristine alkaloids in periwinkle. The findings presented in this paper offer valuable insights for optimizing the production of secondary metabolites with improved quality under in vitro conditions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SNP:
-
Sodium nitroprusside
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- BAP:
-
6-Benzylaminopurine
- G10H :
-
Geraniol 10-hydroxylase
- T16H :
-
Tabersonine 16-hydroxylase
- D4H :
-
Desacetoxyvindoline 4-hydroxylase
- DAT :
-
Deacetylvindoline-4-O-acetyltransferase
- STR :
-
Strictosidine synthase
- CrPRX :
-
Catharanthus roseus Apoplastic Peroxidase
- RPS9 :
-
Ribosomal Protein S9
- TIAs:
-
Terpenoid Indole Alkaloid
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- GR:
-
Glutathione reductase
- qRT-PCR:
-
Quantitative reverse transcription polymerase chain reaction
- PCR:
-
Polymerase chain reaction
- HPLC:
-
High-performance liquid chromatography
- NO:
-
Nitric oxide
- SCV:
-
Settled cell volume
References
Abeles FB, Forrence LE (1970) Temporal and hormonal control of β-1, 3-glucanase in Phaseolus vulgaris L. Plant Physiol 45:395–400. https://doi.org/10.1104/pp.45.4.395
Aebi H (1984) [13] catalase in vitro. Methods in enzymology. Elsevier, pp 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmad A, ul Qamar MT, Shoukat A et al (2021) The effects of genotypes and media composition on callogenesis, regeneration and cell suspension culture of chamomile (Matricaria chamomilla L). PeerJ 9:e11464. https://doi.org/10.7717/peerj.11464
Alhaithloul HA, Soliman MH, Ameta KL et al (2019) Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha Piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 10:43. https://doi.org/10.3390/biom10010043
Amirkavei Najafabadi B, Qavami N, Ebrahimi MA et al (2020) Enhancement of taxol production by applying amino acid complex along with chitosan in suspension culture of Taxus baccata L. J Med Plants 19:99–109. https://doi.org/10.29252/jmp.19.76.99
Amooaghaie R, Korrani FM (2018) Bacillus subtilis and vermicompost suppress damping-off disease in psyllium through nitric oxide-dependent signaling system. Russ J Plant Physiol 65:435–445. https://doi.org/10.1134/S1021443718030093
Aslam J, Mujib A, Fatima Z, Sharma MP (2010) Variations in vinblastine production at different stages of somatic embryogenesis, embryo, and field-grown plantlets of Catharanthus roseus L.(G) Don, as revealed by HPLC. Vitr Cell Dev Biol 46:348–353. https://doi.org/10.1007/s11627-010-9290-y
Baldi A, Srivastava AK, Bisaria VS (2009) Fungal elicitors for enhanced production of secondary metabolites in plant cell suspension cultures. Symbiotic fungi. Springer, pp 373–380. https://doi.org/10.1007/978-3-540-95894-9_23
Birat K, Siddiqi TO, Mir SR et al (2022) Enhancement of vincristine under in vitro culture of Catharanthus roseussupplemented with Alternaria Sesami endophytic fungal extract as a biotic elicitor. Int Microbiol 25:275–284. https://doi.org/10.1007/s10123-021-00213-w
Cai Z, Kastell A, Mewis I et al (2012) Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell, Tissue Organ Culture 108:401-409 https://doi.org/10.1007/s11240-011-0051-3
Chakraborty N, Sarkar A, Acharya K (2021) Biotic elicitor induced nitric oxide production in mitigation of Fusarium wilt of tomato. J Plant Biochem Biotechnol 30:960–972. https://doi.org/10.1007/s13562-021-00705-w
Coste A, Vlase L, Halmagyi A et al (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum Hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Cult 106:279–288. https://doi.org/10.1007/s11240-011-9919-5
Facchini PJ (2001) Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Biol 52:29–66. https://doi.org/10.1146/annurev.arplant.52.1.29
Fan B, Shen L, Liu K et al (2008) Interaction between nitric oxide and hydrogen peroxide in postharvest tomato resistance response to Rhizopus nigricans. J Sci Food Agric 88:1238–1244. https://doi.org/10.1002/jsfa.3212
Farhadi H, Hassanpouraghdam MB, Aazami MA (2021) The induction and development of somatic embryos from the in vitro cultures of Catharanthus roseus (L.) G. Don. Adv Hortic Sci 35. https://doi.org/10.36253/ahsc-9874
Farjaminezhad R, Zare N, Zakaria RA, Farjaminezhad M (2013) Establishment and optimization of cell growth in suspension culture of Papaver bracteatum: a biotechnology approach for thebaine production. Turkish J Biol 37:689–697. https://doi.org/10.3906/biy-1304-54
Fatima S, Mujib A, Tonk D (2015) NaCl amendment improves vinblastine and vincristine synthesis in Catharanthus roseus: a case of stress signalling as evidenced by antioxidant enzymes activities. Plant Cell Tissue Organ Cult 121:445–458. https://doi.org/10.1007/s11240-015-0715-5
Hayat S, Hasan SA, Mori M et al (2010) Nitric oxide: chemistry, biosynthesis, and physiological role. Nitric oxide plant Physiol 1–15. https://doi.org/10.1002/9783527629138.ch1
Hong JK, Yun B-W, Kang JG et al (2008) Nitric oxide function and signalling in plant disease resistance. J Exp Bot 59:147–154. https://doi.org/10.1093/jxb/erm244
Ionkova I (2007) Biotechnological approaches for the production of lignans. Pharmacogn Rev 1:427–443. http://www.phcogrev.com
Junaid A, Mujib A, Bhat MA, Sharma MP (2006) Somatic embryo proliferation, maturation and germination in Catharanthus roseus. Plant Cell Tissue Organ Cult 84:325–332. https://doi.org/10.1007/s11240-005-9041-7
Kellner F, Kim J, Clavijo BJ et al (2015) Genome-guided investigation of plant natural product biosynthesis. Plant J 82:680–692. https://doi.org/10.1111/tpj.12827
Keshvari T, Najaphy A, Kahrizi D, Zebarjadi A (2018) Callus induction and somatic embryogenesis in Stevia rebaudiana Bertoni as a medicinal plant. Cell Mol Biol 64:46–49. https://doi.org/10.14715/cmb/2018.64.2.9
Khan MN, Mobin M, Abbas ZK, Siddiqui MH (2017) Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 68:91–102. https://doi.org/10.1016/j.niox.2017.01.001
Khataee E, Karimi F, Razavi K (2019) Chromium-induced alkaloid production in Catharanthus roseus (L.) G. Don in vitro cultured shoots and related gene expression patterns particularly for the novel gene GS. Acta Agric Slov 113:95–108. https://doi.org/10.14720/aas.2019.113.1.09
Khurana A, Khurana JP, Babbar SB (2011) Nitric oxide induces flowering in the duckweed Lemna aequinoctialis Welw.(syn. L. Paucicostata Hegelm.) Under noninductive conditions. J Plant Growth Regul 30:378–385. https://doi.org/10.1007/s00344-011-9199-7
Li M, Peebles CAM, Shanks JV, San K (2011) Effect of sodium nitroprusside on growth and terpenoid indole alkaloid production in Catharanthus roseus hairy root cultures. Biotechnol Prog 27:625–630. https://doi.org/10.1002/btpr.605
Liang Z-S, Yang D-F, Liang X et al (2012) Roles of reactive oxygen species in methyl jasmonate and nitric oxide-induced tanshinone production in Salvia miltiorrhiza hairy roots. Plant Cell Rep 31:873–883. https://doi.org/10.1007/s00299-011-1208-6
Liang C, Chen C, Zhou P et al (2018) Effect of aspergillus flavus fungal elicitor on the production of terpenoid indole alkaloids in Catharanthus roseus Cambial meristematic cells. Molecules 23:3276. https://doi.org/10.3390/molecules23123276
Liu L-YD, Tseng H-I, Lin C-P et al (2014) High-throughput transcriptome analysis of the leafy flower transition of Catharanthus roseus induced by peanut witches’-broom phytoplasma infection. Plant Cell Physiol 55:942–957. https://doi.org/10.1093/pcp/pcu029
Liu Y, Patra B, Singh SK et al (2021) Terpenoid indole alkaloid biosynthesis in Catharanthus roseus: effects and prospects of environmental factors in metabolic engineering. Biotechnol Lett 43:2085–2103. https://doi.org/10.1007/s10529-021-03179-x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Ma YJ, Li XP, Wang Y, Wang JW (2021) Nitric oxide donor sodium nitroprusside-induced transcriptional changes and hypocrellin biosynthesis of Shiraia sp. S9. Microb Cell Fact 20:1–16. https://doi.org/10.1186/s12934-021-01581-8
Mahendran G, Kumar D, Verma SK et al (2021) Sodium nitroprusside enhances biomass and gymnemic acids production in cell suspension of Gymnema sylvestre (Retz.) R. Br. Ex. Sm. Plant Cell. Tissue Organ Cult 146:161–170. https://doi.org/10.1007/s11240-021-02058-7
Ming Q, Su C, Zheng C et al (2013) Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J Exp Bot 64:5687–5694. https://doi.org/10.1093/jxb/ert342
Mortensen S, Bernal-Franco D, Cole LF et al (2019) EASI transformation: an efficient transient expression method for analyzing gene function in Catharanthus roseus seedlings. Front Plant Sci 10:755. https://doi.org/10.3389/fpls.2019.00755
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Namdeo A, Patil S, Fulzele DP (2002) Influence of fungal elicitors on production of ajmalicine by cell cultures of Catharanthus roseus. Biotechnol Prog 18:159–162. https://doi.org/10.1021/bp0101280
Orbán N, Boldizsár I, Szűcs Z, Dános B (2008) Influence of different elicitors on the synthesis of anthraquinone derivatives in Rubia tinctorum L. cell suspension cultures. Dye Pigment 77:249–257. https://doi.org/10.1016/j.dyepig.2007.03.015
Pan Q, Chen YU, Wang Q et al (2010) Effect of plant growth regulators on the biosynthesis of vinblastine, vindoline and catharanthine in Catharanthus roseus. Plant Growth Regul 60:133–141. https://doi.org/10.1007/s10725-009-9429-1
Pan Y, Lin Y, Yu B et al (2018) Transcriptomics comparison reveals the diversity of ethylene and methyl-jasmonate in roles of TIA metabolism in Catharanthus roseus. BMC Genomics 19:1–14. https://doi.org/10.1186/s12864-018-4879-3
Pandey SS, Singh S, Babu CS et al (2016) Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep 6:1–14. https://doi.org/10.1038/srep26583
Prasad A, Mathur A, Kalra A et al (2013) Fungal elicitor-mediated enhancement in growth and asiaticoside content of Centella asiatica L. shoot cultures. Plant Growth Regul 69:265–273. https://doi.org/10.1007/s10725-012-9769-0
Ramezani A, Haddad R, Sedaghati B, Jafari D (2018) Effects of fungal extracts on vinblastine and vincristine production and their biosynthesis pathway genes in Catharanthus roseus. South Afr J Bot 119:163–171. https://doi.org/10.1016/j.sajb.2018.08.015
Sahu R, Gangopadhyay M, Dewanjee S (2013) Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Solenostemon scutellarioides. Acta Physiol Plant 35:1473–1481. https://doi.org/10.1007/s11738-012-1188-3
Schweizer F, Colinas M, Pollier J et al (2018) An engineered combinatorial module of transcription factors boosts production of monoterpenoid indole alkaloids in Catharanthus roseus. Metab Eng 48:150–162. https://doi.org/10.1016/j.ymben.2018.05.016
Senthil K (2020) Establishment of callus and cell suspension culture of Sophora alopecuroides Linn. For the production of oxymatrine. J Phytol 12:35–39. https://doi.org/10.25081/jp.2020.v12.6308
Sharma A, Verma P, Mathur A, Mathur AK (2018) Genetic engineering approach using early Vinca alkaloid biosynthesis genes led to increased tryptamine and terpenoid indole alkaloids biosynthesis in differentiating cultures of Catharanthus roseus. Protoplasma 255:425–435. https://doi.org/10.1007/s00709-017-1151-7
Siddiqui ZH, Mujib A, Abbas ZK et al (2023) Vinblastine synthesis under the influence of CaCl2 elicitation in embryogenic cell suspension culture of Catharanthus roseus. South Afr J Bot 154:319–329. https://doi.org/10.1016/j.sajb.2023.01.046
Singh NR, Rath SK, Behera S, Naik SK (2018) In vitro secondary metabolite production through fungal elicitation: an approach for sustainability. Fungal nanobionics: principles and applications. Springer, pp 215–242. https://doi.org/10.1007/978-981-10-8666-3_9
Tashackori H, Sharifi M, Ahmadian Chashmi N et al (2016) Induced-differential changes on lignan and phenolic acid compounds in Linum album hairy roots by fungal extract of Piriformospora indica. Plant Cell, Tissue Organ Culture 127:187–194. https://doi.org/10.1007/s11240-016-1041-2
Thakur M, Bhattacharya S, Khosla PK, Puri S (2019) Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants 12:1–12. https://doi.org/10.1016/j.jarmap.2018.11.004
Tonk D, Mujib A, Maqsood M et al (2016) Aspergillus flavus fungus elicitation improves vincristine and vinblastine yield by augmenting callus biomass growth in Catharanthus roseus. Plant Cell Tissue Organ Cult 126:291–303. https://doi.org/10.1007/s11240-016-0998-1
Verma P, Mathur AK, Khan SA et al (2017) Transgenic studies for modulating terpenoid indole alkaloids pathway in Catharanthus roseus: present status and future options. Phytochem Rev 16:19–54. https://doi.org/10.1007/s11101-015-9447-8
Wang C, Wu J, Mei X (2001) Enhancement of taxol production and excretion in Taxus chinensis cell culture by fungal elicitation and medium renewal. Appl Microbiol Biotechnol 55:404–410. https://doi.org/10.1007/s002530000567
Wang JW, Zheng LP, Zhang B, Zou T (2009) Stimulation of artemisinin synthesis by combined cerebroside and nitric oxide elicitation in Artemisia annua hairy roots. Appl Microbiol Biotechnol 85:285–292. https://doi.org/10.1007/s00253-009-2090-9
Xu M, Dong J (2005) Nitric oxide stimulates indole alkaloid production in Catharanthus roseus cell suspension cultures through a protein kinase-dependent signal pathway. Enzyme Microb Technol 37:49–53. https://doi.org/10.1016/j.enzmictec.2005.01.036
Zandi M (2021) Cytotoxicity of taxol in combination with vincristine and vinblastine against A375 cell line. Gene Cell Tissue 8. https://doi.org/10.5812/gct.114359
Zhang XN, Liu J, Liu Y et al (2018) Metabolomics analysis reveals that ethylene and methyl jasmonate regulate different branch pathways to promote the accumulation of terpenoid indole alkaloids in Catharanthus roseus. J Nat Prod 81:335–342. https://doi.org/10.1021/acs.jnatprod.7b00782
Zhou M-L, Zhu X-M, Shao J-R et al (2010) Transcriptional response of the catharanthine biosynthesis pathway to methyl jasmonate/nitric oxide elicitation in Catharanthus roseus hairy root culture. Appl Microbiol Biotechnol 88:737–750. https://doi.org/10.1007/s00253-010-2822-x
Acknowledgements
The support of this research provided by Bu-Ali Sina University (BASU) is acknowledged.
Funding
This work was supported by Bu-Ali Sina university (Grant No: 98–936).
Author information
Authors and Affiliations
Contributions
L.F. conducted the research, prepared the materials and collected the data. M.S. supervised and cooperated in conducting the experiments.
Corresponding author
Ethics declarations
Competing interest
The authors have no main and known competing financial interests or important and personal relationships that could have appeared to influence the study reported in this paper.
Additional information
Communicated by Ali R. Alan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farzaei, L., Sayyari, M. Elicitation with sodium nitroprusside and Trichoderma improves vincristine and vinblastine yield in Catharanthus roseus cell suspension culture by modulating terpenoid indole alkaloid pathway genes. Plant Cell Tiss Organ Cult 157, 37 (2024). https://doi.org/10.1007/s11240-024-02727-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-024-02727-3