Abstract
An understanding of plant secondary metabolites may prove to be important for novel drug development. The objective of this investigation was to enhance the biosynthesis of alkaloid compounds aervine and methylaervine in callus-derived cell suspension cultures of Aerva javanica using biotic and abiotic elicitors. The effect of five different elicitors on the biosynthesis of aervine (AE) and methylaervine (MAE) contents was studied for 20 days to determine the concentrations suitable for their accumulation in A. javanica. The callus obtained from the shoots of A. javanica on Murashige and Skoog (MS) medium containing 2.0 mg L−1 2,4-dichlorophenoxy acetic acid (2,4-D) and 0.5 mg L−1 α-naphthalene acetic acid (NAA), and their cell suspension cultures were used for the elicitation purposes. The result proved the maximum accumulation of AE and MAE contents in cell suspension culture. It was found to be 72.26 ± 0.30 mg g−1 DW of MAE, showing an increase of 8.66-fold, and AE content (7.48 ± 0.39 mg g−1 DW) in sodium carbonate (Na2CO3), whereas AE (34.10 ± 0.84 mg g−1 DW) and MAE (9.69 ± 0.04 mg g−1 DW) showed a 2.51-fold increase in salicylic acid. It was observed that the production of hairy root using Agrobacterium rhizogenes (MTCC 532) helps in improving aervine (42.22 ± 1.04 mg g−1 DW) and methylaervine (8.30 ± 0.09 mg g−1 DW) accumulation. This study will serve as an alternate protocol to improve alkaloid quantity as well as quality by elicitor stimulation. Furthermore, it may help in the sustainable production of A. javanica taxon and thereby helping in rescuing the natural sources recommended to cure several ailments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research on plants has a long-standing tradition in cell culture systems. The systems offer a promising resource for precious secondary metabolites of industrial importance, as well as drugs and food additives (Zhong 2001). In addition, it is also possible to extend our approach to produce antioxidants in medicine, contributing to the sustainable production of industrially important compounds and preserving natural sources (Pyo et al. 2004). Over the years, this field has reached a mature stage through the enhancement of different secondary metabolites using in vitro cell cultures, which could resolve their demands (Buitelaar et al. 1992).
In vitro suspension culture method have proven useful for improving the production of secondary metabolites via the elicitation process. However, the effectiveness of elicitation depends on several parameters such as concentration of biotic and abiotic elicitors, the time of exposure, and the type of plant material (Patel and Krishnamurthy 2013). In several instances, the choice of option for the enhanced production of secondary metabolites would be elicitors such as chitosan, polyamines, abscisic acid, methyl jasmonate, salicylic acid, nitric oxide, and heavy metals (Zhong 2001; Ramirez-Estrada et al. 2016). Khojasteh et al. (2016) reported the use of methyl jasmonate in Satureja khuzistanica improved the production of rosmarinic acid. Similarly, methyl jasmonate and salicylic acid elicited the production of anthocyanin from the callus cultures of Rosa hybrida L. (Ram et al. 2013). Hence, researchers have confirmed the role of elicitors in enhancing the synthesis of secondary metabolites in various cell cultures (Putalun et al. 2010; Udomsuk et al. 2011). The hairy root culture highlights the large-scale biosynthesis of secondary metabolites (Paek et al. 2009). Hairy root culture along with the elicitation mechanism was found to be the best mechanism for enhancing secondary metabolites (Pandey et al. 2015; Nazir et al. 2019).
Aerva javanica is a perennial herb that is used in the traditional medicinal system as it possesses diabetic, demulcent, and diuretic properties (Qasim et al. 2013). Phytochemically, this plant species possesses a variety of bioactive compounds such as alkaloids, steroids, sulfates, triterpenes, lipids, carbohydrates, and glycosides. Studies on the phytochemical nature of the in vitro regenerated A. javanica are lacking in literature. Work in this area is extensive but primarily concerned with enhancing phytocompounds via callus-mediated cell suspension culture, which is essential in producing novel compounds that are helpful in medicinal applications (Choi et al. 2000).
Zapesochnaya et al. (1991) identified four new alkaloids in Aerva lanata. Of them, 10-hydroxycanthin-6-one (aervine) and 10-methoxycanthin-6-one (methylaervine) belong to alkaloids (canthin-6-one). The alkaloids showed a range of effective biological properties such as antiparasitic, antifungal, antibacterial, antiviral, anticancer, and anti-inflammatory activities (Dai et al. 2016; Zhang et al. 2013; Bharitkar et al. 2015; Siveen et al. 2012). Additionally, 24 novel ester derivatives of aervine have been reported to possess in vitro antimicrobial activity (Zhao et al. 2016). Similarly, methylaervine derivatives have also been reported to possess good antimicrobial activity with 22 new quaternarization derivatives (Dai et al. 2018; Li et al. 2019). Given the increasing prevalence of pharmacological profile, there is an urgent need to enhance secondary metabolites for sustainable drug development. Research on plants from the same family can shed light on their ecological roles, interactions with other organisms and adaptations to specific environmental conditions, which is valuable for understanding ecosystem dynamics and conservation efforts. Meanwhile, a recent report of Boobalan and Kamalanathan (2020) provide evidence of enhancing aervine via hairy root cultures of A. lanata. Here, we report a previously neglected aspect of research by analyzing the pharmaceutical role of these alkaloids (aervine and methylaervine), which are enhanced via the elicitation process to achieve rapid production.
Materials and methods
Initiation of callus and establishment of cell suspension culture
The young and elite shoot tips of A. javanica were excised and disinfected, as mentioned in our previous communication (Boobalan and Kamalanathan 2019). The sterile shoot tips were inoculated onto an MS medium provided with 2.0 mg/L of 2, 4-D with 0.5 mg/L NAA, along with 30 g/L sucrose. All the inoculated cultures were maintained in a growth chamber for about 4 weeks until the initiation of callus formation was attained. Subsequently, the fresh callus (about 1 g) was transferred to a sterile 250 ml conical flask containing 100 ml nutrient medium. The medium, now suspended with callus, was then sealed with cotton and kept in a rotary shaker at 80 rpm under a 16-h photoperiod with 3000 lx light intensity. The proliferated callus was then subcultured at regular intervals of 15 days.
Elicitor treatments
After the growth of callus from suspension cultures, the medium was fed with elicitors of different types and concentrations. This study used five types of elicitors viz., chitosan (CHT), salicylic acid (SA), methyl jasmonate (MeJa), sodium dihydrogen orthophosphate (NaH2PO4), and sodium carbonate (Na2CO3), at different concentrations of 10, 25, 50, and 100 mg/L and control (without elicitor). The variously concentrated callus culture was maintained on the rotary shaker at 100 rpm to attain exponential growth until 20 days. The elicited culture was harvested on the 20th day to determine the biomass content (fresh and dry weight).
Determination of biomass accumulation
On the 20th day, the harvested culture was rinsed with sterile distilled water followed by blot drying on a filter paper. The fresh weight (FW) of the biomass concentration was determined after removing the last traces of distilled water. The cells were then placed on a sterile petri dish and stored in a hot-air oven at 60 °C for 2 days, and then the dry weight (DW) of the callus was measured. This experiment was conducted in triplicates, and the fresh and dried weight of callus matter was measured.
Hairy root induction
The generation of transgenic hairy root culture in enhancing the production of secondary metabolites in A. javanica may help to enhance the alkaloids (aervine and methylaervine). All plants were collected from a Cauvery irrigation field situated near Tiruchengode and transported to the botanical garden located in the K. S. Rangasamy College of Arts and Science (A) campus. The samples (leaf and stem explants) from young plants of A. javanica were taken for performing transformation experiments with different strains of Agrobacterium rhizogenes. For culture establishment, the collected explants were surface-sterilized following Zong (2001) and dried up with aseptic filter paper.
Inoculation of A. rhizogenes
Two strains of A. rhizogenes (MTCC 532 and 2364) were procured from IMTECH (Institute of Microbial Technology), Chandigarh, India. The culture was stored in a freshly prepared glycerol stock for further applications. A. rhizogenes from a glycerol stock were subcultured on tryptone agar and incubated overnight at 37 °C. For experiments, the strain was subcultured from overnight incubated plates into 100 ml freshly prepared sterile tryptone broth at 37 °C, with a constant shaking in the orbital shaker (100 rpm). After 16–20 h incubation, the optical density (OD) was determined at 600, and it was observed to be greater than 1. These inoculums were then used for the rest of the study.
Effect of infection and co-cultivation periods on hairy root induction
For the transformation of A. rhizogenes, different types of explants such as leaf, node, and callus were used. The surface-sterilized explants were randomly pierced and clipped. Then, segments of approximately 5 × 5 mm were shifted to an overnight culture of A. rhizogenes MTCC 532 and MTCC 2364. They were kept in the incubator shaker at 25 °C under continuous shaking for different time durations (approximately 10–30 min). After that, any excess liquid was blotted from the explants using Whatman No.1 filter paper. The explants were transferred to petri plates containing hormone-free ½ strength semisolid MS medium, which was incorporated with sodium salts of cefotaxime (250 mg/L). The explants were gently placed on the surface of the medium. Later, they were incubated in the growth chamber maintaining favorable dark conditions at 25 ± 2 °C. Cultures were examined periodically until the emergence of hairy roots. After the establishment of hairy roots, the cultures were subcultured in a 250 ml conical flask containing 100 ml hormone-free ½ strength MS medium with an antibiotic (cefotaxime) every 3 weeks to maintain the freshness of the culture. Similarly, control groups were maintained, and triplicates of biological experiments were performed.
GUS assay and PCR analysis
The GUS assay (histochemical analysis) for T-DNA integration was adapted from the report of Jefferson (1987) with required modifications. First, a solution containing 50 mM phosphate buffer (pH 7.0), 10 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ferricyanide, 1 mM potassium ferrocyanide, 0.05% Triton, and 1 mM X-Gluc was prepared and the hairy roots from MTCC 532 and MTCC 2364 were placed in it at the room temperature for 16 h.
Hairy roots developed by the effect of MTCC 532 and MTCC 2364 were analyzed for rolB, rolC, and virD gene integration by polymerase chain reaction (PCR). Genomic DNA of MTCC 532 and 2364 was transformed to hairy roots of A. javanica, and transformed and non-transformed roots were isolated by using DNeasy® Plant Mini Kit (Qiagen, Germany).
The amplification of rol genes was done by using the following primers of rolB, rolC, and virD genes (forward primers: 5′-GGATCCCAAATTGCTATTCCCC-3′ and reverse primers: 5′-AGGCTTCTTTCATTCGGTTTAC-3′; forward primers: 5′-TGGCTGAAGACGACCTGTGT-3′ and reverse primer: 5′-TAGCCGATTGCAAACTGCA-3′; forward primers: 5′-TGTCGCAAGGCAGTAAG-3′ and reverse primer: 5′-CAAGGAGTCTTTCAGCATG-3′). The PCR reaction mixture constituents were prepared as 2.5 μl of 10 × Taq DNA polymerase buffer in 25 μl reaction automated at 94 °C for 5 min, followed by 30 cycles at 94 °C for 45 s, 57 °C for 30 s, 72 °C for 45 s, and by a final extension at 72 °C for 10 min. The polymerized products were analyzed using 1.0% (w/v) agarose gel electrophoresis and visualized by ethidium bromide staining.
Extraction of aervine and methylaervine content by HP-LC
The method for identification and quantification of bioactive compounds was adopted from the protocol followed by Sivanandhan et al. (2012). The separation was performed on a C18 column (5 mm, 250 × 4.6 mm i.d.) with the column temperature set at 35 °C. Linear gradient elution was used with acetonitrile (A) and aqueous (B), with the procedure flow rate of 0.5 mL/min (gradient change from A to B as followed (v/v): 5%–25% (0–5 min), 25%–55% (5–10 min), and 55%–100% (10–15 min)). The UV detector was used at 254 nm. The quantitative determination of aervine and methylaervine in the hairy root extracts was performed using a calibration graph obtained by plotting the HPLC peak area of the aervine and methylaervine against the concentration of the standard solutions. Standards for aervine and methylaervine were purchased from Chem Faces Wuhan, China (purity ≥ 98%, HPLC) and used for the confirmation of accumulated content.
Statistical analysis
The mean and standard deviation values were calculated by performing all the experiments in triplicates. A statistical analysis system software (SPSS) version 22.0 (Chicago, USA) was used to perform the analysis of variance (ANOVA). Moreover, the differences were considered statistically significant at P < 0.05 by Duncan’s multiple range tests.
Result and discussion
Callus induction
The optimized solid MS medium condition developed yellow-green and pinkish-colored soft textured callus at 2.0 mg/L of 2,4-D, and 0.5 mg/L NAA with the maximum rate of initiation (%). Earlier, a detailed description of callus initiation was reported in A. lanata (Boobalan and Kamalanathan 2019). The initial phase of the study dealt with the growth of friable callus, subsequently transferred to liquid MS medium supplemented with a combination of 2.0 mg/L of 2,4-D and 0.5 mg/L of NAA. Other combinations, including those involving a single hormone, failed to produce a good amount of biomass (Table 1; Fig. 1a). This outcome aligns with the findings of a previously reported study of Kamalanathan and Natarajan (2014), which emphasized the strong correlation observed in the production of pale yellow-green callus mass in A. lanata when treated with a combination of 2, 4-D, and NAA. Similarly, callogenesis was observed with A. javanica in experiments conducted by Chabane and Mouhoub (2017), with 2,4-D in a modified MS medium producing granular yellowish callus.
Effect of biotic and abiotic elicitor on callus culture in A. javanica after 18 days of culture (bar = 1 cm): A control (zero elicitor concentration), B callus response to 50 mg/L of chitosan (CHT); C 25 mg/L of sodium di-hydrogen orthophosphate (NaH2Po4) treated callus, D callus response to 50 mg/L of methyl jasmonate (MeJa), E 50 mg/L of salicylic acid (SA) treated callus, F callus response to 50 mg/L of disodium carbonate (Na2Co3)
Cell suspension culture
In continuation with the A. javanica callus obtained from suspension culture (non-elicitor) and then transferred to elicitor medium, the elicitor treatment process spanned approximately 20 days. Cell growth (FW and DW) was calculated accordingly with attention to the type and concentration of the elicitor treatment. The protocol of the elicitor treatment closely adhered to the guidelines stated in Cai et al. (2017). The process of elicitation was much effective in the exponential phase (after 15 days), exerting a significant influence on biomass accumulation at much higher rates. On the other hand, there was minimal or decrease in growth during the lag phase (1–10 days) (Park et al. 2016). The result of the study depicts the effect of elicitor concentration on influencing the growth of biomass and accumulation of AE and MAE contents.
Effect of elicitor and biomass production in cell suspension culture of A. javanica
This study highlights the remarkable increase in the biomass concentration achieved within a shorter period using elicitors in a liquid MS medium containing CHT, NaH2PO4, MeJa, SA, and Na2CO3. This indirect organogenesis method depicted better growth of proliferated viable cells with all elicitors were used at a concentration of 50 mg/L. In comparison to the biomass concentration of control (1.51 ± 0.07 FW g/flask and 0.21 ± 0.01 DW g/flask), the best response was observed in the medium with NaH2PO4 (6.34 ± 0.22 FW g/flask and 2.37 ± 0.13 DW g/flask), followed by Na2CO3 (4.56 ± 0.22 FW g/flask and 2.40 ± 0.15 DW g/flask). A moderate response was observed with MeJa (3.05 ± 0.06 FW g/flask and 1.88 ± 0.10 DW g/flask) whereas the least considerable growth of callus profusion was recorded on medium containing CHT (2.48 ± 0.23 FW g/flask and 1.86 ± 0.11 DW g/flask). SA, when associated with suspension medium, resulted in the lowest concentration of biomass (2.12 ± 0.02 FW g/flask and 1.55 ± 0.05, 1.86 ± 0.11 DW g/flask) among all five elicitors used in this study (Table 2).
Meanwhile, Madanakumar and Kumaraswamy (2018) reported a lower response to MeJa in Begonia malabarica and Begonia rex-cultorum whereas a favorable response was obtained in the present study. Notably, the elicitation process with a 100 mM CHT and SA, induced by fructose, resulted in a good amount of callus in Rumex vesicarius, showing an increase in the production of flavonoids as dry weight increases. Hence, the efficacy of CHT and SA in enhancing the secondary metabolites production is validated (Gadzovska et al. 2013). Thus, the study showed maximum growth of callus could be achieved with A. javanica species when subjected to suspension culture using the elicitation process. Also, NaH2PO4 proved to be the best suitable elicitor for profuse growth of callus at a significantly higher concentration compared to the other four elicitors used. This study scrutinizes the elicitation protocol for A. javanica that supports future research investigations.
Effect of biotic and different abiotic elicitor concentrations on the production of AE and MAE
Chitosan (CHT)
In this study, it was observed that among different concentrations of CHT treatment, 50 mg/L produced higher biomass (1.86 ± 0.11 FW g/flask and 2.48 ± 0.23 DW g/flask) than the control (1.51 ± 0.07 FW g/flask and 0.21 ± 0.01 DW g/flask) on the 20th day. Meanwhile, the results of CHT elicitation revealed a 5.1-fold increase in AE (16.51 ± 0.09 mg g−1 DW) and a 3.1-fold increase in MAE contents compared to that of control cultures (AE: 2.67 ± 0.42 mg g−1 DW; MAE: 9.42 ± 0.46 mg g−1 DW) (Table 2; Fig. 1b and Fig. S2d). In this study, AE and ME contents significantly increased at moderate concentration and decreased at higher concentration after 20 days of the exposure period. Similarly, Jaisi and Panichayupakaranant (2017); Osman et al. (2018) reported that 150 mg/L CHT resulted in a 1.4-fold increase over gallic acid content in Barringtonia racemosa and a 6.6-fold increase in plumbagin.
The literature study reveals that use of CHT elicitor enhanced secondary metabolites mainly terpenoids in the Ocimum basilicum plant (Kim et al. 2005), flavonoids in Isatis tinctoria (Jiao et al. 2018), phenolic compounds in Vitis vinifera (Cai et al. 2012) as well as Hypericum perforatum (Brasili et al. 2016). CHT elicitation significantly influenced the production of AE content than MAE content. This shows that CHT can be highly effective in enhancing the production of AE accumulation. Several responses observed with CHT elicitation may be due to various factors such as callose opposition, cell death near the site of infection (hypersensitive response, HR), oxidative burst, activation of MAP-kinases, elevation of cytosolic H+ and Ca2+, synthesis of jasmonate and abscisic acid, and induction of proteins related to pathogenesis.
However, the effect of CHT elicitation on biological activities entirely depends on the physicochemical properties (molecular weight, deacetylation degree, and viscosity). These properties collectively establish a threshold condition that spontaneously induces programmed cell death, leading to necrotic cell death (cytotoxicity). This phenomenon has been reviewed by Iriti and Faoro (2009). To be best of our knowledge, so far no work has been reported in the elicitation of A. javanica through callus-derived methods, especially enhancement of AE and MAE contents.
Sodium dihydrogen orthophosphate (NaH2PO4)
This investigation into abiotic elicitation has revealed that the accumulation of AE and MAE contents experienced enhancement at a lower concentration of 25 mg/L NaH2PO4 (7.18 ± 0.05 and 54.13 ± 0.22 mg g−1 DW) whereas declined level was observed with higher concentrations after 20 days of the exposure period. In the case of MAE, the enhancement was a 4.7-fold whereas AE increased only 1.6-fold when compared to control cultures (2.67 ± 0.42 and 9.42 ± 0.46 mg g−1 DW) (Table 2; Fig. 1c and Fig. S2b).
In evidence to this result, the production of 9-hydroxycanthin-6-one was made possible from cultures of Euryco malongifolia at 20 mg/L NaH2PO4 (0.75%) whereas 9-methoxycanthin-6-one was induced at 10 mg/L NaH2PO4 (0. 41%) after 13 days of elicitation period (Keng et al. 2010). Similarly, Toivonen et al. (1991) observed an augmented cell biomass and emphasized the role of NaH2PO4 and production of alkaloid from suspension culture of Catharanthus roseus. Very little literature is available on the elicitation of NaH2PO4, highlighting the need for further works to enhance alkaloid content and several other secondary metabolites, as evident by a previous as well as the present report.
Methyl jasmonate (MeJa)
On the basis of literature, MeJa has been identified to confer resistance against diseases in various fruits (Wang et al. 2015). The use of this abiotic elicitor results in increase in the production of MAE content AE content. MAE accumulation was quantified to be 51.72 ± 0.17 mg g−1 DW showing an increase of 4.49-fold compared to control cultures. In contrast, AE showed only a 0.48-fold increase with an accumulation of 3.15 ± 0.04 mg g−1 DW (Table 2; Fig. 1d and Fig. S2c). Furthermore, MeJa induces an increase in the production of anthocyanin and lignin accumulation couple with enhanced antioxidant activity (Saavedra et al. 2016).
Meanwhile, Saniewski et al. (2006) successfully produced anthocyanins from cultures of Crassula multicava using the MeJa elicitor. Similar studies on MeJa have been reported by many researchers, showing its efficacy in enhancing the accumulation of podophyllotoxin (PTOX) and 6-methoxypodophyllotoxin (6MPTOX) at 100 µM in the Linum album suspension line X4SF (Van Furden et al. 2005). The results surpassed the earlier works in this area, particularly in terms of camptothecin (CPT) production in cultures of Ophiorriza mungos using MeJa and jasmonic acid (Jisha 2006).
Salicylic acid (SA)
Salicylic acid is essential in the expression of genes associated with resistance and plays an indispensable role in the plant defense response. This statement is also supported by the present study, where SA showed a preference for the production of AE (34.10 ± 0.84 mg g−1 DW) at 50 mg/L. In contrast, content was the lowest, with a 0.02-fold increase compared to the control, totaling 9.69 ± 0.04 mg g−1 DW. Notably, AE at 50 mg/L showed an 11.7-fold increase over the control, which happened to be the most significant result among all different concentrations of elicitors tested (Table 2; Fig. 1e and Fig. S2e).
Subsequently, the highest levels of secondary metabolites were derived from cell suspension culture, and this approach can be elicited in diverse medicinal plants such as Withania somnifera (Sivanandhan et al. 2012), Polygonam minus (Shukor et al. 2013), and Gymnema sylvestre (Bhuvaneswari et al. 2015). Conversely, the mechanism that supports enhanced production is not elucidated in the case of colchicine production in cultures of Grivella superb (Ghosh et al. 2006).
Sodium carbonate (Na2CO3)
The increase in MAE content was observed with the addition of Na2CO3, showing a 6.6-fold (72.26 ± 0.30 mg g−1 DW) increase compared to the control culture. On the other hand, AE content showed a 1.8-fold (7.48 ± 0.39 mg g−1 DW) increase than control. A much higher accumulation of MAE content was observed across all the concentrations tested among various elicitors (Table 2; Fig. 1f and Fig. S2a). This study provides clear evidence that the production of MAE content could be highly influenced by Na2CO3 acting as a potential abiotic elicitor. This result is in correlation with the reports suggesting approximately 6 mg/L Na2CO3 in callus culture of E. malongifolia produced a supplementary effect on cell biomass (0.57 g FW and 0.05 g DW). Moreover, the enriched production of alkaloids [9-methoxycanthin-6-one (0.27 ± 0. 06%) and 9-hydroxycanthin-6-one (0.32 ± 0.01%)] was observed in the elicited medium compared to the non-elicitor medium (Keng et al. 2010).
The findings of this study are novel, as no prior reports have detailed increased cell growth along with the increased production of AE and MAE with biotic and abiotic elicitors. This finding is the most noteworthy and perhaps the most significant, describing a higher accumulation of AE content in SA elicitation and elevated MAE content in Na2CO3-induced conditions. Elicitation can be defined in many different ways, with distinction based on the concentration of elicitor that affects the intensity of response across different plant species (Vasconsuelo and Boland 2007).
The chemical elicitor CHT is conducive to the cell growth and production of AE compared to MAE content. MeJa potentially increased production in MAE whereas, although a higher concentration failed to increase the AE content. Notably, NaH2PO4 is presumed to have a negligible effect on AE content, yet, a larger amount was observed in MAE content. These promising results stand out as the main achievement of this work. Extensive data from various plant species show that biotic and abiotic elicitor treatments can increase the production of secondary metabolites content in in vitro culture systems (Vergara Martinez et al. 2017; Ramezannezhad et al. 2019; Rajan et al. 2020). To the best of our knowledge, the potential of the method used in this study has not yet been identified in the literature concerning the effects of CHT, NaH2PO4, MeJa, SA, and Na2CO3 treatments on aervine and methylaervine production in A. javanica cell suspension cultures.
Effect of infection and co-cultivation periods on hairy roots induction A. javanica
Hairy roots were induced from all explants (leaf, node, and callus) after the infection with selected strains of Agrobacterium species (MTCC 532 and MTCC 2364). When the infected explants were co-cultivated for 48 h, it was observed that the increase in the infection period (0 min to 5 h) increases the transformation frequency. It was established that both infection and co-cultivation periods affect the transformation frequency (Table 3). Also, the establishment of proper infection is highly linked to a successive transfer of Ri T-DNA to the host cells (Mariashibu et al. 2013).
Continuing the extension of the prolonged infection period resulted in no transformation of both the strains. The duration of incubation affects the transformation frequency of explants, with a 30-min incubation showing a better frequency of transformation compared to a 20-min incubation. A shorter infection period exhibited a lower frequency of transformation (hairy root induction) whereas a longer infection period was not effective due to hypertonic conditions or hyperactivation of defense mechanism, potentially causing necrosis of the host cells (Mannan et al. 2009). The initiation of protruding hairy roots at wounded sites was spotted within 2–3 weeks after bacterial infection and incubation under dark conditions. A. rhizogenes–mediated transformation was more effective, with the induced roots showing a dynamic growth rate and lively elongation, resulting in the production of numerous branches with a whitish color, varying in thickness. The inoculated leaflets led to the development of numerous adventitious roots and showed the potentiality of hairy root induction (30.33 ± 0.57). The maximum frequency of hairy root induction per single explant was found to be 21.00 ± 1.00 with a 30-min infection with bacterial strain 532 (Fig. 2a–e). Similarly, the frequency of maximum hairy root induction per single explants with strain 2364 was 17.66 ± 0.57 after 17–20 days of incubation, and the infection period of the callus explants was 1 h (Fig. 2f–i; Table 3).
Hairy root culture-Aerva javanica. A Inoculation of sterile leaf, B and C initiation of hairy roots from leaf explants, D hairy root regeneration from nodal explants, E and H development of hairy root lines from callus, F and G formation of transformed roots from leaf explants and, I suspension culture of hairy roots
The net result of comparison showed that the treatment with strain 532 demonstrated the maximum hairy root induction frequency per single explant (22.66 ± 0.57). The uninfected leaves inoculated on ½ MS medium containing cefotaxime sodium salt showed no result even after a 50-day incubation period. Similar results were reported in Cosmos bipinnatus hairy root culture, where 84% rooting efficiency was achieved using half-strength MS media as a co-cultivation medium. On the other hand, the full-strength MS medium failed to reach the maximum transformation efficiency (Jaberi et al. 2018). Recent studies on transformation corroborate with the present study, showing that a decrease in the mineral components significantly increases the efficiency of transformation for root regeneration (Sharafi et al. 2013).
In line with the findings, explants infected with strains MTCC 532 and MTCC 2364 showed earliest root induction after a 30-min infection period, whereas explants infected with 0 min, 10 min, and 5 h of infection period showed no root induction.
Effect of hairy root induction percentage on different explants
The use of different explants showed that leaflets infected with A. rhizogenes strain MTCC 532 for 30 min significantly impacted the percentage response of hairy root induction (72.04 ± 0.92) (Fig. 2). This observation aligns with the earlier reports showing that A. rhizogenes strain can influence frequency of hairy root induction in numerous plant species, as reported earlier (Sujatha et al. 2013). A. rhizogenes strain R1000 has been shown to play a significant role in many plants such as Artemisia vulgaris (Sujatha et al. 2013), Torenia foarnieri (Tao and Li 2006), and Gentiana macrophylla (Tiwari et al. 2007). Leaf explants of Picrorhiza kurroa displayed a higher transformation frequency than the other explants (Verma et al. 2015). Conversely, callus grown in vitro showed only 64.55 ± 0.70 percentage of hairy root response induction. The overall conclusion was that the leaflets and in vitro grown callus segments showed maximum transformation frequencies of 67.29 ± 0.77 and 57.59 ± 0.44, after 48-h of co-cultivation period.
In this study, it was observed that when the explants were infected with the above-mentioned bacterial strains, considering infection period and rooting percentage, the leaf was found to be the best explant compared to callus and nodal segments (Table 3) for inducing hairy roots on semi-solid MS medium. Specifically, the strain MTCC 532 showed an improved and rapid rooting response in comparison to MTCC 2364. Hence, all the hairy roots lines were maintained on ½ MS liquid medium with subcultures carried out at 4-week intervals.
Molecular characterization of transformed hairy roots by GUS assay and PCR analysis
Hairy roots induced from callus, node, and leaf explants under the influence of A. rhizogenes strains (MTCC 532 and 2364) were confirmed through histochemical assay to confirm the state of T-DNA transformation. GUS-transformed roots formed a blue coloration whereas non-transformed roots did not show any blue coloration on staining. Blue coloration after the staining indicated that the GUS gene was transferred successfully to the genome of A. javanica hairy roots (Fig. 3I). Similar reports were documented by many researchers, which is in line with the current study for GUS gene transformation and hairy root induction in species such as Lotus japonicas L. (Xu et al. 2018), Brassica oleracea L. (Kowalczyk et al. 2018), Silene latifolia Poir (Hudzieczek et al. 2019), and Coffea arabica L (Alpizar et al. 2006).
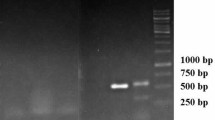
I GUS staining of transformed roots. A Non transformed roots (auxin induced), B transformed roots (before GUS stained) and C transformed roots (after GUS stained). II PCR amplification of the rol B, rol C and virD genes from transformed and non-transformed root lines of A. javanica (a–c). Lane 1: DNA ladder 1000 bp. Lane 2: Negative control (non-transformed or in vitro roots). Lane 3 and 4: Positive control (Template DNA of Agrobacterium Rhizogenes plasmid 532 and 2364). Lane 5 and 6: Hairy root lines developed from Agrobacterium Rhizogenes plasmid 532 and 2364
The molecular confirmation of A. rhizogenes (MTCC 532 and MTCC 2364)-influenced hairy root was achieved by performing PCR amplification with the help of specific forward and reverse primers of rolB, rolC, and virD genes. In this analysis, DNA of the non-transformed roots served as the negative control whereas plasmid DNA from A. rhizogenes (MTCC 532 and MTCC 2364) strain was considered as the positive control. Successive amplification of rol (rolB and rolC) and virD genes was achieved in root lines, resulting in the band at 780 bp (Fig. 3IIa; lanes 3, 4, 5, 6) and 490 bp (Fig. 3IIb; lanes 3, 4, 5, 6) base pairs, respectively. No bands were observed in the negative control (Fig. 3IIa–c; lane 2). Furthermore, Fig. 3IIc, lanes 4–6 shows no band formation, indicating the absence of virD gene expression in transformed roots and confirming the rate of transformation by A. rhizogenes strain MTCC 532.
Likewise, Ri (root inducing) plasmid transformation in hairy roots was confirmed by amplification of the rol genes by PCR in Alpinia galangal (Rao et al. 2012) and P. kurroa (Verma et al. 2015). A. rhizogenes is known to transfer two independent T-DNAs to the plant genome, i.e., TL-DNA and TRDNA (Nilsson and Olsson, 1997). TL-DNA is indispensable to induce hairy roots (Sujatha et al. 2013). Hence, the study confirms A. rhizogenes–mediated transformation of A. javanica by PCR amplification of the rolB, rolC, and virD genes, which was noticed in hairy roots but absent in normal roots.
Efficiency of improved aervine and methylaervine accumulation on the hairy root culture of A. javanica using various Agrobacterium strains by HPLC
The in vivo grown leaf, when infected with strain 532 for 30 min and incubated in half-strength MS liquid medium, showed maximum accumulation of aervine (42.22 ± 1.04 mg g−1 DW) and methylaervine (8.30 ± 0.09 mg g−1 DW). Agrobacterium strain 2364, with a 30-min infection period in in vivo leaf, generated a hairy root line with significantly higher content of aervine (38.80 ± 0.32 mg g−1 DW) and methylaervine (6.42 ± 0.39 mg g−1 DW) compared to other explants used. On the other hand, the hairy root from callus explants showed 31.20 ± 0.19 mg g−1 DW aervine and 5.34 ± 0.39 mg g−1 DW of methylaervine accumulation influenced by strain 532 for 1 h (Fig. S3a, b, c). The peak length and retention time of commercial standards for aervine and methylaervine are displayed in Fig. S1.
Conversely, infection periods of 0, 10 min, and 5 h did not show any biomass production in all the tested explants. The hairy root from the nodal segment exhibited the least accumulation of aervine and methylaervine (5.22 ± 0.22 and 0.92 ± 0.04 mg g−1 DW) respectively, after 4 h infection period (strain 2364). The in vivo leaf showed effective production of aervine and methylaervine content among all the explants and strains tested. This study proved that type of explants and infection period also strongly affect the aervine and methylaervine contents in hairy roots of A. javanica (Table 4). For instance, internodes of V. vinifera showed a better hairy root regeneration than leaf and nodal explants in terms of the number of roots regenerated, regeneration rate, and time are taken for first hairy root development (Hosseini et al. 2017). The results of Zhaogui et al. (2020) showed that the induction of hairy root rate had a unimodal pattern with relation to the infection time, i.e., increased infection period beyond the optimal time duration may lead to explant contamination, whereas reduced infection time may result in an incomplete transfer of Ri plasmid genes to the host genome. Agrobacterium strain 532 showed improved and rapid production of aervine and methylaervine in comparison with strain 2364. It has been reported that the virulence nature of the A. rhizogenes strains directly affects the frequency of Ri plasmid transformation and the rate of hairy root induction (Thilip et al. 2015). These results suggested that a 30-min infection period and a 48 h co-cultivation period of leaf explants were optimal in stimulating effective production of target alkaloids.
Several reports have highlighted the enhanced production of alkaloids through hairy root cultures, particularly for the extraction of catharanthine, vindoline, ajmalicine, and serpentine in C. roseus (Ruiz-May et al. 2009; Almagro et al. 2015). Hairy root cultures showed significant production of bioactive compounds such as camptothecin, verbascoside, scopolamine, and artemisinin (Georgiev et al. 2012). In some cases, accumulation of novel metabolites was not even detected in the wild plants (Pollier et al. 2011). The fast growth rate and biosynthetic stability of hairy roots were considered to be a promising platform for stable production of bioactive components with several pharmaceutical impacts (Ricigliano et al. 2015). Kim et al. (2015) observed rapidly grown hairy root lines from Achyranthes multifora exhibiting enhanced production of the alkaloid (20-hydroxyecdysone).
The primary conclusion of this work highlights the increase in alkaloids (aervine and methylaervine) content through the elicitation process in callus-derived suspension culture and hairy root culture techniques. The biosynthesis of aervine (AE) and methylaervine (MAE) contents was examined in response to five distinct elicitors. The outcome demonstrated the greatest AE and MAE content accumulation in cell suspension culture. It was also discovered that the aervine content and methylaervine in sodium carbonate (Na2CO3) showed an 8.66-fold rise, while the salicylic acid showed an 8.66-fold increase. It has been noted that utilizing A. rhizogenes to produce hairy roots enhances the accumulation of aervine and methylaervine. Through elicitor stimulation, this study will provide an alternative methodology to enhance alkaloid quantity and quality. The optimized protocol established in this study served as a basis for the production of aervine and methylaervine contents on a large-scale using bioreactors in the near future. The use of this procedure can be utilized in industrial scale by approaching the industry for large scale production and commercialization. This is the first well-controlled study providing information on the influence of elicitors on the enhanced production of two pharmaceutically important commercial compounds in A.javanica. The findings underscore the necessity for further research, including preclinical and clinical studies, to explore the enhanced alkaloids’ potential contribution to the development of generic cancer drugs in the pharmaceutical sector.
Abbreviations
- AE:
-
Aervine
- MAE:
-
Methylaervine
- MS:
-
Murashige and Skoog
- 2,4-D:
-
2,4-Dichlorophenoxy acetic acid
- NAA:
-
α-Naphthalene acetic acid
- DW:
-
Dry weight
- SA:
-
Salicylic acid
- MeJa:
-
Methyl jasmonate
- CHT:
-
Chitosan
- GUS:
-
β-Glucuronidase
- PCR:
-
Polymerase chain reaction
- MTCC:
-
Microbial Type Culture Collection and Gene Bank
- EDTA:
-
Ethylenediaminetetraacetic acid
References
Almagro L, Fernandez-Perez F, APedreno MA (2015) Indole alkaloids from Catharanthus roseus: bio production and their effect on human health. Molecules 20(2):2973–3000
Alpizar E, Dechamp E, Espeout S, Royer M, Lecouls AC, Nicole M, Bertrand B, Lashermes P, Etienne H (2006) Efficient production of Agrobacterium Rhizogenes transformed roots and composite plants for studying gene expression in coffee roots. Plant Cell Rep 25(9):959–967
Bharitkar YP, Hazra A, Poduri NSA, Ash A, Maulik PR, Mondal NB (2015) Isolation, structural elucidation and cytotoxicity evaluation of a new pentahydroxy pimarane diterpenoid along with other chemical constituents from Aerva lanata. Nat Prod Res 29(3):253–261
Bhuvaneswari CH, Rao K, Gandi S, Giri A (2015) Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules-methyl jasmonate and salicylic acid. In Vitro Cell Dev Biol-Plant 51:88–92
Boobalan S, Kamalanathan D (2019) Spermidine influences enhanced micropropagation and antibacterial activity in Aerva javanica (Burm. F.) Shult. Ind Crops Prod 137:187–196
Boobalan S, Kamalanathan D (2020) Tailoring enhanced production of aervine in Aerva lanata (L.) Juss. Ex Schult by Agrobacterium rhizogenes-mediated hairy root cultures. Ind Crops Prod 155:112814
Brasili E, Pratico G, Marini F, Valletta A, Capuani G, Sciubba F, Miccheli A, Pasqua G (2014) A non-targeted metabolomics approach to evaluate the effects of biomass growth and chitosan elicitation on primary and secondary metabolism of Hypericum perforatum in vitro roots. Metabolomics 10:1186–1196
Buitelaar RM, Tramper J (1992) Strategies to improve the production of secondary metabolites with plant cell cultures: a literature review. J Biotechnol 23:111–141
Cai J, Ma Y, Hu P, Zhang Y, Chen J, Li X (2017) Elicitation of furanocoumarins in Changium smyrnioides suspension cells. Plant Cell Tiss Organ Cult 130:1–12
Chabanea D, Mouhoub F (2017) Establishment of in vitro plants from female flowers of Aerva javanica (Burm. F) Juss ex Schult. Acta Hortic 1155:607–612
Choi SM, Son SH, Yun SR, Kwon OW, Seon JH, Paek KY (2000) Pilot-scale culture of adventitious roots of ginseng in a bioreactor system. Plant Cell Tiss Organ Cult 62:187–193
Dai JK, Dan WJ, Li N, Du HT, Zhang JW, Wang JR (2016) Synthesis, in vitro antibacterial activities of a series of 3-N-substituted canthin-6-ones. Bioorg Med Chem Lett 26(2):580–583
Dai JK, Dan WJ, Zhang YY, He MF, Wang JR (2018) Design and synthesis of C10 modified and ring-truncated canthin-6-one analogues as effective membrane-active antibacterial agents. Bioorg Med Chem Lett 28(18):3123–3128
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagege D, Courtois D, Joseph C (2013) The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tiss Organ Cult 113:25–39
Georgiev MI, Agostini J, Müller JL, Xu J (2012) Genetically transformed roots: from plant disease to biotechnology. Trends Biotechnol 30(10):528–537
Ghosh S, Ghosh B, Jha S (2006) Aluminium chloride enhances colchicine production in root cultures of Gloriosa superba. Biotechnol Lett 28:497–503
Hosseini SM, Bahramnejad B, Douleti Baneh H, Emamifar A, Goodwin PH (2017) Hairy root culture optimization and resveratrol production from Vitis vinifera sub sp. Sylvesteris. World J Microbiol Biotechnol 33(4):67
Hudzieczek V, Cegan R, Cermak T, Bacovska N, Machalkova Z, Dolezal K, Plihalova L, Voytas D, Hobza R, Vyskot B (2019) Agrobacterium rhizogenes mediated transformation of a dioecious plant model Silene latifolia. N Biotechnol 48:20–28
Iriti M, Faoro F (2009) Chitosan as a MAMP, searching for a PRR. Plant Signal Behav 4:66–68
Jaberi M, Sharafi A, Sharafi AA, Azadi P, Kheiri-Manjili H, Danafar H, Ahmadnia A (2018) Genetically transformed root-based culture technology in medicinal plant Cosmos bipinnatus. Jundishapur J Nat Pharm Prod 13(1):e67182
Jiao J, Gai QY, Wang W, Zang YP, Niu LL, Fu YJ, Wang X (2018) Remarkable enhancement of flavonoid production in a co-cultivation system of Isatis tinctoria L. hairy root cultures and immobilized Aspergillus niger. Ind Crops Prod 112:252–261
Jisha KG (2006) A study on the production of camptothecin from Ophiorrhiza mungos and Nothapodytes foetida using cell and tissue culture. In: Dissertation, Mahatma Gandhi University
Kamalanathan D, Natarajan D (2014) Antiproliferative and antioxidant potential of leaf and leaf derived callus extracts of Aerva lanata (L.) Juss. Ex Schult. against human breast cancer (MCF-7) cell lines. Nat Prod J 4:271–279
Keng CL, Wei AS, Bhatt A (2010) Elicitation effect on cell biomass and production of alkaloids in cell suspension culture of the tropical tree Euryco malongifolia. Res J Costa Rican Dist Edu Univ 2:239–244
Khojasteh A, Mirjalili MH, Palazon J, Eibl R, Cusido RM (2016) Methy-jasmonate enhanced production of rosmarinic acid in cell cultures of Satureja khuzistanica in a bioreactor. Eng Life Sci 16:740–749
Kim HJ, Chen F, Wang X, Rajapakse NC (2005) Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J Agric Food Chem 53:3696–3701
Kim OT, Manickavasagm M, Kim YJ, Jin MR, Kim KS, Seong NS, Hwang B (2015) Genetic Transformation of Ajuga multiflora Bunge with Agrobacterium rhizogenes and 20-Hydroxyecdysone production in hairy roots. J Plant Biol 48:258–262
Kowalczyk T, Gerszberg A, Duranska P, Biłas R, Hnatuszko-Konka K (2018) High efficiency transformation of Brassica oleracea var Botrytis plants by Rhizobium rhizogenes. AMB Expr 8(1):125
Li N, Liu D, Dai JK, Wang JY, Wang JR (2019) Synthesis and in vitro antibacterial activity of quaternized 10-methoxycanthin-6-one derivatives. Molecules 24(8):1553
Madanakumar AJ, Kumaraswamy M (2018) Purified Anthocyanin, its elicitation from cell cultures of Begonia malabarica and Begonia rex-cultorum baby rainbow and it’s In vitro cytotoxicity analysis by MTT Assay. Pharmacogn J 10:553–558
Mannan A, Syed TN, Mirza B (2009) Factors affecting Agrobacterium tumefaciens mediated transformation of Artemisia absinthium Pak. J Bot 41(6):3239–3246
Mariashibu TS, Subramanyam K, Arun M, Mayavan S, Rajesh M, Theboral J, Manickavasagam M, Ganapathi A (2013) Vacuum infiltration enhances the Agrobacterium-mediated genetic transformation in Indian soybean cultivars. Acta Physiol Plant 35:41–54
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nazir M, Tungmunnithum D, Bose S, Drouet S, Garros L, Giglioli-Guivarch N, Abbasi BH, Hano C (2019) Differential production of phenylpropanoid metabolitesin callus cultures of Ocimumbasilicum L. with distinct in vitro antioxidant activities and in vivo protective effects against UV stress. J Agric Food Chem 67(7):1847–1859
Osman NI, Sidik NJ, Awal A (2018) Efficient enhancement of gallic acid accumulation in cell suspension cultures of Barringtonia racemosa L. by elicitation. Plant Cell Tiss Organ Cult 135:203–212
Paek KY, Murthy HN, Hahn EJ, Zhong JJ (2009) Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv Biochem Eng Biotechnol 113:151–176
Pandey H, Pandey P, Singh S, Gupta R, Banerjee S (2015) Production of anti-cancer triterpene (betulinic acid) from callus cultures of different Ocimum species and its elicitation. Protoplasma 252(2):647–655
Park WT, Arasu MV, Al-Dhabi NA, Yeo SK, Jeon J, Park JS, Lee SY, Park SU (2016) Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in Agasta cherugosa cell culture. Molecules 21:426
Patel H, Krishnamurthy R (2013) Elicitors in plant tissue culture. J Pharmacogn Phytochem 2:60–65
Pollier J, Morreel K, Geelen D, Goossens AJ (2011) Metabolite profiling of triterpene saponins in Medicago truncatula hairy roots by liquid chromatography Fourier transform ion cyclotron resonance mass spectrometry. Nat Prod 74(6):1462–1476
Putalun W, Udomsin O, Yusakul G, Juengwatanatrakul T, Sakamoto S, Tanaka H (2010) Enhanced plumbagin production from in vitro cultures of Drosera burmanii using elicitation. Biotechnol Lett 32:721–724
Pyo YH, Lee TC, Logendrac L, Rosen RT (2004) Antioxidant activity and phenolic compounds of Swiss Chard (Beta Vulgaris subspecies Cycla) Extracts. Food Chem 85:19
Qasim SM, Memon S, Bhanger MI, Khan KM (2013) Comparison of chemical composition of Aerva javanica seed essential oils obtained by different extraction methods. Pak J Pharm Sci 26:757–760
Rajan M, Feba KS, Chandran V, Shahena S, Mathew L (2020) Enhancement of rhamnetin production in Vernonia anthelmintica (L.) wild. Cell suspension cultures by eliciting with methyl jasmonate and salicylic acid. Physiol Mol Biol Plants 26:1531–1539
Ram M, Prasad KV, Singh SK, Hada BS, Kumar S (2013) Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrid L. Plant Cell Tiss Organ Cult 113:459–467
Ramezannezhad R, Aghdasi M, Fatemi M (2019) Enhanced production of cichoric acid in cell suspension culture of Echinacea purpurea by silver nanoparticle elicitation. Plant Cell Tiss Organ Cult 139:261–273
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusido RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21:182
Ricigliano V, Javed C, Jingjing T, Andrew A, Dianella GH (2015) Plant hairy root cultures as plasmodium modulators of the slime mold emergent computing substrate Physarumpolycephalum. Front Microbiol 6:1–10
Rao K, Chodisetti B, Mangamoori LN, Giri A (2012) Agrobacterium-mediated transformation in Alpinia galanga (Linn.) Willd. for enhanced acetoxychavicol acetate production. Appl Biochem Biotechnol 168(2):339–347
Ruiz-May E, Galaz-Avalos RM, Loyola-Vargas VM (2009) Differential secretion and accumulation of terpene indole alkaloids in hairy roots of Catharanthus roseus treated with methyl jasmonate. Mol Biotechnol 41(3):278–285
Saavedra GM, Figueroa NE, Poblete LA, Cherian S, Figueroa CR (2016) Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem 190:448–453
Saniewski A, Horbowicz M, Puchalski J (2006) Induction of anthocyanins accumulation by methyl jasmonate in shoots of Crassula multicava Lam. Acta Agrobotanica 59:43–50
Sharafi A, Sohi HH, Mousavi A, Azadi P, Razavi K, Ntui VO (2013) A reliable and efficient protocol for inducing hairy roots in Papaver bracteatum. Plant Cell Tiss Organ Cult 113:1–9
Shukor MFA, Ismail I, Zainal Z, Noor NM (2013) Development of a Polygonum minus cell suspension culture system and analysis of secondary metabolites enhanced by elicitation. Acta Physiol Plant 35:1675–1689
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Mariashibu TS, Manickavasagam M, Ganapathi A (2012) Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crops Prod 37:124–129
Siveen KS, Kuttan G (2012) Modulation of humoral immune responses and inhibition of proinflammatory cytokines and nitric oxide production by 10-methoxycanthin-6-one. Immunopharmacol Immunotoxicol 34(1):116–125
Sujatha G, Zdravkovic-Korac S, Calic D, Flamini G, Ranjitha Kumari BD (2013) High-efficiency Agrobacterium rhizogenes-mediated genetic transformation in Artemisia vulgaris: hairy root production and essential oil analysis. Ind Crops Product 44:643–652
Tao J, Li L (2006) Genetic transformation of Torenia fournieri L. mediated by Agrobacterium rhizogenes. South Afr J Bot 72(2):211–216
Thilip C, Soundar Raju C, Varutharaju K, Aslam A, Shajahan A (2015) Improved Agrobacterium rhizogenes-mediated hairy root culture system of Withania somnifera (L.) Dunal using sonication and heat treatment. 3 Biotech 5(6):949–956
Tiwari RK, Trivedi M, Guang ZC, Guo GQ, Zheng GC (2007) Genetic transformation of Gentiana macrophylla with Agrobacterium rhizogenes: growth and production of secoiridoid glucoside gentiopicroside in transformed hairy root cultures. Plant Cell Rep 26(2):199–210
Toivonen L, Ojala M, Kauppinen V (1991) Studies on the optimization of growth and indole alkaloids production by hairy roots cultures of Catharanthus roseus. Biotechnol Bioeng 37:673–680
Udomsuk L, Jarukamjorn K, Tanaka H, Putalun W (2011) Improved isoflavonoid production in Pueraria candollei hairy root cultures using elicitation. Biotechnol Lett 33:369–374
Vasconsuelo A, Boland R (2007) Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci 172:861–875
Vergara Martinez VM, Estrada Soto SE, Arellano Garcia JJ, Rivera Leyva JC, Castillo Espana P, Flores AF, Cardoso Taketa AT, Perea Arango I (2018) Methyl jasmonate and salicylic acid enhanced the production of ursolic and oleanolic acid in callus cultures of Lepechinia caulescens. Pharmacogn Mag 13:886–889
Van Furden B, Humburg A, Fuss E (2005) Influence of methyl jasmonate on podophyllotoxin and 6-methoxypodophyllotoxin accumulation in Linum album cell suspension cultures. Plant Cell Rep 24(5):312–317
Verma PC, Singh H, Negi AS, Saxena G, Rahman LU, Banerjee S (2015) Yield enhancement strategies for the production of picroliv from hairy root culture of Picrorhiza kurroa Royle ex Benth. Plant Signal Behav 10(5):1–11
Wang K, Liao Y, Kan J, Han L, Zheng Y (2015) Response of direct or priming defense against Botrytis cinerea to methyl jasmonate treatment at different concentrations in grape berries. Int J Food Microbiol 194:32–39
Xu Y, Liu F, Han G, Wang W, Zhu S, Li X (2018) Improvement of Lotus japonicus hairy root induction and development of a mycorrhizal symbiosis system. Appl Plant Sci 6:e1141
Zapesochnaya G, Kurkin V, Okhanov V, Miroshnikov A (1991) Canthin-6-one and β-carboline alkaloids from Aerva lanata. Planta Med 58:192–196
Zhang Y, Liu YB, Li Y, Ma SG, Li L, Qu J, Zhang D, Chen XG, Jiang JD, Yu SS (2013) Sesquiterpenes and alkaloids from the roots of Alangium chinense. J Nat Prod 76(6):1058–1063
Zhao F, Dai JK, Li D, Wang SJ, Wang JR (2016) Synthesis and evaluation of ester derivatives of 10-hydroxycanthin-6-one as potential antimicrobial agents. Molecules 21:390
Zhaogui YAN, Shengyu LIU, Junlian ZHANG, Guan HUANG, Lijun DUAN, Yaomei YE (2020) Optimizing hairy root production from explants of Phyllanthus hainanensis, a shrub used for traditional herbal medicine Front Agr. Sci Eng 7(4):513–522
Zhong JJ (2001) Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. In: Zhong JJ et al. (eds) Plant cells. Adv Bio Chem Eng Biot, vol 72. Springer, Heidelberg, pp 1–26
Acknowledgements
The corresponding author with gratitude acknowledges the Science and Engineering Research Board (SERB, New Delhi) EMEQ—Empowerment and Equity Opportunities for Excellence in Science Scheme, for their financial assistance for this research work (SERB letter No. EEQ/2016/000233, Dt: 14 March 2017). The authors extend the deepest gratitude to the Department of Biotechnology, K. S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode for providing the necessary facilities to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boobalan, S., Srinivasan, R., Keerthanaa, T. et al. Enhancing the aervine and methylaervine production in in vitro cultures of Aerva javanica (Burm. F.) Schult via elicitors and Agrobacterium rhizogenes-mediated hairy root cultures. J. Plant Biochem. Biotechnol. 33, 353–366 (2024). https://doi.org/10.1007/s13562-024-00897-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-024-00897-x