Abstract
Ascorbic acid (AA) is one of the most powerful natural antioxidant able to prevent enzymatic browning after exogenous treatment of minimally-processed products. The specific mechanism by which AA prevents enzymatic browning remains still debated and a direct effect of endogenous AA stimulation and browning has never been studied. The manipulation of AA pathway is a promising approach to study the biochemical mechanism by which AA acts as an anti-browning agent. In this work, cDNA of L-galactono-1,4-lactone dehydrogenase (L-GalLDH), one of the key gene of the Smirnoff–Wheeler’s branch of AA biosynthetic pathway, was isolated and overexpressed in lettuce (Lactuca sativa L. cv ‘Iceberg’), a species highly prone to browning. The hypothesis that the overexpression of L-GalLDH translates to AA accumulation and reduces the browning phenomena in lettuce leaves after cutting was tested. Our results indicate that transgenic lettuce plants, showing about 19-fold overexpression of L-GalLDH as compared to wild type, had about +30 % of AA concentration in mature leaves. Transgenic plants exhibited reduced browning over the leaves, even 10 day after cutting, as demonstrated by higher values of luminosity (L*) and higher values of greenness (a*) compared to control plants. Overall, these findings provide a first evidence of the role of endogenous AA as browning-preventing agent. The obtainment of T2 transgenic lettuce plants is a promising first step to further determine the specific mechanism by which AA acts as an anti-browning preservative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
L-Ascorbic acid (AA), commonly known as vitamin C, is essential for human health and its biosynthetic pathway has been elucidated in animal since 1950s (Ishikawa et al. 2006). Despite the key role that AA plays also in plants, especially under stress condition (Gallie 2013), it is only in the last 15 years that an understanding of its biosynthesis in plants has emerged (Smirnoff et al. 2001; Valpuesta and Botella 2004; Wang et al. 2013).
Among other stressors, the effect of wounding, occurring for example during preparation of minimally-processed produce (or similarly to that induced by pathogens), consists in loss of sub-cellular compartmentalization and release of phenols, which are normally located in cell vacuole. After mechanical cutting, phenols release represents a deleterious effect as those compounds become a high-affinity substrate for browning-related enzymes, such as peroxidases (POXs) or polyphenol oxidases (PPOs) (Saltveit 2000; Degl’Innocenti et al. 2007; Kaewubon et al. 2015). These reactions severely compromise the shelf-life of browning-susceptible products, such as lettuce.
Exogenous AA is commonly utilized as an anti-browning agent, despite its intimal mechanism(s) of action has never yet been clarified. Three main mechanisms have been proposed: (1) AA may act as antioxidant, promoting the regeneration of o-quinones and preserving them from polymerization into brown pigments (Walker 1995; Alscher et al. 1997); (2) AA can bind to histidine residues of PPOs catalytic site, increasing the enzymatic Km of PPO and reducing the turnover of PPO-triggered phenol oxidation (Osuga et al. 1994); (3) as a weak acid, AA accumulation may lower cytosolic pH, thus down-regulating the activity of browning-promoting enzymes (POXs and PPOs) after cutting (Vamos-Vigyazo 1981; Landi et al. 2013). Among these three possible hypotheses, the latter appears less probable in lettuce as both PPOs and POXs maintain high activity under a wide range of pH (Landi et al. 2013).
It has been found that leaf vegetables with constitutive high level of AA (such as rocket salad and spinach) result less prone to browning phenomena than do low-containing leaf vegetables, such as lettuce (Degl’Innocenti et al. 2007; Bottino et al. 2009). Thus, the attempt to increase the AA content in a browning-sensitive commodity represents a promising first step to clarify the involvement of AA as anti-browning compounds after cutting. In addition, despite in some countries (especially in Europe) transgenic plants are not permitted as human and/or animal food source, outside these countries private companies may nevertheless be interested in the possibility of producing AA-enhanced plants for niche markets (Ishikawa et al. 2006; Cruz-Rus et al. 2012; Rai and Shekhawat 2014; Gerszberg et al. 2015).
Despite many works have demonstrated that AA is synthetized from hexose sugars in plants, some steps of AA pathway still remain uncertain. It seems established that AA can be synthetized following three alternative pathways: (1) the myo-inisitol pathway; (2) the galacturonate pathway, and (3) the Smirnoff–Wheeler’s pathway, which involves the synthesis of AA from L-galactose (Wheeler et al. 1998) (Fig. 1). L-galactose is generated from mannose-1-phosphate by the conversion of guanosine diphosphate (GDP)-mannose to GDP-L-galactose by GDP-mannose-3′,5′-epimerase which is then converted to L-galactose. It has been demonstrated that the GDP-L-galactose guanyltransferase (GGT) converts GDP-L-galactose to L-galactose-1-phosphate (Fig. 1). Then, L-galactono-1,4-lactone is synthesized from the oxidation of L-galactose by the NADH-dependent L-galactose dehydrogenase (Wolucka et al. 2001; Laing et al. 2007; Linster et al. 2007; Linster and Clarke 2008; Zhou et al. 2012). Finally, L-galactono-1,4-lactone is oxidized to AsA by L-galactono-1,4-lactone dehydrogenase (L-GalLDH) (EC 1.3.2.3).
Three postulated pathways for ascorbic acid biosynthesis in plants. Among them, only the Smirnoff-Wheeler’s branch has been confirmed through studies with transgenic plants and almost all the enzymes have been characterized in some species. The only missing step remains the conversion of GDP-L-galactose to L-galactose-1-phosphate even though a GDP-L-galactose guanyltransferase has been proposed by Dowdle et al. (2007) to catalyze this reaction
Many genes involved in AA biosynthesis and recycling have been cloned, and transgenic plants containing modified levels of AA have been generated (reviewed in Hancock and Viola 2002; Zhang et al. 2007; Cruz-Rus et al. 2012). Silencing/overexpression of genes encoding various enzymes in the AA biosynthesis and metabolic network lead to a decrease/increase in AA content (Alhagdow et al. 2007; Pineau et al. 2008; Badejo et al. 2009; Hemavathi-Upadhyaya et al. 2010; Yu et al. 2010; Bulley et al. 2012; Liu et al. 2013). A supported relationship between L-GalLDH activity and AA biosynthesis (Ôba et al. 1994; Wheeler et al. 1998; Tabata et al. 2001; 2002; Tamaoki et al. 2003) has led to suggestion that this step may be a suitable target for manipulation of AA biosynthesis in plants (Hancock and Viola 2005). Although no clear relationships among the AA content and L-GalLDH protein amount have been observed in wheat (Bartoli et al. 2005), tomato (Alhagdow et al. 2007) and peach (Imai et al. 2009), the overexpression of tobacco L-GalLDH in BY-2 cells under the constitutive CAMV35S promoter resulted in up to fourfold increased enzyme activity and a 60 % increase in the AA pool size (Tokunaga et al. 2005). In addition, antisense suppression of L-GalLDH mRNA led to a significant decline in both L-GalLDH activity and AA levels (−30 %) in the transgenic tobacco BY-2 cells (Tabata et al. 2001). Even in romain lettuce the overexpression of L-GalLDH cDNA isolated from Arabidopsis lead to a 3.2-fold increase of AA content when compared with wild type (WT) lettuce (Guo et al. 2013).
In this study we overexpressed the endogenous L-GalLDH in L. sativa L. cv ‘Iceberg’ to test whether it leads to an accumulation of AA and if it is correlated to a decrease in processed leaf browning. This work represents the first report in which the AA content has been manipulated by overexpression of endogenous L-GalLDH cDNA in Compositae, a large family which numbers several edible crops, and the first clear evidence that endogenous AA can act as anti-browning compound.
Materials and methods
Plant material and growth condition
L. sativa seeds (cv ‘Iceberg’, purchased from Blumen, Milan, Italy) were germinated in Petri dishes, on filter papers moistened with distilled water at 23 ± 1 °C in the dark. After 3–4 days, germinated seeds were transferred to 8 cm diameter pots containing a 60:40 mixture of potting soil and sand, respectively. Seedlings were grown in a growth chamber at 23 ± 1 °C under a 16-h photoperiod. Irradiances at the top of the seedlings were 500 μmol photons m−2 s−1 provided by High Pressure Sodium Lamps HPST 400W/E40/H0 (Venture Lighting Italia S.r.l., Milan, Italy).
Isolation of complete L-GalLDH mRNA in lettuce
Two expressed sequence tags (ESTs) of L. sativa (DY974309) and L. serriola (BQ987137) from the TIGR Plant Transcript Assembly (http://blast.jcvi.org/euk-blast/plantta_blast.cgi) and corresponding to fragments of L-GalLDH-related sequences were identified. The EST sequences were used to choose the primers (LAC1F and LAC4R) for PCR amplification of the L-GalLDH cDNA in lettuce (Online Resource 1). The primer LAC1F is placed 36 bp before the putative start codon of the L-GalLDH gene.
Total RNA was extracted from young leaf blades of 20-day-old lettuce plants with the TriPure Isolation Reagent, according to the manufacturer’s instructions (Roche Diagnostics GmbH, Germany). Total RNA (4 μg), was used with the Superscript™ II pre-amplification kit (Invitrogen S.R.L., Life Technologies, Carlsbad, CA), to produce the first strand cDNA in conditions recommended by manufacturer. One cDNA fragment was obtained with the primer combination LAC1F-LAC4R. The following PCR conditions were used: 94 °C for 4 min, 35 cycles (30 s at 94 °C, 30 s at 64 °C, 60 s at 72 °C), 72 °C for 7 min. A 3′RACE was conducted using the L-GalLDH-specific primer LAC5F and the Universal Amplification Primer (UAP) 9 (Online Resource 1) with the following PCR conditions: 94 °C for 4 min, 35 cycles (30 s at 94 °C, 30 s at 60 °C, 30 s at 72 °C), 72 °C for 7 min.
To obtain a full-length L-GalLDH CDS a PCR was performed with the specific primers LAC1F and LAC6R (Online Resource 1). The PCR conditions were: 94 °C for 4 min, 35 cycles (30 s at 94 °C, 30 s at 58 °C, 100 s at 72 °C), 72 °C for 10 min. The PCRs were performed with a Phusion® high-fidelity DNA polymerase (Thermo Scientific, St. Leon, Germany), according to the manufacturer’s instructions.
Detailed information on LsL-GalLDH cloning sequences are reported on Online Resource 2. Sequence data from this article have been deposited in GenBank under the accession number HG810915.2.
Database searches and phylogenetic analysis
Database searches were carried out using the BLAST program at the National Center for Biotechnology Information (NCBI) (Altschul et al. 1997). PROSITE and PFAM databases were searched to identify conserved domains (Bateman et al. 2002; Falquet et al. 2002). Mito ProtII-v1.101 software at the ExPASy Bioinformatics Resource Portal was used for the prediction of putative mitochondrial targeting sequences and cleavage (Claros and Vincens 1996). The deduced L-GalLDH amino acid sequence of lettuce was compared to L-GalLDH sequences of other higher plants. The amino acid sequence from Volvox carteri (GenBank accession No. XM002947966) was used as out-group in the phylogenetic analysis. The evolutionary history was inferred using the minimum evolution (ME) method (Rzhetsky and Nei 1992). The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (100 replicates) are shown next to the branches (Felsenstein 1985). The evolutionary distances were computed using the Poisson correction method (Zuckerkandl and Pauling 1965) and are expressed as units of amino acid substitutions per site. The ME tree was searched using the close-neighbor-interchange (CNI) algorithm (Nei and Kumar 2000) at a search level of 1. The Neighbour-joining algorithm (Saitou and Nei 1987) was used to generate the initial tree. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). A total of 543 positions were found in the final dataset. Phylogenetic analyses were conducted in MEGA4 (Tamura et al. 2007).
Semi-quantitative RT-PCR analysis
To analyse LsL-GalLDH transcript levels, semi-quantitative RT-PCR analysis were carried out from cotyledons of 5-day-old WT plants (C), internodal stem of 60-day-old plants (ST), young leaf blade (2 cm long) of 20-day-old plants (YL), completely expanded leaf blades of about 30-day-old plants (mature leaf, ML), vegetative shoots (VS) of 20-day-old plants, young inflorescences (YI) of 60-day-old plants. Detailed information on semi-quantitative RT-PCR methods are reported on Online Resource 2.
Material for the production of transgenic plants
Lettuce seeds (L. sativa cv ‘Iceberg’) were immersed in 70 % ethanol for 1 min and then rinsed with sterile distilled water. Later on, seeds were surface sterilized in 10 % (v/v) ‘ACE’ bleach (Procter & Gamble S.r.l., Rome, Italy) for 15 min under a pressure of 400 mm Hg, followed by three washes in sterile distilled water. The seeds were placed on solidified agar (0.8 % w/v) Murashige and Skoog (MS; 1962) medium with 3 % (w/v) sucrose (pH 5.7). Seeds were germinated at 23 ± 1 °C (16-h photoperiod, 100 μmol m−2 s−1, daylight fluorescent tubes). Cotyledons were excised after 3–5 days for bacterial inoculation.
Construction of PetE::LsL-GalLDH cassette and growth of Agrobacterium tumefaciens strain
Forward and reverse primers, LATF and LATR (Online Resource 1) able to insert SalI restriction sites at the end of the LsL-GalLDH cDNA were used. The PCR conditions were: 95 °C for 4 min, 35 cycles (30 s at 94 °C, 30 s at 64 °C, 100 s at 72 °C), 72 °C for 10 min. The PCRs were performed with a Phusion® high-fidelity DNA polymerase (Thermo Scientific), according to the manufacturer’s instructions. The PCR products were separated using electrophoresis on a 1 % TAE-agarose gel and visualized with Gel Red™ Nucleic Acid Stain under UV light. The selected amplified product was purified as reported on Online Resource 2, inserted into the pGEM®-T easy vector (Promega), and transformed in Escherichia coli JM109 competent cells (Promega). Plasmid cDNA was prepared as reported on Online Resource 2 and both strands of several clones were automatically sequenced.
The transcript was then ligated in a pBIN19 derivative binary vector pVDH282 (Frugis et al. 2001) containing an expression cassette (pea plastocyanin promoter PetE-NOS terminator). The cDNA was inserted exploiting a SalI restriction site downstream of pPetE and upstream of tNOS (Online Resource 3). The resulting binary vector was named pBINLsL-GalLDH.
The pBINLsL-GalLDH construct was inserted in LBA4404 A. tumefaciens strain. Bacteria were grown from −70 °C glycerol stocks at 28 °C on Luria broth (LB) (Sambrook and Russell 2001) semi-solidified with 1.5 % (w/v) Bactoagar (Oxoid) and supplemented with kanamycin sulphate (100 mg L−1) and rifampicin (50 mg L−1). Overnight liquid cultures were incubated at 28 °C on a horizontal rotary shaker (180 rpm) and were initiated by inoculating 20 mL of liquid LB medium, containing kanamycin sulphate (50 mg L−1) and rifampicin (40 mg L−1), into 100 cm3 conical flasks. Bacterial cultures were grown to an O.D.600 of 1.0–1.5 prior to inoculation of explants.
Plant transformation
Cotyledons excised from 3–5-day-old seedlings were inoculated with A. tumefaciens and transgenic shoots regenerated using a modified procedure described by Curtis et al. (1994). Detailed information on plant transformation and recovering of T0, T1 and T2 transgenic plants are reported on Online Resource 2.
Gene expression analysis by real-time RT-PCR (qPCR)
Total RNA was extracted from mature leaf blades of about 30-day-old of both WT and T2 homozygous lettuce transgenic plants (PetE::LsL-GalLDH) with the TriPure Isolation Reagent, according to the manufacturer’s instructions (Roche Diagnostics GmbH, Germany). The total RNA was isolated from sample collected immediately (t0) or 24 h (t1), 48 h (t2) and 72 h (t3) after the cutting. The RNA integrity was checked by gel electrophoresis and quantified with a microdrop and treated with RQ1 RNase-Free DNase (Promega) following the manufacturer’s instructions. First strand cDNA was synthesized using iScript™cDNA synthesis Kit (Bio-Rad) following the manufacturer’s instructions. Real-time quantitative RT-PCR (qPCR) was performed using an ABI Prism 7300 sequence detection system (Applied Biosystems). Relative quantification was done using internal standard curves and normalizing with the reference gene Lsβtub3 gene (Rosales-Mendoza et al. 2010 and Online Resource 2). Lsβtub3 resulted the most stable housekeeping genes among the other we tested (e.g. Lsβactin). The sequences of gene-specific primers for LsL-GalLDH (LsL-GalLDHF and LsL-GalLDHR) and Lsβtub3 (Lsβtub3F and Lsβtub3R) are reported in Online Resource 1. Quantitative PCR was performed using 50 ng of cDNA and iQSYBRGreen Supermix (Bio-Rad Laboratories), according to the manufacturer’s instructions. The thermal cycling conditions of RT-PCR were as follows: stage I 10 s at 50 °C, stage II 3 min at 95 °C, stage III (×40) 5 s at 95 °C + 30 s at 60 °C. Three independent biological replicates were analyzed per each treatment. Relative quantification of specific mRNA levels was performed using the comparative 2−ΔΔCt method (Livak and Schmittgen 2001).
AA determination
Total ascorbate (AA), reduced ascorbate (AsA), and dehydroascorbate (DHA) were spectrophotometrically determined as described by Kampfenkel et al. (1995). The assay is based on the reduction of Fe3+ to Fe2+ by AsA and the spectrophotometric detection of Fe2+ complexed with 2,2′-dipyridyl. DHA is calculated as the difference between AA and AsA. AA, AsA, and DHA were determined immediately (t0) or 24 h (t1), 48 h (t2) and 72 h (t3) after cutting in leaves of T2 plants (derived from self-pollinated T1 03 plants as reported below) stored under dark condition at 4 °C in 0.5-L polyethylene terephthalate boxes. AA content was also determined in fully expanded leaves of T1 plants (T1 22, T1 48, T1 01, T1 02 and T1 03). Data were expressed as µg AA g−1 FW.
Colour determination
Leaf surface colour measurements were carried out at each time after cutting (0, 1, 2, 3, 10 days) in 5 randomly selected leaves of T2 plants. In each selected leaf, colour was monitored in three spots by using standard CIE Lab* colour space coordinates determined by an Ocean Optic HR2000-UV-VIS-NIR spectrometer coupled whit a tungsten halogen DH2000 light source (Ocean Optics, USA). L* represents the lightness of colours (lightness index scale) and ranged from 0 for black to 100 for white; a* value represents redness and greenness (a* and –a*, respectively). After cutting, leaves were stored under dark condition at 4 °C in 0.5-L polyethylene terephthalate boxes for monitoring color changes over time.
Statistical analysis
The experiment was repeated twice with similar results; a representative run is reported herein. Reported data for semi-quantitative RT-PCR, real-time RT-PCR (qPCR), AA content, and colour parameters represent at least the mean ± SD of five biological replications (n = 5). Homogeneity of variance among data was evaluated using Bartlett’s test (p = 0.05). The percentage data were analyzed after arcsine transformation. Means were subjected to two-way analyses of variance (ANOVA) with genotype (G) and storage (S) as variability factor. One-way ANOVA was applied for statistical analyses of AA in T1 plants. Means were separated after Tukey’s test (p = 0.05).
Results
Isolation and sequence analysis of LsL-GalLDH cDNA
LsL-GalLDH cDNA contains a complete open reading frame (ORF) of 1,833 bp, flanked by 5′- and 3′-untranslated regions (UTR) of 36 and 88 bp, respectively (Online Resource 4). The putative peptide LsL-GalLDH is 610 amino acids long with a theoretical pI of 8.68 and a calculated molecular weight of 69.2 kDa (Fig. 2a). It contains a mitochondrial targeting sequence (probability of export to mitochondria: 0.73) with the cleavage site FR/YA similar to other known L-GalLDHs (Fig. 2a). It is likely that LsL-GalLDH is processed to a mature protein by removal of an N-terminal peptide of 107 amino acids, which probably takes place during transport of L-GalLDH into mitochondria. Therefore, the mature LsL-GalLDH protein consist of 503 amino acid residues having a calculated molecular weight of 57.2 kDa and a pI of 6.68. Analysis of LsL-GalLDH amino acid sequence identified a putative N-terminal FAD-binding domain between residues 128 and 264, wherein is located a motif 157VGSGLSP163 common to L-GalLDHs characterized from plants (Fig. 2a). From the alignment, it is evident that L-GalLDHs in plants lack the histidine residue involved in covalent flavinylation in GUO (D-gluconolactone oxidase), ALO (D-arabinono-γ-lactone oxidase), and GLO (L-gulono-1,4-lactone oxidase), but contains a leucine residue instead (Leu161 in LsL-GalLDH, Fig. 2a, Online Resource 4 and Online Resource 5), indicating that the flavin cofactor is non-covalently bound to the protein (Leferink et al. 2008). The essential Glu-Arg pair found in the active site of L-GalLDH from Arabidopsis thaliana (Leferink et al. 2009) is also present in LsL-GalLDH (Fig. 2a, Online Resource 4 and Online Resource 5). The arginine 487 is crucial for the stabilization of the anionic form of the reduced FAD cofactor (Leferink et al. 2009) while the glutamic acid 485 is involved in productive substrate binding (Leferink et al. 2009).
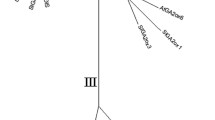
Sequence analysis of L-galactono-1,4-lactone dehydrogenase (L-GalLDH) from Lactuca sativa (LsL-GalLDH). a Predicted amino acids sequence of LsL-GalLDH. The cleavage site FR/YA, amino acids for mitochondrial targeting (Fraaije et al. 1998), is in red and bold characters (amino acid residues 105–108). A motif (157VGSGLSP163), common to other L-GalLDHs, is in orange characters (amino acid residues 157–163). Within this motif the Leu (L)161 residue is boxed. The FAD-binding domain is in blue and bold characters (amino acid residues 133–293 and 581–602). The Glu (E)485 and the Asp (R)487 residues are boxed and in bold-brown character. The domain specific to D-arabinono-1,4-lactone oxidase is underlined. b Dendrogram between 14 L-GalLDH proteins. The L-GalLDH amino acid sequences and the relative GenBank accession numbers are reported in Online Resource 6. Consensus tree was inferred using the Minimum Evolution (ME) method. The Neighbor-joining algorithm was used to generate the initial tree. The optimal tree with the sum of branch length = 1.82969450 is shown. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). A total of 543 positions in the final dataset were found. The L. sativa L-GalLDH is underlined and the percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (100 replicates) are shown next to the branches. Phylogenetic analyses were conducted using the on line open tool MEGA4. The L-GalLDH amino acid sequence from Volvox carteri (GenBank accession no. XM002947966) was used as outgroup in the phylogenetic analysis. (Color figure online)
Although plant L-GalLDHs have been identified as integral membrane proteins of the inner mitochondrial membrane (Siendones et al. 1999; Bartoli et al. 2000; Hancock and Viola 2005; Schertl et al. 2012; Szarka et al. 2013), we did not find any transmembrane regions in the sequence of mature LsL-GalLDH. Analogous results were reported for the sequence of Arabidopsis L-GalLDH (Leferink et al. 2008).The domain specific to D-arabinono-1,4-lactone oxidase, which is involved in the final step of the D-erythroascorbic acid biosynthesis pathway, has been also identified (Fig. 2a and Online Resource 4).
Sequence and phylogenetic analyses revealed that LsL-GalLDH was related with L-GalLDH genes of other species (Fig. 2b; Online Resource 6). A search in GenBank database with the BLAST program showed that LsL-GalLDH shared a query coverage of 95–99 % and the highest amino acid identity (73–76 %) with L-GalLDH proteins of Camellia sinensis (GenBank accession numbers KF619448), N. tabacum (GenBank accession number BAB13368), Malus domestica (GenBank accession number FJ752244), Fragaria vesca subsp. vesca (GenBank accession number XP_004303609) and Ipomoea batatas (GenBank accession number BAA34995). LsL-GalLDH cluster in the same sub-group of the C. sinensis L-GalLDH within a major monophyletic clade of the eudicot asterids and separated from members of the rosid clade.
Transcription analysis of LsL-GalLDH in lettuce organs
The semi-quantitative RT-PCR was used to analyse the steady state levels of LsL-GalLDH mRNA in various organs of WT lettuce (Online Research 7). LsL-GalLDH was consistently transcribed in all samples analyzed (i.e., cotyledons, internodal stem, young and mature leaves, vegetative shoots and young inflorescences), and the highest mRNA levels occurred in internodal stem and expanded leaf blades (Online Research 7).
Regeneration of PetE::LsL-GalLDH transgenic plants
Detailed information on regeneration of transformed plants are reported on Online Resource 2. The frequency of independent putatively transgenic plants was low (24/600, 4.0 %). Twelve putative transformed plants (T0) were selected on the basis of the presence of the PetE::LsL-GalLDH construct assessed by a PCR-based method (Fig. 3a). The twelve plants, heterozygous for the transgene PetE::LsL-GalLDH, were grown until anthesis to seed set (Online Resource 8). Among the twelve T0 lines we randomly selected, only five lines produced a sufficient amount of seeds to obtain T1 plants. Hence, these five lines were assessed for AA content. The T1 03 line was selected as one of the best AA producer as reported below. The T1 03 plants were grown on medium supplemented with kanamycin sulphate until maturity and self-pollinated for obtaining homozygous T2 seeds. The homozygous T2 transgenic progeny was tested by a PCR-based approach (Fig. 3b; Online Resource 8).
Screening of putatively transformed plants conducted by a PCR approach. The size (1176 bp) of expected PCR product is indicated. a T0 putatively transformed plants: three plants (lanes 1, 2 and 3) showed a clear signal as well the positive control (lane 5, DNA of vector); lane 4, T0 non-transformed plants (escape); lane 6, DNA from non-transformed wild type (WT) Lactuca sativa (L); lane 7, sterile distilled water (H2O). M indicates the PhiX 174 DNA HaeIII Digest DNA ladder. b T2 transformed plants. Six randomly chosen plants from a homozygous progeny selected in a kanamycin-supplemented medium that showed the expected amplified PCR-product (lanes 1, 2, 3, 4, 5 and 6). L, DNA from non-transformed WT L. sativa; H2O, sterile distilled water. M indicates the 1 Kb XL ladder (5 PRIME)
Real-time RT-PCR LsL-GalLDH expression in mature leaves of WT and transgenic plants
Previous transformation experiments in lettuce with gene under the control of the CaMV 35S promoter failed, resulting in no constitutive expression of the gene of interest (data not shown). Hence, we decided to overexpress the LsL-GalLDH gene under the control of the pea plastocyanin promoter, which is considered a constitutive promoter, although not as strong as the CaMV 35S, and slightly light dependent (Brown et al. 2005; Pwee and Gray 2008). Transgenic plants exhibited an increase (p < 0.05) in the expression of the gene of almost 19-fold change with respect to WT plants, and this result proved the successful transformation (Fig. 4a). Moreover, although there was a decrease of the expression levels of LsL-GalLDH after leaf-cutting, transgenic lettuce showed almost eightfold change higher levels in comparison to WT.
LsL-GalLDH transcription levels (a), and total ascorbate (AA), reduced ascorbate (grey bars), and oxidized ascorbate (white bars) (b) in a WT line (n = 5) and in an homozygote overexpressing line (n = 5) of Lactuca sativa at different time points: t0, immediately after cutting; t1, 24 h after cutting; t2, 48 h after cutting; t3, 72 h after cutting. For LsL-GalLDH transcription level, data were normalized using β-tubulin 3 (Lsβtub3) as housekeeping gene. Luminosity (L*) level (c) and greenness (a*) (d) evaluated in WT (white circles) and transgenic homozygote plants (dark circles) at 1, 2, 3 and 10 days after cutting. Means were subjected to two-way analyses of variance (ANOVA) with genotype (G) and storage (S) as variability factor. Means were separated after Tukey’s test (p = 0.05)
AA content and colour determination
The T1 LsL-GalLDH overexpressing plants (line 01, 02 and 03) had about +29 % of AA as compared to WT plants and T1 line 22 and 48 (Online resource 9). The T2 LsL-GalLDH overexpressing plants had about +32 % of constitutive (t0) AA content in mature leaves as compared to WT plants (p < 0.001; Fig. 4b). Similar enhancement of AA was maintained in transgenic lettuce leaves after cutting (t1–t3 values averaged 127.9 µg g−1 FW vs 96.6 found in WT; p < 0.01). In both the genotypes, the proportion of DHA increased at t2 (23.4 vs. 27.0 in WT and T2 plants, respectively) and even more at t3 (26.2 vs. 34.75 in WT and T2 plants, respectively).
CIE Lab* colour values highlight that LsL-GalLDH overexpressing plants had higher values of L* (Fig. 4c) and lower values of a* (Fig. 4d) as compared to the WT counterpart. Although the effect of the storage was significant only for a*, likely due to the high variability found for L* values, the T-test evidenced as both WT and T2 plants had similar values of L* and a* immediately after cutting (t0), but transgenic plants had higher values of L* and lower values of a* (p < 0.001) 3 and 10 d after cutting. Higher development of browning appearance in WT plants compared to LsL-GalLDH overexpressing plants was evident also to naked eye, especially closer the cutting surfaces (Online Resource 10).
Discussion
The manipulation of AA biosynthesis in plants is a useful strategy to add new insight both in basic and applicative plant research, such instance that oriented toward reduction of loss of minimally-processed products due to browning phenomena. Obtained results offer the evidence that transformation of lettuce with L-GalLDH cDNA effectively lead to: (1) overexpression of LsL-GalLDH, even 24, 48 and 72 h after cutting, (2) incremented concentration of AA (about +30 %) accumulated in mature leaves and (3) reduced browning phenomena after cutting. In addition, as L-GalLDH complete CDS have never been isolated in a member of Compositae hence sequence investigation and phylogenetic analyses among other plant families are provided here.
The putative LsL-GalLDH polypeptide sequence presented a mitochondrial targeting signal in the amino terminal end, rich in Ala, Leu, Arg, and Ser residues (6, 13, 7, and 18, respectively) and with relatively few Asp, Glu, Ile, and Val residues (0, 3, 3, and 2, respectively). This composition is similar to that reported for other known L-GalLDHs and matched the characteristics of mitochondrial target peptides (von Heije 1986; Pateraki et al. 2004). The existence of a FAD binding domain suggests that the flavin group is involved in the reaction catalysed by L-GalLDH (Ôba et al. 1995). LsL-GalLDH lacks the histidine involved in covalent attachment of the FAD cofactor, but contains a leucine (Leu161) at this position. Replacement of Leu into His in Arabidopsis L-GalLDH revealed that the presence of a histidine at this position does not initiate covalent binding of the cofactor (Leferink et al. 2008). Covalent coupling of the FAD cofactor is likely an autocatalytic process, requiring a pre-organized binding site (Fraaije et al. 2000).
Although it has been established that L-GalLDH catalyses the last step of AA biosynthetic pathway in several plant species (Østergaard et al. 1997; Ioannidi et al. 2009; Li et al. 2010; Cocetta et al. 2012; Xu et al. 2013), no clear correlation between AA content and expression of L-GalLDH has been always detected. An extensive analysis performed in cabbage showed that the expression pattern of the major genes in the D-Man/L-Gal branch of AA pathway, including L-GalLDH, have a higher expression levels in cultivar with higher AA content (Ren et al. 2013). Similarly, during apple fruit formation, a greater transcription and activity of L-GalLDH in young fruit contributed to increase the AA content (Li et al. 2011). Post-transcriptional regulation of L-GalLDH has also been proposed in different organs of some species or under stress condition (Bartoli et al. 2005; Loscos et al. 2008). In our work, we found that in all tested organs the mRNA was consistently transcribed suggesting that in lettuce L-GalLDH is not a tissue-specific prerogative.
Controversial results were obtained by using transgenic plants, in which AA pathway has been manipulated trough overexpression/silencing of the L-GalLDH gene. Alhagdow et al. (2007) observed that tomato L-GalLDH silencing did not exhibit clear changes in AA contents compared with WT plants. In contrast, in A. thaliana GGT transgenic lines had the highest AA accumulation with a 2.9-fold increase to the WT, which was followed by L-GalLDH (1.8-fold) and L-galactose-1-phosphate phosphatase transgenic lines (1.5-fold) (Zhou et al. 2012). In our study, the overexpression of L-GalLDH in lettuce plants is consistent with higher AA content in agreement with that observed by Tokunaga et al. (2005) by the overexpression of the same gene in BY-2 cells of N. tabacum and by Guo et al. (2013) by the overexpression of Arabidopsis L-GalLDH in romain lettuce. However, Guo et al. (2013) found a 3.2-fold increment of AA content while in this study AA levels were incremented only by about +30 % in Ls-GalLDH overexpressing plants.
The higher AA concentration found in transformed lettuce plants correlated with reduced browning development over the leaves after wounding (3 and 10 days later; Online Resource 10), proved by higher levels of L* and lower intensities of a* found in T2 LsL-GalLDH overexpressing plants (Fig. 4c, d) as compared to WT. Higher values of L* are indicative of low levels of browning since browning development is commonly associated with the oxidation of phenolics and their polymerization into dark-brown pigments (King et al. 1991; Ke and Saltveit 1989). The level of redness is also to be considered related to the browning phenomena (Castañer et al. 1999) as an increment of this value is mainly associated with the breakdown of chlorophyll, and hence, to the reduction of leaf greenness. In agreement, Martin-Diana et al. (2005) found that high temperature treatment of lettuce leaves (50 °C) leading to degradation of browning-related enzyme (such as polyphenol oxidase and peroxidase) reduced the browning appearance after cutting and leaves maintained higher L*, lower a* values as compared to lettuce leaves treated with lower temperatures (25 and 4 °C). In addition, it has been demonstrated that exogenous AA sprayed over lettuce leaves induces L* to decrease less markedly than unsprayed leaves, and immersion of lettuce leaves in a solution containing 1 % AA exhibited a less pronounced increment in a* values (Rivera et al. 2006). Furthermore, Altunkaya and Gökmen (2009) demonstrated in lettuce that exogenous AA was a very effective anti-browning compound and treated leaves maintained high level of phenolic components.
Of note, in both WT and T2 LsL-GalLDH overexpressing plants the proportion of the oxidized form of ascorbate (DHA) decreased significantly leading to the hypothesis that AA could act directly as antioxidant promoting the regeneration of o-quinones. Given these data, we speculate that the higher level of DHA found in T2 leaves than in WT at 3 and 10 days after cutting can partially explain the reduction of browning appearance over T2 lettuce leaves.
In conclusion, these LsL-GalLDH transgenic plants represent a first promising model which has revealed the role of endogenous AA in the prevention of browning, but further experiments are necessary to clarify the mechanism by which AA counteracts the enzymatic browning. In particular, the AA competitive inhibition for PPO catalytic site and the pH-dependent theory for which AA accumulation may lower cytosolic pH, thus down-regulating the activity of browning-promoting enzymes, need to be tested.
Abbreviations
- AA :
-
L-Ascorbic acid (sum of reduced and oxidized form of ascorbic acid)
- ALO:
-
D-Arabinono-γ-lactone oxidase
- AsA:
-
Reduced form of ascorbic acid
- BAP:
-
6-Benzylaminopurine
- DHA:
-
Dehydroascorbate
- DTT:
-
Dithiothreitol
- ESTs:
-
Expressed sequence tags
- GDP:
-
Guanosine diphosphate
- GGT:
-
GDP-L-galactose guanyltransferase
- L-GalLDH:
-
L-galactono-1,4-lactone dehydrogenase
- GLO:
-
L-gulono-1,4-lactone oxidase
- GUO:
-
D-gluconolactone oxidase
- LsL-GalLDH:
-
Lactuca sativa L-galactono-1,4-lactone dehydrogenase
- MS:
-
Murashige and Skoog medium
- NAA:
-
α-Naphthalene-acetic-acid
- POXs:
-
Peroxidases
- PPOs:
-
Polyphenol oxidases
- RACE:
-
Rapid amplification of cDNA ends
References
Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, Baldet P (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145:1408–1422
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationship in green cells. Physiol Plant 100:224–233
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Altunkaya A, Gökmen V (2009) Effect of anti-browning agents on phenolic compounds profile of fresh lettuce (L. sativa). Food Chem 117:122–126
Badejo AA, Eltelib HA, Fukunaga K, Fujikawa Y, Esaka M (2009) Increase in ascorbate content of transgenic plants overexpressing the acerola (Malpighia glabra) phosphomannomutase gene. Plant Cell Physiol 50:423–428
Bartoli CG, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123:335–343
Bartoli CG, Guiamet JJ, Kiddle G, Pastori GM, Di Cagno R, Theodoulou FL, Foyer CH (2005) Ascorbate content of wheat leaves is not determined by maximal L-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ 28:1073–1081
Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer ELL (2002) The pfam protein families database. Nucleic Acids Res 30:276–280
Bottino A, Degl’Innocenti E, Guidi L, Graziani G, Fogliano V (2009) Bioactive compounds during storage of fresh-cut spinach: the role of endogenous ascorbic acid in the improvement of produce quality. J Agric Food Chem 57:2925–2931
Brown NJ, Sullivan JA, Gray JC (2005) Light and plastid signals regulate the expression of the pea plastocyanin gene through a common region at the 5′ end of the coding region. Plant J 4:541–552
Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K, Andre C, Brewster D, Karunairetnam S, Allan AC, Laing WA (2012) Enhancing ascorbate in fruits and tubers through over-expression of the L-galactose pathway gene GDP-L-galactose phosphorylase. Plant Biotechnol J 10:390–397
Castañer M, Gil MI, Ruiz MV, Artes F (1999) Browning susceptibility of minimally processed Baby and Romaine lettuces. Eur Food Res Technol 209:52–56
Claros MG, Vincens P (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241:779–786
Cocetta G, Karppinen K, Suokas M, Hohtola A, Häggman H, Spinardi A, Mignani I, Jaakola L (2012) Ascorbic acid metabolism during bilberry (Vaccinium myrtillus L.) fruit development. J Plant Physiol 169:1059–1065
Cruz-Rus E, Amaya I, Valpuesta V (2012) The challenge of increasing vitamin C content in plant foods. Biotechnol J 7:1110–1121
Curtis IS, Power JB, Blackhall NW, de Laat AMM, Davey MR (1994) Genotype-independent transformation of lettuce using Agrobacterium tumefaciens. J Exp Bot 45:1441–1449
Degl’Innocenti E, Pardossi A, Tognoni F, Guidi L (2007) Physiological basis of sensitivity to enzymatic browning in ‘lettuce’, ‘escarole’ and ‘rocket salad’ when stored as fresh-cut products. Food Chem 104:209–215
Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDPp-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52:673–689
Falquet L, Pagni M, Bucher P, Hulo N, Sigrist CJ, Hofmann K, Bairoch A (2002) The PROSITE database, its status in 2002. Nucleic Acids Res 30:235–238
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fraaije MW, Van Berkel WJH, Benen JAE, Visser J, Mattevi A (1998) A novel oxidoreductase family sharing a conserved FAD-binding domain. Trends Biochem Sci 23:206–207
Fraaije MW, van den Heuvel RHH, van Berkel WJH, Mattevi A (2000) Structural analysis of flavinylation in vanillyl-alcohol oxidase. J Biol Chem 275:38654–38658
Frugis G, Giannino D, Mele G, Nicolodi C, Chiappetta A, Bitonti MB, Innocenti AM, Dewitte W, Van Onckelen H, Mariotti D (2001) Overexpression of KNAT1 in lettuce shifts leaf determinate growth to a shoot-like indeterminate growth associated with an accumulation of isopentenyl-type cytokinins. Plant Physiol 126:1370–1380
Gallie DR (2013) The role of L-ascorbic acid recycling in responding to environmental stress and promoting plant growth. J Exp Bot 64:433–443
Gerszberg A, Hnatuszko-Konka K, Kowalczyk T, Kononowicz AK (2015) Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant Cell Tiss Organ Cult 120:881–902
Guo X, Liu RH, Fu X, Sun X, Tang K (2013) Over-expression of L-galactono-γ-lactone dehydrogenase increases vitamin C, total phenolics and antioxidant activity in lettuce through bio-fortification. Plant Cell Tiss Organ Cult 114:225–236
Hancock RD, Viola R (2002) Biotechnological approaches for L-ascorbic acid production. Trends Biotechnol 20:299–305
Hancock RD, Viola R (2005) Improving the nutritional value of crops through enhancement of L-ascorbic acid (vitamin C) content: rationale and biotechnological opportunity. J Agric Food Chem 58:5248–5257
Hemavathi-Upadhyaya CP, Akula N, Young KE, Chun SC, Kim DH, Park SW (2010) Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett 32:321–330
Imai T, Ban Y, Terakami S, Yamamoto T, Moriguchi T (2009) L-Ascorbate biosynthesis in peach: cloning of six L-galactose pathway-related genes and their expression during peach fruit development. Physiol Plant 136:139–149
Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK (2009) Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J Exp Bot 60:663–678
Ishikawa T, Dowdle J, Smirnoff N (2006) Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol Plant 126:343–355
Kaewubon P, Hutadilok-Towatana N, Teixeira da Silva J, Meesawat U (2015) Ultrastructural and biochemical alterations during browning of pigeon orchid (Dendrobium crumenatum Swartz) callus. Plant Cell Tiss Organ Cult 121:53–69
Kampfenkel K, Van Montagu M, Inzé D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Ke D, Saltveit ME (1989) Wound induced ethylene production, phenolic metabolism and susceptibility to russet spotting in Iceberg lettuce. Physiol Plant 76:412–418
King AD, Magnuson JA, Torok T, Godman N (1991) Microflora and storage quality of partially processed lettuce. J Food Sci 56:459–461
Laing W, Wright M, Cooney J, Bulley S (2007) The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci USA 104:9534–9539
Landi M, Degl’Innocenti E, Guglielminetti L, Guidi L (2013) Role of ascorbic acid in polyphenol-oxidase inhibition and browning prevention in different browning-sensitive Lactuca sativa var. capitata (L.) and Eruca sativa (Mill.) stored as fresh-cut products. J Sci Food Agric 93:1814–1819
Leferink NGH, van den Berg WAM, van Berkel WJH (2008) L-Galactono-γ-lactone dehydrogenase from Arabidopsis thaliana, a flavoprotein involved in vitamin C biosynthesis. FEBS J 275:713–726
Leferink NGH, Mac Donald FJ, van den Berg WAM, van Berkel WJH (2009) Functional assignment of Glu386 and Arg388 in the active site of L-Galactono-γ-lactone dehydrogenase. FEBS Lett 583:3199–3203
Li MJ, Ma FW, Guo CM, Liu J (2010) Ascorbic acid formation and profiling of genes expressed in its synthesis and recycling in apple leaves of different ages. Plant Physiol Biochem 48:216–224
Li M, Chen X, Wang P, Ma F (2011) Ascorbic acid accumulation and expression of gene involved in its biosynthesis and recycling in developing apple fruit. J Am Soc Hortic Sci 136:231–238
Linster CL, Clarke SG (2008) L-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends Plant Sci 13:567–573
Linster CL, Adler LN, Webb K, Christensen KC, Brenner C, Clarke SG (2007) A second GDP-L-galactose phosphorylase in Arabidopsis en route to vitamin C. Covalent intermediate and substrate requirements for the conserved reaction. J Biol Chem 282:18482–18492
Liu Y, Yu L, Tong J, Ding J, Wang R, Lu Y, Xiao L (2013) Tiller number is altered in the ascorbic acid-deficient rice suppressed for L-galactono-1,4-lactone dehydrogenase. J Plant Physiol 170:389–396
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Loscos M, Matamoros A, Becana M (2008) Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol 146:1282–1292
Martin-Diana AB, Rico D, Barry-Ryan C, Frias JM, Mulcahy J, Henehan GTM (2005) Calcium lactate washing treatments for salad-cut Iceberg lettuce: effect of temperature and concentration on quality retention parameters. Food Res Int 38:729–740
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New York
Ôba K, Fukui M, Imai Y, Iriyama S, Nogami K (1994) L-Galactono-γ-lactone dehydrogenase: partial characterization, induction of activity and role in synthesis of ascorbic acid in wounded white potato tuber tissue. Plant Cell Physiol 35:473–478
Ôba K, Ishikawa S, Nishikawa M, Mizuno H, Yamamoto T (1995) Purification and properties of L-galactono-γ-lactone dehydrogenase, a key enzyme for ascorbic acid biosynthesis, from sweet potato roots. J Biochem 117:120–124
Østergaard J, Persiau G, Davey M, Bauw G, Van Montagu M (1997) Isolation of a cDNA coding for L-galactono-γ-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants: purification, characterization, cDNA cloning, and expression in yeast. J Biol Chem 272:30009–30016
Osuga D, Van der Schaaf A, Whitaker JR (1994) Control of PPO activity using a catalytic mechanism. In: Yada RY, Jackman RL, Smith JL (eds) Protein structure-function relationship in foods. Springer, New York, pp 62–88
Pateraki I, Sanmartin M, Kalamaki M, Gerasopoulos D, Kanellis AK (2004) Molecular characterization and expression studies during melon fruit development and ripening of L-galactono-1,4-lactone dehydrogenase. J Exp Bot 55:1623–1633
Pineau B, Layoune O, Danon A, De Paepe R (2008) L-Galactono-1,4-lactone dehydrogenase is required for the accumulation of plant respiratory complex I. J Biol Chem 283:32500–32505
Pwee KH, Gray JC (2008) The pea plastocyanin promoter direct cell-specific but not full light-regulated expression in transgenic plants. Plant J 3:437–449
Rai MK, Shekhawat NS (2014) Recent advances in genetic engineering for improvement of fruit crops. Plant Cell Tiss Organ Cult 116:1–15
Ren J, Chen Z, Duan W, Somg X, Liu T, Wang J, Hou X, Li Y (2013) Comparison of ascorbic acid biosynthesis in different tissues of three non-heading Chinese cabbage cultivars. Plant Physiol Biochem 73:229–236
Rivera JRE, Stone MB, Stushnoff C, Pilon-Smith E, Kendall PA (2006) Effects of ascorbic acid applied by two hydrocooling methods on physical and chemical properties of green leaf lettuce stored at 5 °C. J Food Sci 71:S270–S276
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Alpuche-Solís ÁG, Martínez-Gonzáles L, Korban SS (2010) Expression o fan immunogenic F1-V fusion protein in lettuce as a plant-based vaccine against plague. Planta 232:409–416
Rzhetsky A, Nei M (1992) A simple method for estimating and testing minimum evolution trees. Mol Biol Evol 9:945–967
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Saltveit ME (2000) Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol Technol 21:61–69
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schertl P, Sunderhaus S, Klodmann J, Gergoff Grozeff GE, Bartoli CG, Braun H-P (2012) L-Galactono-1,4-lactone dehydrogenase (GLDH) forms a part of three subcomplexes of mitochondrial complex I in Arabidopsis thaliana. J Biol Chem 287:14412–14419
Siendones E, González-Reyes JA, Santos-Ocaña C, Navas P, Córdoba F (1999) Biosynthesis of ascorbic acid in kidney bean. L-Galactono-γ-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane. Plant Physiol 120:907–912
Smirnoff N, Conklin PL, Loewus FA (2001) Biosynthesis of ascorbic acid in plants: a renaissance. Annu Rev Plant Physiol Plant Mol Biol 52:437–467
Szarka A, Bánhegyi G, Asard H (2013) The inter-relationship of ascorbate transport, metabolism and mitochondrial, plastidic respiration. Antioxid Redox Signal 19:1036–1044
Tabata K, Ôba K, Suzuki K, Esaka M (2001) Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. Plant J 27:139–148
Tabata K, Takaoka T, Esaka M (2002) Gene expression of ascorbic acid-related enzymes in tobacco. Phytochemistry 61:631–635
Tamaoki M, Mukai F, Asai N, Nakajima N, Kubo A, Aono M, Saji H (2003) Light-controlled expression of a gene encoding L-galactono-γ-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci 164:1111–1117
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tokunaga T, Miyahara K, Tabata K, Esaka M (2005) Generation, and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for L-galactono-1,4-lactone dehydrogenase. Planta 220:854–863
Valpuesta V, Botella MA (2004) Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant. Trends Pant Sci 9:573–577
Vamos-Vigyazo L (1981) Polyphenol oxidase and peroxidase in fruits and vegetables. Crit Rev Food Sci Nutr 15:49–127
von Heije G (1986) Mitochondrial targeting sequences may form amphiphilic helices. EMBO J 5:1335–1342
Walker JRL (1995) Enzymatic browning in fruits: its biochemistry and control. In: Lee CY, Whitaker JR (eds) Enzymatic browning and its prevention. ASC symposium series 600, Washington, DC, pp 8–22
Wang J, Yu Y, Zhang Z, Quan R, Zhang H, Ma L, Deng XW, Huang R (2013) Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell 25:625–636
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Wolucka BA, Persiau G, Van Doorsselaere J, Davey MW, Demol H, Vandekerckove J, Van Montagu M, Zabeau M, Boerjan W (2001) Partial purification and identification of GDP-mannose 3′,5′-epimerase of Arabidopsis thaliana, a key enzyme of the plant vitamin C pathway. Proc Natl Acad Sci USA 98:14843–14848
Xu Y, Zhu X, Chen Y, Gong Y, Liu L (2013) Expression profiling of genes involved in ascorbate biosynthesis recycling during fleshy root development in radish. Plant Physiol Biochem 70:269–277
Yu L, Jiang J, Zhang C, Jiang L, Ye N, Nu Y, Yang G, Liu E, Peng C, He Z, Peng X (2010) Glyoxylate rather than ascorbate is an efficient precursor of oxalate biosynthesis in rice. J Exp Bot 61:1625–1634
Zhang L, Wang Z, Xia Y, Kai G, Chen W, Tang K (2007) Metabolic engineering of plant L-ascorbic acid biosynthesis: recent trends and application. Crit Rev Biotechnol 27:173–182
Zhou Y, Tao QC, Wang ZN, Fan R, Li Y, Sun XF, Tang KX (2012) Engineering ascorbic acid biosynthetic pathway in Arabidopsis leaves by single and double gene transformation. Biol Plant 56:451–457
Zuckerkandl E, Pauling L (1965) Evolutionary divergence and convergence in proteins. In: Bryson V, Vogel HJ (eds) Evolving genes and proteins. Academic Press, New York, pp 97–166
Acknowledgments
We are indebted to Donato Giannino for providing the derivative binary vector pVDH282 and for the useful suggestions about transformation protocol of L. sativa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Landi, M., Fambrini, M., Basile, A. et al. Overexpression of L-galactono-1,4-lactone dehydrogenase (L-GalLDH) gene correlates with increased ascorbate concentration and reduced browning in leaves of Lactuca sativa L. after cutting. Plant Cell Tiss Organ Cult 123, 109–120 (2015). https://doi.org/10.1007/s11240-015-0819-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0819-y