Abstract
The phenomenon of browning can be a major limitation for orchid callus culture, causing a loss of regenerative capacity and subsequent cell death. This research was conducted to determine the effect of in vitro culture period (1–3 months) on the appearance of tissue browning. Biological alterations at the cellular and subcellular levels, as well as biochemical aspects, were examined. Callus derived from bisected pigeon orchid (Dendrobium crumenatum Swartz) protocorms were cultured on modified Vacin and Went solid medium supplemented with 0.5 mg L−1 1-naphthaleneacetic acid and 1 mg L−1 6-benzyladenine. Callus that was not subcultured was collected at 1-month intervals and examined for structural (using scanning electron microscopy and transmission electron microscopy) and biochemical alterations associated with browning. Three-month-old unsubcultured callus cells were loosely arranged and major organelles were deformed, exhibiting nuclear envelope breakage, dysfunctional mitochondria, tannin-filled vesicles and swollen chloroplasts, relative to 1-month-old green callus, which served as the control. Ultrastructural disorganization involving the nucleus, mitochondria and chloroplasts typified enzymatic oxidative browning. Browning 3-month-old callus had significantly higher polyphenol oxidase (PPO) activity and total phenolic content than control callus. The levels of PPO and total phenolics may serve as useful biochemical markers when selecting suitable callus for subsequent regeneration trials or for particle bombardment in genetic transformation experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue browning is a serious obstacle in the maintenance of plant tissue cultures, and is believed to be caused by the accumulation and oxidation of phenolic compounds (Jones and Saxena 2013) resulting in the inhibition of growth and a decrease in the regeneration ability of plant cells (Arnaldos et al. 2001; Ling et al. 2007; Chugh et al. 2009; Mondal et al. 2013). Yoruk and Marshall (2003) revealed many factors responsible for tissue browning, including the presence and action of reactive oxygen species (ROS), phenolic compounds and oxidative enzymes, all of which are involved in the process of phenolic oxidation.
In general, ROS can be generated during normal plant metabolic processes, namely photosynthesis and respiration, and their levels can increase in response to abiotic stresses such as cold, UV light, salinity and drought (Sharma et al. 2012; Tripathy and Oelmüller 2012). Moreover, mechanical damage in vitro, such as wounding during explant preparation, is a key factor attributed to browning during callus induction as a result of in an increase in ROS (Wang et al. 2011). Various organelles and cellular structures such as chloroplasts, mitochondria, peroxisomes, plasmalemma, the endoplasmic reticulum (ER) and cell walls are the sites of production and scavenging of ROS in response to stress (Gill and Tuteja 2010). This excessive generation of ROS can induce oxidative damage in various cellular components such as the deformation of chloroplasts, disintegration of the plasmalemma, and breakage of the nuclear membrane, all of which are ultimately caused by the oxidation of proteins, the peroxidation of lipids, and hydroxylation of nucleic acids (Mittler 2002; Gill and Tuteja 2010). The appearance of excess ROS, which is accompanied by organellar disorganization, results in cell death (Kratsch and Wise 2000; Wi et al. 2005; Wahid et al. 2007; Sharma et al. 2012), which can be visualized by the browning colour of cells.
Liu and Chen (2010) reported that the production of ROS in Phalaenopsis spp., including the hydroxyl radical (·OH), superoxide anion (O2·−) and hydrogen peroxide (H2O2) increased significantly during callus induction. This overproduction of ROS indicated the presence of oxidative stress negatively affected plant growth and development (de Pinto and de Gara 2004; Pitzschke et al. 2009). Zhou et al. (2009) had earlier shown that the presence of gibberellic acid repressed Phalaenopsis callus browning as a result of repressed phenylanine ammonia lyase (PAL; EC 4.3.1.5) activity and gene expression.
Several oxidative enzymes are involved in enzymatic browning, namely PAL, peroxidase (POD, EC 1.11.1.7) and polyphenol oxidase (PPO, EC 1.10.3.1) (Xu and Li 2006; Yingsanga et al. 2008). In particular, PPO plays a more crucial role than other enzymes (He et al. 2009). PPO is a copper-containing enzyme implicated in two oxidative reactions associated with the oxidation of phenolic compounds, including the hydroxylation of monophenols to o-diphenols and the oxidation of o-diphenols to o-quinones (Mayer 2006). These highly reactive quinones can then polymerize with other quinones or amino acids to form brown melanin-like pigments which are the major cause of enzymatic browning and are extremely harmful to plant cells (Gacche et al. 2006; Oren-Shamir 2009). These pigments cause the visual symptoms commonly termed “browning” in plant tissue culture. PPO is localized on the thylakoid membrane of chloroplasts while phenolic substrates are present in the vacuole (Toivonen and Brummell 2008). This enzyme-substrate compartmentation can prevent the occurrence of in vivo oxidation. However, the latent form of PPO can be activated when it is released from plastids after degradation of the thylakoid membrane followed by chloroplast breakdown caused by oxidative damage (Dai 1993; Wang et al. 2010). In addition, physical damage such as explant preparation or wounding can break down this compartmentation, increasing oxidation and thus browning (North et al. 2012). Incidentally, root growth of tree peony in vitro also increased the activity of PPO (Fu et al. 2011). This active PPO then mixes with the vacuolar substrates (i.e., phenolic compounds) inducing the formation of toxic o-quinone which leads to the production of a brown complex pigment (Ahmad et al. 2013).
Tang and Newton (2004) reported that maximum PPO activity, which was observed in a non-browning callus line of Pinus virginiana Mill., gradually declined over a 3-week culture period. However, PPO activity increased dramatically in the first 3 weeks and increased continuously during culture of a browning callus line. It might, in the latter case, have been due to the presence of oxidative stress during the extended culture period, but ultimately proving, through a simple experimental design, that PPO activity is unequivocally associated with browning.
In various plant species, embryogenic callus has totipotency and is thus usually utilized as the target material for studying somaclonal variation, somatic hybridization and genetic transformation (Belarmino and Mii 2000; Hossain et al. 2013). In particular, indirect somatic embryogenesis via an intervening callus phase can provide a high rate of formation and conversion of somatic embryos into plantlets (Zhao et al. 2008). This callus-mediated regeneration is also one of the favourite techniques for rapid in vitro propagation and for biotechnological applications that are widely used in many plants, including orchids (Tokuhara and Mill 2001; Debnath et al. 2006). Embryogenic callus is in fact, in Cymbidium, a cluster of immature protocorm-like bodies, or somatic embryos (Teixeira da Silva and Tanaka 2006). Successful callus culture is based on its ability to be maintained for an extended period of time—ideally without changes in regenerative ability—and to regenerate whole plants when needed. Unfortunately, in certain orchids, callus culture is still limited due to its slow growth, difficulties in maintaining callus cultures and their tendency to brown and become necrotic (Begum et al. 1994; Chang and Chang 1998; Khosravi et al. 2008). Although callus can be maintained by frequent subcultures onto fresh media, this technique can give rise to somaclonal variation (Bairu et al. 2011). Furthermore, culturing callus without subculture can increase the accumulation of free radicals, lipid peroxidation and also arrest the morphogenic transition of plant cells (Benson 2000; George 2008). Therefore, efficient callus culture without signs of browning is a key requirement in orchid tissue culture, and thus genetic transformation. To better understand the phenomenon of browning during orchid tissue culture, the incubation period and how it affects the biological response at the biochemical and cellular and/or subcellular levels must be determined. This is important since the timing of sampling can influence the interpretation of results (Teixeira da Silva and Dobránszki 2013), and thus negative aspects that hinder the in vitro development of callus will also thus reflect themselves in the quantitative output of that study.
Dendrobium crumenatum Swartz (pigeon orchid), a common epiphytic orchid widely distributed in South East Asia such as India, Sri Lanka, Myanmar, Indo-China, Malay Peninsula, Philippines and Taiwan (Leong and Wee 2013), has a very unique and attractive white and fragrant flower. This orchid also exhibits synchronous flowering which is triggered by a sudden drop in temperature (about 10 °C), usually after heavy rainfall and then blooms 9 days later (Meesawat and Kanchanapoom 2007). This orchid species can be a good choice for studying the phenomenon of browning because it displays rapid growth and provides a high rate of callus induction and proliferation (Meesawat and Kanchanapoom 2002). Meesawat and Kanchanapoom (2002) reported that axillary bud-derived callus could be induced on a modified Vacin and Went (VW) solid medium (Vacin and Went 1949) containing 0.1 mg L−1 1-naphthaleneacetic acid (NAA) and 1 mg L−1 6-benzyladenine (BA) within 4 weeks of culture. Other than these studies, the in vitro culture of this orchid has not been described.
The effects of oxidative stress induced by an extended period of culture in vitro at the biochemical level have been revealed (Xu and Li 2006; Zhao et al. 2010; Shi 2011), to a very limited extent in orchids (Phalaenopsis and Cymbidium), but alteration at the subcellular level associated with browning phenomenon are still poorly characterized even though the brown tissue is easy to visualize.
Therefore, the aim of this study was to determine the level of browning in callus cultures of a potential model orchid in response to subculture period during callus culture to better understand the ultrastructural dynamics underlying callus browning. This process was assessed by ultrastructural and biochemical analyses. A clearer understanding of the phenomenon of browning provides valuable insight about the browning process and could be used in a practical way to prevent or reduce tissue browning during callus culture of D. crumenatum or other important orchids that show similar browning of callus or other tissues in vitro. Knowledge of the biochemical nature of browning callus would also allow for the selection of the most suitable callus for genetic transformation experiments, for example, particle bombardment.
Materials and methods
Plant material
Dendrobium crumenatum Swartz plants were cultivated in a shaded greenhouse at a photosynthetic photon flux density (PPFD) of 30 µmol m−2 s−1 under natural conditions (approximately 28 ± 2 °C) at the Department of Biology, Faculty of Science, Prince of Songkla University, Thailand. Blooming flowers of more than five-year-old mother plants were hand cross-pollinated and 6-week-old capsules were surface-sterilized in 1.2 % sodium hypochlorite (NaOCl) solution (1.14 % active chlorine) containing 1-2 drops of Tween 20 for 20 min and rinsed three times with sterile distilled water. Each capsule was then cut longitudinally and seeds were scooped out and placed into 30 mL of Vacin and Went (VW) (Vacin and Went 1949) liquid medium containing 2 % sucrose and 2 % peptone. The pH of the media was adjusted to 5.3 with 1 N NaOH or HCl prior to autoclaving at 121 °C for 20 min. Seeds, which were subcultured monthly, were maintained under a 16 h photoperiod provided by cool daylight fluorescent lamps (36 Watts, Philips, Bangkok, Thailand) at 23 µmol m−2 s−1 on an orbital shaker (120 rpm) at 25 ± 2 °C for 3 months. The protocorms that formed were used as explants for callus induction.
Callus induction
For callus induction, 3-month-old protocorms (approximately 2–3 mm in diameter) at stage 3 of developmental growth (Stewart and Zettler 2002) were transversely bisected without excising the shoot tip and placed on 10 mL of modified VW solid medium consisting of VW macro salts (full strength), Murashige and Skoog (1962; MS) micro salts (half strength), 2 % sucrose, 2 % peptone, 0.5 mg L−1 1-naphthaleneacetic acid (NAA) (Fluka Chemie GmbH, Buchs, Switzerland) and 1 mg L−1 6-benzyladenine (BA) (Sigma-Aldrich Co., St. Louis, MO, USA). The medium was solidified with 0.2 % Phytagel (Sigma-Aldrich Co.) and is referred to hereafter as callus induction medium (CIM). The pH of the media was adjusted to 5.3 with 1 N NaOH or HCl prior to autoclaving at 121 °C for 20 min. The transversely bisected protocorms (4 segments per 60 mL glass bottle) were then cultured under conditions of in vitro seed culture as described previously. Green compact callus derived from bisected protocorms obtained after 8 weeks of culture and subcultured at 1-month intervals served as the material for subsequent analyses. All CIM-related media constituents were based on optimized in-house trials.
Callus culture
In order to determine the effect of culture period on callus culture, callus [100 mg, fresh weight (FW)] was transferred onto 60 mL glass bottle contained 10 mL of CIM. Callus was maintained continually on the same CIM without subculturing under the same culture conditions indicated above. One-month-old proliferated callus served as the control. Samples in which callus was predominant (>80 % of the explant) were collected at monthly intervals for 3 months and subjected to ultrastructural and biochemical analyses.
Histological and histochemical analyses
Ten pieces of callus at the end of each culture period were fixed in FAA II [formaldehyde (Ajax Finechem, Taren Point, Australia): glacial acetic acid (J.T. Baker, Phillipsburg, NJ, USA): 70 % ethyl alcohol (Merck, Billerica, MA, USA); 5:5:90 v/v/v] for 48 h (Ruzin 1999). Fixed tissues were dehydrated in a tertiary-butyl-alcohol series and embedded in paraffin wax (Histoplast PE; Richard-Allan Scientific, Kalamazoo, MI, USA). Sections 6 µm thick were cut with a rotary microtome (Shandon Southern Product Ltd., Cheshire, UK). Callus sections were stained with Delafield’s hematoxylin and safranin (Ruzin 1999) to investigate general structures. To indicate the characteristic meristematic cells based on qualitative observations, histochemical analyses, namely the periodic acid-Schiff (PAS) reaction (Feder and O’Brien 1968) and toluidine blue O (TBO) staining (Ruzin 1999), were performed to examine the presence of carbohydrates (insoluble polysaccharide) and phenolic compounds, respectively. All sections were viewed under an Olympus BX 51 TRF light microscope (Olympus Optical Co. Ltd., Tokyo, Japan) and photographs were captured on an Olympus DP 72 digital camera (Olympus Optical Co. Ltd.).
Scanning electron microscopy
Scanning electron microscopy (SEM) was performed to investigate alterations in cellular structures during callus culture. Six samples were fixed in SEM fixative [10 % formaldehyde (Ajax Finechem), 5 % acetic acid (J.T. Baker), 45 % ethanol (Merck) and 1 % Triton X-100 (Panreac, Barcelona, Spain)] at 4 °C for 2 h. Samples were washed in 0.1 M phosphate buffer (pH 7.2) and dehydrated through an ethanol series including 30, 50, 70, 80, 90, 95 and 100 % ethanol (15 min each wash). Samples were dried with a Polaron CPD 7501 critical point dryer (VG. Microtech, East Sussex, UK), covered with gold by a SPI-MODULE sputter coater (SPI Supplies Division of Structure Probe Inc., West Chester, PA, USA), and examined with a Quanta 400 scanning electron microscope (FEI, Brno, Czech Republic) at an accelerating voltage of 10 kV.
Ultrastructural analysis
To determine the influence of browning on subcellular structures, six intact callus clumps were fixed overnight in a cold (4 °C) solution of 2.5 % glutaraldehyde (Sigma-Aldrich Co.) in 0.1 M phosphate buffer (pH 7.2) to which 1 % caffeine (Sigma-Aldrich Co.) was added. Caffeine was added to precipitate phenolics, i.e., to localize phenolic compounds within plant cells (Mueller and Greenwood 1978). Pre-fixed samples were washed three times with 0.1 M phosphate buffer (pH 7.2) and post-fixed with 1 % osmium tetroxide (OsO4) (Electron Microscopy Sciences, Hatfield, PA, USA) in 0.1 M phosphate buffer (pH 7.2) for 1 h at room temperature. The post-fixed tissues were washed three times with distilled water, dehydrated through a graded ethanol series (70, 80, 90 and 100 % ethanol) for 15 min each and infiltrated with EMbed 812 (Electron Microscopy Sciences). Ultrathin sections (60 nm thick) were cut using a RMC MTXL ultramicrotome (Boeckeler Instruments, Arizona, TX, USA) and stained in 2 % uranyl acetate (Electron Microscopy Sciences) for 10 min followed by lead citrate (Electron Microscopy Sciences) for 5 min to obtain optimum contrast (Reynolds 1963). Lead citrate was prepared by mixing solution A (1.33 g Pb(Na3)2 in 15 mL of distilled water) with solution B (1.7 g Na3C6H5O7·2H2O in 15 mL of distilled water) (at a 1:1 ratio). Sections were viewed with a JEM-2010 transmission electron microscope (JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 160 kV.
Preparation of extract
Cultured callus at each collection (1, 2 and 3 months of inoculation) were harvested and prepared as aliquots for the determination of PPO activity and total phenolic content. The extraction was carried out as described by Tang and Newton (2004) with some modifications. Callus (400 mg FW) was homogenized in 400 µL of 0.1 M sodium phosphate buffer (pH 7.2) supplemented with 0.1 % (w/v) sodium dodecyl sulfate (SDS) (EMD Millipore Co., Billerica, MA, USA). The homogenate was centrifuged at 12,000×g for 10 min at 4 °C in a Mikro 200R refrigerated centrifuge (Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany). The supernatant was collected, and aliquots (100 µL of supernatant) were stored at -20 °C for the determination of PPO activity and total phenolic content.
PPO activity assay
To evaluate PPO activity using a modified method of Dai (1993), 10 µL of crude extract was mixed with a reaction mixture containing 150 µL of 0.5 M catechol (Sigma-Aldrich Co.) and 590 µL of 0.1 M sodium phosphate buffer (pH 7.2). The absorbance of samples was measured at 490 nm for 3 min at 15-s intervals with a Genesys 20 visible spectrophotometer (Thermo Fisher Scientific, Somerset, NJ, USA). One unit of PPO activity was defined as the amount of enzyme that caused a change of 0.01 in the absorbance per min (Dai 1993).
Determination of total phenolic content
The assay of total phenolic content was performed according to Park et al. (2006) with some modifications to determine the total phenolic content during callus culture without subculture. Briefly, 10 µL of the supernatant was mixed with 200 µL of Folin-Ciocalteu phenol reagent (Sigma-Aldrich Co.) and 1 mL of 10 % sodium carbonate (Ajax Finechem). After incubating the solution for 20 min at room temperature, the absorbance of the solution was measured at 735 nm with a Genesys 20 visible spectrophotometer (Thermo Fisher Scientific). The total phenolic content was calculated based on a gallic acid calibration curve and expressed as milligrams of gallic acid equivalents (GAE) per gram of fresh weight (mg GAE g−1 FW).
Determination of chlorophyll and carotenoid contents
Callus (100 mg FW) was ground on ice with a chilled mortar and pestle in 2.3 mL of 80 % acetone in the dark. Homogenized tissue was centrifuged at 10,000×g for 10 min at room temperature with a Sorvall Biofuge PICO centrifuge (Kendro Laboratory Products, Osterode, Germany). The optical density of the supernatant was measured with an HP-8453E UV–visible spectrophotometer (Hewlett Packard, Palo Alto, CA, USA) at 480, 510, 645 and 663 nm. Total chlorophyll (chl), chl a, chl b and carotenoid contents were calculated in mg g−1 FW using the following formulae (Misra et al. 2010):
where A480, A510, A645 and A663 are the absorbance values (optical density) at 480, 510, 645 nm and 663 nm, respectively; V is the volume of extracted solution (mL); FW is the fresh weight of callus (g).
Statistical analyses
The effects of culture period on PPO activity, total phenolic content and pigment content were determined. Each experiment in the biochemical assays was designed according to a completely randomized design and repeated twice with three replicates per treatment. Data were analyzed statistically using a one-way analysis of variance (ANOVA) and significant differences between means were determined by Duncan’s multiple range test at P ≤ 0.05 (Bewick et al. 2004) using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) software.
Results and discussion
Morphological, histological and histochemical features during callus culture
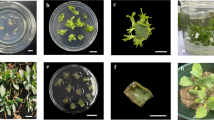
Bisected protocorm-derived callus (Fig. 1a) was cultured on modified VW medium. Callus discoloration was observed after 3–4 weeks as small brown spots on the surface of the callus mass (Fig. 1b). This callus gradually turned pale green after 2 months in culture (Fig. 1c) and finally turned dark brown within 3 months (Fig. 1d). Callus discoloration was attributed to oxidative browning which is a consequence of phenolic oxidation that induces the formation of brown pigments (Sapers et al. 2002).
Changes at the cellular and subcellular levels were also detected and monitored during the 3-month callus culture period. The histological study demonstrated that the 1-month-old callus of D. crumenatum consisted of uniform and tightly arranged parenchymatous cells (Fig. 2a, b). However, there were no obvious differences between the 2-month-old callus (Fig. 2c, d) and 1-month-old green callus. In contrast, a loose and disordered cell arrangement was clearly noticed in 3-month-old callus displaying browning (Fig. 2e, f). Laukkanen et al. (2000) reported that yellow-brown callus of Scots pine exhibited a thick cell wall and the absence of cytoplasm compared with green callus. Changes in colour and cell structure of callus were also examined in brown and non-oxidized callus of Jatropha curcas (He et al. 2009). In the latter study, it was revealed that cells of non-oxidized callus were spheroid and compactly arranged while irregular and loosely arranged cells were observed in brown callus.
Cellular structure of bisected protocorm-derived callus of Dendrobium crumenatum. a, b 1- and c, d 2-month-old callus exhibiting uniform and tightly arranged parenchyma cells (arrows) compared to e, f 3-month-old browning callus showing variable and disordered cells with partially collapsed cells (arrows)
Histochemical observations showed the accumulation of ergastic substances such as tannin (TBO staining)—a phenolic compound—and insoluble carbohydrate (PAS reaction) during callus culture. One- and 2-month-old callus exhibited a blue to dark blue colour which indicated clear nuclei from which tannin was absent (Fig. 3a, b). In contrast, 3-month-old callus with a greenish-blue colour (Fig. 3c) after TBO staining revealed an increase in tannin accumulation more than that of 1-month-old callus. Tannin is a phenolic by-product and is considered to be a waste product that has adverse physiological effects on plant cells, contributing to cell growth inhibition and eventually cell death (Santiago et al. 2000). Moreover, the 1- and 2-month-old callus showed a greater accumulation of insoluble carbohydrate (Fig. 3d, e) than in 3-month-old callus (Fig. 3f). This corresponds to the histological detection of carbohydrates in the embryogenic callus of Vanilla planifolia (Palama et al. 2010). Thus, the present results indicate that browning callus had accumulated little insoluble carbohydrates leading to a lack of an energy sources required for further development which could result in the loss of totipotency in morphogenically competent cells (George 2008).
Histochemical observations in a, d 1-month-old callus, b, e 2-month-old callus and c, f 3-month-old callus obtained from bisected Dendrobium crumenatum protocorms. After TBO staining, a 1- and b 2-month-old callus show a blue colour indicating the absence/or a low amount of tannin-like material whereas c 3-month-old browning callus exhibits a green colour showing the accumulation of tannin (arrow). PAS reaction detects the presence of insoluble polysaccharides with a magenta colour (arrow) in the d 1- and e 2-month-old callus compared to f negative PAS staining observed in the browning callus. (Color figure online)
Ultrastructural analysis
Alterations to subcellular characteristics during callus culture are described next based on the age of the callus. Throughout this paper, the term callus has been used to represent the singular and plural forms of callus (Teixeira da Silva 2012).
One-month-old callus
In the 1-month-old callus showing a strong green colour, a thin cell wall, tonoplast and plasmodesmata were clearly observed (Fig. 4a, b). The well-organized organelles, including a nucleus, mitochondria and chloroplasts, were very apparent in the cytoplasm (Fig. 4c–g). Globular chromoplasts (Fig. 4c) and other plastids, including chloro-chromoplasts (Fig. 4d), were noticed. These plastids contained small plastoglobuli, which are lipoprotein particles in plastids that contain lipid isoprenoid-derived metabolites (Bréhélin et al. 2007). Plastoglobuli have been shown to play a key role in the chloroplast to chromoplast transition and carotenoid metabolism (Bian et al. 2011).
TEM micrographs of 1-month-old callus cultured on modified VW medium. a Panoramic view of 1-month-old cell showing distinct tonoplast (t), thinning cell wall (cw), regular nucleus (n) and numerous mitochondria (m) (bar 2 µm). Magnified views of b plasmodesmata (arrows) (bar 1.25 µm), c chromoplast (chr) with plastoglobuli (arrow heads) (bar 1 µm), d plastoglobuli (arrow heads) in chloro-chromoplast (bar 500 nm), e chloroplasts (chl) containing starch grain (s) (bar 5 µm), f amyloplast (bar 500 nm), g dictyosome (d), free ribosomes (r) and rough ER (RER) within the cytoplasm (bar 500 nm)
This study exhibited two types of starch-containing plastids: chloroplasts (Fig. 4e) and amyloplast (Fig. 4f). This peculiarity was consistent with the ability of plastids to differentiate or redifferentiate between these and several other forms, depending on the function in the cell, as shown in duckweed (Spirodela polyrhiza), a monocotyledonous plant (Wang and Messing 2012). Moreover, this starch-containing amyloplast could function as a source and sink organelle for starch biosynthesis as it still retained a simple thylakoid membrane (Wang and Messing 2012). The appearance of this organelle at the subcellular level coincided with the presence of carbohydrates after PAS staining, indicating that large numbers of amyloplasts in plant cells exist, representing active metabolism of cells, and might play an important role in a cell’s ability to regenerate from non-browning callus of pigeon orchid (Zenkteler and Kwaśna 2007).
Furthermore, a dense cytoplasm that was rich in free ribosomes, cisternae of rough or granular ER and dictyosomes was also present (Fig. 4g). Appezzato-da-Glória and Machado (2004) revealed that a ribosome-rich cytoplasm, a conspicuous dictyosome and rough ER (RER) were observed in the meristemoid cells of Bauhinia forficata Link and Glycine max (L.) Merrill. The presence of these organelles indicated that these cells had high metabolic activity during plant cell growth as they were able to synthesize protein and cell wall components (Pihakaski-Maunsbach et al. 1993; Appezzato-da-Glória and Machado 2004).
These well-organized organelles, including a clearly defined nucleus, rounded mitochondria, plastids (chloroplasts, chromoplasts and amyloplasts), numerous free ribosomes, RER and an active dictyosome suspended in dense cytoplasm reported in our study (Fig. 4) are consistent with observations made by others for embryogenic and organogenic callus of banana and peach palm which could develop further to form plantlets (Steinmacher et al. 2011; de Oliveira Ribeiro et al. 2012).
Two-month-old callus
Two-month-old callus was mostly pale green. The plasmalemma and organellar membranes were evident. In addition, an empty double-membranous vesicle which was very similar to an autophagosome-like vesicle detected in Arabidopsis cells undergoing programmed cell death (PCD) (Hofius et al. 2009) was obviously present (Fig. 5a, b). The secretion of cytoplasmic material to be deposited at the cell wall was observed (Fig. 5c). The appearance of these membranous vesicles was also prevalent in the cortical cells of cucumber (Cucumis sativus L.) after exposure to chilling stress (Lee et al. 2002). The alteration and initial degeneration of crucial organelles, particularly the nucleus, mitochondria and chloroplasts—relative to 1-month-old callus—were also noticed in 3-month-old callus (Fig. 7).
TEM micrographs of browning callus after a 2-month culture period on modified VW medium. Callus cells exhibiting a a simple double-membranous structure (bar 200 nm) and b concentric membranous whorls (bar 250 nm). c Membranous vesicle showing the secretion of cytoplasmic material to be deposited at the walls (bar 100 nm)
Three-month-old callus
Three-month-old callus was light to dark brown in colour. Various organelles in 3-month-old callus cells were completely disorganized. Brown cells had minimal cytoplasm, numerous degraded thylakoid-containing plastids and shrunken plasmalemma resulting in detachment of the plasmalemma from the cell wall (Fig. 6a). A cell with a shrunken membrane indicates membrane dysfunction, an increase in cell membrane permeability and the leakage of ions which are distinguishing features of the response to oxidative stress (Sunkar 2010), similar to the alteration of organelles in (oxidative) stress-induced cells of other plants and in the browning callus of D. crumenatum in this study. Oxidative stress caused by the over-production of ROS can occur in in vitro culture (Cassells and Curry 2001). Thus, explant wounding and various environmental stresses such as hormone, mineral composition, water and light can lead to enhanced ROS production (Cassells and Curry 2001). When D. crumenatum callus is not subcultured onto fresh medium, factors leading to oxidative stress in vitro might have been caused by wounding of the callus mass since the beginning of callus culture on CIM, prolonged culture period with nutrient deficiency, environmental stress, long-term oxidative stress and negatively impacted metabolic processes of plant cells, including photosynthesis and respiration. Factors such as these contribute to ROS production resulting in an imbalance between ROS and ROS scavengers (Adelberg et al. 1997; Apel and Hirt 2004; Liu and Chen 2010). Three-month-old callus also had distortions in thickened cell walls (Fig. 6a), similar to malformed cell walls observed by Gunawardena et al. (2007) in lace plant where cell wall degradation was caused by the action of ROS that lead to rapid cell death. Cell wall thickening also causes the arrest of cell growth and is attributed to reduced extensibility of the cell wall (Singh and Prasad 2009).
TEM micrographs of browning callus after a 3-month culture period on modified VW medium. Necrotic cells exhibiting signs of cell damage and eventually cell death. a Brown cell showing disorganized organelles and twisted cell wall with shrunken membrane (arrow) (bar 5 µm). Magnified images of (b), an area of the cytoplasm showing numerous small vesicles (arrows) and osmiophilic material (arrow head) (bar 1 µm). c Magnified view of osmiophilic deposition (arrow) that appears to be discharged into the vacuole (v) and attached to the inner surface of the tonoplast (arrow head) (bar 500 nm). d Dense fibrillar structures (asterisk) between the cell wall (bar 1 µm), e, f numerous smooth ER (SER) (bar 500, 100 nm), g peroxisomes (p) and lipid bodies (lb) (bar 500 nm)
Three-month-old callus exhibited numerous microvesicles compared with 1-month-old callus, in which such vesicles were absent (Fig. 6b). These vesicles contained dense pigmentation consisting of a dark osmiophilic material, tannin, which was attached to the inner surface of the vacuolar membrane (Fig. 6c). Tannin was also deposited in the cytoplasm (Fig. 6c, arrow). Laukkanen et al. (2000) reported that tannin accumulated to a high level in the cytoplasm and intercellular spaces and could lead to tissue browning in Pinus sylvestris L. callus cells. In particular, the accumulation of condensed tannin in the cytoplasm was one factor causing browning and death of plant cells due to the oxidation of tannin and other polyphenolic compounds (Laukkanen et al. 2000; Ahmad et al. 2013). In addition, condensed tannin can be deposited in the browning cells of Phalaenopsis leaves when cultured in vitro (Xu et al. 2005).
Laukkanen et al. (2000) also reported the presence of dense fibrillar structures between neighboring cell walls in P. sylvestris browning callus, indicating high oxidative stress in these cells. Similar fibrillar components could be found in 3-month-old callus cells of D. crumenatum (Fig. 6d). Three-month-old callus displayed more smooth ER (SER) (Fig. 6e, f), peroxisomes with a large crystal-like structure and lipid bodies (Fig. 6g) compared to 1-month-old callus.
Peroxisomes are the site of H2O2 scavenging in response to H2O2 that is formed by photorespiration and β-oxidation of fatty acids (Sandalio et al. 2013). These peroxisomes, which contain an antioxidant enzyme catalase, form by budding off from a specific segment of the SER (Hu et al. 2012). The SER also plays a role in the formation of lipid bodies both in the cytoplasm and plastids, and are produced in response to oxidative stress (Chapman et al. 2012). Moreover, increased dilation and vesiculation of SER are evidence of the response to oxidative stress (Lee et al. 2002). However, dictyosomes were not clearly observed in 2-month-old callus or in 3-month-old callus in this study. The disappearance of dictyosomes in browning callus may imply that the formation of vesicles may be mainly involved with ER although previous reports revealed that vesicles could originate from both the ER and dictyosomes (Matile and Moor 1968; Stefanowska et al. 2002; Mauseth 2014).
Ultrastructural alteration in important organelles
Various organelles underwent changes during callus culture. However, the important organelles, particularly the nucleus, mitochondria and chloroplasts showed distinct alterations.
One-month-old callus exhibited a nucleus with a nucleolus, a distinct nuclear envelope and two types of chromatin (heterochromatin and euchromatin) (Fig. 7a). These structures are related to the characteristics of totipotent embryogenic cells that have a nucleus containing less perinuclear heterochromation but more euchromatin (Verdeil et al. 2007). The 1-month-old callus still exhibited numerous round mitochondria, each with a distinct double-membrane envelope (Fig. 7b). The isodiametric shape of the mitochondria is an important site of both high metabolic activity and respiration rate, which could be observed in the embryogenic cells of Inga vera Willd. subsp. Affinis (DC.) T.D. Penn. (Stein et al. 2010). The mitochondrion is an important organelle involved in a wide range of biosynthetic reactions that maintain eukaryotic life and that is involved with the response of a plant to oxidative stress (Bi et al. 2009). Chloroplasts containing either a large starch grain or several starch grains were clearly observed (Fig. 7c, d). Forters and Pais (2000) reported that starch accumulated in callus cells and prenodular structures of Humulus lupulus var. Nugget which could provide large amounts of energy required for organ initiation and further development. One or more starch grain-containing chloroplasts were clearly observed during organogenesis of Glycine max (L.) Merrill and Bauhinia forficata Link (Appezzato-da-Glória and Machado 2004) and Zea mays (Marín-Méndez et al. 2009), indicating the relationship between these plastids and the organogenic potential of plant cells. Moreover, the presence of starch grains was also related to the acquisition of embryogenic potential in the plant species Drosera spathulata Labill. (Bobák et al. 2004), Picea abies and Picea omorika (Hazubska-Przybył et al. 2008) and embryogenic cells of Inga vera (Stein et al. 2010). Thus, starch accumulation might be an important factor for supporting a morphogenic pathway, both organogensis and embryogenesis, which are energy-requiring processes (George 2008).
TEM micrographs of ultrastructural alteration of important organelles during callus culture. One-month-old callus exhibits a a nucleus with a clear nuclear envelope (ne), nucleolus (nu), euchromatin (eu) and heterochromatin (het) (bar 1 µm). b Well-organized mitochondrion (m) with a mitochondrial double-membrane system (arrow) and numerous cristae (bar 100 nm). High magnification of chloroplast with c one or d several starch grains (s) and a well-developed thylakoid membrane system (th) (bar 500 nm, 2 µm). Two-month-old callus showing e peripheral and nucleoplasmic heterochromatin (bar 1 µm). f Magnified images of nuclear envelope exhibiting invagination of double-layered nuclear membrane (bar 500 nm). g Spherical mitochondria with swollen cristae and less dense matrix material (bar 200 nm). h Chloroplast containing lipid bodies (arrows) and no starch grains; the thylakoid membrane starts to swell (bar 500 nm). Three-month-old callus showing i disintegrated nucleus (arrow) (bar 1 µm). j Irregular mitochondrion with unclear cristae (bar 200 nm). k Chloroplast with strongly dilated thylakoid membranes (bar 1 µm)
Two-month-old callus showed a nucleus with a nucleolus, a nuclear pore and two distinct types of chromatins, peripheral and nucleoplasmic heterochromatins, which were detected more than in the euochromatin state (Fig. 7e). The nuclear envelope showed minimal changes with invagination into the nucleus causing an unusual arrangement of nuclear pores (Fig. 7f). Spherical mitochondria showing enlarged cristae and less matrix material were also detected (Fig. 7g). Moreover, alterations in chloroplasts, a major site of PPO (Yoruk and Marshall 2003; Mayer 2006), were observed. Chloroplasts appeared to lack starch grains. A swollen thylakoid membrane and lipid droplets were also observed in some chloroplasts (Fig. 7h). These facets were consistent with the characteristics of plant cells after being exposed to chilling stress (Kratsch and Wise 2000). These injured cells, caused by chilling stress, exhibited swelling chloroplasts with dilated thylakoids, reduced size and number of starch grains as well as the appearance of lipid droplet-containing chloroplasts which were similar to 3-month-old D. crumenatum callus. It is possible that browning callus could be a consequence of oxidative stress due to organelle disorganization and increased lipid peroxidation during callus culture without subculture. An increase in lipid peroxidation as a result of oxidative stress has been attributed to an imbalance between ROS generation and antioxidative enzymes (Tripathy and Oelmüller 2012).
Three-month-old callus showed the condensation of chromatin at the periphery of the nucleus resulting from breakage in some areas of the nuclear envelope (Fig. 7i). Peripheral nuclear chromatin was also noted in plant cells both undergoing PCD (Gunawardena et al. 2005) and plants induced by oxidative stress (Speranza et al. 2007). Swollen mitochondria with deformed inner and outer membranes were noticed (Fig. 7j). These changes were consistent with the typical structure of dysfunctional mitochondria in necrotic cells as a consequence of lipid peroxidation and over-generate ROS (Lee et al. 2002; Yoshinaga et al. 2005). Swollen chloroplasts with swollen thylakoid membranes were still evident (Fig. 7k). These malformed chloroplasts might be attributed to an excess production of ROS during long-term callus culture under stress conditions which was indicated by an increased amount of lipid peroxidation (data not shown). Dilation of thylakoid membranes and the loss of granal stacking in chloroplasts were also observed in Arabidopsis seedlings (Wi et al. 2005) maintained under growth cabinet conditions and in the callus of Nicotiana bigelovii var. bigelovii (Bennici and Tani 2009) after gamma irradiation and salinity stress. These alterations to chloroplasts could reduce the photosynthetic capacity of that organ or tissue (Wu et al. 2009). In contrast, callus that was subcultured at a suitable interval showed chloroplasts with a normal shape since ROS-induced damage could be reduced by subculturing (Peng and Zhang 2009). Unfortunately, subcultures can still induce somaclonal variation (Bairu et al. 2011). Moreover, Brillouet et al. (2013) revealed that swollen chloroplasts containing unstacked thylakoids are the origin of tannosome, an organelle involved in the formation of tannin storage, and the production of condensed tannin. As mentioned above, tannin is a polyphenolic compound that participates in enzymatic browning caused by its oxidation, and resulting in the formation of toxic o-quinone (Ahmad et al. 2013).
These ultrastructural features indicate that browning-related changes involve a wide range of organelles. In particular, chloroplasts, the nucleus and mitochondria are the primary organelles affected by oxidative stress and alterations to these organelles subsequently indicate signs of necrosis. These finding are consistent with ultrastructural changes caused by many oxidative stresses, as described above. Thus, a browning-induced incubation period is a (new) form of abiotic stress.
PPO activity
PPO is a crucial enzyme for defense against oxidative stress in plant cells and is located in the thylakoid membrane of the chloroplast (Mayer 2006). PPO is involved in the enzymatic oxidation of phenolic compounds that result in discoloration and eventual death of explants (Mayer 2006). In D. crumenatum callus cultures, PPO activity increased gradually and continuously throughout the 3-month incubation period. However, maximum enzyme activity was observed in 3-month-old callus and was significantly higher than 1-month-old callus (Table 1). This is in agreement with an increase in PPO activity observed in the browning of bamboo shoots (Huang et al. 2002), Pinus virginiana Mill callus (Tang and Newton 2004), and tree peony roots (Fu et al. 2011). Moreover, changes in PPO activity might be noticed after wounding (Mayer 2006) and several other oxidative stress-inducing situations such as salinity (Weisany et al. 2012) and drought (Terzi et al. 2013).
Total phenolic content
Phenolics are one of the important factors in the browning reaction since they act as substrates of oxidative enzymes. The total phenolic content of 3-month-old D. crumenatum callus was significantly higher than that of the 1- and 2-month-old callus (Table 1). The over-production of phenolics adversely influenced callus growth and a high amount of phenolics resulted in tissue browning as reported in European and Canadian yew (Taxus baccata L. and T. Canadensis Marsh.) (Dubravina et al. 2005) and Cicer arietinum (Naz et al. 2008). Leng et al. (2009) also reported that the total phenolic content and percentage browning in Pistacia vera L. callus gradually increased and was approximately 2- and 2.3-fold higher than the controls, respectively. Thus, the long-term culture of D. crumenatum callus showed a positive relation between the quantity of total phenolic content and the degree of browning. This is consistent with previous reports on browning in Phalaenopsis tissue culture (Xu and Li 2006; Xu et al. 2010; Yin et al. 2006).
Chl and carotenoid contents
A decrease in Chl and carotenoid contents is associated with the deterioration of callus caused by tissue browning (Laukkanen et al. 2000; He et al. 2009). The degradation of Chl, one of the major causes of changes in colour, is a consequence of Chl oxidation which is directly oxidized by the phenoxy radical derived from phenolic oxidation (Yamauchi et al. 2004; Toivonen and Brummell 2008). Moreover, carotenoids are important pigments that act as antioxidants by scavenging singlet oxygen, the triplet state of Chl and other harmful free radicals, thus preventing the destruction of Chl (Zhang et al. 2005; Boguszewska and Zagdanska 2012). Chlorophyll is an essential pigment for photosynthesis, which is one of the most central biological processes. The reduction of Chl is attributed to the degradation of Chl and the inhibition of Chl biosynthesis results in the arrest of photosynthesis and cell growth (Najafi and Jamei 2014). The contents of total Chl, Chl a, Chl b and carotenoids decreased significantly when callus was maintained on CIM for 3 months without subculture (Table 2). A gradual decrease in Chl and carotenoid concentrations were also observed during Jatropha curcas callus culture (He et al. 2009) and shoot proliferation (Misra et al. 2010). In addition, the loss of Chl content is normally considered to be the consequence of Chl degradation resulting from oxidative stress and this incidence also increased ROS production (Misra et al. 2010). The reduction of Chl content induced by abiotic stress, which then stimulates the overproduction of ROS and increased activity of Chl-degrading enzymes (Sevengor et al. 2011), would support a possible mechanism at work in D. crumenatum callus cultures in which the lack of subcultures would be equivalent to an abiotic stress. Water stress also caused a reduction in Chl content in wheat (Triticum durum) by inhibiting Chl synthesis (Ghozlène et al. 2013).
Carotenoids play an important role in overcoming oxidative stress and preventing lipid peroxidation in membranes by decreasing carotenoid content (Amirjani 2010; Boguszewska and Zagdanska 2012) and are effective scavengers that eliminate singlet oxygen and lipid radicals (Ramel et al. 2012). This concept is fortified by studies that observed a significant decrease in carotenoid content with increasing level of salt stress in barley (Hordeum vulgare) grains (El-Tayeb 2005). Yang et al. (2013) revealed that the carotenoid content of wheat (Triticum aestivum L.) seedlings decreased after exposure to UV-B radiation.
Conclusion
Bisected D. crumenatum protocorm-derived callus gradually turns brown as culture period is prolonged, which is a common phenomenon for many orchid callus cultures. The occurrence of browning was confirmed by biochemical assays and by cellular and subcellular observations. These findings demonstrate that tissue browning is related to the period of in vitro tissue culture which can serve as oxidative abiotic stress if no sub-cultures are performed, resulting in alterations to the shape and appearance of many organelles and cellular structures. Early tissue browning can also serve as a sign for the need to sub-culture, before callus dies. Understanding the causes of observed changes during callus browning can provide important clues for predicting the onset of browning. In the future, investigations of enzymatic browning at the molecular level should be done to establish ways to prevent it.
Abbreviations
- BA:
-
6-Benzyladenine
- Chl:
-
Chlorophyll
- CIM:
-
Callus induction medium
- NAA:
-
1-Naphthaleneacetic acid
- PAL:
-
Phenylanine ammonia lyase
- PAS:
-
Periodic acid-Schiff
- PCD:
-
Programmed cell death
- POD:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- RER:
-
Rough endoplasmic reticulum (ER)
- ROS:
-
Reactive oxygen species
- SER:
-
Smooth ER
- SDS:
-
Sodium dodecyl sulfate
- TBO:
-
Toluidine blue O
- VW:
-
Vacin and Went medium
References
Adelberg JW, Desamero NV, Hale SA, Young RE (1997) Long-term nutrient and water utilization during micropropagation of Cattleya on a liquid/membrane system. Plant Cell Tissue Org Cult 48:1–7
Ahmad I, Hussain T, Ashraf I, Nafees M, Maryam RM, Iqbal M (2013) Lethal effects of secondary metabolites on plant tissue culture. Am-Eurasian J Agric Environ Sci 13:539–547
Amirjani MR (2010) Effect of NaCl on some physiological parameters of rice. Eur J Biol Sci 3(1):6–16
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Appezzato-da-Glória B, Machado SR (2004) Ultrastructural analysis of in vitro direct and indirect organogenesis. Revista Brasil Bot 27(3):429–437
Arnaldos TL, Muñoz R, Ferrer MA, Calderón AA (2001) Changes in phenol content during strawberry (Fragaria × ananassa, cv. Chandler) callus culture. Physiol Plant 113:315–322
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173
Begum AA, Tamaki M, Tahara M, Kako S (1994) Somatic embryogenesis in Cymbidium through in vitro culture of inner tissue of protocorm-like bodies. J Jpn Soc Hortic Sci 63(2):419–427
Belarmino MM, Mii M (2000) Agrobacterium-mediated genetic transformation of a Phalaenopsis orchid. Plant Cell Rep 19:435–442
Bennici A, Tani C (2009) Ultrastructural effects of salinity in Nicotiana bigelovii var. bigelovii callus cells and Allium cepa roots. Caryologia 62(2):124–133
Benson EE (2000) In vitro plant recalcitrance: an introduction. In Vitro Cell Dev Biol Plant 36:141–148
Bewick V, Cheek L, Ball J (2004) Statistics review 9: one-way analysis of variance. Crit Care 8(2):130–136
Bi YH, Chen WL, Zhang WN, Zhou Q, Yun LJ, Xing D (2009) Production of reactive oxygen species, impairment of photosynthetic function and dynamic changes in mitochondria are early events in cadmium-induced cell death in Arabidopsis thaliana. Biol Cell 101:629–643
Bian W, Barsan C, Egea I, Purgatto E, Chervin C, Zouine M, Latché A, Bouzayen M, Pech JC (2011) Metabolic and molecular events occurring during chromoplast biogenesis. J Bot. Article ID 289859, 13 pp
Bobák M, Šamaj J, Pretová A, Blehová A, Hlinková E, Ovecka M, Hlavacka A, Kutarnová Z (2004) The histological analysis of indirect somatic embryogenesis on Drosera spathulata Labill. Acta Physiol Plant 26(3):353–361
Boguszewska D, Zagdańska B (2012) ROS as signaling molecules and enzymes of plant response to unfavorable environmental conditions. In: Lushchak W, Semchyshyn HM (eds) Oxidative stress-molecular mechanisms and biological effects. InTech, Rijeka, pp 341–362
Bréhélin C, Kessler F, van Wijk KJ (2007) Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci 12:260–266
Brillouet JM, Romieu C, Schoefs B, Solymosi K, Cheynier V, Fulcrand H, Verdeil JL, Conéjéro G (2013) The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Ann Bot 112:1003–1014
Cassells AC, Curry RF (2001) Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture: implications for micropropagators and genetic engineers. Plant Cell Tissue Org Cult 64:145–157
Chang C, Chang WC (1998) Plant regeneration from callus culture of Cymbidium ensifolium var. misericors. Plant Cell Rep 17:251–255
Chapman KD, Dyer JM, Mullen RT (2012) Biogenesis and functions of lipid droplets in plants. J Lipid Res 53(2):215–226
Chugh S, Guha S, Rao IU (2009) Micropropagation of orchids: a review on the potential of different explants. Sci Hortic 122:507–520
Dai J (1993) Postharvest leaf blackening in Protea neriifolia R. Br. Ph.D. Dissertation, University of Hawaii
de Oliveira Ribeiro L, Paiva LV, Pádua MS, Santos BR, Alves E, Stein VC (2012) Morphological and ultrastructural analysis of various types of banana callus, cv. Prata anã. Acta Sci Agron 34:423–429
de Pinto MC, de Gara L (2004) Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J Exp Bot 55(408):2559–2569
Debnath M, Malik CP, Bisen PS (2006) Micropropagation: a tool for the production of high quality plant-based medicines. Curr Pharm Biotechnol 7:33–49
Dubravina GA, Zaytseva SM, Zagoskina NV (2005) Changes in formation and localization of phenolic compounds in the tissues of European and Canadian yew during dedifferentiation in vitro. Russ J Plant Physiol 52(5):672–678
El-Tayeb MA (2005) Response of barley gains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 45:215–225
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142
Forters AM, Pais MS (2000) Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): histological studies and changes in the starch content. Am J Bot 87(7):971–979
Fu Z, Xu P, He S, Teixeira da Silva JA, Tanaka M (2011) Dynamic changes in enzyme activities and phenolic content during in vitro rooting of tree peony (Paeonia suffruticosa Andr) plantlets. Maejo Int J Sci Technol 5(2):252–265
Gacche RN, Shete AM, Dhole NA, Ghole VS (2006) Reversible inhibition of polyphenol oxidase from apple using L-cysteine. Indian J Chem Technol 13:459–463
George EF (2008) Plant tissue culture procedure - background. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, vol 1, 3rd edn., The BackgroundSpringer, Dordrecht, pp 1–28
Ghozlène I, Mohammed-Réda D, Nedjoud G, Houria B, Ali C (2013) Oxidative stress, chlorophyll content and ROS production and localization in Triticum durum seed. Ann Biol Res 4(5):11–15
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gunawardena AHLAN, Sault K, Donnelly P, Greenwood JS, Dengler NG (2005) Programmed cell death and leaf morphogenesis in Monstera obliqua (Araceae). Planta 221:607–618
Gunawardena AHLAN, Greenwood JS, Dengler NG (2007) Cell wall degradation and modification during programmed cell death in lace plant, Aponogeton madagascariensis (Aponogetonaceae). Am J Bot 94(7):1116–1128
Hazubska-Przybył T, Bojarczuk K, Guzicka M (2008) Structure of embryogenic tissues and accumulation of storage materials in somatic embryos of Picea abies and P. omorika. Dendrobiology 60:19–28
He Y, Guo X, Lu R, Niu B, Pasapula V, Hou P, Cai F, Xu Y, Chen F (2009) Changes in morphology and biochemical indices in browning callus from Jatropha curcas hypocotyls. Plant Cell Tissue Org Cult 98:11–17
Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis DI, Petersen NHT, Mattsson O, Jørgensen LB, Jones JDG, Mundy J, Petersen M (2009) Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell 137:773–783
Hossain MM, Kant R, Van PT, Winarto B, Zeng S, Teixeira da Silva JA (2013) The application of biotechnology to orchids. Crit Rev Plant Sci 32(2):69–139
Hu J, Baker A, Bartel B, Linka N, Mullen RT, Reumann S, Zolman BK (2012) Plant peroxisomes: biogenesis and function. Plant Cell 24:2279–2303
Huang LC, Lee YL, Huang BL, Kuo CI, Shaw JF (2002) High polyphenol oxidase activity and low titratable acidity in browning bamboo tissue culture. In Vitro Cell Dev Biol Plant 38:358–365
Jones AMP, Saxena PK (2013) Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: a novel approach to reduce oxidative browning in plant tissue culture. PLoS ONE 8(10):e76802
Khosravi AR, Kadir MA, Kazemin SB, Zaman FQ, de Silva AE (2008) Establishment of a plant regeneration system from callus of Dendrobium cv. Serdang beauty. Afr J Biotechnol 7(22):4093–4099
Kratsch HA, Wise RR (2000) The ultrastructure of chilling stress. Plant, Cell Environ 23:337–350
Laukkanen H, Rautiainen L, Taulavuori E, Hohtola A (2000) Changes in cellular structures and enzymatic activities during browning of Scots pine callus derived from mature buds. Tree Physiol 20:467–475
Lee SH, Singh AP, Chung GC, Kim YS, Kong IB (2002) Chilling root temperature causes rapid ultrastructural changes in cortical cells of cucumber (Cucumis sativus L.) root tips. J Exp Bot 53:2225–2237
Leng P, Su S, Wei F, Yu F, Duan Y (2009) Correlation between browning, total phenolic content, polyphenol oxidase and several antioxidation enzymes during pistachio tissue culture. Acta Hort 829:127–132
Leong TM, Wee YC (2013) Observations of pollination in the pigeon orchid, Dendrobium crumenatum Swartz (Orchidaceae) in Singapore. Nat Singap 6:91–96
Ling ACK, Yap CP, Shaib JM, Vilasini P (2007) Induction and morphogenesis of Phalaenopsis callus. J Trop Agric Food Sci 35(1):147–152
Liu FP, Chen LX (2010) Redox dynamics during embryogenic callus induction of Phalaenopsis spp. J Wuhan Bot Res 28(6):737–743
Marín-Méndez W, Sanchéz-Chacón E, Gatica-Arias AM, Ramírez-Fonseca P, Freer-Bustamante E, Valdez-Melara M (2009) Ultrastructure and histology of organogenesis induced from shoot tips of maize (Zea mays, Poaceae). Rev Biol Trop 57(1):129–139
Matile PH, Moor H (1968) Vacuolation: origin and development of the lysosomal apparatus in root-tip cells. Planta 80:159–175
Mauseth JD (2014) Botany: an introduction to plant biology, 5th edn. Jones & Bartlett Learning, Burlington
Mayer AM (2006) Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry 67:2318–2331
Meesawat U, Kanchanapoom K (2002) In vitro plant regeneration through embryogenesis and organogenesis from callus culture of pigeon orchid (Dendrobium crumenatum Sw.). Thammasart Int J Sci Tech 7(2):9–17
Meesawat U, Kanchanapoom K (2007) Understanding the flowering behavior of pigeon orchid (Dendrobium crumenatum Swartz). Orchid Sci Biotech 1:6–14
Misra P, Toppo DD, Gupta N, Chakrabarty D, Tuli R (2010) Effect of antioxidants and associate changes in antioxidant enzymes in controlling browning and necrosis of proliferating shoots of elite Jatropha curcas L. Biomass Bioenerg 34:1861–1869
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mondal T, Aditya S, Banerjee N (2013) In vitro axillary shoot regeneration and direct protocorm-like body induction from axenic shoot tips of Doritis pulcherrima Lindl. Plant Tissue Cult Biotechnol 23(2):251–261
Mueller WC, Greenwood AD (1978) The ultrastructure of phenolic-storing cells fixed with caffeine. J Exp Bot 29(110):757–764
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Najafi S, Jamei R (2014) Effect of silver nanoparticles and Pb(NO3)2 on the yield and chemical composition of mung bean (Vigna radiata). J Stress Physiol Biochem 10(1):316–325
Naz S, Ali A, Iqbal J (2008) Phenolic content in vitro cultures of chickpea (Cicer arietinum L) during callogenesis and organogenesis. Pak J Bot 40(6):2525–2539
North JJ, Ndakidemi PA, Laubscher CP (2012) Effects of antioxidants, plant growth regulators and wounding on phenolic compound excretion during micropropagation of Strelitzia reginae. Int J Phys Sci 7(4):638–646
Oren-Shamir M (2009) Does anthocyanin degradation play a significant role in determining pigment concentration in plants? Plant Sci 177:310–316
Palama TL, Menard P, Fock I, Choi YH, Bourdon E, Govinden-Soulange J, Bahut M, Payet B, Verpoorte R, Kodja H (2010) Shoot differentiation from protocorm callus cultures of Vanilla planifolia (Orchidaceae): proteomic and metabolic responses at early stage. BMC Plant Biol 10(82):1–18
Park SY, Shin KS, Paek KY (2006) Increased ethylene and decreased phenolic compound stimulate somatic embryo regeneration in leaf thin section cultures of Doritaenopsis hybrid. J Plant Biol 49(5):358–363
Peng H, Zhang J (2009) Plant genomic DNA methylation in response to stresses: potential applications and challenges in plant breeding. Prog Nat Sci 19:1037–1045
Pihakaski-Maunsbach K, Brauner Nygaard K, Jensen KH, Rasmussen O (1993) Cellular changes in early development of regenerating thin cell layer-explants of rapeseed analyzed by light and electron microscopy. Physiol Plant 87:167–176
Pitzschke A, Djamei A, Bitton F, Hirt H (2009) A major role of the MEKK1–MKK1/2–MPK4 pathway in ROS signalling. Mol Plant 2(1):120–137
Ramel F, Birtic S, Cuiné S, Triantaphylidès C, Ravanat JL, Havaux M (2012) Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 158:1267–1278
Reynolds S (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Ruzin S (1999) Plant microtechnique and microscopy. Oxford University Press, New York
Sandalio LM, Rodríguez-Serrano M, Romero-Puertas MC, del Río LA (2013) Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. In: del Río LA (ed) Peroxisomes and their key role in cellular signaling and metabolism. Springer, Dordrecht, pp 231–255
Santiago LJM, Louro RP, de Oliveira DE (2000) Compartmentation of phenolic compounds and phenylalanine ammonia-lyase in leaves of Phyllanthus tenellus Roxb. and their induction by copper sulphate. Ann Bot 86:1023–1032
Sapers GM, Hicks KB, Miller RL (2002) Antibrowning agents. In: Brane AL, Davidson PM, Salminen S, Thorngate JH III (eds) Food additives, 2nd edn. Marcel Dekker, New York, pp 543–561
Sevengor S, Yasar F, Kusvuran S, Ellialtioglu S (2011) The effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidative enzymes of pumpkin seedling. Afr J Agric Res 6(21):4920–4924
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. Article ID 217037, 26 pp
Shi Q (2011) Studies on preventive technique and browning mechanism in the tissue culture of Cymbidium longibracteatum ‘Cui-xian’ flower stalks. M.Sc. Dissertation, Sichuan Agricultural University
Singh A, Prasad R (2009) Salt stress effects growth and cell wall bound enzymes in Arachis hypogaea L. seedlings. Int J Integr. Biol 7(2):117–123
Speranza A, Ferri P, Battistelli M, Falcieri E, Crinelli R, Scoccianti V (2007) Both trivalent and hexavalent chromium strongly alter in vitro germination and ultrastructure of kiwifruit pollen. Chemosphere 66(7):1165–1174
Stefanowska M, Kuraś M, Kacperska A (2002) Low temperature-induced modifications in cell ultrastructure and localization of phenolics in water oilseed rape (Brassica napus L. var. oleifera L.) leaves. Ann Bot 90:637–645
Stein VC, Paiva R, Vargas DP, Soares FP, Alves E, Nogueira GF (2010) Ultrastructural calli analysis of Inga vera Willd. subsp. Affinis (DC.) T.D. Penn Rev Árvore 34:789–796
Steinmacher DA, Guerra MP, Saare-Surminski K, Lieberei R (2011) A temporary immersion system improves in vitro regeneration of peach palm through secondary somatic embryogenesis. Ann Bot 108:1463–1475
Stewart SL, Zettler LW (2002) Symbiotic germination of three semi-aquatic rein orchids (Habenaria repens, H. quinquiseta, H. macroceratitis) from Florida. Aquat Bot 72:25–35
Sunkar R (2010) Plant stress tolerance methods and protocols. Humana Press, New York
Tang W, Newton RJ (2004) Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.). Plant Sci 167:621–628
Teixeira da Silva JA (2012) Callus, calluses or calli: multiple plurals? Asian Australasian J Plant Sci Biotechnol 6(Special issue 1):125–126
Teixeira da Silva JA, Tanaka M (2006) Multiple regeneration pathways via thin cell layers in hybrid Cymbidium (Orchidaceae). J Plant Growth Reg 25(3):203–210
Teixeira da Silva JA, Dobránszki J (2013) How timing of sampling can affect the outcome of the quantitative assessment of plant organogenesis. Sci Hortic 159:59–66
Terzi R, Saruhan Güler N, Kutlu Çalişkan N, Kadioğlu A (2013) Lignification response for rolled leaves of Ctenanthe setosa under long-term drought stress. Turk J Biol 37:614–619
Toivonen PMA, Brummell DA (2008) Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol Technol 48:1–14
Tokuhara K, Mill M (2001) Induction of embryogenic callus and cell suspension culture from shoot tips excised from flower stalk buds of Phalaenopsis (Orchidaceae). In Vitro Cell Dev Biol Plant 37:457–461
Tripathy BC, Oelmüller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7(12):1621–1633
Vacin E, Went F (1949) Some pH changes in nutrient solution. Bot Gaz 110:605–613
Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ (2007) Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci 12(6):245–252
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang W, Messing J (2012) Analysis of ADP-glucose pyrophosphorylase expression during turion formation induced by abscisic acid in Spirodela polyrhiza (greater duckweed). BMC Plant Biol 12(5):1–14
Wang JB, Wang XS, Jin ZQ (2010) Enzymatic browning of postharvest litchi: a review. Acta Hortic 863:613–618
Wang XD, Nolan KE, Irwanto RR, Sheahan MB, Rose RJ (2011) Ontogeny of embryogenic callus in Medicago truncatula: the fate of the pluripotent and totipotent stem cells. Ann Bot 107:599–609
Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K (2012) Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics J 5(2):60–67
Wi SG, Chung BY, Kim JH, Baek MH, Yan DH, Lee JW, Kim JS (2005) Ultrastructural changes of cell organelles in Arabidopsis stems after gamma irradiation. J Plant Biol 48(2):195–200
Wu Z, Chen LJ, Long YJ (2009) Analysis of ultrastructure and reactive oxygen species of hyperhydric garlic (Allium sativum L.) shoots. In Vitro Cell Dev Biol Plant 45:483–490
Xu CJ, Li L (2006) Changes of total phenol content and the activities of PPO, POD and PAL during the browning in Phalaenopsis explant in vitro. Acta Hortic Sin 33(3):671–674 (in Chinese with English abstract)
Xu CJ, Li L, Li H, Zhang MG (2005) Preliminary studies on the elements of browning and the changes in cellular texture of leaf explant browning in Phalaenopsis. Acta Hortic Sin 32(6):1111–1113 (in Chinese with English abstract)
Xu CJ, Tan RF, Chen DY, Lai YY, Li L (2010) Ultrastructure and distribution of phenol in Phalaenopsis browning leaf explants. North Hortic 21:90–92 (in Chinese with English abstract)
Yamauchi N, Funamoto Y, Shigyo M (2004) Peroxidase-mediated chlorophyll degradation in horticultural crops. Phytochem Rev 3:221–228
Yang L, Han R, Sun Y (2013) Effects of exogenous nitric oxide on wheat exposed to enhanced ultraviolet-B radiation. Am J Plant Sci 4:1285–1290
Yin F, Ge H, Peng K, Zhao L, Zhou Y, Li Q (2006) The influence of phenols on tissue browning of Phalaenopsis. Acta Hortic Sin 33(5):1137–1140 (in Chinese with English abstract)
Yingsanga P, Srilaonga V, Kanlayanarat S, Noichindab S, McGlassonc WB (2008) Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol Technol 50:164–168
Yoruk R, Marshall MR (2003) Physicochemical properties and function of polyphenol oxidase: a review. J Food Biochem 27:361–422
Yoshinaga K, Arimura SI, Niwa Y, Tsutsumi N, Uchimiya H, Kawai-Yamada M (2005) Mitochondrial behaviour in the early stages of ROS stress leading to cell death in Arabidopsis thaliana. Ann Bot 96:337–342
Zenkteler E, Kwaśna H (2007) Pre-treatment of Dryopteris cristata (L.). A Gray rhizome as a method of elimination of contaminants and explant browning micropropagation. Biodivers Res Conserv 5(8):81–86
Zhang X, Ervin EH, Schmidt RE (2005) The role of leaf pigment and antioxidant levels in UV-B resistance of dark- and light-green Kentucky bluegrass cultivars. J Am Soc Hortic Sci 130(6):836–841
Zhao P, Wu F, Feng FS, Wang WJ (2008) Protocorm-like body (PLB) formation and plant regeneration from the callus culture of Dendrobium candidum Wall ex Lindl. In Vitro Cell Dev Biol Plant 44:178–185
Zhao Y, Yang SH, Ge WY, Li QX, Chen HX, Ge H (2010) The metabolism of phenolics and reactive oxygen species in relation to the explant browning differences among the varieties of Phalaenopsis during the tissue culture. Acta Hortic Sin 37:963–970 (in Chinese with English abstract)
Zhou W, Tan R, Xu C, Lai Y, Chen D, Li L (2009) Gibberellic acid inhibits browning, enzyme activity and gene expression of phenylalanine ammonia-lyase in Phalaenopsis leaf explants. Genes Genom Genomics 3:68–71
Acknowledgments
This work was supported by the Department of Biology at Faculty of Science, Graduate School of Prince of Songkla University and by a grant from Science Achievement Scholarship of Thailand (SAST) (28/2554). We thank Dr. Brian Hodgson from the Faculty of Pharmaceutical Science, Prince of Songkla University, for assistance with the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaewubon, P., Hutadilok-Towatana, N., Teixeira da Silva, J.A. et al. Ultrastructural and biochemical alterations during browning of pigeon orchid (Dendrobium crumenatum Swartz) callus. Plant Cell Tiss Organ Cult 121, 53–69 (2015). https://doi.org/10.1007/s11240-014-0678-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0678-y