Abstract
Behçet syndrome (BS) is a unique type of vasculitis that affects veins and arteries of all sizes, leading to recurrent vascular events, mostly venous thrombosis. The prevalence of venous thromboembolism in BS patients ranges between 15 and 40%. Thrombosis is usually an early manifestation leading to diagnosis of BS in up to 40% of patients. BS is per se a model of inflammation-induced thrombosis. The primary autoimmune response activates lymphocytes that in turn produce a cytokine cascade that activates neutrophils, which modify the secondary structure of fibrinogen making it less susceptible to plasmin-induced lysis. This leads to endothelial dysfunction, platelet activation and overexpression of tissue factor leading to inflammatory thrombi, usually attached to the wall. The pathogenesis of thrombosis is especially relevant to direct the specific treatment, that is based on immunosuppression rather than anticoagulation. Superficial vein thrombosis (SVT) and deep vein thrombosis (DVT) are the most common form of thrombosis in BS, but thrombosis in atypical sites (cava vein, suprahepatic veins, intracardiac thrombus) and arterial involvement can also occur. We assessed the latest update of the European League Against Rheumatism recommendations for the management of BS. Vascular Behçet treatment is usually based of immunosuppressants, and the role of anticoagulation remains controversial. The use of interventional and surgical procedures should be carefully evaluated, due to the risk of triggering a vascular pathergy phenomenon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
The importance of vascular involvement lies on its high frequency among BS patients (15-40%) and high rates of recurrences (30–50%).
-
Vascular involvement is the main predictor of morbi-mortality in BS.
-
Vascular involvement is an early manifestation of the disease, emerging frequently before the diagnosis criteria have been fulfilled.
-
Deep vein thrombosis should be treated with glucocorticoids and immunosuppressants in BS patients. The role of anticoagulation remains controversial.
Introduction
Behçet’s syndrome (BS) is a complex condition of unknown etiology that generally affects mucosa and skin but. However, the eyes, joints, blood vessels, central nervous system and gastrointestinal tract might also be affected [1]. BS has a peculiar geographic distribution in Mediterranean countries (Turkey has the highest prevalence, up to 42 per 10,000), Middle-East and Far-East [1]. The onset of BS usually occurs in third decade and both genders are equally affected, with more severe course among men [1,2,3].
Since a syndrome describes a collection of signs and symptoms that usually occur together and might not always have a definite cause, Behçet should be considered a syndrome rather than a disease [1, 4]. Based on the tendency of certain features to be associated, several phenotypes (or “clusters”) of BS might be distinguished [1, 5, 6], including skin-mucosa involvement alone, joint involvement, vascular involvement, eye involvement and parenchymal neurological involvement [1].
BS is a multifactorial condition with an unknown etiology. Previously considered an autoimmune disorder, BS shows no sex differences is not usually related with other autoimmune conditions. It seems reasonable to consider BS a polygenic autoinflammatory disease [6]. Pro-inflammatory Il-1β levels are increased in patients with active BS; also, some variants of the Mediterranean Fever gene and genes encoding Toll-like receptors have been described [6]. An association with the Human Leukocyte Antigen (HLA) B51 has been described, and it is considered a risk factor present in 60% of cases of BS, suggesting a genetic predisposition in some cases [6, 7]. However, its presence alone cannot explain the pathophysiology of BS [6]. This led to the concept “MHC-opathy” [6, 8], which could explain some clusters of BS, but not all the spectrum of the syndrome. Classically, the prevalence of BS has been higher in the Silk Route, but due to migration it spread to other countries [6]. In that way, environmental factors might modify its clinical expression, due to environment-gene interaction [6]. Epigenetics have been proved to be important in BS, since some methylation patterns might be useful as biomarkers or therapeutical targets. The role of epigenetics in the geographical and environmental differences of BS needs to be clarified [6].
Diagnosis of BS is clinical and there are no laboratory, histological or imaging tests that might help [1]. Clinical criteria for the diagnosis have been established, including oral and genital apthosis, ocular lesions, skin lesions, neurological and vascular manifestations. The course of the disease is characterized by frequent relapses and spontaneous remissions, which are usually more active during the first years of BS and tend to decrease over time [1]. BS can cause important morbidity, including physical disability, cognitive impairment and blindness; besides, an increased mortality has been described, associated with vascular involvement [2].
Vascular tree involvement in BS occurs in a subgroup of patients who suffer from recurrent inflammatory thrombosis involving veins and, more rarely, arteries. Specific diagnostic and management strategies might be required in these patients [9]. Of note, vascular manifestations are the main predictor of mortality and morbidity in BS [10].
Epidemiology of vascular involvement in BS
Around 20–40% of venous involvement in BS patients has been reported [3], being more frequent in men [2], with a 14-fold increased risk of venous thrombosis [11]. Venous thrombosis in BS presents mainly as deep vein thrombosis (DVT) of lower extremities, but other vascular territories such as cerebral venous sinus, pulmonary artery and vena cava might also be affected [2, 7]. On the other hand, arterial involvement, although affecting only 3–5% of patients, represents a unique feature of BS, with aneurysms affecting peripheral, visceral and pulmonary territories [9].
Histological features and pathophysiology of vascular involvement in BS
BS is a unique vasculitis since the entire vascular tree might be compromised, including both veins and arteries of all sizes [12]. The vascular involvement includes neutrophil and lymphocyte infiltrates predominantly around the vessels, without granulomatous inflammatory lesions [9]. Fibrous thickening in all layers of the vessel wall and aneurysm formation (particularly in the pulmonary arteries) are often seen in biopsies of patients with vascular affectation [13], and no increased risk of atherosclerosis compared to other inflammatory diseases has been reported [14].
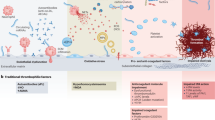
The pathogenesis of vascular involvement (Fig. 1) consists of inflammatory thrombi formation that is usually attached to the inflamed vessel wall, a phenomenon also known as “thromboinflammation” [2]. Correlation between inflammatory response and thrombosis is the result of endothelial dysfunction, tissue factor overexpression and platelet activation mediated by cytokine production (Th1 phenotype: TNF (tumor necrosis factor) α, IL (interleukin)1, IL-6, and Th17 phenotype: IL-17), or by molecules that activate neutrophils chemotaxis (CXCL [chemokine ligand] 8 and G-CSF [granulocyte colony-stimulating factor]) and by the interaction of fibrinogen, thrombin, factor Xa and factor VIIa with immune cells through integrins, Toll like receptors and PARs (protease-activated receptor) 1,2,3,4 [9]. Neutrophils have an important role in the pathogenesis of thrombosis, through NADPH oxidase (specifically NOX2), mediated by an excess of reactive oxygen species (ROS) that modify the secondary structure of fibrinogen making it less susceptible to plasmin-induced lysis [9, 15]. Programmed neutrophil death releases granules or structures that kill extracellular microorganisms such as adenylated chromatin, also called Neutrophils Extracellular Traps (NETs). NETs are implicated in maintaining inflammation and causing vascular damage seen in BS [16]. These findings suggest that BS is per se a model of inflammation-induced thrombosis [17].
Pathophysiology of vascular involvement in Behçet’s syndrome. HSP heat shock protein; TNF-α tumor necrosis factor alpha; INF-ɣ interferon gamma; IL-1 interleukin-1; IL-6 interleukin-6; IL-17 interleukin-17; CXCL-8 chemokine ligand 8; G-CSF granulocyte colony-stimulating factor; NOX2 NADPH oxidase 2; NETs neutrophil extracellular traps
Venous thrombosis
Vascular involvement includes venous and arterial territory. DVT and superficial venous thrombosis (SVT) of lower limbs are the most frequent manifestations of venous involvement [9]. VTE typically affects males in the fourth decade of life and it is usually an early manifestation (5 years within the disease onset) [2] leading to diagnosis of BS in up to 40% of patients [1, 7]. Erythema nodosum, fever and constitutional symptoms are associated with a higher risk of VTE [7]. Approximately 30–50% of patients with vascular BS have recurrent vascular episodes, with a mean of 1.1–2.2 episodes per patient [7, 18,19,20].
There are no tools to identify BS patients at higher risk for venous thrombosis. In a recent study [21], venous wall thickness (VWT) was significantly higher in BS patients compared to ankylosing spondylitis patients and healthy controls, and the measures were higher in BS patients with vascular involvement. Seyahi et al. [22] demonstrated that VWT is increased in BS, with venous wall inflammation being a key feature. Therefore, VWT measurement might be useful to identify patients (mainly young males) presenting with a first idiopathic episode of VTE who are at risk of developing BS.
Here, we review both the typical venous involvement in BS (superficial and deep venous thrombosis) and the thrombosis in atypical sites. Figure 2 and Table 1 summarize the management of vascular involvement in BS.
Treatment of venous thromboembolism in atypical sites and arterial involvement in Behçet’s syndrome [23]. IFN interferon; CYP cyclophosphamide; AZA azathioprine; CyA cyclosporine A; MTX methothrexate; Thal thalidomide; Anti-TNF anti-tumor necrosis factor; EULAR European League Against Rheumatism
Superficial and deep vein thrombosis
SVT and DVT are the most common vascular manifestation in BS, accounting for up to 70% of vascular Behçet [9]. SVT and DVT can affect superior or, most commonly, lower limbs. Long-term complications are frequent, leading to moderate or severe post thrombotic syndrome (PTS) in up to 25%, especially in patients with recurrent DVT [7, 9, 23, 24]. If anticoagulation is initiated in BS patients, a thoracic computed tomography should be performed to rule out pulmonary artery aneurysms due to the high risk of bleeding [23, 25].
In the latest update of the European League Against Rheumatism (EULAR), all the recommendations regarding vascular Behçet (acute DVT, refractory venous thrombosis and arterial involvement) were based in scarce evidence (level of evidence III, strength of recommendation C) [23]. DVT in BS should be treated with glucocorticoids and immunosuppressants (IS) as azathioprine, cyclophosphamide or cyclosporine A, with no data to mandate the preference of one IS agent over the others [23].
DVT in BS is the result of inflammation of the vessel wall rather than hypercoagulability, which questions the role of anticoagulation in these patients [23]. A meta-analysis of three retrospective studies analyzed the efficacy of IS and/or anticoagulation for preventing DVT recurrences [9, 19, 26, 27]. The risk of VTE recurrence was higher in patients treated with anticoagulation alone compared to those treated with IS plus anticoagulation and compared to those with IS alone. Also, IS agents have shown a protective role for the development of a first VTE episode in BS patients [7]. In addition, a retrospective study reported good results with the use of combined treatment: corticosteroids, cyclosporine (CYP), azathioprine (AZA), and anticoagulation [28]. In these studies, all patients were treated with vitamin K antagonists, and there is no evidence regarding the role of direct oral anticoagulants in the treatment of venous thrombosis in BS. On the other hand, a study by Seyahi et al. [25] showed that the absence of anticoagulation could increase the risk of PTS, although Aksoy et al. could not demonstrate that in another retrospective study [29]. The role of anticoagulation in BS [30] remains an issue of debate, and more studies are required to clarify it.
DVT in BS patients is associated with less recanalization of the thrombi and more collateral circulation [31]. A prospective study [24] showed that the lack of recanalization of the thrombi is a risk factor predicting recurrence of DVT in lower limbs. IS decrease the risk of PTS, and interferon-alpha seems to increase recanalization rate, although it could not be compared to other IS [24, 29].
Monoclonal anti-tumor necrosis factor (anti-TNF) antibodies may be used in BS patients with refractory VTE. Anti-TNF agents showed benefits in arterial and venous involvement in retrospective studies [23, 32,33,34]. Anti-TNF agents could also be effective in BS with vascular involvement refractory to conventional IS and some data suggest they could also decrease the risk of VT recurrence [32]. In a retrospective study, adalimumab was effective inducing remission on venous thrombosis when compared to other IS [34].
To sum up, the mechanisms underlying thrombosis in BS are multifactorial including immune, inflammatory, tissue factor and impaired fibrinolysis. Accordingly, the question of management would not be immune modulation or anticoagulants, but probably a combination therapy for clearly defined periods of time. Thus, the optimal therapeutic strategy in terms of drugs and duration remains to be defined.
Venous thrombosis in atypical sites
Thrombosis in atypical sites, while uncommon, might be characteristic of vascular BS and its associated with a high morbidity and mortality. Figure 2 summarizes the treatment of thrombosis in atypical sites in BS patients.
Superior and inferior vena cava thrombosis
Involvement of the great veins in BS is rare (around 1–17%), affecting the inferior and superior vena cava equally [7, 19, 20, 27, 28, 35, 36]. Besides, BS is the main non-neoplastic cause of vena cava (VC) syndrome [28, 37], and exceptionally might cause VC syndrome by inflammatory thickening of the vascular wall without the presence of thrombus [38].
The EULAR recommendations [23] for the management of VC thrombosis and lower limb DVT are similar. However, cyclophosphamide is reserved for cases of extensive thrombosis or VC involvement, due to potential adverse effects.
Budd–Chiari syndrome
BS is a frequent cause of Budd–Chiari syndrome (BCS) in countries with a high prevalence of BS, reaching up to 10–15% of the causes of BCS [39, 40]. On the other hand, BCS is a rare vascular complication of BS, even among patients of the “vascular Behçet” cluster, affecting about 1–5% of patients [39, 40]. BCS, pulmonary aneurysms, and neuro-behçet constitute the main burden of morbidity and mortality related to BS. BCS in BS may present in two different clinical pictures: acute or symptomatic BCS presents with abdominal pain, ascites, or hepatic failure whose mortality reaches up to 60%; and silent or chronic BCS which is often asymptomatic with efficient collateral formation, showing a better prognosis (10% mortality) [40, 41]. Also, thrombosis of the VC is usually associated [39, 42, 43]. Portal vein is rarely affected [40].
Due to this particularity, the management of BCS in BS is complex [44, 45]. The cornerstone of treatment is based on immunomodulatory therapy including corticosteroids, immunosuppressants (cyclophosphamide, cyclosporine-A or azathioprine), and biological therapy (infliximab) (level of recommendation III). Anticoagulation is still a matter of debate. On the other hand, interventional therapy is reserved for the chronic form, and it should be avoided in the acute phase due to the potential vascular inflammatory reactivity [40, 41, 46].
Intracardiac thrombosis
Only 100 cases of intracardiac thrombosis (ICT) in patients with BS have been reported, with a mean age of 30–40 years old and 87% males. ICT affects mainly the right chambers (right ventricle) with a low relapse rate [47, 48]. Importantly, in the presence of an intracardiac thrombus in the right chambers in a young male patient, BS should always be considered [47, 49]. ICT is usually associated with cardiac, pulmonary, or vascular involvement. Due to its high association, pulmonary aneurysms should be ruled out before considering anticoagulation [23, 47,48,49,50].
Recommendations for the management of ICT in BS patients are based on retrospective studies, and include colchicine [51], corticosteroids [42, 47, 49, 50], interferon-alpha [50], cyclophosphamide [42, 49], cyclosporine-A [42, 49, 50], azathioprine [42, 49], thalidomide [42], and methotrexate [42]. Anticoagulant therapy alone is not recommended; however, positive results have been reported in combination therapy in individualized cases with low hemorrhagic risk [42, 46, 49]. In some refractory cases anti-TNF such as infliximab or adalimumab should be considered [32]. Surgical treatment might be considered in severe and refractory cases despite optimized medical treatment. However, surgery can trigger a vascular pathergy phenomenon consisting of venous thrombosis recurrence and early surgical complications, with high mortality [46, 48]. After treatment, resolution rates of 70–80% have been reported [42, 47,48,49,50].
Central venous thrombosis
Central nervous system (CNS) involvement in BS is rare (around 5–10% of vascular BS) and is more common in young men. Neuro-Behçet (NB) can affect the brain parenchyma, the vascular system, or both [52,53,54]. Simultaneous parenchymal and vascular involvement in the same patient is rare [55]. Central venous sinus thrombosis (CVST) varies according to the series from 2 to 40% of patients with NB [53, 54], and ranges from 0 to 13% in patients with vascular Behçet [7, 19, 20, 27, 36]. Shi et al. found that 52% of vascular-NB patients presented thrombosis in more than one location [55]. Hence, in the setting of CVST, screening for extracranial vascular involvement is strongly recommended [23, 52,53,54].
EULAR recommendations for vascular NB treatment include high doses of corticosteroids with slow tapering. IS are not recommended since no clear benefits have been reported. Furthermore, cyclosporine-A is recommended against in patients with acute or previous NB episode, due to potential neurological impairment [23]. Anticoagulant treatment seems relatively safe in retrospective studies [53,54,55,56].
Arterial involvement
Arterial involvement (AI) in BS represents a unique feature [6]. BS is probably the only chronic inflammatory disease that causes aneurysms that can affect peripheral, visceral and pulmonary arteries [9]. Aneurysms are the most common type of arterial lesion in BS but occlusions and stenosis can also occur [57, 58]. One-third of AI are multiple [57, 58]. Pathergy phenomenon, arterial stenosis or occlusion and arterial thrombosis are risk factors associated with aneurysm formation [58].
AI affects 1–7% of BS patients [9, 57] and is more common in males in the fourth decade of life [9, 57, 58]. It can be the first manifestation of the disease in some patients although it frequently appears few years after the diagnosis [57, 58]. AI can be consequence of traumatic injury including surgery, arterial puncture, arteriography or even lung biopsy [57, 58]. Although fever is an uncommon symptom of BS, it is associated with AI [7, 57, 59], especially in aneurysm formation [57].
Long-term mortality and morbidity are increased if arterial manifestations are present, especially pulmonary aneurysms [9, 33, 58, 59]. The rate of mortality can reach up to 15% [58]. Hughes–Stovin syndrome, considered a cardiovascular subtype of BS, is defined by the presence of arterial pulmonary aneurysms and peripheral venous thrombosis [1, 9].
The main locations of AI are the aorta, femoral, pulmonary, and iliac arteries [57, 58, 60]. More uncommon locations include digestive, coronary and cerebral arteries [57]. Thoracic aorta and pulmonary arteries involvement have the highest mortality rate (up to 40% of deaths) [57].
IS are the cornerstone of treatment in AI [23]. The EULAR recommendation includes high-dose glucocorticoids and cyclophosphamide. Anti-TNF might be used in refractory cases, as they have shown efficacy in vascular BS in some retrospective studies [61]. Desbois et al. [32] demonstrated that anti-TNF led to remission in 89% of patients previously treated with IS and high dose glucocorticoids. The discontinuation of anti-TNF agents after initiated is still an unresolved question [61].
Pulmonary artery involvement
Two types of pulmonary artery involvement (PAI) have been described in BS: pulmonary artery aneurysms (PAA) and pulmonary artery thrombosis (PAT) [59]. The frequency of PAA in BS ranges from 1 to 18% and is more common in men [61, 62]. Pulmonary symptoms include hemoptysis, dyspnea, fever, cough or chest pain [59, 62]. By the time of diagnosis of PAI many patients have signs of active BS [59]. PAI is associated with venous thrombosis but not with peripheral artery disease [59, 63].
The first level of treatment of PAA are high-dose glucocorticoids and cyclophosphamide, followed by long-term azathioprine [6, 23, 61, 64]. With this treatment, 70% of patients achieve clinical and radiologic remission, but mortality rate and relapses remain high (26% mortality and 20% relapses during 7-year follow-up) [61]. Etanercept was superior to placebo in suppressing mucocutaneous lesions in the only randomized controlled trial developed with anti-TNF in BS [65], although it was not effective in suppressing the pathergy phenomenon. EULAR recommends the use of anti-TNF agents in refractory PAI cases. Except for life threatening situations, surgery is not recommended and endovascular treatment (embolization) is preferred [23, 61]. Anticoagulation is contraindicated due to high risk of bleeding [23, 25].
Aortic and peripheral involvement
The aorta is the most affected artery, especially abdominal aorta followed by coronary arteries, superficial femoral artery, subclavian artery and popliteal artery [58].
Treatment of aneurysmal lesions consists of glucocorticoids and immunosuppressants (cyclophosphamide) according to EULAR recommendations [23], and this treatment is necessary before intervention to decrease the risk of postoperative complications [23]. The choice of surgical intervention can be made according to aneurysm size and surgeon experience [23]. The risk of rupture is related to aneurysm diameter, but severe spontaneous rupture in BS is unusual [66].
Coronary arteries
Coronary arteries can be affected in BS, reaching up to 10% of all AI cases, with a mean age of 30 ± 11 years [58, 67]. Most patients with coronary artery involvement present with acute coronary syndrome [58]. Lesions can include stenosis, occlusion and pseudoaneurysm, with or without myocardial infarction. Patients with coronary involvement have poor prognosis compared with other patients with AI [67]. Treatment of coronary artery aneurysms agree with the AI medical recommendations and general recommendations of myocardial infarction.
In conclusion, the vascular involvement of Behçet syndrome can affect both veins and arteries of all sizes. Manifestations can include thrombosis and aneurysm formation. Vascular Behçet treatment is based on immunosuppressants and the role of anticoagulation is not clearly established.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Seyahi E (2019) Phenotypes in Behçet’s syndrome. Intern Emerg Med 14(5):677–689
Seyahi E (2016) Behçet’s disease: how to diagnose and treat vascular involvement. Best Pract Res Clin Rheumatol 30(2):279–295
Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V et al (2003) The long-term mortality and morbidity of Behçet syndrome: a 2-decade outcomesurvey of 387 patients followed at a dedicated center. Medicine (Baltimore) 82(1):60–76
Yazici H (2020) Behçet syndrome as a construct. Turk J Med Sci 50(SI-2):1585–1586
Bettiol A, Prisco D, Emmi G (2020) Behçet: the syndrome. Rheumatology (Oxford) 59(Suppl 3):iii101–iii107
Yazici H, Seyahi E, Hatemi G, Yazici Y (2018) Behçet syndrome: a contemporary view. Nat Rev Rheumatol 14(2):107–119
Toledo-Samaniego N, Galeano-Valle F, Pinilla-Llorente B, Del-Toro-Cervera J, Marra A, Proietti M et al (2020) Clinical features and management of venous thromboembolism in patients with Behçet’s syndrome: a single center case-control study. Intern Emerg Med 15(4):635–644
McGonagle D, Aydin SZ, Gül A, Mahr A, Direskeneli H (2015) ‘MHC-I-opathy’—unified concept for spondyloarthritis and Behçet disease. Nat Rev Rheumatol 11:731–740
Emmi G, Bettiol A, Silvestri E, Di Scala G, Becatti M, Fiorillo C, Prisco D (2019) Vascular Behçet’s syndrome: an update. Intern Emerg Med 14(5):645–652
Fei Y, Li X, Lin S, Song X, Wu O, Zhu Y et al (2013) Major vascular involvement in Behçet’s disease: a retrospective study of 796 patients. Clin Rheumatol 32(6):845–852
Tayer-Shifman OE, Seyahi E, Nowatzky J, Ben-Chetrit E (2012) Major vessel thrombosis in Behçet’s disease: the dilemma of anti-coagulant therapy—the approach of rheumatologists from different countries. Clin Exp Rheumatol 30(5):735–740
Kiraz S, Ertenli I, Oztürk MA, Haznedaroğlu IC, Celik I, Calgüneri M (2002) Pathological haemostasis and “prothrombotic state” in Behçet’s disease. Thromb Res 105(2):125–133
Demirkesen C, Oz B, Goksel S (2010) Behçet’s disease: pathology. In: Yazici Y, Yazici H (eds) Behçet’s syndrome, 1st edn. Springer, New York, p 215e42
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F (2013) 2012 revised inter-national Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65(1):1–11
Emmi G, Becatti M, Bettiol A, Hatemi G, Prisco D, Fiorillo C (2019) Behçet’s syndrome as a model of thrombo-infammation: the role of neutrophils. Front Immunol 10:1085
Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11(8):519–531
Becatti M, Emmi G, Bettiol A, Silvestri E, Di Scala G, Taddei N, Prisco D, Fiorillo C (2019) Behçet’s syndrome as a tool to dissect the mechanisms of thrombo-inflammation: clinical and pathogenetic aspects. Clin Exp Immunol 195(3):322–333
Zeidan MJ, Saadoun D, Garrido M, Klatzmann D, Six A, Cacoub P (2016) Behçet’s disease physiopathology: a contemporary review. Autoimmun Highlights 7:4
Alibaz-Oner F, Karadeniz A, Yılmaz S, Balkarlı A, Kimyon G, Yazıcı A et al (2015) Behçet disease with vascular involvement: effects of different therapeutic regimens on the incidence of new relapses. Medicine (Baltimore) 94(6):e494
Tascilar K, Melikoglu M, Ugurlu S, Sut N, Caglar E, Yazici H et al (2014) Vascular involvement in Behçet’s syndrome: a retrospective analysis of associations and the time course. Rheumatology (Oxford) 53(11):2018–2022
Alibaz-Oner F, Ergelen R, Mutis A, Erturk Z, Asadov R, Mumcu G, Ergun T, Direskeneli H (2019) Venous vessel wall thickness in lower extremity is increased in male patients with Behcet’s disease. Clin Rheumatol 38(5):1447–1451
Seyahi E (2020) Venous involvement in inflammatory disorders. Curr Opin Rheumatol 32(1):29–34
Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F et al (2018) 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis 77(6):808–818
Ozguler Y, Hatemi G, Cetinkaya F, Tascilar K, Hamuryudan V, Ugurlu S et al (2020) Clinical course of acute deep vein thrombosis of the legs in Behçet’s syndrome. Rheumatology (Oxford) 59(4):799–806
Seyahi E, Cakmak OS, Tutar B, Arslan C, Dikici AS, Sut N et al (2015) Clinical and ultrasonographic evaluation of lower-extremity vein thrombosis in Behcet syndrome: an observational study. Medicine (Baltimore) 94(44):e1899
Ahn JK, Lee YS, Jeon CH, Koh EM, Cha HS (2008) Treatment of venous thrombosis associated with Behcet’s disease: immunosuppressive therapy alone versus immunosuppressive therapy plus anticoagulation. Clin Rheumatol 27(2):201–205
Desbois AC, Wechsler B, Resche-Rigon M, Piette JC, Huong Dle T, Amoura Z et al (2012) Immunosuppressants reduce venous thrombosis relapse in Behçet’s disease. Arthritis Rheum 64:2753–2760
Hamzaoui A, Fatima J, Thouraya BS, Monia SK, Imed BG, Mounir L et al (2014) Vena cava thrombosis in Behçet’s disease. Anadolu Kardiyol Derg 14:292–293
Aksoy A, Colak S, Yagiz B, Coskun BN, Omma A, Yildiz Y et al (2021) Predictors for the risk and severity of post-thrombotic syndrome in vascular Behçet’s disease. J Vasc Surg Venous Lymphat Disord 9(6):1451–1459
Seyahi E, Yazici H (2016) To anticoagulate or not to anticoagulate vascular thrombosis in Behçet’s syndrome: an enduring question. Clin Exp Rheumatol 34(1 Suppl 95):S3-4
Seyahi E, Gjoni M, Durmaz EŞ, Akbaş S, Sut N, Dikici AS, Mihmanli I, Yazici H (2019) Increased vein wall thickness in Behçet disease. J Vasc Surg Venous Lymphat Disord 7(5):677-684.e2
Desbois AC, Biard L, Addimanda O, Lambert M, Hachulla E, Launay D, Ackermann F, Pérard L, Hot A, Maurier F, Mausservey C, Bernard F, Noel N, Alric L, Mirault T, Cohen F, Boussouar S, Resche-Rigon M, Cacoub P, Saadoun D (2018) Efficacy of anti-TNF alpha in severe and refractory major vessel involvement of Behcet’s disease: a multicenter observational study of 18 patients. Clin Immunol 197:54–59
Lin CH, Luo D, Ma HF, Shen Y, Zou J, Guan JL (2020) Clinical characteristics and factors influencing the prognosis of Behçet’s disease complicated with vascular involvement. Vasa 49(4):309–318
Emmi G, Vitale A, Silvestri E, Boddi M, Becatti M, Fiorillo C, Fabiani C, Frediani B, Emmi L, Di Scala G, Goldoni M, Bettiol A, Vaglio A, Cantarini L, Prisco D (2018) Adalimumab-based treatment versus disease-modifying antirheumatic drugs for venous thrombosis in Behçet’s syndrome: a retrospective study of seventy patients with vascular involvement. Arthritis Rheumatol 70(9):1500–1507
Düzgün N, Ateş A, Aydintuğ OT, Demir O, Olmez U (2006) Characteristics of vascular involvement in Behçet’s disease. Scand J Rheumatol 35:65–68
Wu X, Li G, Huang X, Wang L, Liu W, Zhao Y et al (2014) Behçet’s disease complicated with thrombosis: a report of 93 Chinese cases. Medicine (Baltimore) 93(28):e263
Thomas I, Helmold ME, Nychay S (1992) Behçet’s disease presenting as superior vena cava syndrome. J Am Acad Dermatol 26:863–865
Vandergheynst F, Francois O, Laureys M, Decaux G (2008) Superior vena cava syndrome without thrombosis revealing Behçet’s disease: two cases. Jt Bone Spine 75:359–361
Sakr MA, Reda MA, Ebada HE, Abdelmoaty AS, Hefny ZM, Ibrahim ZH, Aboelmaaty ME (2020) Characteristics and outcome of primary Budd–Chiari syndrome due to Behçet’s syndrome. Clin Res Hepatol Gastroenterol 44(4):503–512
Seyahi E, Caglar E, Ugurlu S, Kantarci F, Hamuryudan V, Sonsuz A et al (2015) An outcome survey of 43 patients with Budd–Chiari syndrome due to Behçet’s syndrome followed up at a single, dedicated center. Semin Arthritis Rheum 44:602–609
Desbois AC, Rautou PE, Biard L, Belmatoug N, Wechsler B, Resche-Rigon M et al (2014) Behcet’s disease in Budd–Chiari syndrome. Orphanet J Rare Dis 9:104
Ben Ghorbel I, Belfeki N, Houman MH (2016) Intracardiac thrombus in Behçet’s disease. Reumatismo 68:148–153
Cansu DU, Temel T, Erturk A, Kasifoglu T, Acu B, Korkmaz C (2016) The long-term outcomes for patients with Budd–Chiari syndrome caused by Behcet’s disease: a case series on the results, from cirrhosis to death. Hepat Mon 16:e32457
Oblitas CM, Galeano-Valle F, Toledo-Samaniego N, Pinilla-Llorente B, Del Toro-Cervera J, Álvarez-Luque A et al (2019) Budd–Chiari syndrome in Behçet’s disease successfully managed with immunosuppressive and anticoagulant therapy: a case report and literature review. Intractable Rare Dis Res 8:60–66. https://doi.org/10.5582/irdr.2018.01128
Oblitas CM, Toledo-Samaniego N, Fernández-Yunquera A, Díaz-Fontenla F, Galeano-Valle F, Del-Toro-Cervera J et al (2020) Chronic Budd–Chiari syndrome in Behçet’s disease successfully managed with transjugular intrahepatic portosystemic shunt: a case report and literature review. Clin J Gastroenterol 13(4):572–578
Galeano-Valle F, Demelo-Rodriguez P, Álvarez-Sala-Walther L, Pinilla-Llorente B, Echenagusia-Boyra MJ, Rodriguez-Abella H et al (2018) Intracardiac thrombosis in Behçet’s Disease successfully treated with immunosuppressive agents: a case of vascular pathergy phenomenon. Intractable Rare Dis Res 7(1):54–57
Aksu T, Tufekcioglu O (2015) Intracardiac thrombus in Behçet’s disease: four new cases and a comprehensive literature review. Rheumatol Int 35:1269–1279
Emmungil H, Yaşar Bilge NŞ, Küçükşahin O, Kılıç L, Okutucu S, Gücenmez S et al (2014) A rare but serious manifestation of Behçet’s disease: intracardiac thrombus in 22 patients. Clin Exp Rheumatol 32:S87-92
Wang H, Guo X, Tian Z, Liu Y, Wang Q, Li M et al (2016) Intracardiac thrombus in patients with Behcet’s disease: clinical correlates, imaging features, and outcome: a retrospective, single-center experience. Clin Rheumatol 35:2501–2507
Zhu YL, Wu QJ, Guo LL, Fang LG, Yan XW, Zhang FC, Zhang X (2012) The clinical characteristics and outcome of intracardiac thrombus and aortic valvular involvement in Behçet’s disease: an analysis of 20 cases. Clin Exp Rheumatol 30(3 Suppl 72):S40–S45
Stack J, Ryan J, McCarthy G (2015) Colchicine: new insights to an old drug. Am J Ther 22:e151–e157
Uluduz D, Midi I, Duman T, Colakoglu S, Tüfekci A, Bakar M et al (2019) Behçet’s disease as a causative factor of cerebral venous sinus thrombosis: subgroup analysis of data from the VENOST study. Rheumatology (Oxford) 58:600–608
Houman MH, Bellakhal S, Ben Salem T, Hamzaoui A, Braham A, Lamloum M et al (2013) Characteristics of neurological manifestations of Behçet’s disease: a retrospective monocentric study in Tunisia. Clin Neurol Neurosurg 115:2015–2018
Toledo-Samaniego N, Galeano-Valle F, Ascanio-Palomares P, González-Martínez B, Valencia-Kruszyna A, Demelo-Rodríguez P (2020) Neurological manifestations of Behçet’s disease: study of 57 patients. Med Clin (Barc) 154(12):488–492 (English, Spanish)
Shi J, Huang X, Li G, Wang L, Liu J, Xu Y et al (2018) Cerebral venous sinus thrombosis in Behçet’s disease: a retrospective case-control study. Clin Rheumatol 37:51–57
Saadoun D, Wechsler B, Resche-Rigon M, Trad S, Le Thi HD, Sbai A et al (2009) Cerebral venous thrombosis in Behçet’s disease. Arthritis Rheum 61:518–526
Saadoun D, Asli B, Wechsler B, Houman H, Geri G, Desseaux K et al (2012) Long-term outcome of arterial lesions in Behçet disease: a series of 101 patients. Medicine (Baltimore) 91(1):18–24
Zhou J, Shi J, Liu J, Sun L, Li L, Li C et al (2019) The clinical features, risk factors, and outcome of aneurysmal lesions in Behcet’s disease. J Immunol Res 2019:9198506
Seyahi E, Melikoglu M, Akman C, Hamuryudan V, Ozer H, Hatemi G et al (2012) Pulmonary artery involvement and associated lung disease in Behçet disease: a series of 47 patients. Medicine (Baltimore) 91(1):35–48
Balcioglu O, Ertugay S, Bozkaya H, Parildar M, Posacioglu H (2015) Endovascular repair and adjunctive immunosuppressive therapy of aortic involvement in Behçet’s disease. Eur J Vasc Endovasc Surg 50(5):593–598
Hamuryudan V, Seyahi E, Ugurlu S, Melikoglu M, Hatemi G et al (2015) Pulmonary artery involvement in Behçet’s syndrome: effects of anti-Tnf treatment. Semin Arthritis Rheum 45(3):369–373
Zhang X, Dai H, Ma Z, Yang Y, Liu Y (2015) Pulmonary involvement in patients with Behçet’s disease: report of 15 cases. Clin Respir J 9(4):414–422
Hamuryudan V, Er T, Seyahi E, Akman C, Tüzün H, Fresko I et al (2004) Pulmonary artery aneurysms in Behçet syndrome. Am J Med 117(11):867–870
Saba D, Saricaoğlu H, Bayram AS, Erdoğan C, Dilek K, Gebitekin C et al (2003) Arterial lesions in Behçet’s disease. Vasa 32(2):75–81
Melikoglu M, Fresko I, Mat C, Ozyazgan Y, Gogus F, Yurdakul S et al (2005) Short-term trial of etanercept in Behçet’s disease: a double blind, placebo controlled study. J Rheumatol 32(1):98–105
Sahutoglu T, Artim Esen B, Aksoy M, Kurtoglu M, Poyanli A, Gul A (2019) Clinical course of abdominal aortic aneurysms in Behçet disease: a retrospective analysis. Rheumatol Int 39(6):1061–1067
Geri G, Wechsler B, Thi Huong DL, Isnard R, Piette JC, Amoura Z et al (2012) Spectrum of cardiac lesions in Behçet disease: a series of 52 patients and review of the literature. Medicine (Baltimore) 91(1):25–34
Dincses E, Esatoglu SN, Fresko I, Melikoglu M, Seyahi E (2019) Outcome of invasive procedures for venous thrombosis in Behçet’s syndrome: case series and systematic literature review. Clin Exp Rheumatol 37 Suppl 121(6):125–131
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toledo-Samaniego, N., Oblitas, C.M., Peñaloza-Martínez, E. et al. Arterial and venous involvement in Behçet’s syndrome: a narrative review. J Thromb Thrombolysis 54, 162–171 (2022). https://doi.org/10.1007/s11239-022-02637-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-022-02637-1